Molecular Survey of Hemopathogens in Dogs, Including Blood Donors, from Central-Western Brazil
Abstract
1. Introduction
2. Materials and Methods
2.1. Ethics Committee
2.2. Study Site
2.3. DNA Extraction from Canine Blood Samples
2.4. Conventional PCR (cPCR) for the Endogenous Mammalian Gene Glyceraldehyde-3-Phosphate Dehydrogenase (gapdh)
2.5. Detection of Bartonella spp.
2.5.1. Bartonella spp. Culture
2.5.2. Quantitative Real-Time PCR (qPCR) for Bartonella spp.
2.6. Molecular Detection and Characterization of Piroplasmids
2.7. Molecular Detection and Characterization of Ehrlichia spp. and Anaplasma spp.
2.8. Molecular Detection and Characterization of Hemoplasmas
2.9. Agarose Gel Electrophoresis
2.10. Sequencing and Phylogenetic Analyses
2.11. Comparison of Molecular Occurrence of Hemopathogens Among Dog Groups and Sex
3. Results
3.1. Microbiological and Molecular Investigation of Bartonella spp.
3.2. Molecular Detection of Piroplasmids
3.3. Molecular Detection of Ehrlichia spp. and Anaplasma spp.
3.4. Hemoplasmas
3.5. Statistical Analysis
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lucas, R.L.; Lentz, K.D.; Hale, A.S. Collection and preparation of blood products. Clin. Tech. Small Anim. Pract. 2004, 19, 55–62. [Google Scholar] [CrossRef]
- Helm, J.; Knottenbelt, C. Blood transfusions in dogs and cats 1. Indications. In Pract. 2010, 32, 184–189. [Google Scholar] [CrossRef]
- Morikawa, M.K.; Bochio, M.M.; Pincelli, V.A.; Freire, R.L.; Pereira, P.M. Monitoração e avaliação clínica da eficácia da transfusão de sangue total e concentrado de hemácias em cães. Pesq. Vet. Bras. 2010, 30, 665–669. [Google Scholar] [CrossRef]
- American Veterinary Medical Association. Animal Blood Shortage Deepens During Pandemic; AVMA: Schaumburg, IL, USA, 2021; Available online: https://www.avma.org/news/animal-blood-shortage-deepens-during-pandemic (accessed on 17 July 2025).
- Descamps, M.; Humm, K. Why some canine and feline blood donors do not make the cut: A cohort study. Vet. Rec. 2023, 193, e2993. [Google Scholar] [CrossRef]
- Associação Brasileira de Hematologia e Medicina Transfusional Veterinária (ABVHMT). Nota Técnica ABVHMT nº 3: Cães e Gatos Doadores de Sangue: Requisitos e Cuidados. Available online: https://abvhmt.org/wp-content/uploads/2024/02/Nota-Tecnica-ABVHMT-DOADORES-REQUISITOS-E-CUIDADOS.pdf (accessed on 10 May 2025).
- Kuo, K.W.; McMichael, M. Small animal transfusion medicine. Vet. Clin. N. Am. Small Anim. Pract. 2020, 50, 1203–1214. [Google Scholar] [CrossRef]
- Wardrop, K.J.; Birkenheuer, A.; Blais, M.C.; Callan, M.B.; Kohn, B.; Lappin, M.R.; Sykes, J. Update on canine and feline blood donor screening for blood-borne pathogens. J. Vet. Intern. Med. 2016, 30, 15–35. [Google Scholar] [CrossRef]
- Cruz, F.D.; Otsubo, A.A.F.; Trevisan, Y.P.A.; Cruz, T.D.; Almeida, A.B.D.; Mendonça, A.J.; Nakazato, L.; Sousa, V.R.F. Occurrence of Leishmania chagasi, Trypanosoma cruzi, Babesia canis vogeli, Anaplasma platys, and Ehrlichia canis in canine blood donors. Semin. Ciênc. Agrár. 2017, 38, 295–304. [Google Scholar] [CrossRef]
- Stegeman, J.R.; Birkenheuer, A.J.; Kruger, J.M.; Breitschwerdt, E.B. Transfusion-associated Babesia gibsoni infection in a dog. J. Am. Vet. Med. Assoc. 2003, 222, 959–963. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Lee, D.; Yoon, E.; Bae, H.; Chun, D.; Kang, J.G.; Jung, D.-I.; Yu, D.H. Clinical case of a transfusion-associated canine Mycoplasma haemocanis infection in the Republic of Korea: A case report. Korean J. Parasitol. 2020, 58, 565–569. [Google Scholar] [CrossRef]
- Vascellari, M.; Ravagnan, S.; Carminato, A.; Cazzin, S.; Carli, E.; Da Rold, G.; Lucchese, L.; Natale, A.; Otranto, D.; Capelli, G. Exposure to vector-borne pathogens in candidate blood donor and free-roaming dogs of northeast Italy. Parasites Vectors 2016, 9, 1–10. [Google Scholar] [CrossRef]
- Nury, C.; Blais, M.-C.; Arsenault, J. Risk of transmittable blood-borne pathogens in blood units from blood donor dogs in Canada. J. Vet. Intern. Med. 2021, 35, 1316–1324. [Google Scholar] [CrossRef] [PubMed]
- Castillo, M.A.C.; de Almeida, B.A.; Wissmann, D.; Moreira, R.F.; Okano, F.Y.; Gonzalez, F.H.; Soares, J.F.; de Faria Valle, S. Viability of erythrocytes in canine packed red blood cells stored in CPDA-1 is related to the presence of Mycoplasma haemocanis. Comp. Immunol. Microbiol. Infect. Dis. 2023, 97, 101982. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.H.; Latham, S.M.; Woolhouse, M.E. Risk factors for human disease emergence. Philos. Trans. R. Soc. Lond. B Biol. Sci. 2001, 356, 983–989. [Google Scholar] [CrossRef]
- Ryu, S.; Kim, B.I.; Lim, J.S.; Tan, C.S.; Chun, B.C. One health perspectives on emerging public health threats. J. Prev. Med. Public Health 2017, 50, 411–419. [Google Scholar] [CrossRef] [PubMed]
- Chala, B.; Hamde, F. Emerging and re-emerging vector-borne infectious diseases and the challenges for control: A review. Front. Public Health 2021, 9, 715759. [Google Scholar] [CrossRef] [PubMed]
- Feldman, B.F.; Zinkl, J.G.; Jain, N.C. Schalm’s Veterinary Hematology, 5th ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2000. [Google Scholar]
- Birkenheuer, A.J.; Levy, M.G.; Breitschwerdt, E.B. Development and evaluation of a seminested PCR for detection and differentiation of Babesia gibsoni (Asian genotype) and B. canis DNA in canine blood samples. J. Clin. Microbiol. 2003, 41, 4172–4177. [Google Scholar] [CrossRef]
- Maggi, R.G.; Duncan, A.W.; Breitschwerdt, E.B. Novel chemically modified liquid medium that will support the growth of seven Bartonella species. J. Clin. Microbiol. 2005, 43, 2651–2655. [Google Scholar] [CrossRef]
- Duncan, A.W.; Maggi, R.G.; Breitschwerdt, E.B. Bartonella DNA in dog saliva. Emerg. Infect. Dis. 2007, 13, 1948. [Google Scholar] [CrossRef]
- Keim, P.; Price, L.B.; Klevytska, A.M.; Smith, K.L.; Schupp, J.M.; Okinaka, R.; Jackson, P.J.; Hugh-Jones, M.E. Multiple-locus variable-number tandem repeat analysis reveals genetic relationships within Bacillus anthracis. J. Bacteriol. 2000, 182, 2928–2936. [Google Scholar] [CrossRef]
- Oteo, J.A.; Maggi, R.; Portillo, A.; Bradley, J.; García-Álvarez, L.; San-Martín, M.; Roura, X.; Breitschwerdt, E. Prevalence of Bartonella spp. by culture, PCR and serology, in veterinary personnel from Spain. Parasites Vectors 2017, 10, 1–9. [Google Scholar] [CrossRef]
- Breitschwerdt, E.B.; Maggi, R.G. Bartonella quintana and Bartonella vinsonii subsp. vinsonii bloodstream co-infection in a girl from North Carolina, USA. Med. Microbiol. Immunol. 2019, 208, 101–107. [Google Scholar] [CrossRef]
- Bustin, S.A.; Benes, V.; Garson, J.A.; Hellemans, J.; Huggett, J.; Kubista, M.; Mueller, R.; Nolan, T.; Pfaffl, M.W.; Shipley, G.L.; et al. The MIQE guidelines: Minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 2009, 55, 611–622. [Google Scholar] [CrossRef]
- Jefferies, R.; Ryan, U.M.; Jardine, J.; Robertson, I.D.; Irwin, P.J. Babesia gibsoni: Detection during experimental infections and after combined atovaquone and azithromycin therapy. Exp. Parasitol. 2007, 117, 115–123. [Google Scholar] [CrossRef]
- Greay, T.L.; Zahedi, A.; Krige, A.S.; Owens, J.M.; Rees, R.L.; Ryan, U.M.; Oskam, C.L.; Irwin, P.J. Endemic, exotic and novel apicomplexan parasites detected during a national study of ticks from companion animals in Australia. Parasites Vectors 2018, 11, 1–20. [Google Scholar] [CrossRef]
- Kawabuchi, T.; Tsuji, M.; Sado, A.; Matoba, Y.; Asakawa, M.; Ishihara, C. Babesia microti-like parasites detected in feral raccoons (Procyon lotor) captured in Hokkaido, Japan. J. Vet. Med. Sci. 2005, 67, 825–827. [Google Scholar] [CrossRef]
- Soares, J.F.; Girotto, A.; Brandão, P.E.; Da Silva, A.S.; Franca, R.T.; Lopes, S.T.; Labruna, M.B. Detection and molecular characterization of a canine piroplasm from Brazil. Vet. Parasitol. 2011, 180, 203–208. [Google Scholar] [CrossRef] [PubMed]
- Corduneanu, A.; Hrazdilová, K.; Sándor, A.D.; Matei, I.A.; Ionică, A.M.; Barti, L.; Ciocănău, M.-A.; Măntoiu, D.S.; Coroiu, I.; Hornok, S.; et al. Babesia vesperuginis, a neglected piroplasmid: New host and geographical records, and phylogenetic relations. Parasites Vectors 2017, 10, 1–8. [Google Scholar] [CrossRef]
- Barbosa, A.D.; Austen, J.; Portas, T.J.; Friend, J.A.; Ahlstrom, L.A.; Oskam, C.L.; Ryan, U.M.; Irwin, P.J. Sequence analyses at mitochondrial and nuclear loci reveal a novel Theileria sp. and aid in the phylogenetic resolution of piroplasms from Australian marsupials and ticks. PLoS ONE 2019, 14, e0225822. [Google Scholar] [CrossRef] [PubMed]
- Schreeg, M.E.; Marr, H.S.; Tarigo, J.L.; Cohn, L.A.; Bird, D.M.; Scholl, E.H.; Levy, M.G.; Wiegmann, B.M.; Birkenheuer, A.J. Mitochondrial genome sequences and structures aid in the resolution of Piroplasmida phylogeny. PLoS ONE 2016, 11, e0165702. [Google Scholar] [CrossRef]
- Duarte, S.C.; Linhares, G.F.C.; Romanowsky, T.N.; da Silveira Neto, O.J.; Borges, L.M.F. Assessment of primers designed for the subspecies-specific discrimination among Babesia canis canis, Babesia canis vogeli and Babesia canis rossi by PCR assay. Vet. Parasitol. 2008, 152, 16–20. [Google Scholar] [CrossRef] [PubMed]
- Campos, J.B.; André, M.R.; Gonçalves, L.R.; Freschi, C.R.; Santos, F.M.; de Oliveira, C.E.; Piranda, E.M.; Andrade, G.B.; Macedo, G.C.; Machado, R.Z.; et al. Assessment of equine piroplasmids in the Nhecolândia sub-region of Brazilian Pantanal wetland using serological, parasitological, molecular, and hematological approaches. Ticks Tick Borne Dis. 2019, 10, 714–721. [Google Scholar] [CrossRef]
- Secato, C.T.; Gonçalves, L.R.; Ramos, I.A.S.; Calchi, A.C.; Mongruel, A.C.B.; da Silva, T.M.; Machado, R.Z.; André, M.R. Molecular detection of vector-borne agents in water buffaloes (Bubalus bubalis) and associated ectoparasites from Brazil. Trop. Anim. Health Prod. 2025, 57, 267. [Google Scholar] [CrossRef]
- Benevenute, J.L.; Dumler, J.S.; Ogrzewalska, M.; Roque, A.L.R.; Mello, V.V.C.; de Sousa, K.C.M.; Gonçalves, L.R.; D’Andrea, P.S.; Lemos, E.R.S.; Machado, R.Z.; et al. Assessment of a quantitative 5′ nuclease real-time polymerase chain reaction using groEL gene for Ehrlichia and Anaplasma species in rodents in Brazil. Ticks Tick Borne Dis. 2017, 8, 646–656. [Google Scholar] [CrossRef]
- Doyle, C.K.; Labruna, M.B.; Breitschwerdt, E.B.; Tang, Y.W.; Corstvet, R.E.; Hegarty, B.C.; Bloch, K.C.; Li, P.; Walker, D.H.; McBride, J.W. Detection of medically important Ehrlichia by quantitative multicolor TaqMan real-time polymerase chain reaction of the dsb gene. J. Mol. Diagn. 2005, 7, 504–510. [Google Scholar] [CrossRef]
- Massung, R.F.; Slater, K.; Owens, J.H.; Nicholson, W.L.; Mather, T.N.; Solberg, V.B.; Olson, J.G. Nested PCR assay for detection of granulocytic ehrlichiae. J. Clin. Microbiol. 1998, 36, 1090–1095. [Google Scholar] [CrossRef]
- De Sousa, K.C.M.; Calchi, A.C.; Herrera, H.M.; Dumler, J.S.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Anaplasmataceae agents among wild mammals and ectoparasites in Brazil. Epidemiol. Infect. 2017, 145, 3424–3437. [Google Scholar] [CrossRef] [PubMed]
- Willi, B.; Meli, M.L.; Lüthy, R.; Honegger, H.; Wengi, N.; Hoelzle, L.E.; Reusch, C.E.; Lutz, H.; Hofmann-Lehmann, R. Development and application of a universal hemoplasma screening assay based on the SYBR Green PCR principle. J. Clin. Microbiol. 2009, 47, 4049–4054. [Google Scholar] [CrossRef] [PubMed]
- Di Cataldo, S.; Kamani, J.; Cevidanes, A.; Msheliza, E.G.; Millán, J. Hemotropic mycoplasmas in bats captured near human settlements in Nigeria. Comp. Immunol. Microbiol. Infect. Dis. 2020, 70, 101448. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Compton, S.M.; Trull, C.L.; Mascarelli, P.E.; Mozayeni, B.R.; Breitschwerdt, E.B. Infection with hemotropic Mycoplasma species in patients with or without extensive arthropod or animal contact. J. Clin. Microbiol. 2013, 51, 3237–3241. [Google Scholar] [CrossRef]
- Mongruel, A.C.B.; Spanhol, V.C.; Valente, J.D.M.; Porto, P.P.; Ogawa, L.; Otomura, F.H.; Marquez, E.S.; André, M.R.; Vieira, T.S.W.J.; Vieira, R.F.D.C. Survey of vector-borne and nematode parasites involved in the etiology of anemic syndrome in sheep from Southern Brazil. Rev. Bras. Parasitol. Vet. 2020, 29, e007320. [Google Scholar] [CrossRef]
- Sanger, F.; Nicklen, S.; Coulson, A.R. DNA sequencing with chain-terminating inhibitors. Proc. Natl. Acad. Sci. USA 1977, 74, 5463–5467. [Google Scholar] [CrossRef]
- Hall, T.A. BioEdit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. In Nucleic Acids Symposium Series; Oxford Academic: Oxford, UK, 1999; Volume 41, No. 41; pp. 95–98. [Google Scholar]
- Ewing, B.; Green, P. Base-calling of automated sequencer traces using phred. II. Error probabilities. Genome Res. 1998, 8, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Ewing, B.; Hillier, L.; Wendl, M.C.; Green, P. Base-calling of automated sequencer traces using Phred. I. Accuracy assessment. Genome Res. 1998, 8, 175–185. [Google Scholar] [CrossRef]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef] [PubMed]
- Thompson, J.D.; Higgins, D.G.; Gibson, T.J. CLUSTAL W: Improving the sensitivity of progressive multiple sequence alignment through sequence weighting, position-specific gap penalties and weight matrix choice. Nucleic Acids Res. 1994, 22, 4673–4680. [Google Scholar] [CrossRef] [PubMed]
- Glez-Peña, D.; Gómez-Blanco, D.; Reboiro-Jato, M.; Fdez-Riverola, F.; Posada, D. ALTER: Program-oriented conversion of DNA and protein alignments. Nucleic Acids Res. 2010, 38, W14–W18. [Google Scholar] [CrossRef]
- Nguyen, L.-T.; Schmidt, H.A.; von Haeseler, A.; Minh, B.Q. IQ-TREE: A fast and effective stochastic algorithm for estimating maximum-likelihood phylogenies. Mol. Biol. Evol. 2015, 32, 268–274. [Google Scholar] [CrossRef]
- Trifinopoulos, J.; Nguyen, L.T.; von Haeseler, A.; Minh, B.Q. W-IQ-TREE: A fast online phylogenetic tool for maximum likelihood analysis. Nucleic Acids Res. 2016, 44, W232–W235. [Google Scholar] [CrossRef]
- Felsenstein, J. Confidence limits on phylogenies: An approach using the bootstrap. Evolution 1985, 39, 783–791. [Google Scholar] [CrossRef]
- Stöver, B.C.; Müller, K.F. TreeGraph 2: Combining and visualizing evidence from different phylogenetic analyses. BMC Bioinform. 2010, 11, 7. [Google Scholar] [CrossRef]
- Pitassi, L.H.U.; de Paiva Diniz, P.P.V.; Scorpio, D.G.; Drummond, M.R.; Lania, B.G.; Barjas-Castro, M.L.; Gilioli, R.; Colombo, S.; Sowy, S.; Breitschwerdt, E.B.; et al. Bartonella spp. bacteremia in blood donors from Campinas, Brazil. PLoS Negl. Trop. Dis. 2015, 9, e0003467. [Google Scholar] [CrossRef]
- Drummond, M.R.; Dos Santos, L.S.; de Almeida, A.R.; Lins, K.D.A.; Barjas-Castro, M.L.; Diniz, P.P.V.D.P.; Velho, P.E.N.F. Comparison of molecular methods for Bartonella henselae detection in blood donors. PLoS Negl. Trop. Dis. 2023, 17, e0011336. [Google Scholar] [CrossRef]
- Crawford, K.; Walton, J.; Lewis, D.; Tasker, S.; Warman, S.M. Infectious agent screening in canine blood donors in the United Kingdom. J. Small Anim. Pract. 2013, 54, 414–417. [Google Scholar] [CrossRef]
- Furquim, M.E.C.; do Amaral, R.; Dias, C.M.; Gonçalves, L.R.; Perles, L.; de Paula Lima, C.A.; Barros-Battesti, D.M.; Machado, R.Z.; André, M.R. Genetic diversity and Multilocus Sequence Typing Analysis of Bartonella henselae in domestic cats from Southeastern Brazil. Acta Trop. 2021, 222, 106037. [Google Scholar] [CrossRef]
- Do Amaral, R.B.; Cardozo, M.V.; de Mello Varani, A.; Gonçalves, L.R.; Furquim, M.E.C.; Dias, C.M.; Santana, M.Z.; Assis, W.O.; da Silva, A.R.; Herrera, H.M.; et al. Bartonella machadoae sp. nov. isolated from wild rodents in the Pantanal wetland. Acta Trop. 2022, 229, 106368. [Google Scholar] [CrossRef]
- Dias, C.M.; do Amaral, R.B.; Perles, L.; dos Santos Muniz, A.L.; Rocha, T.F.G.; Machado, R.Z.; André, M.R. Multi-locus Sequencing Typing of Bartonella henselae isolates reveals coinfection with different variants in domestic cats from Midwestern Brazil. Acta Trop. 2023, 237, 106742. [Google Scholar] [CrossRef] [PubMed]
- Diniz, P.P.; Maggi, R.G.; Schwartz, D.S.; Cadenas, M.B.; Bradley, J.M.; Hegarty, B.; Breitschwerdt, E.B. Canine bartonellosis: Serological and molecular prevalence in Brazil and evidence of co-infection with Bartonella henselae and Bartonella vinsonii subsp. berkhoffii. Vet. Res. 2007, 38, 701–710. [Google Scholar]
- das Neves, L.F.; Dias, C.M.; Mongruel, A.C.B.; de Oliveira Lopes, G.; do Rosario Batista, L.M.; Araujo, F.A.A.; Pereira, G.T.; Machado, R.Z.; André, M.R. Zoonotic variants of Bartonella henselae in domesticated cats, including blood donors, in central-western Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2025, 123, 102398. [Google Scholar] [CrossRef]
- André, M.R.; Canola, R.A.M.; Braz, J.B.; Perossi, I.F.S.; Calchi, A.C.; Ikeda, P.; Machado, R.Z.; Vasconcelos, R.O.; Camacho, A.A. Aortic valve endocarditis by Bartonella clarridgeiae in a dog in Brazil. Rev. Bras. Parasitol. Vet. 2019, 28, 661–670. [Google Scholar] [CrossRef] [PubMed]
- Mendonça, C.S.; Mundim, A.V.; Costa, A.S.; Moro, T.V. Erliquiose canina: Alterações hematológicas em cães domésticos naturalmente infectados. Biosci. J. 2005, 21, 167–174. [Google Scholar]
- Holanda, L.C.D.; Almeida, T.L.A.C.D.; Mesquita, R.M.D.; Oliveira Júnior, M.B.D.; Oliveira, A.A.D.F. Hematological observations in the blood and bone marrow of dogs naturally infected by Ehrlichia spp. and Anaplasma spp. Ciênc. Anim. Bras. 2019, 20, e47686. [Google Scholar] [CrossRef]
- Assad, R.Q.; Junior, E.S.; Lambert, M.M.; Corrêa, C.B.S.; Lemos, T.D.; Toma, H.K.; Scott, F.B.; Almosny, N.R.P. Detecção molecular e alterações hematológicas de amostras de cães naturalmente infectados por Babesia vogeli na região metropolitana do Rio de Janeiro. Braz. J. Vet. Med. 2020, 42, e032120. [Google Scholar] [CrossRef]
- Correia, B.; Magalhães, A.; Rocha, L.; Cardoso, I.; Ferreira, R.R.F.; Mesa-Sanchez, I. Prevalence of subclinical infectious agents in a blood donor population tested on every donation. J. Small Anim. Pract. 2024, 65, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Jefferies, R.; Ryan, U.M.; Jardine, J.; Broughton, D.K.; Robertson, I.D.; Irwin, P.J. Blood, bull terriers and babesiosis: Further evidence for direct transmission of Babesia gibsoni in dogs. Aust. Vet. J. 2007, 85, 459–463. [Google Scholar] [CrossRef]
- Irwin, P.J. Canine babesiosis: From molecular taxonomy to control. Parasites Vectors 2009, 2 (Suppl. S1), S4. [Google Scholar] [CrossRef] [PubMed]
- Silaghi, C.; Kohn, B.; Chirek, A.; Thiel, C.; Nolte, I.; Liebisch, G.; Pfister, K. Relationship of molecular and clinical findings on Anaplasma phagocytophilum involved in natural infections of dogs. J. Clin. Microbiol. 2011, 49, 4413–4414. [Google Scholar] [CrossRef]
- Lasta, C.S.; Santos, A.P.; Messick, J.B.; Oliveira, S.T.; Biondo, A.W.; Vieira, R.F.D.C.; Dalmolin, M.L.; González, F.H.D. Molecular detection of Ehrlichia canis and Anaplasma platys in dogs in Southern Brazil. Rev. Bras. Parasitol. Vet. 2013, 22, 360–366. [Google Scholar] [CrossRef] [PubMed]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasites Vectors 2015, 8, 75. [Google Scholar] [CrossRef]
- Willi, B.; Novacco, M.; Meli, M.; Wolf-Jäckel, G.; Boretti, F.; Wengi, N.; Lutz, H.; Hofmann-Lehmann, R. Haemotropic mycoplasmas of cats and dogs: Transmission, diagnosis, prevalence and importance in Europe. Schweiz. Arch. Tierheilkd. 2010, 152, 237–244. [Google Scholar] [CrossRef]
- Zobba, R.; Corda, A.; Ballocco, I.; Sotgiu, F.; Alberti, A.; Antognoni, M.T.; Pinna Parpaglia, M.L. Immune-Mediated Hemolytic Anemia Associated with Mycoplasma haematoparvum in a Splenectomized Dog in Italy. Acta Vet. Beogr. 2019, 70, 277–284. [Google Scholar] [CrossRef]
- Tasker, S. Hemotropic Mycoplasma. In Clinical Small Animal Internal Medicine; Wiley: Hoboken, NJ, USA, 2020; pp. 927–930. [Google Scholar] [CrossRef]
- Compton, S.M.; Maggi, R.G.; Breitschwerdt, E.B. ‘Candidatus Mycoplasma haematoparvum’ and Mycoplasma haemocanis infections in dogs from the United States. Comp. Immunol. Microbiol. Infect. Dis. 2012, 35, 557–562. [Google Scholar] [CrossRef]
- Messick, J.B. Hemotrophic mycoplasmas (hemoplasmas): A review and new insights into pathogenic potential. Vet. Clin. Pathol. 2004, 33, 2–13. [Google Scholar] [CrossRef] [PubMed]
- Lester, S.J.; Hume, J.B.; Phipps, B. Haemobartonella canis infection following splenectomy and transfusion. Can. Vet. J. 1995, 36, 444–445. [Google Scholar]
- Beaufils, J.P. Anémie hémolytique chez un chien infecté par Mycoplasma haemocanis. Prat. Méd. Chir. Anim. Comp. 2012, 47, 43–47. [Google Scholar] [CrossRef]
- Chalker, V.J. Canine mycoplasmas. Res. Vet. Sci. 2005, 79, 1–8. [Google Scholar] [CrossRef]
- Dagnone, A.S.; Souza, A.I.; André, M.R.; Machado, R.Z. Molecular diagnosis of Anaplasmataceae organisms in dogs with clinical and microscopical signs of ehrlichiosis. Rev. Bras. Parasitol. Vet. 2009, 18, 20–25. [Google Scholar] [CrossRef]
- Ramos, C.A.; Ramos, R.A.; Araújo, F.R.; Guedes, D.S., Jr.; Souza, I.I.; Ono, T.M.; Vieira, A.S.; Pimentel, D.S.; Rosas, E.O.; Faustino, M.A.G.; et al. Comparison of nested-PCR with blood smear examination in detection of Ehrlichia canis and Anaplasma platys in dogs. Rev. Bras. Parasitol. Vet. 2009, 18, 58–62. [Google Scholar] [CrossRef]
- Vieira, R.F.D.C.; Biondo, A.W.; Guimarães, A.M.S.; Santos, A.P.; Santos, R.P.; Dutra, L.H.; Diniz, P.P.V.P.; Morais, H.A.; Messick, J.B.; Labruna, M.B.; et al. Ehrlichiosis in Brazil. Rev. Bras. Parasitol. Vet. 2011, 20, 1–12. [Google Scholar] [CrossRef]
- Araujo, A.C.; Silveira, J.A.; Azevedo, S.S.; Nieri-Bastos, F.A.; Ribeiro, M.F.; Labruna, M.B.; Horta, M.C. Babesia canis vogeli infection in dogs and ticks in the semiarid region of Pernambuco, Brazil. Pesq. Vet. Bras. 2015, 35, 456–461. [Google Scholar] [CrossRef]
- Soares, R.; Ramos, C.A.; Pedroso, T.; Babo-Terra, V.; Cleveland, H.; Araújo, F. Molecular survey of Anaplasma platys and Ehrlichia canis in dogs from Campo Grande, Mato Grosso do Sul, Brazil. An. Acad. Bras. Cienc. 2017, 89, 301–306. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, J.; Hiura, E.; Sobral, S.A.; de Toledo Vieira, F.; Braga, F.R.; Tobias, F.L.; Aguiar, D.M.; Langoni, H. Ocorrência de Babesia sp., Ehrlichia canis e Hepatozoon canis em cães domiciliados em dois municípios do estado do Espírito Santo–Brasil. Vet. Zootec. 2022, 29, 1–9. [Google Scholar] [CrossRef]
- Kmetiuk, L.B.; Shaw, P.; Wallington, A.; Kattoor, J.J.; Johnson, M.; Wilkes, R.P.; Biondo, L.M.; Giuffrida, R.; Santarém, V.A.; Figueiredo, F.B.; et al. Hemotropic mycoplasmas (hemoplasmas) in indigenous populations and their dogs living in reservation areas, Brazil. Sci. Rep. 2025, 15, 7973. [Google Scholar] [CrossRef]
- Fernandes, A.J.; Elshafie, N.O.; Kmetiuk, L.B.; Ullmann, L.S.; Brandão, A.P.D.; Haisi, A.; van Wilpe Bach, R.; de Barros-Filho, I.R.; Araújo Junior, J.P.; Barbosa, D.S.; et al. Hemotropic mycoplasmas (hemoplasmas) in wild boars, hunting dogs, and hunters from two Brazilian regions. Transbound. Emerg. Dis. 2022, 69, 908–912. [Google Scholar] [CrossRef] [PubMed]
- Valle, S.F.; Messick, J.B.; Dos Santos, A.P.; Kreutz, L.C.; Duda, N.C.; Machado, G.; Corbellini, L.G.; Biondo, A.W.; González, F.H.D. Identification, occurrence and clinical findings of canine hemoplasmas in southern Brazil. Comp. Immunol. Microbiol. Infect. Dis. 2014, 37, 259–265. [Google Scholar] [CrossRef]
- Vieira, R.F.D.C.; Vidotto, O.; Vieira, T.S.W.J.; Guimaraes, A.M.S.; Santos, A.P.; Nascimento, N.C.; dos Santos, N.J.R.; Martins, T.F.; Labruna, M.B.; Marcondes, M.; et al. Molecular investigation of hemotropic mycoplasmas in human beings, dogs and horses in a rural settlement in southern Brazil. Rev. Inst. Med. Trop. São Paulo 2015, 57, 353–357. [Google Scholar] [CrossRef]
- Kmetiuk, L.B.; Shaw, P.; Wallington, A.; Panazzolo, G.K.; Domingues, O.J.; Kattoor, J.J.; Johnson, M.; Biondo, L.M.; Wilkes, R.; Figueiredo, F.B.; et al. One Health approach to hemotropic mycoplasmas (hemoplasmas): Molecular detection in quilombola communities and their dogs in Brazil. One Health 2025, 20, 101024. [Google Scholar] [CrossRef] [PubMed]
- Wardrop, K.J.; Reine, N.; Birkenheuer, A.; Hale, A.; Hohenhaus, A.; Crawford, C.; Lappin, M.R. Canine and feline blood donor screening for infectious disease. J. Vet. Intern. Med. 2005, 19, 135–142. [Google Scholar] [CrossRef]
- Bouza-Mora, L.; Dolz, G.; Solórzano-Morales, A.; Romero-Zuñiga, J.J.; Salazar-Sánchez, L.; Labruna, M.B.; Aguiar, D.M. Novel genotype of Ehrlichia canis detected in samples of human blood bank donors in Costa Rica. Ticks Tick Borne Dis. 2017, 8, 36–40. [Google Scholar] [CrossRef] [PubMed]
- Maggi, R.G.; Mascarelli, P.E.; Havenga, L.N.; Naidoo, V.; Breitschwerdt, E.B. Co-infection with Anaplasma platys, Bartonella henselae and ‘Candidatus Mycoplasma haematoparvum’ in a veterinarian. Parasites Vectors 2013, 6, 103. [Google Scholar] [CrossRef]
- Louly, C.C.B.; Fonseca, I.N.; Oliveira, V.F.D.; Linhares, G.F.C.; Menezes, L.B.D.; Borges, L.M.F. Seasonal dynamics of Rhipicephalus sanguineus (Acari: Ixodidae) in dogs from a police unit in Goiânia, Goiás, Brazil. Ciênc. Rural. 2007, 37, 464–469. [Google Scholar] [CrossRef]

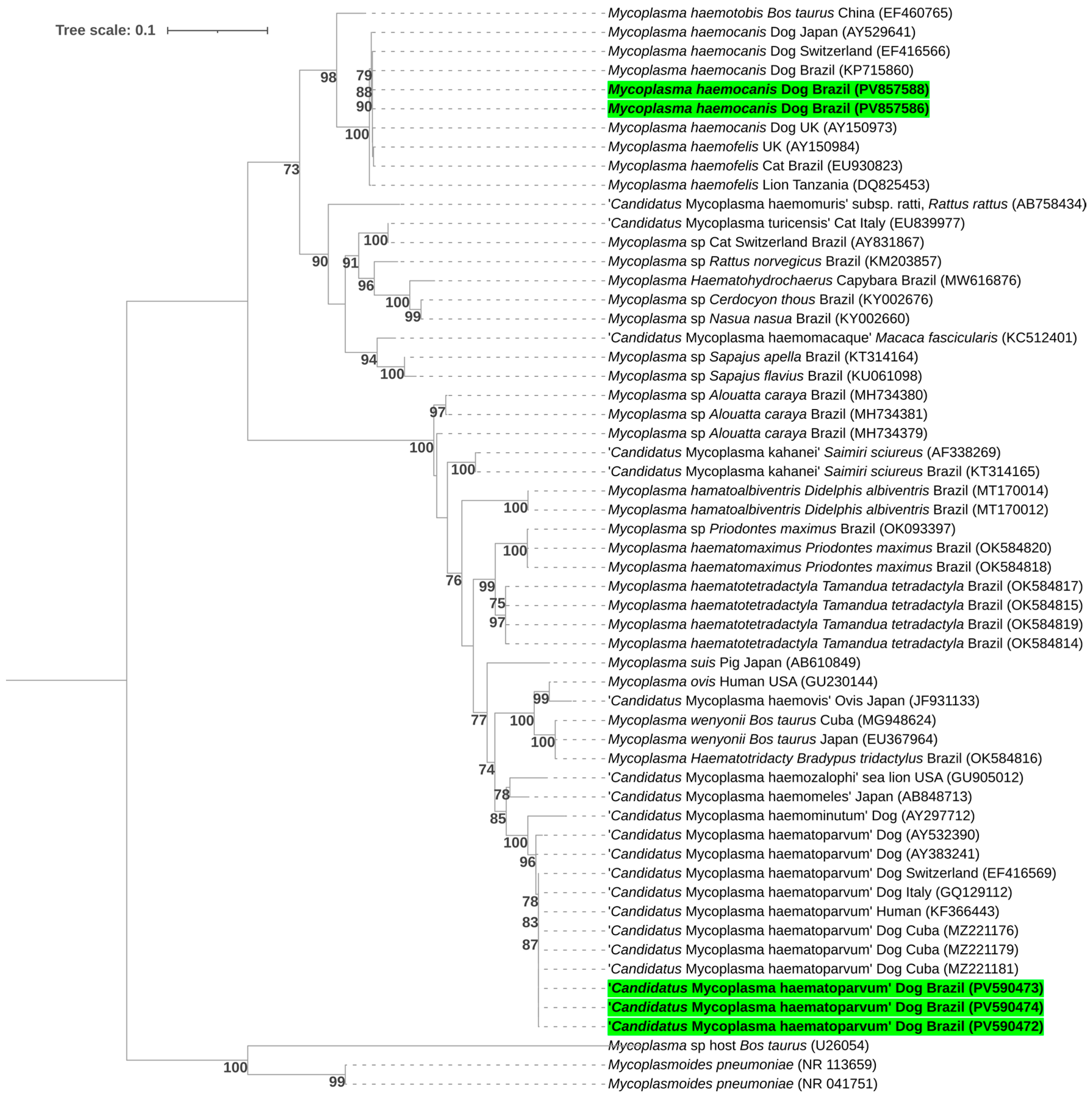
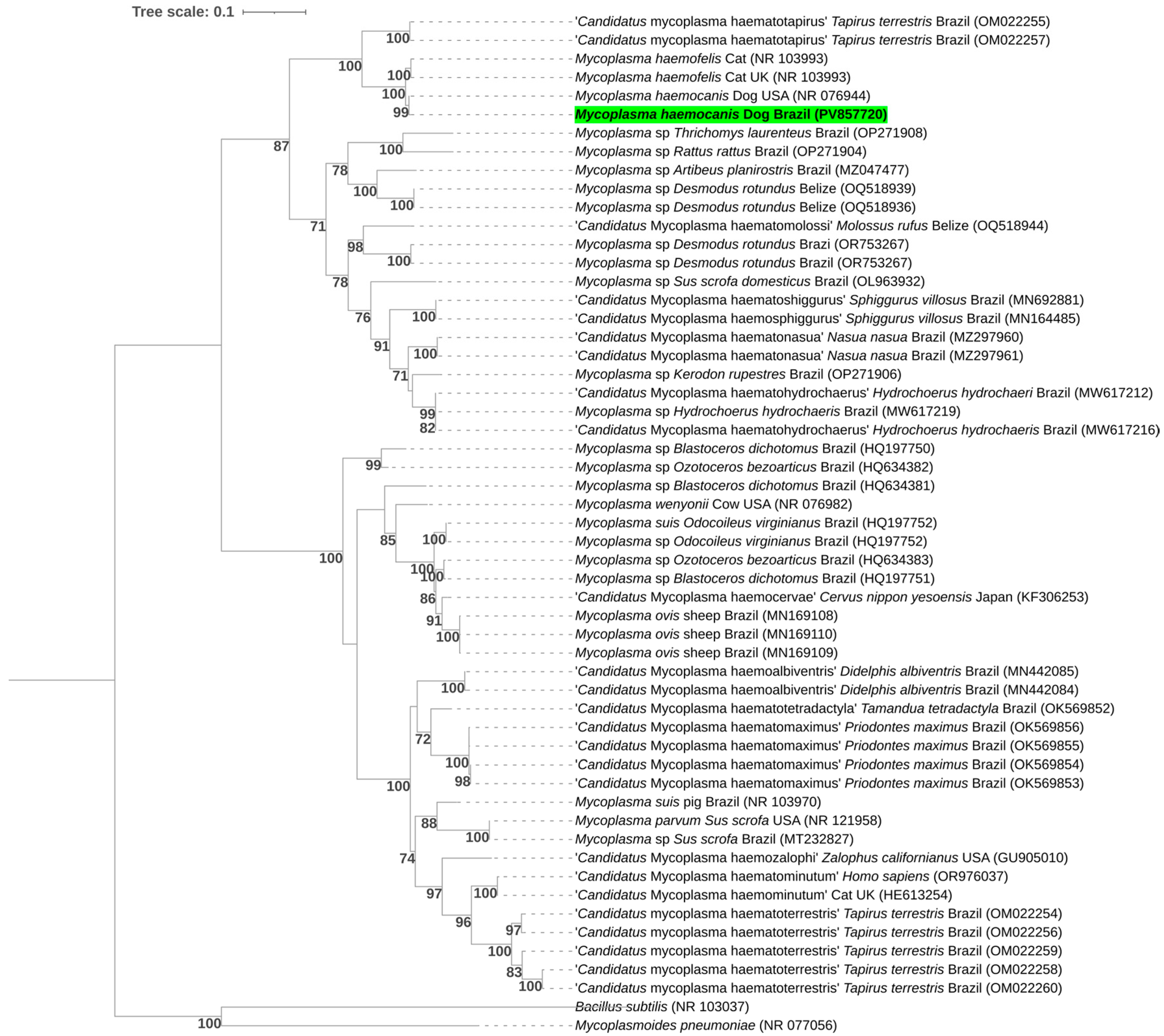
| GenBank Accession Number | Group | Target Gene | Sequence Length (bp) | Query Coverage | E-Value | Closest Match in GenBank | Host/Country |
|---|---|---|---|---|---|---|---|
| PV603924 | Clinical routine | 18S rRNA | 776 | 100% | 0.0 | 99.74%—Babesia vogeli (MK881089) | Dog/China |
| PV603925 | Clinical routine | 18S rRNA | 658 | 100% | 0.0 | 100%—Babesia vogeli (MN823199.1) | Dog/Brazil |
| PV608394 | Clinical routine | cox-1 | 515 | 100% | 0.0 | 100%—Babesia vogeli (KC207825) | Dog/USA |
| PV608395 | Clinical routine | cox-1 | 523 | 100% | 0.0 | 100%—Babesia vogeli (MZ577094) | Dog/Brazil |
| PV608396 | Clinical routine | cox-1 | 584 | 100% | 0.0 | 100%—Babesia vogeli (KC207825) | Dog/USA |
| PV608397 | Clinical routine | hsp70 | 633 | 100% | 0.0 | 100.00%—Babesia vogeli (AB248733) | Dog/Japan |
| PV608398 | Clinical routine | hsp70 | 602 | 100% | 0.0 | 98.69%—Babesia vogeli (AB248733) | Dog/Japan |
| PV608399 | Clinical routine | dsb | 308 | 100% | 6 × 10−158 | 100% Ehrlichia canis (MT005829) | Rhipicephalus linnaei/Australia |
| PV575161 | Clinical routine | 16S rRNA | 482 | 100% | 0.0 | 100% Anaplasma platys (MK814414) | Dog/South Africa |
| PV575162 | Clinical routine | 16S rRNA | 513 | 100% | 0.0 | 100% Anaplasma platys (MK814414) | Dog/South Africa |
| PV590472 | Clinical routine | 16S rRNA | 1027 | 100% | 0.0 | 99.52%—‘Candidatus Mycoplasma haematoparvum’ (AY383241) | Dog/USA |
| PV590473 | Blood donor | 16S rRNA | 978 | 100% | 0.0 | 100%—‘Candidatus Mycoplasma haematoparvum’ (MZ221179) | Dog/Cuba |
| PV590474 | Blood donor | 16S rRNA | 897 | 100% | 0.0 | 100%—‘Candidatus Mycoplasma haematoparvum’ (MZ221181) | Dog/Cuba |
| PV857586 | Clinical routine | 16S rRNA | 739 | 100% | 0.0 | 100%—Mycoplasma haemocanis (GQ129116) | Dog/Italy |
| PV857587 | Blood donor | 16S rRNA | 423 | 100% | 0.0 | 100%—Mycoplasma haemocanis (MT816510) | Dog/Portugal |
| PV857588 | Blood donor | 16S rRNA | 701 | 100% | 0.0 | 100%—Mycoplasma haemocanis (GQ129116) | Dog/Italy |
| PV857719 | Clinical routine | 23S rRNA | 256 | 100% | 4 × 10−129 | 100%—Mycoplasma haemocanis (NR_076944) | Dog/USA |
| PV857720 | Blood donor | 23S rRNA | 435 | 100% | 0.0 | 99.77%—Mycoplasma haemocanis (NR_076944) | Dog/USA |
| Hemopathogen | Dog Group | N | Positive | Prevalence (%) | CI_Low | CI_High |
|---|---|---|---|---|---|---|
| Anaplasma spp. | CL | 100 | 6 | 6 | 0.028 | 0.125 |
| CB | 100 | 0 | 0 | 0 | 0.037 | |
| Babesia spp. | CL | 100 | 3 | 3 | 0.010 | 0.085 |
| CB | 100 | 0 | 0 | 0 | 0.037 | |
| Bartonella spp. | CL | 100 | 0 | 0 | 0 | 0.037 |
| CB | 100 | 0 | 0 | 0 | 0.037 | |
| Ehrlichia spp. | CL | 100 | 15 | 15 | 0.093 | 0.233 |
| CB | 100 | 0 | 0 | 0 | 0.037 | |
| Mycoplasma spp. | CL | 100 | 2 | 2 | 0.006 | 0.070 |
| CB | 100 | 5 | 5 | 0.022 | 0.112 |
| Hemopathogen | Dog Sex | N | Positive | Prevalence (%) | CI_Low | CI_High |
|---|---|---|---|---|---|---|
| Anaplasma spp. | M | 114 | 4 | 3.51 | 0.014 | 0.087 |
| F | 86 | 2 | 2.33 | 0.006 | 0.081 | |
| Babesia spp. | M | 114 | 0 | 0 | 0 | 0.033 |
| F | 86 | 3 | 3.49 | 0.012 | 0.098 | |
| Bartonella spp. | M | 114 | 0 | 0 | 0 | 0.033 |
| F | 86 | 0 | 0 | 0 | 0.043 | |
| Ehrlichia spp. | M | 114 | 8 | 7.02 | 0.036 | 0.132 |
| F | 86 | 7 | 8.14 | 0.040 | 0.159 | |
| Mycoplasma spp. | M | 114 | 3 | 2.63 | 0.009 | 0.075 |
| F | 86 | 4 | 4.65 | 0.018 | 0.114 |
| Hemopathogen | p_Value | Odds_Ratio | CI_Low | CI_High |
|---|---|---|---|---|
| Anaplasma spp. | 0.02893028 | 0 | 0 | 0.826 |
| Babesia spp. | 0.24623116 | 0 | 0 | 2.406 |
| Bartonella spp. | 1 | 0 | 0 | Inf |
| Ehrlichia spp. | 3.4634 × 10−5 | 0 | 0 | 0.248 |
| Mycoplasma spp. | 0.44475403 | 2.56747153 | 0.408 | 27.586 |
| Hemopathogen | p_Value | Odds_Ratio | CI_Low | CI_High |
|---|---|---|---|---|
| Anaplasma spp. | 0.70137731 | 0.65609672 | 0.058 | 4.702 |
| Babesia spp. | 0.0779199 | Inf | 0.553 | Inf |
| Bartonella spp. | 1 | 0 | 0 | Inf |
| Ehrlichia spp. | 0.79169103 | 1.17309712 | 0.346 | 3.876 |
| Mycoplasma spp. | 0.46605801 | 1.7994358 | 0.296 | 12.621 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
da Silva, J.V.d.S.A.; das Neves, L.F.; Bolzan, M.E.; Batista, L.M.d.R.; Araujo, F.A.A.; Machado, R.Z.; André, M.R. Molecular Survey of Hemopathogens in Dogs, Including Blood Donors, from Central-Western Brazil. Pathogens 2025, 14, 1180. https://doi.org/10.3390/pathogens14111180
da Silva JVdSA, das Neves LF, Bolzan ME, Batista LMdR, Araujo FAA, Machado RZ, André MR. Molecular Survey of Hemopathogens in Dogs, Including Blood Donors, from Central-Western Brazil. Pathogens. 2025; 14(11):1180. https://doi.org/10.3390/pathogens14111180
Chicago/Turabian Styleda Silva, João Vitor dos Santos Alves, Lorena Freitas das Neves, Maria Eduarda Bolzan, Liliane Maria do Rosario Batista, Francisco Anilton Alves Araujo, Rosangela Zacarias Machado, and Marcos Rogério André. 2025. "Molecular Survey of Hemopathogens in Dogs, Including Blood Donors, from Central-Western Brazil" Pathogens 14, no. 11: 1180. https://doi.org/10.3390/pathogens14111180
APA Styleda Silva, J. V. d. S. A., das Neves, L. F., Bolzan, M. E., Batista, L. M. d. R., Araujo, F. A. A., Machado, R. Z., & André, M. R. (2025). Molecular Survey of Hemopathogens in Dogs, Including Blood Donors, from Central-Western Brazil. Pathogens, 14(11), 1180. https://doi.org/10.3390/pathogens14111180





