Integrated Analysis of Salmonella Infantis in Chicken Meat: Epidemiological Surveillance, Antibiotic Resistance, and Potential Bioactive Control Agents
Abstract
1. Introduction
2. Materials and Methods
2.1. Salmonella spp. Isolation and Identification
2.1.1. Sample Collection and Isolation
2.1.2. Salmonella spp. Identification
DNA Extraction and Real-Time PCR Assay
2.2. Antimicrobial Susceptibility Test
2.3. Detection of Resistance Genes by PCR
2.4. The Antimicrobial Effects of Selected Natural Bioactive Compounds (Carvacrol, Eugenol, and α-Terpineol) on Salmonella Isolates
2.4.1. Minimum Inhibitory (MIC) and Bactericidal Concentration (MBC) Assay
2.4.2. Agar Well Diffusion Method
2.5. Statistical Analysis
3. Results
3.1. Salmonella spp. Identification
3.2. Determination of Antimicrobial Susceptibility
Determination of Antibiotic Resistance Genes
3.3. The Antimicrobial Effects of Carvacrol, Eugenol, and α-Terpineol on Salmonella Isolates
3.3.1. Minimum Inhibitory (MIC) and Bactericidal Concentration (MBC) Assay Values
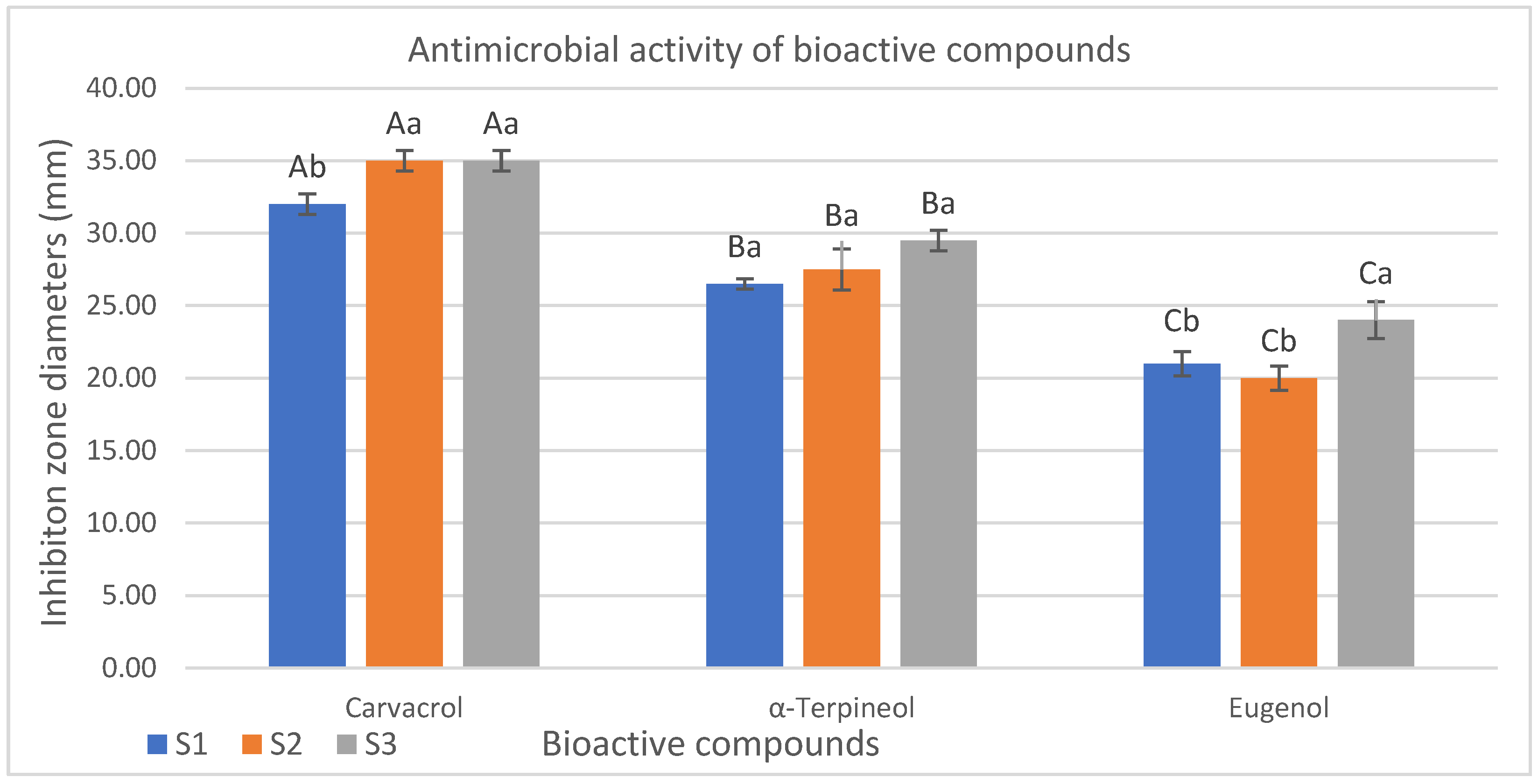
3.3.2. Results of Agar Well Diffusion Method
4. Discussion
4.1. Salmonella Infantis
4.2. Determination of Antimicrobial Susceptibility and Antibiotic Resistance Genes
4.3. Evolution of the Antimicrobial Effects of Carvacrol, Eugenol, and α-Terpineol on Salmonella Isolates
4.3.1. Evolution of Minimum Inhibitory (MIC) and Bactericidal Concentration (MBC) Assay Values
4.3.2. Evolution of Agar Well Diffusion Method Results
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Talukder, H.; Roky, S.A.; Debnath, K.; Sharma, B.; Ahmed, J.; Roy, S. Prevalence and antimicrobial resistance profile of Salmonella isolated from human, animal and environment samples in South Asia: A 10-year Meta-analysis. J. Epidemiol. Glob. Health 2023, 13, 637–652. [Google Scholar] [CrossRef]
- Chanam’e Pinedo, L.; Mughini-Gras, L.; Franz, E.; Hald, T.; Pires, S.M. Sources and trends of human salmonellosis in Europe, 2015–2019: An analysis of outbreak data. Int. J. Food Microbiol. 2022, 379, 109850. [Google Scholar] [CrossRef] [PubMed]
- Papoula-Pereira, R.; Alvseike, O.; Cenci-Goga, B.T.; Grispoldi, L.; Nagel-Alne, G.E.; Ros-Lis, J.V.; Thomas, L.F. Economic evidence for the control of Salmonella in animal-derived food systems: A scoping review. Food Control 2025, 175, 111275. [Google Scholar] [CrossRef]
- Lamichhane, B.; Mawad, A.M.M.; Saleh, M.; Kelley, W.G.; Harrington, P.J.; Lovestad, C.W.; Amezcua, J.; Sarhan, M.M.; El Zowalaty, M.E.; Ramadan, H.; et al. Salmonellosis: An Overview of Epidemiology, Pathogenesis, and Innovative Approaches to Mitigate the Antimicrobial Resistant Infections. Antibiotics 2024, 13, 76. [Google Scholar] [CrossRef]
- Bermudez-Aguirre, D.; Carter, J.; Niemira, B.A. An investigation about the historic global foodborne outbreaks of Salmonella spp. in eggs: From hatcheries to tables. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70202. [Google Scholar] [CrossRef]
- Adley, C.C.; Ryan, M.P. The nature and extent of foodborne disease. In Antimicrobial Food Packaging; Academic Press: New York, NY, USA, 2025; pp. 3–14. [Google Scholar]
- European Food Safety Agency/European Centre for Disease Prevention and Control (EFSA/ECDC). Rapid Outbreak Assessment: Multi- CountryOutbreak of Salmonella Enteritidis Sequence Type (ST)11 Infections Linked to Eggs and Egg Products; Third Update; EFSA/ECDC: Stockholm, Sweden, 2022.
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H.; et al. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- Cawthraw, S.; Wales, A.; Guzinski, J.; Trew, J.; Ring, I.; Huby, T.; Hussaini, A.; Petrovska, L.; Martelli, F. Salmonella Infantis outbreak on six broiler units in Great Britain: Investigation, epidemiology, and control. J. Appl. Microbiol. 2025, 136, lxaf040. [Google Scholar] [CrossRef]
- Nazari Moghadam, M.; Rahimi, E.; Shakerian, A.; Momtaz, H. Prevalence of Salmonella typhimurium and Salmonella Enteritidis isolated from poultry meat: Virulence and antimicrobial-resistant genes. BMC Microbiol. 2023, 23, 168. [Google Scholar] [CrossRef]
- Canning, M.; Birhane, M.G.; Dewey-Mattia, D.; Lawinger, H.; Cote, A.; Gieraltowski, L.; Schwensohn, C.; Tagg, K.A.; Watkins, L.K.F.; Robyn, M.P.; et al. Salmonella outbreaks linked to beef, United States, 2012–2019. J. Food Prot. 2023, 86, 100071. [Google Scholar] [CrossRef] [PubMed]
- Strickland, A.J.; Sampedro, F.; Hedberg, C.W. Quantitative risk assessment of salmonella in ground beef products and the resulting impact of risk mitigation strategies on public health. J. Food Prot. 2023, 86, 100093. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.; Mourão, J.; Peixe, L.; Antunes, P. Non-typhoidal Salmonella in the pig production chain: A comprehensive analysis of its impact on human health. Pathogens 2019, 8, 19. [Google Scholar] [CrossRef] [PubMed]
- Broadway, P.R.; Brooks, J.C.; Mollenkopf, D.F.; Calle, M.A.; Loneragan, G.H.; Miller, M.F.; Carroll, J.A.; Sanchez, N.C.B.; Wittum, T.E. Prevalence and antimicrobial susceptibility of Salmonella Serovars isolated from U.S. retail ground pork. Foodborne Pathog. Dis. 2021, 18, 219–227. [Google Scholar] [CrossRef]
- EFSA. Report of the task force on zoonoses data collection on the analysis of the baseline survey on the prevalence of Salmonella in broiler flocks of Gallus gallus, in the EU, 2005–2006. EFSA J. 2007, 98, 1–85. [Google Scholar]
- Shahada, F.; Chuma, T.; Kosugi, G.; Okamoto, K. Genetic Characteristics of Antimicrobial Resistance of Salmonella Isolated from Chicken in Japan. J. Vet. Med. Sci. 2006, 68, 115–120. [Google Scholar]
- European Food Safety Authority (EFSA). The EU Summary Report on AMR in Zoonotic and Indicator Bacteria from Humans, Animals and Food in 2021. EFSA J. 2023, 21, e07784. [Google Scholar]
- Dishan, A.; Hizlisoy, H.; Onmaz, N.E.; Yildirim, Y.; Gonulalan, Z.; Al, S. Comprehensive analysis of Salmonella in poultry meat and products in Türkiye: Prevalence, antibiotic susceptibility and genomic characterisation. Int. J. Food Sci. Technol. 2024, 59, 3412–3422. [Google Scholar] [CrossRef]
- USDA. Cost Estimates of Foodborne Illnesses. U. S. Department of Agriculture (USDA) Economic Research Service; 2021. Available online: https://www.usda.gov (accessed on 18 August 2025).
- Li, L.; McWhorter, A.; Chousalkar, K. Ensuring Egg Safety: Salmonella Survival, Control, and Virulence in the Supply Chain. Compr. Rev. Food Sci. Food Saf. 2025, 24, e70075. [Google Scholar] [CrossRef]
- Simpson, K.M.J.; Hill-Cawthorne, G.A.; Ward, M.P.; Mor, S.M. Diversity of Salmonella Serotypes From Humans, Food, Domestic Animals and Wildlife in New South Wales, Australia. BMC Infect. Dis. 2018, 18, 623. [Google Scholar] [CrossRef]
- Kumar, G.; Kumar, S.; Jangid, H.; Dutta, J.; Shidiki, A. The rise of non-typhoidal Salmonella: An emerging global public health concern. Front. Microbiol. 2025, 16, 1524287. [Google Scholar] [CrossRef]
- Osivand, Z.; Rahimi, E.; Shakerian, A.; Khamesipour, F. Prevalence, Antibiotic Resistance, Virulence and Antimicrobial Resistance Gene Profiles of Salmonella Species Recovered from Retail Beef and Poultry Processing Environments. BMC Microbiol. 2025, 25, 174. [Google Scholar] [CrossRef]
- Rodrigues, G.L.; Panzenhagen, P.; Ferrari, R.G.; Paschoalin, V.M.F.; Conte-Junior, C.A. Antimicrobial resistance in nontyphoidal Salmonella isolates from human and swine sources in Brazil: A systematic review of the past three decades. Microb. Drug Resist. 2020, 26, 1260–1270. [Google Scholar] [CrossRef]
- Chiou, C.S.; Hong, Y.P.; Wang, Y.W.; Chen, B.H.; Teng, R.H.; Song, H.Y.; Liao, Y.S. Antimicrobial resistance and mechanisms of azithromycin resistance in nontyphoidal Salmonella isolates in Taiwan, 2017 to 2018. Microbiol. Spectr. 2023, 11, e03364-22. [Google Scholar] [CrossRef] [PubMed]
- Nuanmuang, N.; Leekitcharoenphon, P.; Njage, P.M.K.; Gmeiner, A.; Aarestrup, F.M. An overview of antimicrobial resistance profiles of publicly available Salmonella genomes with sufficient quality and metadata. Foodborne Pathog. Dis. 2023, 20, 405–413. [Google Scholar] [CrossRef]
- Wang, C.-Y.; Chen, Y.-W.; Hou, C.-Y. Antioxidant and Antibacterial Activity of Seven Predominant Terpenoids. Int. J. Food Prop. 2019, 22, 230–238. [Google Scholar] [CrossRef]
- Yazgan, H.; Kuley, E.; Özogul, Y. Investigation of Bioactive Compounds and Antimicrobial Properties of Aqueous Garlic Extracts. Biomass Convers. Biorefin. 2024, 14, 16673–16680. [Google Scholar] [CrossRef]
- Imran, M.; Aslam, M.; Alsagaby, S.A.; Saeed, F.; Ahmad, I.; Afzaal, M.; Arshad, M.U.; Abdelgawad, M.A.; El-Ghorab, A.H.; Khames, A.; et al. Therapeutic application of carvacrol: A comprehensive review. Food Sci. Nutr. 2022, 10, 3544–3561. [Google Scholar] [CrossRef]
- Mączka, W.; Twardawska, M.; Grabarczyk, M.; Wińska, K. Carvacrol—A natural phenolic compound with antimicrobial properties. Antibiotics 2023, 12, 824. [Google Scholar] [CrossRef]
- Ozogul, Y.; Karsli, G.T.; Yazgan, H.; Kuley, E.; Oztop, H.M.; Ozogul, F.; Esatbeyoglu, T. Enhanced pathogen control through thymol and carvacrol nanoemulsions: A microfluidization approach. Food Bioprocess Technol. 2025, 18, 5377–5387. [Google Scholar] [CrossRef]
- Ulanowska, M.; Olas, B. Biological Properties and Prospects for the Application of Eugenol—A Review. Int. J. Mol. Sci. 2021, 22, 3671. [Google Scholar] [CrossRef]
- de Oliveira, M.G.; Marques, R.B.; de Santana, M.F.; Santos, A.B.; Brito, F.A.; Barreto, E.O.; De Sousa, D.P.; Almeida, F.R.C.; Badauê-Passos, D., Jr.; Antoniolli, Â.; et al. α-Terpineol Reduces Mechanical Hypernociception and Inflammatory Response. Basic Clin. Pharmacol. Toxicol. 2012, 111, 120–125. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Quintans, L., Jr.; de Almeida, R.N. Evaluation of the Anticonvulsant Activity of α-Terpineol. Pharm. Biol. 2007, 45, 69–70. [Google Scholar] [CrossRef]
- Hassan, S.B.; Gali-Muhtasib, H.; Göransson, H.; Larsson, R. Alpha-Terpineol: A Potential Anticancer Agent. Anticancer Res. 2010, 30, 1911–1919. [Google Scholar]
- Hussein, K.N.; Csehi, B.; József, S.; Ferenc, H.; Kiskó, G.; Dalmadi, I.; Friedrich, L. Effect of α-Terpineol on Chicken Meat Quality during Refrigerated Conditions. Foods 2021, 10, 1855. [Google Scholar] [CrossRef]
- Khaleel, C.; Tabanca, N.; Buchbauer, G. α-Terpineol, a natural monoterpene: A review of its biological properties. Open Chem. 2018, 16, 349–361. [Google Scholar] [CrossRef]
- Knez Hrnčič, M.; Cör, D.; Knez, Ž.; Hrnčič, M.K. Extraction techniques and analytical methods for characterization of active compounds in Origanum species. Molecules 2020, 25, 4735. [Google Scholar] [CrossRef]
- ISO 6579-1:2017; Microbiology of the Food Chain—Horizontal Method for the Detection, Enumeration and Serotyping of Salmonella—Part 1: Detection of Salmonella spp. International Organization for Standardization: Geneva, Switzerland, 2017. Available online: https://www.eucast.org (accessed on 23 September 2025).
- EUCAST (European Committee on Antimicrobial Susceptibility Testing). Breakpoint Tables for Interpretation of MICs and Zone Diameters, Version 15.0. 2025. Available online: https://www.eucast.org/clinical_breakpoints (accessed on 23 September 2025).
- Ng, L.K.; Martin, I.; Alfa, M.; Mulvey, M. Multiplex PCR for the Detection of Tetracycline Resistant Genes. Mol. Cell. Probes 2001, 15, 209–215. [Google Scholar] [CrossRef]
- Woodford, N.; Fagan, E.J.; Ellington, M.J. Multiplex PCR for Rapid Detection of Genes Encoding CTX-M ESBLs. J. Antimicrob. Chemother. 2006, 57, 154–155. [Google Scholar] [CrossRef]
- Dallenne, C.; Da Costa, A.; Decré, D.; Favier, C.; Arlet, G. Development of a Set of Multiplex PCR Assays for the Detection of Genes Encoding Important β-Lactamases in Enterobacteriaceae. J. Antimicrob. Chemother. 2010, 65, 490–495. [Google Scholar] [CrossRef]
- Reischl, U.; Linde, H.J.; Metz, M.; Leppmeier, B.; Lehn, N. Rapid Identification of MRSA and Species Confirmation Using Real-Time Fluorescence PCR. J. Clin. Microbiol. 2000, 38, 2429–2433. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Li, L.; Shirtliff, M.E.; Alam, M.J.; Yamasaki, S.; Shi, L. Expression of Antibiotic Resistance Genes in Salmonella Isolates from Poultry. Appl. Environ. Microbiol. 2007, 73, 7386–7389. [Google Scholar]
- Cattoir, V.; Poirel, L.; Rotimi, V.; Soussy, C.J.; Nordmann, P. Multiplex PCR for Detection of Plasmid-Mediated qnr Genes in ESBL-Producing Enterobacterial Isolates. J. Antimicrob. Chemother. 2007, 60, 394–397. [Google Scholar] [CrossRef] [PubMed]
- Cavaco, L.M.; Aarestrup, F.M.; Agersø, Y. Quinolone-Resistant Salmonella Hadar/Enteritidis from Danish Poultry. J. Antimicrob. Chemother. 2009, 64, 304–306. [Google Scholar]
- Wang, M.; Tran, J.H.; Jacoby, G.A.; Zhang, Y.; Wang, F.; Hooper, D.C. Plasmid-Mediated Quinolone Resistance in Clinical Escherichia coli from Shanghai, China. Antimicrob. Agents Chemother. 2003, 47, 2242–2248. [Google Scholar] [CrossRef]
- Antunes, P.; Machado, J.; Peixe, L. Characterization of Antimicrobial Resistance and Class 1 and 2 Integrons in Salmonella enterica Isolates from Different Sources in Portugal. J. Antimicrob. Chemother. 2005, 56, 1025–1029. [Google Scholar] [CrossRef]
- Sutcliffe, J.; Tait-Kamradt, A.; Wondrack, L. Detection of Erythromycin-Resistant Determinants by PCR. Antimicrob. Agents Chemother. 1996, 40, 2562–2566. [Google Scholar] [CrossRef]
- CLSI (Clinical and Laboratory Standards Institute). Performance Standards for Antimicrobial Susceptibility Testing, 34th ed.; CLSI Supplement M100; CLSI: Wayne, PA, USA, 2024. [Google Scholar]
- Aytar, F. Doğal Bileşiklerin Antibakteriyel Etkisinin Salmonella spp. Üzerine Karşılaştırmalı Olarak Araştırılması. Master’s Thesis, İstanbul Üniversitesi, İstanbul, Turkey, 2019. [Google Scholar]
- Antunes, P.; Mourão, J.; Campos, J.; Peixe, L. Salmonellosis: The Role of Poultry Meat. Clin. Microbiol. Infect. 2016, 22, 110–121. [Google Scholar] [CrossRef]
- Mattock, J.; Chattaway, M.A.; Hartman, H.; Dallman, T.J.; Smith, A.M.; Keddy, K.; Langridge, G.C. A One Health Perspective on Salmonella enterica Serovar Infantis, an Emerging Human Multidrug-Resistant Pathogen. Emerg. Infect. Dis. 2024, 30, 701–710. [Google Scholar] [CrossRef]
- Kürekçi, C.; Şahin, S. Salmonella infantis. J. Turk. Vet. Med. Soc. 2023, 94, 73–83. [Google Scholar]
- Raji, M.A.; Kazeem, H.M.; Magyigbe, K.A.; Ahmed, A.O.; Lawal, D.N.; Raufu, I.A. Salmonella Serovars, Antibiotic Resistance, and Virulence Factors Isolated from Chickens and Ready-to-Eat Gizzards in Nigeria. Int. J. Food Sci. 2021, 2021, 8872137. [Google Scholar] [CrossRef]
- Telli, A.E.; Biçer, Y.; Kahraman, H.A.; Telli, N.; Doğruer, Y. Presence and Antibiotic Resistance of Salmonella spp. Isolated from Chicken Meat and Giblets Consumed in Konya, Turkey. Eurasian J. Vet. Sci. 2018, 34, 164–170. [Google Scholar] [CrossRef]
- Hassan, A.H.; Salam, H.S.; Abdel-Latef, G.K. Identification of Virulence Genes, β-Lactams and Quinolone Resistance Genes, and Integrons in Salmonella from Retail Chicken in Egypt. J. Microbiol. Biotechnol. Food Sci. 2021, 10, 1320–1325. [Google Scholar]
- Sırıken, B.; Türk, H.; Yıldırım, T.; Durupınar, B.; Erol, İ. Prevalence and Characterization of Salmonella Isolated from Chicken Meat in Turkey. J. Food Sci. 2015, 80, M1044–M1050. [Google Scholar] [CrossRef]
- Babacan, O.; Karadeniz, H. Çiğ Tavuk Etlerinden İzole Edilen Salmonella spp. Suşlarının Antibiyotik Duyarlılıklarının Araştırılması. Vet. Hekimler Derneği Derg. 2019, 90, 105–114. [Google Scholar] [CrossRef]
- Ishihara, K.; Nakazawa, C.; Nomura, S.; Elahi, S.; Yamashita, M.; Fujikawa, H. Effects of Climatic Elements on Salmonella Contamination in Broiler Meat in Japan. J. Vet. Med. Sci. 2020, 82, 646–652. [Google Scholar] [CrossRef]
- Wardhana, D.K.; Haskito, A.E.P.; Purnama, M.T.E.; Safitri, D.A.; Annisa, S. Detection of Microbial Contamination in Chicken Meat from Local Markets in Surabaya, Indonesia. Vet. World 2021, 14, 3138–3144. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA); European Centre for Disease Prevention and Control (ECDC). The European Union One Health 2018 Zoonoses Report. EFSA J. 2019, 17, e05926. [Google Scholar] [CrossRef]
- Sodagari, H.R.; Shrestha, R.D.; Agunos, A.; Gow, S.P.; Varga, C. Comparison of Antimicrobial Resistance among Salmonella enterica Serovars Isolated from Canadian Turkey Flocks, 2013–2021. Poult. Sci. 2023, 102, 102655. [Google Scholar] [CrossRef] [PubMed]
- Bush, K.; Jacoby, G.A. Updated Functional Classification of β-Lactamases. Antimicrob. Agents Chemother. 2010, 54, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Bradford, P.A. Extended-Spectrum β-Lactamases in the 21st Century: Characterization, Epidemiology, and Detection. Clin. Microbiol. Rev. 2001, 14, 933–951. [Google Scholar] [CrossRef]
- Carattoli, A. Resistance Plasmid Families in Enterobacteriaceae. Antimicrob. Agents Chemother. 2009, 53, 2227–2238. [Google Scholar] [CrossRef]
- Guerra, B.; Junker, E.; Schroeter, A.; Malorny, B.; Lehmann, S.; Helmuth, R. Phenotypic and Genotypic Characterization of Antimicrobial Resistance in German Escherichia coli Isolates from Cattle, Swine and Poultry. J. Antimicrob. Chemother. 2003, 52, 489–492. [Google Scholar] [CrossRef]
- Wasyl, D.; Zając, M.; Lalak, A.; Skarżyńska, M.; Samcik, I.; Kwit, R.; Jabłoński, A.; Bocian, Ł.; Woźniakowski, G.; Hoszowski, A.; et al. Antimicrobial Resistance in Commensal Escherichia coli and Salmonella enterica from Food Animals in Poland. Microb. Drug Resist. 2018, 24, 807–815. [Google Scholar] [CrossRef]
- Acar, S.; Bulut, E.; Stasiewicz, M.J.; Soyer, Y. Genome Analysis of Antimicrobial Resistance, Virulence, and Plasmid Presence in Turkish Salmonella Serovar Infantis Isolates. Int. J. Food Microbiol. 2019, 307, 108275. [Google Scholar] [CrossRef]
- Nógrády, N.; Király, M.; Davies, R.; Nagy, B. Multidrug-Resistant Salmonella Infantis in Broiler Chickens and Humans in Hungary: The Need for Coordinated Monitoring. Food Res. Int. 2012, 45, 387–392. [Google Scholar]
- Hindermann, D.; Gopinath, G.; Chase, H.; Negrete, F.; Stephan, R. Salmonella enterica subsp. enterica Serovar Infantis from Meat Products and Human Infections in Switzerland: Phenotypic and Genotypic Antimicrobial Resistance and Phylogenetic Analysis. Food Control 2017, 73, 19–26. [Google Scholar]
- Rahmani, H.; Mardani, K.; Madani, M. Antimicrobial Resistance Patterns and Distribution of Resistance Genes in Salmonella Isolates from Iranian Poultry Farms. Vet. Res. Forum 2013, 4, 157–163. [Google Scholar]
- Bacci, C.; Torpdahl, M.; Nielsen, E.M. Resistance Genes in Salmonella Isolates from Food and Human Sources in Denmark. Microb. Drug Resist. 2014, 20, 146–153. [Google Scholar]
- Archer, J.R.; Otchere, J.; Osei, G.Y.; Pappoe, M.K.; Agyapong, A.; Newman, M.J.; Andoh, L.A.; Dickens, C.; Asante-Poku, A.; Harrison, E.M.; et al. Prevalence and Resistance Genes of Salmonella spp. Isolated from Poultry in Ghana. Front. Microbiol. 2023, 14, 1153205. [Google Scholar]
- Hall, R.M.; Collis, C.M. Mobile Gene Cassettes and Integrons: Capture and Spread of Genes by Site-Specific Recombination. Mol. Microbiol. 1995, 15, 593–600. [Google Scholar] [CrossRef]
- Seiffert, S.N.; Hilty, M.; Perreten, V.; Endimiani, A. Extended-Spectrum Cephalosporin-Resistant Gram-Negative Organisms in Livestock: An Emerging Problem for Human Health? Drug Resist. Updates 2013, 16, 22–45. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Tolmasky, M.E. Aminoglycoside Modifying Enzymes. Drug Resist. Updates 2010, 13, 151–171. [Google Scholar] [CrossRef]
- Shaw, K.J.; Rather, P.N.; Hare, R.S.; Miller, G.H. Molecular Genetics of Aminoglycoside Resistance Genes and Familial Relationships of the Aminoglycoside-Modifying Enzymes. Microbiol. Rev. 1993, 57, 138–163. [Google Scholar] [CrossRef] [PubMed]
- van Hoek, A.H.; Mevius, D.; Guerra, B.; Mullany, P.; Roberts, A.P.; Aarts, H.J. Acquired Antibiotic Resistance Genes: An Overview. Front. Microbiol. 2011, 2, 203. [Google Scholar] [CrossRef]
- Abbasoğlu, D.; Akçelik, M. Phenotypic and Genetic Characterization of Multidrug-Resistant Salmonella Infantis Strains Isolated from Broiler Chicken Meats in Turkey. Biologia 2011, 66, 406–410. [Google Scholar] [CrossRef]
- Leverstein-van Hall, M.A.; Blok, H.E.; Donders, A.R.T.; Paauw, A.; Fluit, A.C.; Verhoef, J. Multidrug Resistance among Enterobacteriaceae Is Strongly Associated with the Presence of Integrons. J. Infect. Dis. 2003, 187, 251–259. [Google Scholar] [CrossRef]
- Peng, Y.; Wang, Z.; Li, X. Antimicrobial Resistance in Salmonella Isolated from Frozen Chicken Meat in China. Poult. Sci. 2024, 103, 102567. [Google Scholar]
- Telsaç, R.; Tuncay, M. Prevalence and Antibiotic Resistance Profiles of Salmonella spp. in Chicken Meat. Turk. J. Vet. Anim. Sci. 2022, 46, 708–717. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oils’ Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef] [PubMed]
- Angane, M.; Swift, S.; Huang, K.; Butts, C.A.; Quek, S.Y. Essential Oils and Their Major Components: Updated Review on Antimicrobial Activities, Mechanism of Action and Potential Food Industry Applications. Foods 2022, 11, 464. [Google Scholar] [CrossRef]
- Ma, M.; Zhao, J.; Yan, X.; Zeng, Z.; Wan, D.; Yu, P.; Xia, J.; Zhang, G.; Gong, D. Synergistic Effects of Monocaprin and Carvacrol against Escherichia coli O157:H7 and Salmonella Typhimurium in Chicken Meat Preservation. Food Control 2022, 132, 108480. [Google Scholar] [CrossRef]
- Khan, I.; Bahuguna, A.; Shukla, S.; Aziz, F.; Chauhan, A.K.; Ansari, M.B.; Bajpai, V.K.; Huh, Y.S.; Kang, S.C. Antimicrobial Potential of Carvacrol against Uropathogenic E. coli. Microb. Pathog. 2020, 142, 104046. [Google Scholar] [CrossRef]
- Heckler, C.; Brandelli, A.; Malheiros, P.S. Combined Effect of Carvacrol, Thymol and Nisin against Staphylococcus aureus and Salmonella Enteritidis. An. Acad. Bras. Cienc. 2021, 93, e20210550. [Google Scholar] [CrossRef]
- Keyvan, E.; Tutun, H.; Kahraman, H.A.; Şen, E.; Demirtaş, A.; Dönmez, S.; Akyüz, A.Ö. Determination of Time-Dependent Antibacterial Activities of Curcumin, Carvacrol and Styrax Liquidus on Salmonella Enteritidis. Ankara Univ. Vet. Fak. Derg. 2022, 69, 149–158. [Google Scholar]
- Boyer, E.; Galán-Relaño, Á.; Romero-Salmoral, A.; Barraza, P.; Gómez-Gascón, L.; Tarradas, C.; Luque, I.; de Aguiar, F.C.; Lorenzo, B.H. Post-Antibiotic and Post-Sub-MIC Effects of Carvacrol against Salmonella Typhimurium. Animals 2024, 14, 2631. [Google Scholar] [CrossRef]
- Cui, H.; Chen, X.; Aziz, T.; Mohamed, R.A.E.H.; Al-Asmari, F.; Alshammari, J.M.; Al-Joufi, F.A.; Shi, C.; Lin, L. Inactivation Mechanisms of Carvacrol on Salmonella Typhimurium and Its Combined Inhibitory Effects with 405 nm Blue Light. Int. J. Food Microbiol. 2025, 414, 111276. [Google Scholar]
- Zhao, X.; Zheng, S.; Wei, S.; Tian, Q.; Tao, Y.; Bo, R.; Liu, M.; Li, J. The Protective Effect and Potential Mechanisms of Eugenol against Salmonella Infections. Poult. Sci. 2022, 101, 101801. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Wei, S.; Tian, Q.; Peng, W.; Tao, Y.; Bo, R.; Liu, M.; Li, J. Eugenol Exposure in vitro Inhibits T3SS and TIF Virulence Genes in Salmonella Typhimurium. Microb. Pathog. 2022, 162, 105314. [Google Scholar] [CrossRef] [PubMed]
- Kim, Y.; Nahar, S.; Cho, A.J.; Mahamud, A.S.U.; Park, S.H.; Ha, S.D. Synergistic Antibacterial Effect of DNase I and Eugenol against Salmonella Enteritidis Biofilm. Food Control 2023, 150, 109764. [Google Scholar] [CrossRef]
- Balyan, S.; Dadwal, V.; Jha, D.K.; Patil, B.S. Innovative Food Safety Strategy: Eugenol Nanoemulsion with Post-Biotic Biopolymer for Biofilm Inhibition. Food Control 2025, 176, 111348. [Google Scholar] [CrossRef]
- Gökmen, T.G. Investigation of Antibacterial Activity of Carvacrol, Alpha-Terpineol and Eugenol on ESBL-Producing Escherichia coli Strains Isolated from Chicken Meat. Turk. J. Agric. Food Sci. Technol. 2025, 13, 1625–1630. [Google Scholar]
- Akermi, S.; Smaoui, S.; Chaari, M.; Elhadef, K.; Gentile, R.; Hait, M.; Roymahapatra, G.; Mellouli, L. Combined In Vitro/In Silico Approaches and Safety Assessment of Thymol and Carvacrol. Chem. Biodivers. 2024, 21, e202301575. [Google Scholar] [CrossRef]
- Asadi, S.; Nayeri-Fasaei, B.; Zahraei-Salehi, T.; Yahya-Rayat, R.; Shams, N.; Sharifi, A. Antibacterial and Anti-Biofilm Properties of Carvacrol Alone and in Combination with Cefixime against E. coli. BMC Microbiol. 2023, 23, 55. [Google Scholar] [CrossRef]
- Devi, K.P.; Nisha, S.A.; Sakthivel, R.; Pandian, S.K. Eugenol (an Essential Oil of Clove) Acts as an Antibacterial Agent against Salmonella Typhi. J. Ethnopharmacol. 2010, 130, 107–115. [Google Scholar] [CrossRef] [PubMed]
- Evrendilek, G. Empirical Prediction and Validation of Antibacterial Inhibitory Effects of Various Plant Essential Oils on Common Pathogenic Bacteria. Int. J. Food Microbiol. 2015, 202, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Jeyakumar, G.E.; Lawrence, R. Mechanisms of Bactericidal Action of Eugenol against Escherichia coli. J. Herb. Med. 2021, 26, 100406. [Google Scholar] [CrossRef]

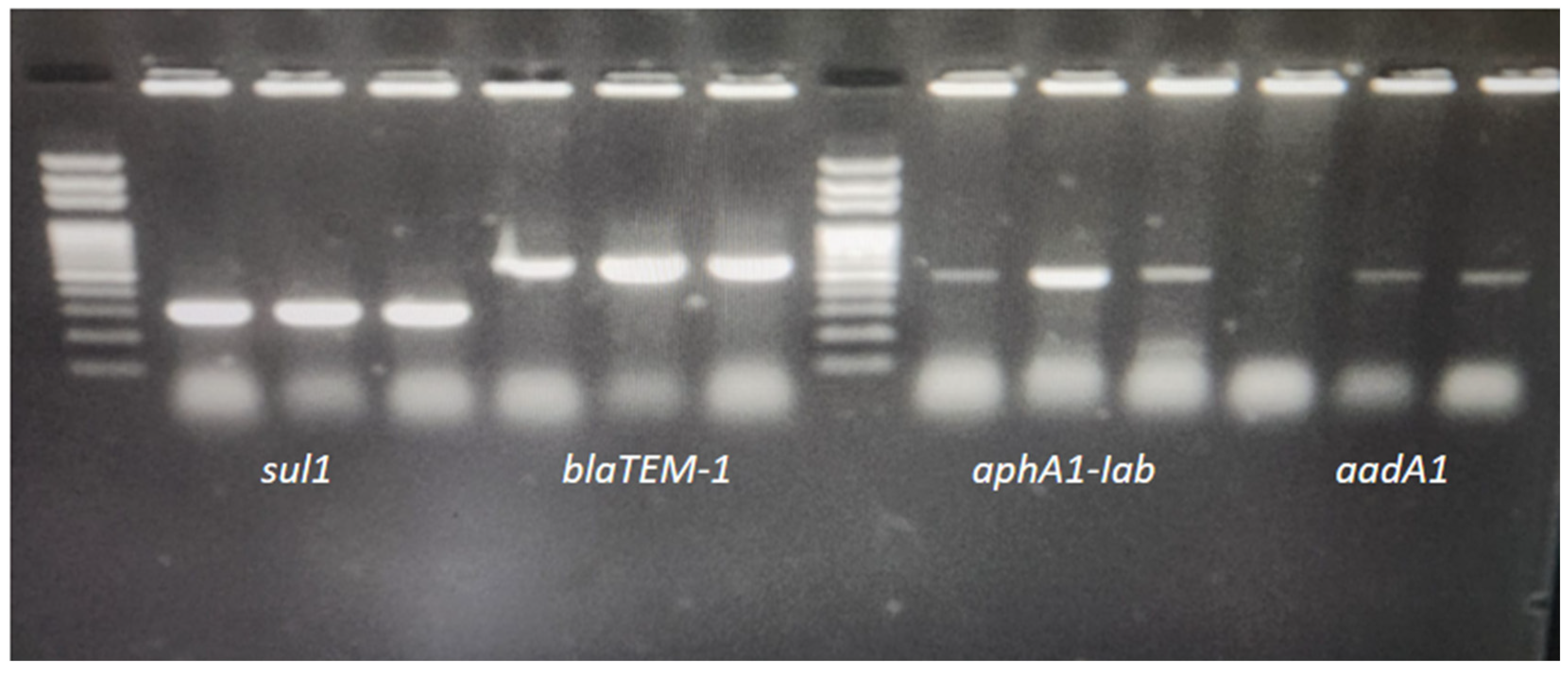
| Gene | Sequences | °C * | bp | References |
|---|---|---|---|---|
| TEM | F: CATTTCCGTGTCGCCCTTATTC R: CGTTCATCCATAGTTGCCTGAC | 52 °C | 800 bp | [41,42,43] |
| SHV | F: AGCCGCTTGAGCAAATTAAAC R: ATCCCGCAGATAAATCACCAC | 52 °C | 713 bp | |
| OXA | F: GGCACCAGATTCAACTTTCAAG R: GACCCCAAGTTTCCTGTAAGTG | 52 °C | 564 bp | |
| CTXM1 | F: TTAGGAARTGTGCCGCTGYA R: CGATATCGTTGGTGGTRCCAT | 52 °C | 688 bp | |
| CTXM2 | F: CGTTAACGGCACGATGAC R: CGATATCGTTGGTGGTRCCAT | 52 °C | 404 bp | |
| CTXM9 | F: TCAAGCCTGCCGATCTGGT R: TGATTCTCGCCGCTGAAG | 52 °C | 561 bp | |
| CTXM 8/25 | F: AACRCRCAGACGCTCTAC R: TCGAGCCGGAASGTGTYAT | 52 °C | 326 bp | |
| TEM-1 | F: CAGCGGTAAGATCCTTGAGA R: ACTCCCCGTCGTGTAGATAA | 46 °C | 643 bp | [44] |
| tetA | F: GGTTCACTCGAACGACGTCA R: CTGTCCGACAAGTTGCATGA | 55 °C | 210 bp | [41] |
| tetB | F: CCTCAGCTTCTCAACGCGTG R: GCACCTTGCTGATGACTCTT | 55 °C | 659 bp | |
| cat1 | F: ATGAGAAAAAATCACTGGATATACC R: TTACGCCCCGCCCTGCC | 56 °C | 547 bp | [44,45] |
| cat2 | F: TCCGGGCCTGCTGACAGGCATC R: GAGTTGAGCGTCAGCGGGTG | 56 °C | 352 bp | |
| qnrA | F:GGATGCCAGTTTCGAGGA R:TGCCAGGCACAGATCTTG | 50 °C | 492 bp | [46,47,48] |
| qnrB | F:GGMATHGAAATTCGCCACTG R:TTTGCYGYYCGCCAGTCGAA | 50 °C | 264 bp | |
| qnrS | F:TCGACGTGCTAACTTGCG R:GATCTAAACCGTCGAGTTCGG | 50 °C | 466 bp | |
| qnrC | F:GGGTTGTACATTTATTGAATCG R:CACCTACCCATTTATTTTCA | 50 °C | 307 bp | |
| qnrD | F:CGAGATCAATTTACGGGGAATA R:AACAAGCTGAAGCGCCTG | 50 °C | 582 bp | |
| aadA1 | F:TATCAGAGGTAGTTGGCGTCAT R:GTTCCATAGCGTTAAGGTTTCATT | 45 °C | 484 bp | [41] |
| aphA1-IAB | F: AAACGTCTTGCTCGAGGC R: CAAACCGTTATTCATTCGTGA | 46 °C | 500 bp | |
| Sul1 | F: TCACCGAGGACTCCTTCTTC R: CAGTCCGCCTCAGCAATATC | 45 °C | 331 bp | [49] |
| ermB | F:GAAAAGGTACTCAACCAAATA R:AGTAACGGTACTTAAATTGTTTAC | 52 °C | 639 bp | [50] |
| Sample | Salmonella spp (n, %) | S. Typhimurium (n, %) | S. Infantis (n, %) | S. Enteritidis (n, %) |
|---|---|---|---|---|
| Wing (n: 25) | ND | ND | ND | ND |
| Breasts (n: 25) | ND | ND | ND | ND |
| Drumstick (n: 25) | 1, 4% | ND | %4 | ND |
| Thigh (n: 25) | 2, 8% | ND | %8 | ND |
| Antibiotics | Salmonella Infantis 1 (S1) | Salmonella Infantis 2 (S2) | Salmonella Infantis 3 (S3) |
|---|---|---|---|
| Ampicillin-AM | R | R | R |
| Amoxicillin-clavulanic acid-AMC | R | R | R |
| Ceftriaxone-CRO | R * | R | S |
| Ceftazidime-CAZ | S | S | S |
| Aztreonam-ATM | S | S | S |
| Cefotaxime-CTX | S | S | S |
| Gentamicin-CN | S | S | S |
| Amikacin-AK | S | S | S |
| Ciprofloxacin-CIP | S | S | S |
| Levofloxacin-LEV | S | S | S |
| Enrofloxacin-ENR | S | S | S |
| Trimethoprim-sulfamethoxazole-TMZ | R | R | R |
| Tetracycline-T | R | R | R |
| Chloramphenicol-C | S | S | S |
| Streptomycin-S | R | R | R |
| Kanamycin-K | R | R | R |
| Salmonella Infantis Isolate | Minimum Inhibitory Concentration (MIC) | Minimum Bactericidal Concentration (MBC) | ||||
|---|---|---|---|---|---|---|
| Carvacrol | Eugenol | α-Terpineol | Carvacrol | Eugenol | α-Terpineol | |
| S1 | 1.56 µL/mL | 3.125 µL/mL | 6.25 µL/mL | 6.25 µL/mL | 12.5 µL/mL | 25 µL/mL |
| S2 | 1.56 µL/mL | 6.25 µL/mL | 6.25 µL/mL | 3.125 µL/mL | 12.5 µL/mL | 25 µL/mL |
| S3 | 1.56 µL/mL | 6.25 µL/mL | 12.50 µL/mL | 3.125 µL/mL | 12.5 µL/mL | 25 µL/mL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tekin, Y.; Yazgan, H.; Gokmen, T.G.; Gungor, N.; Uprak, N.S. Integrated Analysis of Salmonella Infantis in Chicken Meat: Epidemiological Surveillance, Antibiotic Resistance, and Potential Bioactive Control Agents. Pathogens 2025, 14, 1178. https://doi.org/10.3390/pathogens14111178
Tekin Y, Yazgan H, Gokmen TG, Gungor N, Uprak NS. Integrated Analysis of Salmonella Infantis in Chicken Meat: Epidemiological Surveillance, Antibiotic Resistance, and Potential Bioactive Control Agents. Pathogens. 2025; 14(11):1178. https://doi.org/10.3390/pathogens14111178
Chicago/Turabian StyleTekin, Yasin, Hatice Yazgan, Tulin Guven Gokmen, Nuri Gungor, and Nur Sima Uprak. 2025. "Integrated Analysis of Salmonella Infantis in Chicken Meat: Epidemiological Surveillance, Antibiotic Resistance, and Potential Bioactive Control Agents" Pathogens 14, no. 11: 1178. https://doi.org/10.3390/pathogens14111178
APA StyleTekin, Y., Yazgan, H., Gokmen, T. G., Gungor, N., & Uprak, N. S. (2025). Integrated Analysis of Salmonella Infantis in Chicken Meat: Epidemiological Surveillance, Antibiotic Resistance, and Potential Bioactive Control Agents. Pathogens, 14(11), 1178. https://doi.org/10.3390/pathogens14111178






