Clinical Presentation, Management and Outcome of Cerebral Echinococcosis in Children: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
- To evaluate the clinical presentations, diagnostic approaches, and treatment strategies used in pediatric patients (0–18 years) with cerebral echinococcosis.
- To analyze post-treatment outcomes including treatment-related complications.
- To identify prognostic factors, such as cyst burden/location, treatment timing, the correlation between specific treatments and outcomes and between instrumental diagnosis and outcomes.
2. Materials and Methods
2.1. Systematic Review and Meta-Analysis
2.2. Search Strategy
2.3. Eligibility Criteria
- Population
- Patients in pediatric age (0–18 years) with echinococcal infection and neurological involvement
- Intervention/Exposure
- Research documenting natural history, diagnostic techniques, treatment procedures, or clinical outcomes in echinococcosis with CNS or intracranial manifestations.
- Comparison
- Inclusion of studies without comparator groups was permitted, reflecting the variability in clinical practice and the overall rarity of cerebral echinococcosis.
- Outcomes
- Neurological features (e.g., location of cysts, elevated intracranial pressure, seizures, focal deficits).
- Treatment outcomes (e.g., cyst regression or resolution, recurrence, cyst’s complications).
- Other clinically relevant measures, such as mortality or outcome.
- Study Design
- Eligible designs encompassed case reports, case series, cohort analyses, case–control studies, and interventional trials (both randomized and non-randomized) presenting outcome data.
- Systematic reviews of primary studies were considered if they contributed unique, original insights or unpublished findings.
- Language
- Only studies published in English or those with sufficiently detailed English summaries were included.
2.4. Study Selection
2.5. Data Extraction
2.6. Statistical Analysis
2.7. PROSPERO Registration
3. Results
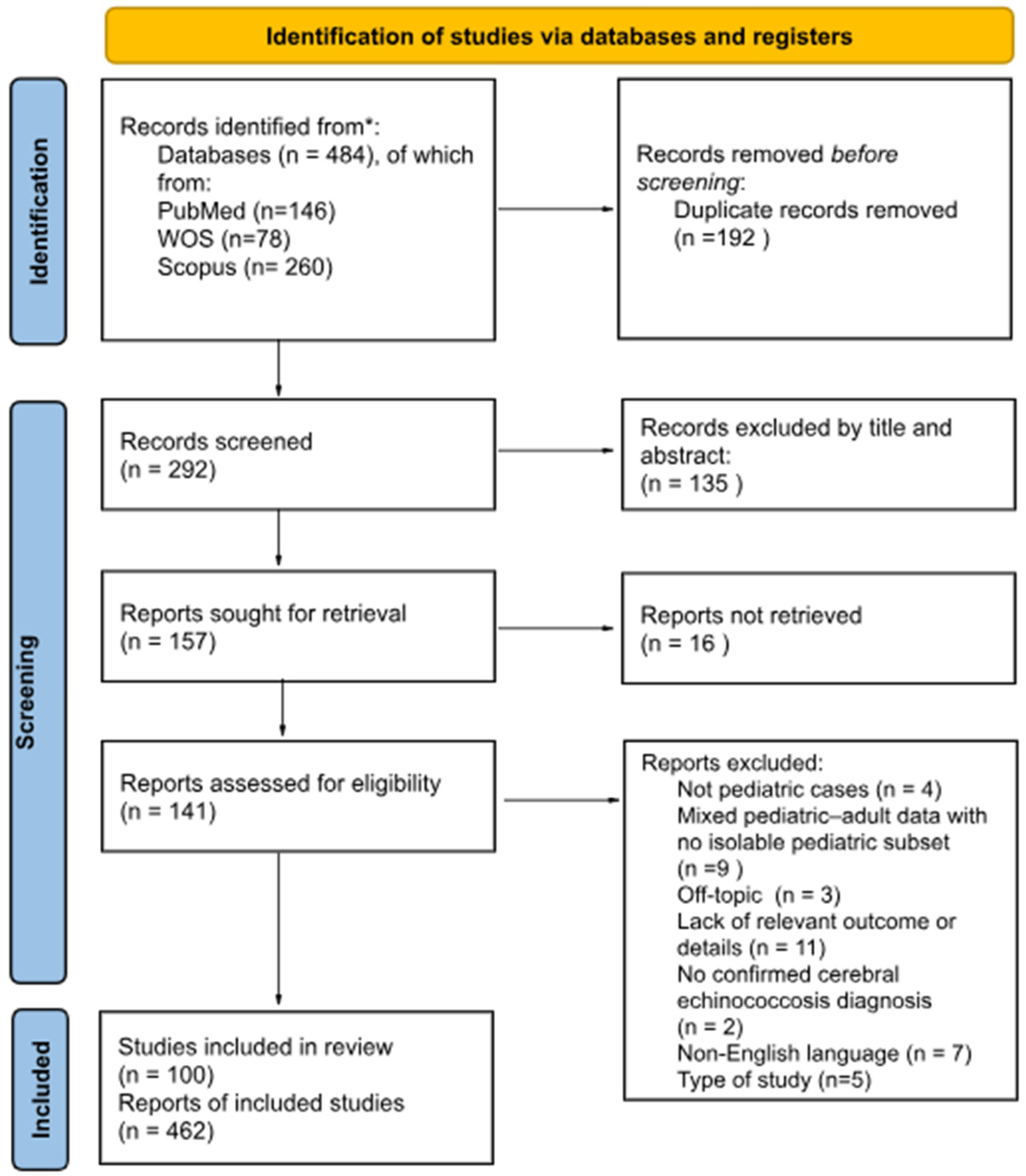
3.1. Systematic Review and Characteristics of Included Studies
3.2. Population Characteristics
3.3. Clinical Presentation
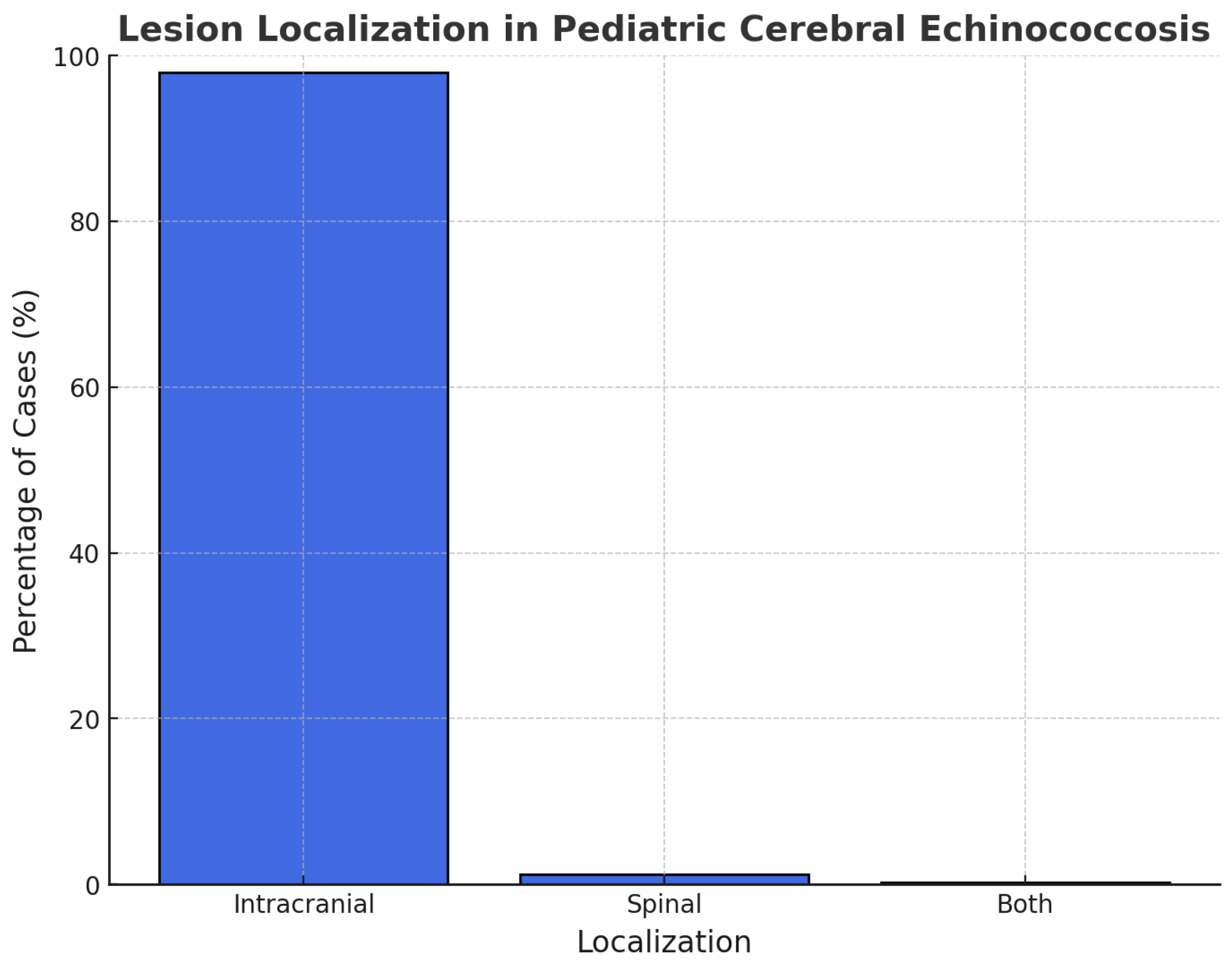
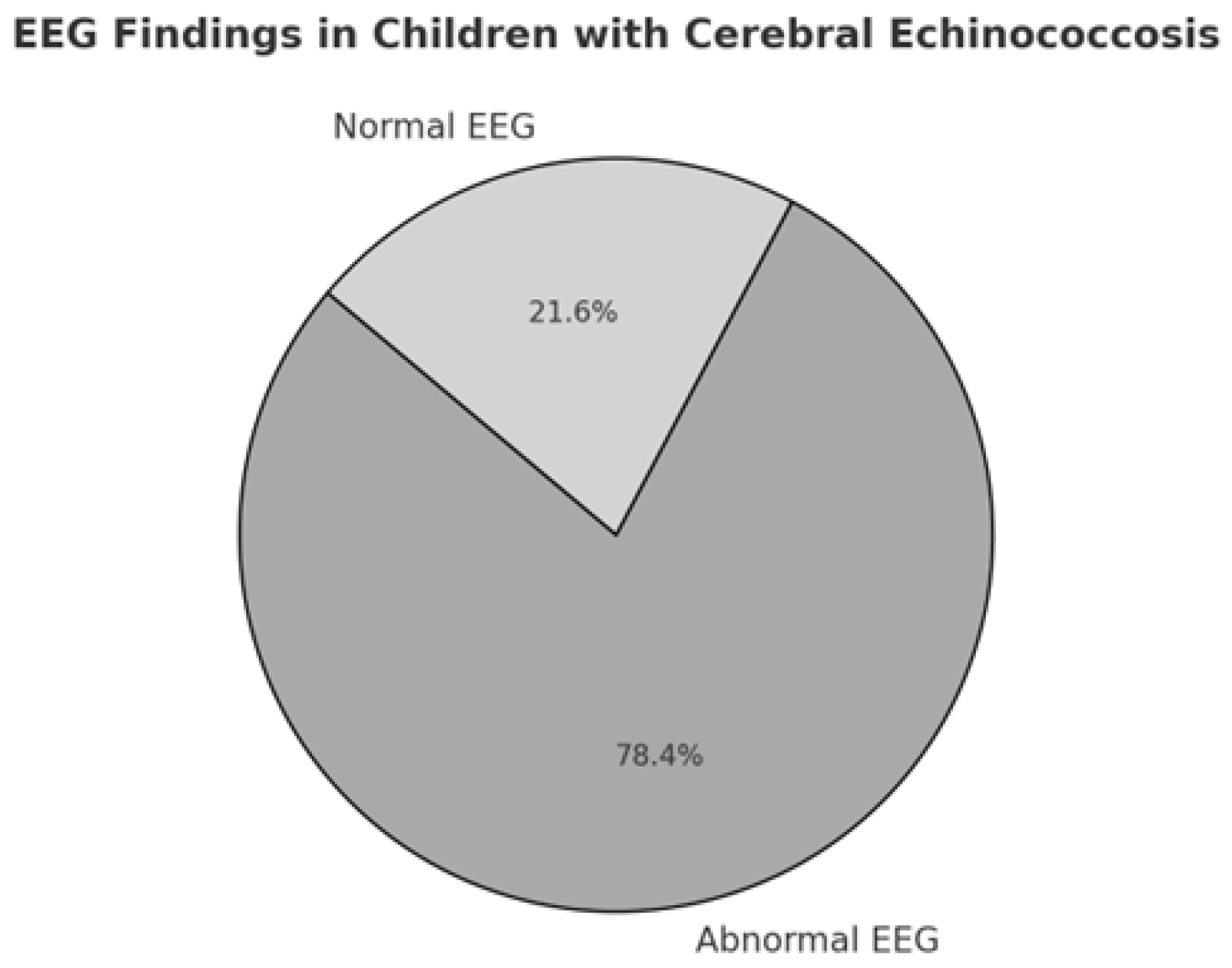
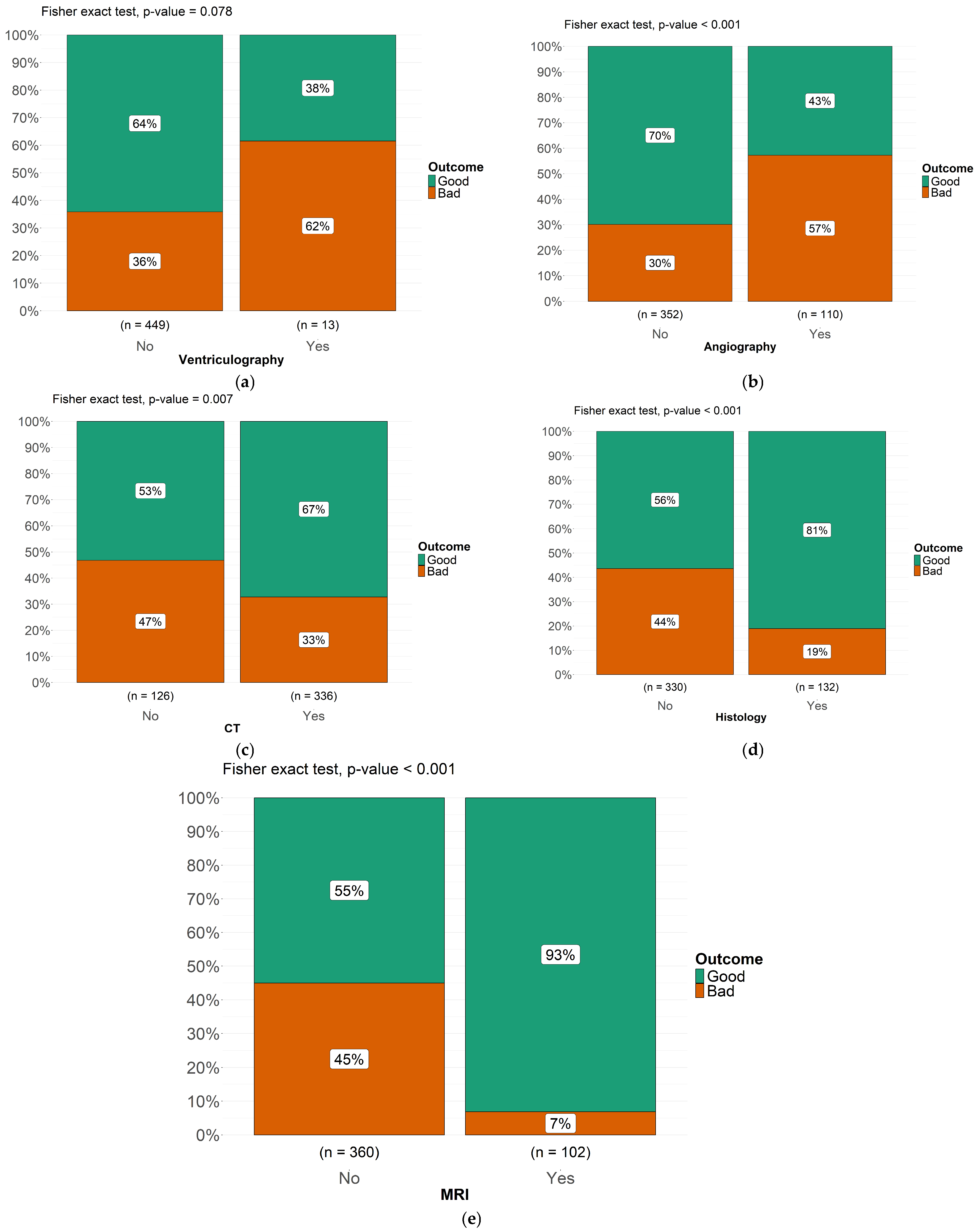
3.4. Instrumental Diagnosis
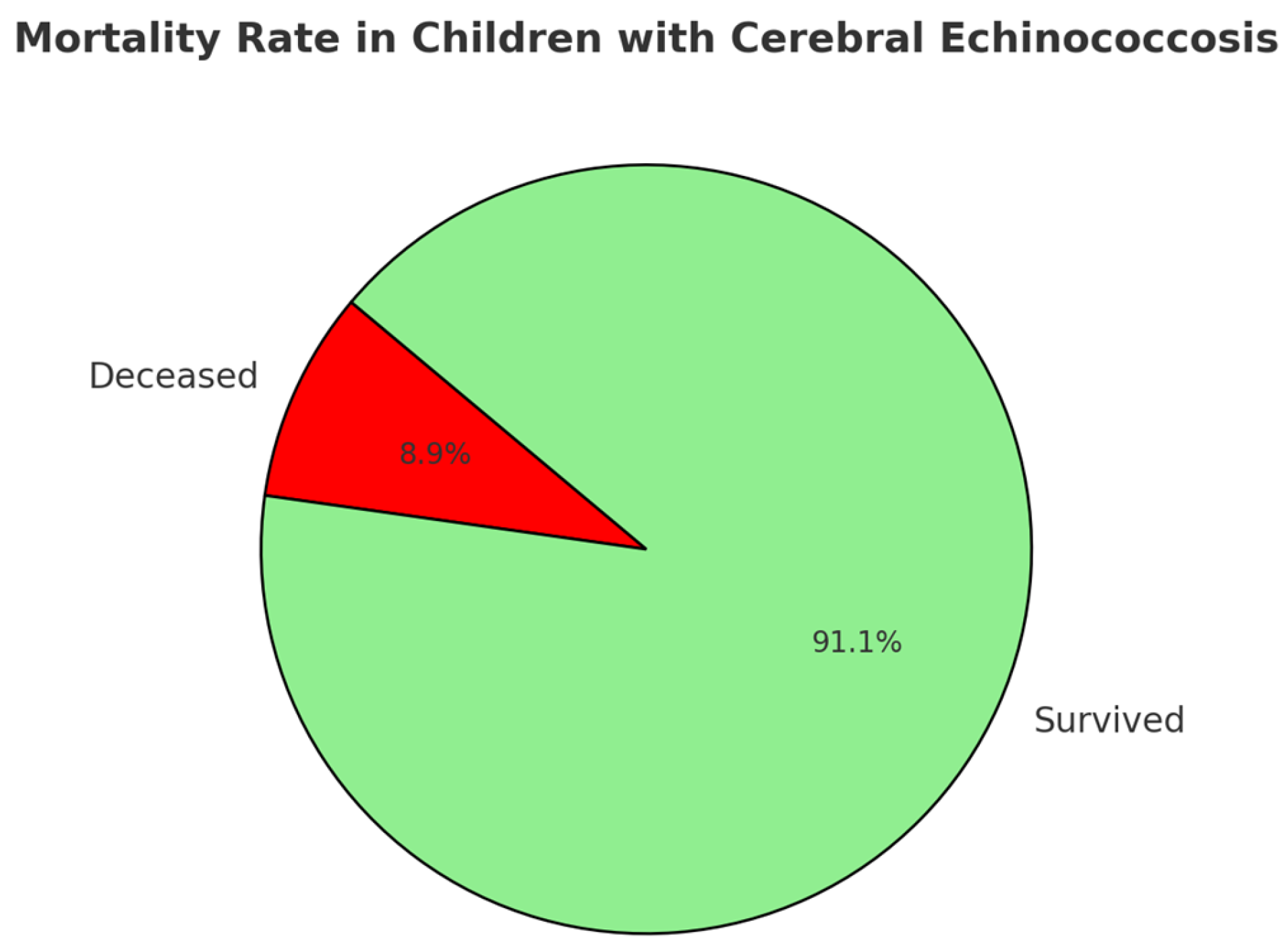
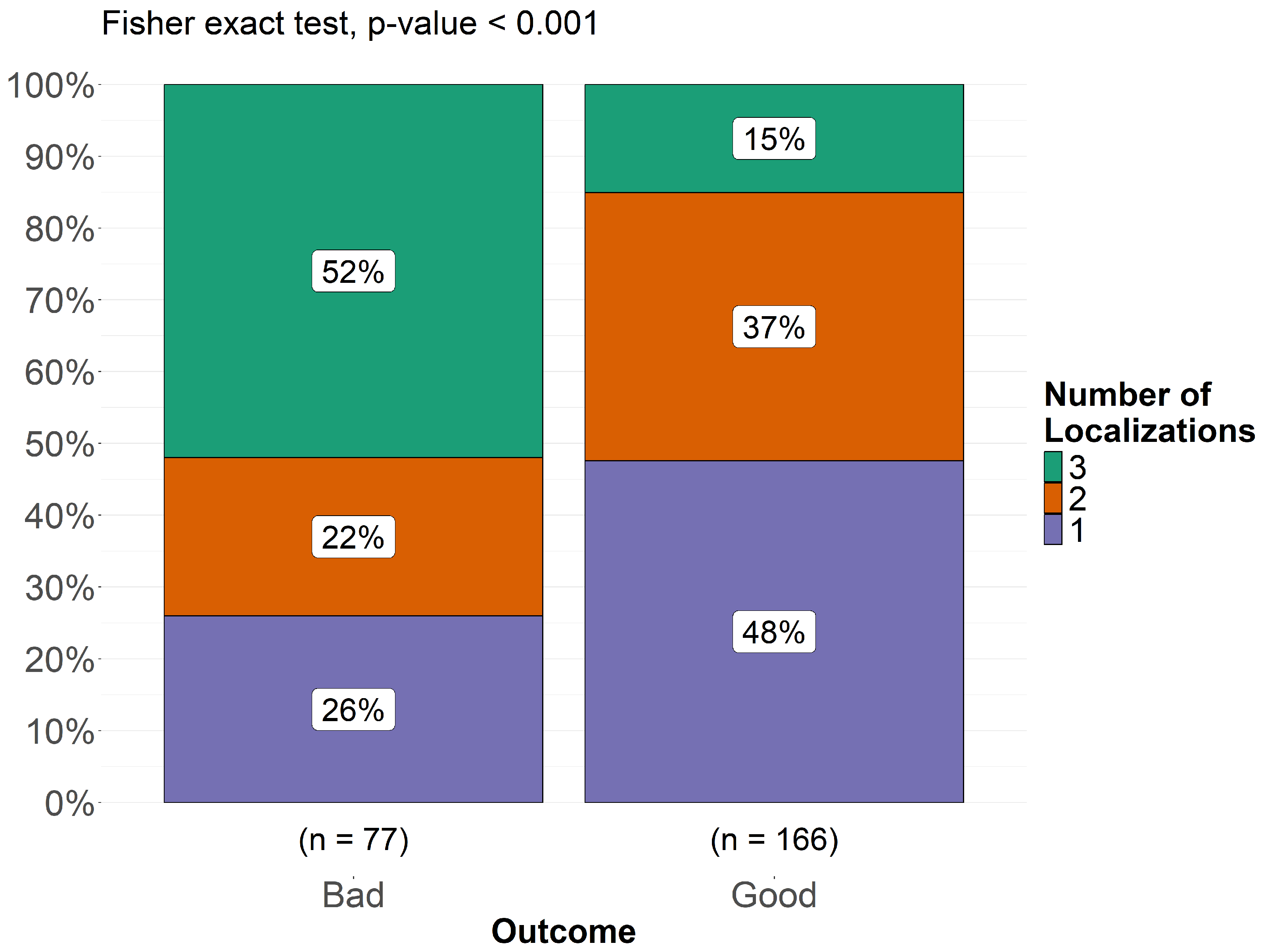
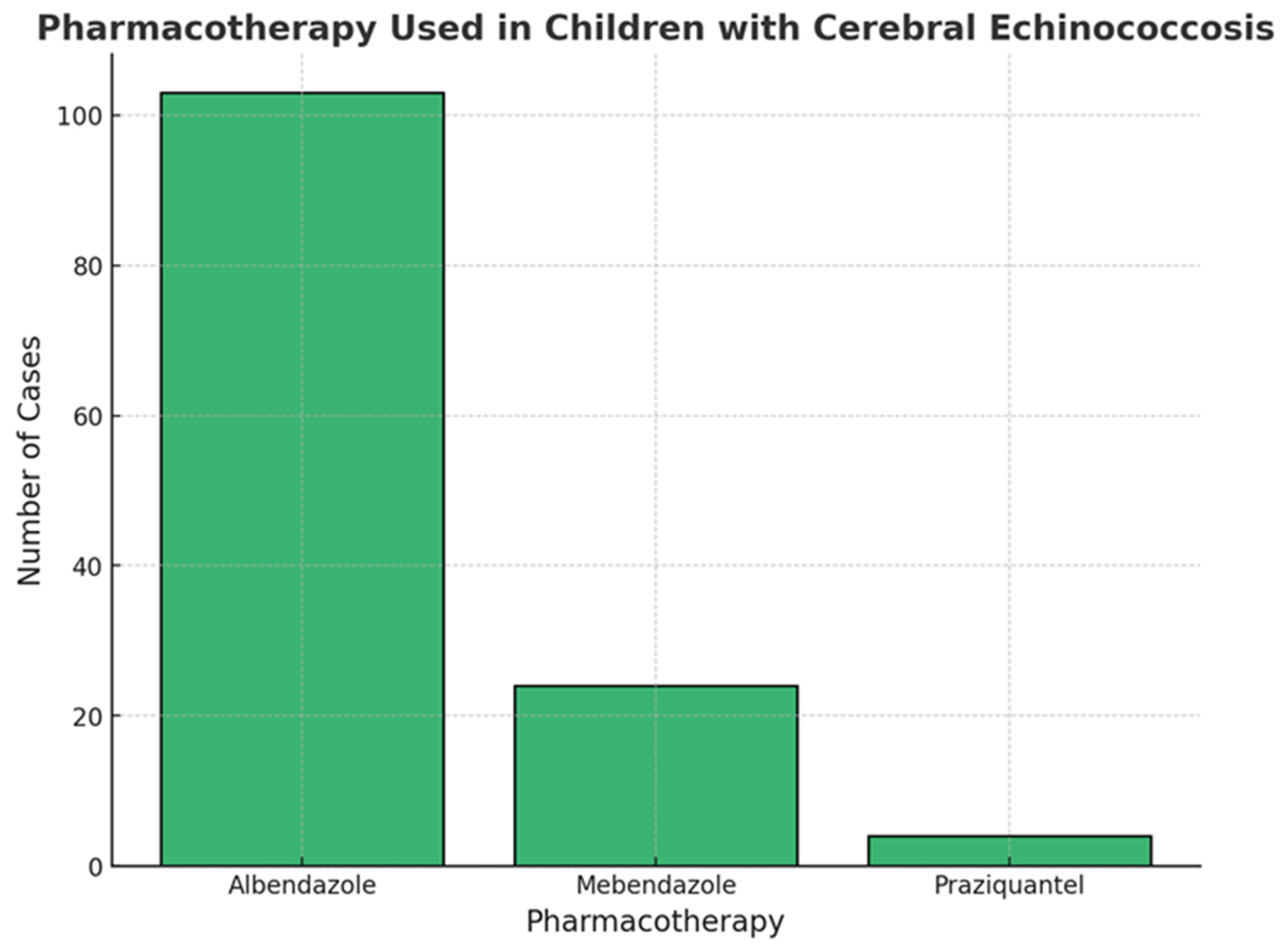
3.5. Treatment and Outcome
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CNS | Central nervous system |
| CSF | Cerebrospinal fluid |
| CT | Computed tomography |
| EEG | Electroencephalography |
| GCS | Glasgow Coma Scale |
| HIV | Human immunodeficiency virus |
| IQR | Interquartile range |
| MRI | Magnetic resonance imaging |
| p | p-value |
| PRISMA | Preferred Reporting Items for Systematic Reviews and Meta-Analyses |
| PROSPERO | International Prospective Register of Systematic Reviews |
| SD | Standard deviation |
References
- World Health Organization. Echinococcosis. Available online: https://www.who.int/news-room/fact-sheets/detail/echinococcosis (accessed on 7 November 2025).
- Garcia, H.H. Parasitic infections of the nervous system. Continuum 2021, 27, 943–962. [Google Scholar] [CrossRef]
- Carpio, A.; Romo, M.L.; Parkhouse, R.M.; Short, B.; Dua, T. Parasitic diseases of the central nervous system: Lessons for clinicians and policy makers. Expert Rev. Neurother. 2016, 16, 401–414. [Google Scholar] [CrossRef]
- Elsheikha, H.M.; Khan, N.A. Protozoa traversal of the blood–brain barrier to invade the central nervous system. FEMS Microbiol. Rev. 2010, 34, 532–553. [Google Scholar] [CrossRef]
- McManus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef]
- Eckert, J.; Deplazes, P. Biological, epidemiological, and clinical aspects of echinococcosis: A zoonosis of increasing concern. Clin. Microbiol. Rev. 2004, 17, 107–135. [Google Scholar] [CrossRef]
- Assamadi, M.; Benantar, L.; Hamadi, H.; Ksiks, O.; El Hadwe, S.; Aniba, K. Cerebral hydatid cyst in children: A case series of 21 patients and review of literature. Neurochirurgie 2022, 68, 618–626. [Google Scholar] [CrossRef]
- Padayachy, L.C.; Dattatraya, M. Hydatid disease (Echinococcus) of the central nervous system. Childs Nerv. Syst. 2018, 34, 1967–1971. [Google Scholar] [CrossRef]
- Kemaloğlu, S.; Ozkan, U.; Bükte, Y.; Acar, M.; Ceviz, A. Growth rate of cerebral hydatid cyst, with a review of the literature. Childs Nerv. Syst. 2001, 17, 743–745. [Google Scholar] [CrossRef]
- Duransoy, Y.K.; Mete, M.; Barutçuoğlu, M.; Unsal, Ü.; Selçuki, M. Intracranial hydatid cyst is a rare cause of midbrain herniation: A case report and literature review. J. Pediatr. Neurosci. 2013, 8, 224–227. [Google Scholar] [CrossRef]
- Siyadatpanah, A.; Brunetti, E.; Emami Zeydi, A.; Moghadam, Y.D.; Agudelo Higuita, N.I. Cerebral cystic echinococcosis. Case Rep. Infect. Dis. 2020, 2020, 1754231. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef] [PubMed]
- Page, M.J.; Moher, D.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. PRISMA 2020 explanation and elaboration: Updated guidance and exemplars for reporting systematic reviews. BMJ 2021, 372, n160. [Google Scholar] [CrossRef] [PubMed]
- Gomori, J.M.; Cohen, D.; Eyd, A.; Pomeranz, S. Water lily sign in CT of cerebral hydatid disease: A case report. Neuroradiology 1988, 30, 358. [Google Scholar] [CrossRef] [PubMed]
- Işık, A.D.; Sönmez, Ö.; Erdemli, P.C.; Kepenekli, E.; Ergenç, Z.; Yılmaz, S.; Tuncay, S.A.; Parlak, B.; Dağçınar, A. A 4-year-old child with a giant cerebral hydatid cyst: A case report. Iran. J. Parasitol. 2024, 19, 113–116. [Google Scholar] [CrossRef]
- Borni, M.; Abdelmouleh, S.; Taallah, M.; Blibeche, H.; Ayadi, A.; Boudawara, M.Z. A case of pediatric primary osteolytic extradural and complicated hydatid cyst revealed by a skull vault swelling. Childs Nerv. Syst. 2024, 40, 335–343. [Google Scholar] [CrossRef]
- Erşahin, Y.; Mutluer, S.; Dermirtaş, E.; Yurtseven, T. A case of thalamic hydatid cyst. Clin. Neurol. Neurosurg. 1995, 97, 321–323. [Google Scholar] [CrossRef]
- Waziri, T.M.; Rao Bollineni, V.; Kadi, R.; De Mey, J. A giant intracranial hydatid cyst in an eleven-year-old boy. J. Belg. Soc. Radiol. 2017, 101, 6. [Google Scholar] [CrossRef]
- Bakaris, S.; Sahin, S.; Yuksel, M.; Karabiber, H. A large cerebral hydatid cyst associated with liver cyst. Ann. Trop. Paediatr. 2003, 23, 313–317. [Google Scholar] [CrossRef]
- Ashraf, M.; Ahmed, S.; Ahmad, S.; Ahmad, A. A large hydatid cyst in the brain of a 10-year-old child. J. Coll. Physicians Surg. Pak. 2022, 32, 538–540. [Google Scholar] [CrossRef]
- Karakoç, Z.C.; Kasimcan, M.O.; Pipia, A.P.; Tore, G.; Alberti, A.; Varcasia, A.; Sav, A.; Oruçkaptan, H. A life-threatening brainstem compression by cerebral Echinococcus granulosus. Infez. Med. 2016, 24, 62–66. [Google Scholar]
- Elvan-Tuz, A.; Karadag-Oncel, E.; Kara-Aksay, A.; Sarioglu, F.C.; Karadag, A.; Yilmaz-Ciftdogan, D. A rare case series of central nervous system cystic echinococcosis. J. Trop. Pediatr. 2021, 67, fmab056. [Google Scholar] [CrossRef]
- Kadri, H.; Dughly, M.; Abouharb, R.; Bakleh, S.; Mackieh, R. A rare entity of primary hydatid cyst located between the two layers of the intracranial dura in a child: A case report. Oxf. Med. Case Rep. 2023, 2023, omad107. [Google Scholar] [CrossRef]
- Al-Ihribat, A.R.; Qunaibi, Y.N.; Amro, R.B.; Ahmed, J.; Rije, R.F.; Shalalfeh, H.; Ibedo, F.; Itkaidek, S. A single giant frontoparietal intra-axial hydatid cyst in a 5-year-old female: A case report and review of literature. Oxf. Med. Case Rep. 2025, 2025, omae180. [Google Scholar] [CrossRef]
- Daskas, N.; Aggelopoulos, E.; Tzoufi, M.; Kosta, P.; Siamopoulou, A.; Argyropoulou, M.I. Accidental drainage of a cerebral hydatid cyst into the peritoneal cavity. Pediatr. Infect. Dis. J. 2004, 23, 685–686. [Google Scholar] [CrossRef]
- Çavuş, G.; Açik, V.; Çavuş, Y.; Bilgin, E.; Gezercan, Y.; Ökten, A.I. An extraaxially localized intrasellar giant hydatid cyst with hypophyseal insufficiency. Childs Nerv. Syst. 2018, 34, 1391–1396. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, A.; Trebini, F.; Torta, R. Brain hydatidosis: Report of two cases. J. Neurol. Neurosurg. Psychiatry 1980, 43, 91–94. [Google Scholar] [CrossRef] [PubMed]
- Khaldi, M.; Mohamed, S.; Kallel, J.; Khouja, N. Brain hydatidosis: Report on 117 cases. Childs Nerv. Syst. 2000, 16, 765–769. [Google Scholar] [CrossRef] [PubMed]
- Khalatbari, M.R.; Brunetti, E.; Shobeiri, E.; Moharamzad, Y. Calcified mass on brain CT in a teenager with refractory seizures. Neuroradiol. J. 2014, 27, 691–696. [Google Scholar] [CrossRef]
- Menschaert, D.; Daron, A.; Frere, J. Case report of cerebral cystic echinococcosis in a 5-year-old child. Front. Trop. Dis. 2023, 4, 1090644. [Google Scholar] [CrossRef]
- Zhang, Q.; Hang, X.; Yang, M. Cerebral cystic echinococcosis among children in Qinghai, China: A case series. Southeast Asian J. Trop. Med. Public Health 2019, 50, 621–627. [Google Scholar]
- Jabbar, M.N. Cerebral cystic echinococcosis: A child with a giant hydatid cyst. New Emir. Med. J. 2021, 2, 38–41. [Google Scholar] [CrossRef]
- Boles, D.M. Cerebral echinococciasis. Surg. Neurol. 1981, 16, 280–282. [Google Scholar] [CrossRef] [PubMed]
- Ciurea, A.V.; Fountas, K.N.; Coman, T.C.; Machinis, T.G.; Kapsalaki, E.Z.; Fezoulidis, N.I.; Robinson, J.S. Long-term surgical outcome in patients with intracranial hydatid cyst. Acta Neurochir. 2006, 148, 421–426. [Google Scholar] [CrossRef] [PubMed]
- Kandemirli, S.G.; Cingoz, M.; Olmaz, B.; Akdogan, E.; Cengiz, M. Cerebral hydatid cyst with intraventricular extension: A case report. J. Trop. Pediatr. 2019, 65, 514–519. [Google Scholar] [CrossRef]
- Krajewski, R.; Stelmasiak, Z. Cerebral hydatid cysts in children. Childs Nerv. Syst. 1991, 7, 154–155. [Google Scholar] [CrossRef]
- Kocaman, S.; Ersahin, Y.; Mutluer, S. Cerebral hydatid cysts in children. J. Neurosci. Nurs. 1999, 31, 270–275. [Google Scholar] [CrossRef]
- Anderson, M.; Bickerstaff, E.R.; Hamilton, J.G. Cerebral hydatid disease in Britain. J. Neurol. Neurosurg. Psychiatry 1975, 38, 1104–1108. [Google Scholar] [CrossRef]
- Şengül, G.; Çakir, M.; Çalikoğlu, Ç.; Duman, S.; Zeynal, M.; Duman, A. Cerebral hydatid disease: Clinical analysis of ten cases. J. Neurol. Sci. 2012, 29, 754–760. [Google Scholar]
- Lunardi, P.; Missori, P.; Di Lorenzo, N.; Fortuna, A. Cerebral hydatidosis in childhood: A retrospective survey with emphasis on long-term follow-up. Neurosurgery 1991, 29, 515–517. [Google Scholar] [CrossRef]
- Braunsdorf, E.W.; Schmidt, D.; Rautenberg, M. Cerebral manifestation of hydatid disease in a child. Childs Nerv. Syst. 1988, 4, 249–251. [Google Scholar] [CrossRef]
- Naderzadeh, A.; Ghanim, S.M.; Keikhosravi, E.; Shojaeian, R. Childhood refractory headache: Alarming sign of hydatid disease in endemic area. J. Pediatr. Surg. Case Rep. 2020, 61, 101610. [Google Scholar] [CrossRef]
- Wani, N.A.; Kousar, T.L.; Gojwari, T.; Robbani, I.; Singh, M.; Ramzan, A.; Khan, Q.; Kirmani, A.; Wani, A. Computed tomography findings in cerebral hydatid disease. Turk. Neurosurg. 2011, 21, 347–351. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fleta, J.; Sarria, A.; Villagrasa, J.; Usón, A.; Ramos, F.J.; Bueno, M.; Calatayud, V. Computed tomography in the diagnosis of brain hydatidosis in children. Acta Paediatr. Scand. 1987, 76, 835–836. [Google Scholar] [CrossRef] [PubMed]
- McCorkell, S.J.; Lewall, D.B. Computed tomography of intracerebral echinococcal cysts in children. J. Comput. Assist. Tomogr. 1985, 9, 514–518. [Google Scholar] [CrossRef] [PubMed]
- Ekici, M.A.; Ekici, A.; Per, H.; Tucer, B.; Kumandaş, S.; Kurtsoy, A. Concomitant heart and brain hydatid cyst without other organ involvement: A case report. Dusunen Adam J. Psychiatry Neurol. Sci. 2011, 24, 155–159. [Google Scholar] [CrossRef]
- Samiy, E. Involvement of the central nervous system by hydatid cyst. Br. J. Clin. Pract. 1966, 20, 143–151. [Google Scholar] [CrossRef]
- Hilmani, S.; Bertal, A.; Lakhdar, A.; Ouboukhlik, A.; Elkamar, A.; Elazhari, A. Craniocerebral hydatid cyst. Case illustration. J. Neurosurg. 2006, 105, 77. [Google Scholar] [CrossRef]
- Mingde, Q.; Zhesheng, H. Echinococcosis of the central nervous system. Chin. Med. J. 1980, 93, 269–274. [Google Scholar] [CrossRef]
- Hajhouji, F.; Aniba, K.; Laghmari, M.; Lmejjati, M.; Ghannane, H.; Benali, S.A. Epilepsy: Unusual presentation of cerebral hydatid disease in children. Pan Afr. Med. J. 2016, 25, 58. [Google Scholar] [CrossRef]
- Mehrizi, M.A.A.; Tavakolian, A.; Rezaee, H.; Keykhosravi, E.; Salahshoor, Y. Giant cerebral hydatid cyst in a five-year-old child: A case report. Int. J. Pediatr. 2020, 8, 11485–11491. [Google Scholar]
- Ganjeifar, B.; Ghafouri, M.; Shokri, A.; Rahbarian Yazdi, F.; Hashemi, S.A. Giant cerebral hydatid cyst: A rare case report. Clin. Case Rep. 2021, 9, 1774–1778. [Google Scholar] [CrossRef] [PubMed]
- Ghasemi, A.A.; Mohammadzade, H.; Mohammadi, R. Giant hydatid cyst of the brain: Intact cyst removal in an 8-year-old child. Int. J. Surg. Case Rep. 2023, 106, 108172. [Google Scholar] [CrossRef] [PubMed]
- Rashid, M.; Kirmani, S.; Rashid, M. Giant intradural extramedullary spinal hydatid cyst: A rare presentation. Clin. Imaging 2012, 36, 881–883. [Google Scholar] [CrossRef] [PubMed]
- Arora, S.K.; Aggarwal, A.; Datta, V. Giant primary cerebral hydatid cyst: A rare cause of childhood seizure. J. Pediatr. Neurosci. 2014, 9, 73–75. [Google Scholar] [CrossRef]
- Gök, H.; Başkurt, O. Giant primary intracranial hydatid cyst in a child with hemiparesis. World Neurosurg. 2019, 129, 404–406. [Google Scholar] [CrossRef]
- Magoha, M.A.; Mohan, N.K.; Musau, C.K.; Okemwa, M.P. Giant solitary cerebral hydatid cyst in a child, complicated with postoperative subdural haematoma: Case report and review of literature. East Afr. Med. J. 2016, 93, 626–631. [Google Scholar]
- Ozkan, U.; Kemaloglu, M.S.; Selçuki, M. Gigantic intracranial mass of hydatid cyst. Childs Nerv. Syst. 2001, 17, 623–625. [Google Scholar] [CrossRef]
- Anvari, M.; Amirjamshidi, A.; Abbassioun, K. Gradual and complete delivery of a hydatid cyst of the brain through a single burr hole, a wrong happening! Childs Nerv. Syst. 2009, 25, 1639–1642. [Google Scholar] [CrossRef]
- Opperman, C.J.; Enslin, J.M.N.; Nuttall, J.; Brink, A.J.; Da Fonseca, S.P.; Tootla, H.D. Hydatid brain cyst: A delayed diagnosis in a rural setting during COVID-19. S. Afr. Med. J. 2021, 111, 1050–1054. [Google Scholar] [CrossRef]
- Maamri, K.; Cherif, I.; Trifa, A.; Nessib, N.; Elkahla, G.; Darmoul, M. Hydatid cyst in the third ventricle of the brain: Case report of an exceptionally rare condition. Childs Nerv. Syst. 2022, 38, 1637–1641. [Google Scholar] [CrossRef]
- Gader, G.; Slimane, A.; Sliti, F.; Kherifech, M.; Belhaj, A.; Bouhoula, A.; Kallel, J. Hydatid cyst of the brainstem: The rarest of the rare locations for echinococcosis. Radiol. Case Rep. 2024, 19, 4422–4425. [Google Scholar] [CrossRef] [PubMed]
- Villarejo, F.; Blazquez, M.G.; Arcas, J.; Pascual-Castroviejo, I.; Esteban, F. Hydatid cyst of the posterior fossa: Case report. Neurosurgery 1983, 12, 228–229. [Google Scholar] [CrossRef]
- Ndondo, A.P.; Fieggen, G.; Wilmshurst, J.M. Hydatid disease of the spine in South African children. J. Child Neurol. 2003, 18, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Peter, J.C.; Domingo, Z.; Sinclair-Smith, C.; de Villiers, J.C. Hydatid infestation of the brain: Difficulties with computed tomography diagnosis and surgical treatment. Pediatr. Neurosurg. 1994, 20, 78–83. [Google Scholar] [CrossRef] [PubMed]
- Majumdar, K.; Saran, R.K.; Sakhuja, P.; Jagetia, A.; Sinha, S. Intact protoscolices and hooklets in cytospin preparation of intra-operative cyst fluid allow rapid confirmation of rare cerebral intraventricular hydatid infestation. Cytopathology 2013, 24, 277–279. [Google Scholar] [CrossRef]
- Sardi, I.; Sanzo, M.; Buccoliero, A.M.; Mortilla, M.; de Martino, M.; Genitori, L. Intracerebral atypical presentation of echinococcosis in a child. Pediatr. Neurosurg. 2008, 44, 350–352. [Google Scholar] [CrossRef]
- Jangir, H.; Roy, C.; Goyal, S.; Goindi, A.S.; Kedia, S.; Suri, V. Intracranial hydatid cyst in a pediatric patient: A rare mimic of brain malignancy. Childs Nerv. Syst. 2025, 41, 84. [Google Scholar] [CrossRef]
- Duishanbai, S.; Jiafu, D.; Guo, H.; Liu, C.; Liu, B.; Aishalong, M.; Mijiti, M.; Wen, H. Intracranial hydatid cyst in children: Report of 30 cases. Childs Nerv. Syst. 2010, 26, 821–827. [Google Scholar] [CrossRef]
- Gautam, S.; Sharma, A. Intracranial hydatid cyst: A report of three cases in North-West India. J. Pediatr. Neurosci. 2018, 13, 91–95. [Google Scholar] [CrossRef]
- Erşahin, Y.; Mutluer, S.; Güzelbağ, E. Intracranial hydatid cysts in children. Neurosurgery 1993, 33, 219–224. [Google Scholar] [CrossRef]
- Erman, T.; Tuna, M.; Göçer, I.; Ildan, F.; Zeren, M.; Cetinalp, E. Intracranial intraosseous hydatid cyst: Case report and review of literature. Neurosurg. Focus 2001, 11, ECP1. [Google Scholar] [CrossRef] [PubMed]
- Karak, P.K.; Mittal, M.; Bhatia, S.; Mukhopadhyay, S.; Berry, M. Isolated cerebral hydatid cyst with pathognomonic CT sign. Neuroradiology 1992, 34, 9–10. [Google Scholar] [CrossRef] [PubMed]
- Bartosch, C.; Reis, C.; Castro, L. Large solitary cerebral hydatid cyst. Arch. Neurol. 2011, 68, 946–947. [Google Scholar] [CrossRef] [PubMed]
- Borkar, S.A.; Verma, N.; Joseph, S.L.; Kale, S.S.; Mahapatra, A.K. Magnetic resonance imaging of pediatric primary cerebral hydatidosis. J. Pediatr. Neurosci. 2017, 12, 298–299. [Google Scholar] [CrossRef]
- Erongun, U.; Ozkal, E.; Acar, O.; Kocaoğullar, Y. Multiple and infected cerebral hydatid cysts: Case report. Neurosurg. Rev. 1994, 17, 77–81. [Google Scholar] [CrossRef]
- Khan, M.B.; Riaz, M.; Bari, M.E. Multiple cerebral hydatid cysts in an 8-year-old boy: A case report and literature review of a rare presentation. Surg. Neurol. Int. 2015, 6, 125. [Google Scholar] [CrossRef]
- Iplikçioğlu, A.C.; Ozer, A.F.; Benli, K.; Işik, N.; Erbengi, A. Multiple cerebral hydatid cysts: Report of two cases. Br. J. Neurosurg. 1989, 3, 217–219. [Google Scholar] [CrossRef]
- Nurchi, G.; Floris, F.; Montaldo, C.; Mastio, F.; Peltz, T.; Coraddu, M. Multiple cerebral hydatid disease: Case report with magnetic resonance imaging study. Neurosurgery 1992, 30, 436–438. [Google Scholar] [CrossRef]
- Akdemir, G.; Ergün, R.; Gezici, A.R.; Ökten, A.I.; Ergüngör, F.M. Multiple hydatid cysts of the aqueduct of Sylvius: A case report with MRI study. Turk. Neurosurg. 2000, 10, 142–144. [Google Scholar]
- Yüceer, N.; Güven, M.B.; Yilmaz, H. Multiple hydatid cysts of the brain: A case report and review of the literature. Neurosurg. Rev. 1998, 21, 181–184. [Google Scholar] [CrossRef]
- Gordillo, I.; Millán, J.M.; Escudero, L.; Orduna, M.; Roger, R.; de la Fuente, M. Multiple intracranial hydatid cysts. Neuroradiology 1986, 28, 285. [Google Scholar] [CrossRef] [PubMed]
- Iyigun, O.; Uysal, S.; Sancak, R.; Hokelek, M.; Uyar, Y.; Bernay, F.; Ariturk, E. Multiple organ involvement hydatid cysts in a 2-year-old boy. J. Trop. Pediatr. 2004, 50, 374–376. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.R.; Lu, Y.J.; Bao, Y.H.; Zhang, T.R. Parasellar epidural hydatid cysts. Neurosurgery 1993, 32, 662–665. [Google Scholar] [CrossRef] [PubMed]
- Palani, A. Partially calcified giant intracerebral hydatid cyst in a pediatric child. Neurol. India 2012, 60, 260–262. [Google Scholar] [CrossRef]
- Hmada, S.; Mesbahi, T.; Jehri, A.; Jouida, A.; Naja, A.; Amenzoui, N.; Lakhdar, A. Pediatric brain hydatid cyst: About two cases. Ann. Med. Surg. 2022, 78, 103–107. [Google Scholar] [CrossRef]
- Menekşe, G.; Özsoy, K.M.; Dağlıoğlu, E.; Güzel, E.; Güzel, A.; Belen, D. Pediatric giant-sized intracerebral hydatid cyst: Reports of two cases. J. Neurol. Sci. 2012, 29, 841–846. [Google Scholar]
- Iplikçioğlu, A.C.; Ozek, M.M.; Ozer, A.F.; Ozgen, T. Periventricular hydatid cyst presenting with hemichorea. Childs Nerv. Syst. 1992, 8, 292–293. [Google Scholar] [CrossRef]
- Evliyaoğlu, C.; Keskil, S. Possible spontaneous “birth” of a hydatid cyst into the lateral ventricle. Childs Nerv. Syst. 2005, 21, 425–428. [Google Scholar] [CrossRef]
- Altibi, A.M.; Qarajeh, R.A.; Belsuzarri, T.A.; Maani, W.; Kanaan, T.M. Primary cerebral echinococcosis in a child: Case report—Surgical technique, technical pitfalls, and video atlas. Surg. Neurol. Int. 2016, 7, S893–S898. [Google Scholar] [CrossRef]
- Kartikueyan, R.; Patel, S.M.; Chattopadhyay, A.; Krishnan, P. Primary cerebral hydatid cyst: An unusual cause of very slowly progressive hemiparesis in a child. J. Neurosci. Rural Pract. 2016, 7, 603–604. [Google Scholar] [CrossRef]
- Radmenesh, F.; Nejat, F. Primary cerebral hydatid cyst: Two case reports. Iran. J. Pediatr. 2008, 18, 83–86. [Google Scholar]
- Guzel, A.; Tatli, M.; Maciaczyk, J.; Altinors, N. Primary cerebral intraventricular hydatid cyst: A case report and review of the literature. J. Child Neurol. 2008, 23, 585–588. [Google Scholar] [CrossRef] [PubMed]
- Imperato, A.; Consales, A.; Ravegnani, M.; Castagnola, E.; Bandettini, R.; Rossi, A. Primary hydatid cyst of the brain in a child: A case report. Pol. J. Radiol. 2016, 81, 578–582. [Google Scholar] [CrossRef][Green Version]
- Cataltepe, O.; Tahta, K.; Colak, A.; Erbengi, A. Primary multiple cerebral hydatid cysts. Neurosurg. Rev. 1991, 14, 231–234. [Google Scholar] [CrossRef] [PubMed]
- Yaka, U.; Aras, Y.; Aydoseli, A.; Akcakaya, M.O.; Sencer, A.; Imer, M.; Hepgul, K. Primary multiple cerebral hydatid disease: Still symptomatic despite pathologically confirmed death of the cyst. Turk. Neurosurg. 2013, 23, 505–508. [Google Scholar] [CrossRef]
- Ray, M.; Singhi, P.D.; Pathak, A.; Khandelwal, N.K. Primary multiple intracerebral echinococcosis in a young child. J. Trop. Pediatr. 2005, 51, 59–61. [Google Scholar] [CrossRef][Green Version]
- Çelebi, S.; Bozdemir, Ş.E.; Taşkapılıoğlu, Ö.; Hacımustafaoğlu, M. Primary solitary and multiple intracranial hydatid cyst disease: Report of four cases. Çocuk Enfeksiyon Derg. 2018, 12, e110–e114. [Google Scholar] [CrossRef]
- Per, H.; Kumandaş, S.; Gümüş, H.; Kurtsoy, A. Primary solitary and multiple intracranial hydatid disease: Report of five cases. Brain Dev. 2009, 31, 228–233. [Google Scholar] [CrossRef]
- Kalkan, E.; Cengiz, S.L.; Ciçek, O.; Erdi, F.; Baysefer, A. Primary spinal intradural extramedullary hydatid cyst in a child. J. Spinal Cord Med. 2007, 30, 297–300. [Google Scholar] [CrossRef]
- Ijaz, L.; Mirza, B.; Nadeem, M.M.; Saleem, M. Simultaneous giant hydatid cysts of brain and liver. J. Coll. Physicians Surg. Pak. 2015, 25, S53–S55. [Google Scholar]
- Carrea, R.; Dowling, E., Jr.; Guevara, J.A. Surgical treatment of hydatid cysts of the central nervous system in the pediatric age (Dowling’s technique). Childs Brain 1975, 1, 4–21. [Google Scholar] [CrossRef]
- Tzili, N.; Ahbeddou, S.; Ahmimech, J.; Abboud, H.; Boutarbouch, M.; El Hassan, A.; Berraho, A. Swollen eyelid reveals multiple intracranial hydatid cysts associated with a palpebral cyst. J. Fr. Ophtalmol. 2016, 39, 210–212. [Google Scholar] [CrossRef]
- Tuzun, Y.; Kadioglu, H.H.; Izci, Y.; Suma, S.; Keles, M.; Aydin, I.H. The clinical, radiological and surgical aspects of cerebral hydatid cysts in children. Pediatr. Neurosurg. 2004, 40, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Onal, C.; Barlas, O.; Orakdögen, M.; Hepgül, K.; Izgi, N.; Unal, F. Three unusual cases of intracranial hydatid cyst in the pediatric age group. Pediatr. Neurosurg. 1997, 26, 208–213. [Google Scholar] [CrossRef] [PubMed]
- Eljebbouri, B.; Elmostarchid, B. Unusual cause of acute intracranial hypertension. Emerg. Med. J. 2013, 30, 1011. [Google Scholar] [CrossRef] [PubMed]
- González-Ruiz, C.A.; Isla, A.; Pérez-Higueras, A.; Blázquez, M.G. Unusual CT image of a cerebral hydatid cyst. Pediatr. Radiol. 1990, 20, 283–284. [Google Scholar] [CrossRef]
- Dagtekin, A.; Koseoglu, A.; Kara, E.; Karabag, H.; Avci, E.; Torun, F.; Bagdatoglu, C. Unusual locations of hydatid cysts in pediatric patients. Pediatr. Neurosurg. 2009, 45, 379–383. [Google Scholar] [CrossRef]
- Thakur, S.H.; Joshi, P.C.; Kelkar, A.B.; Seth, N. Unusual presentation of hydatid cyst—Ruptured intraventricular hydatid. Indian J. Radiol. Imaging 2017, 27, 282–285. [Google Scholar] [CrossRef]
- Copley, I.B.; Fripp, P.J.; Erasmus, A.M.; Otto, D.D. Unusual presentations of cerebral hydatid disease in children. Br. J. Neurosurg. 1992, 6, 203–210. [Google Scholar] [CrossRef]

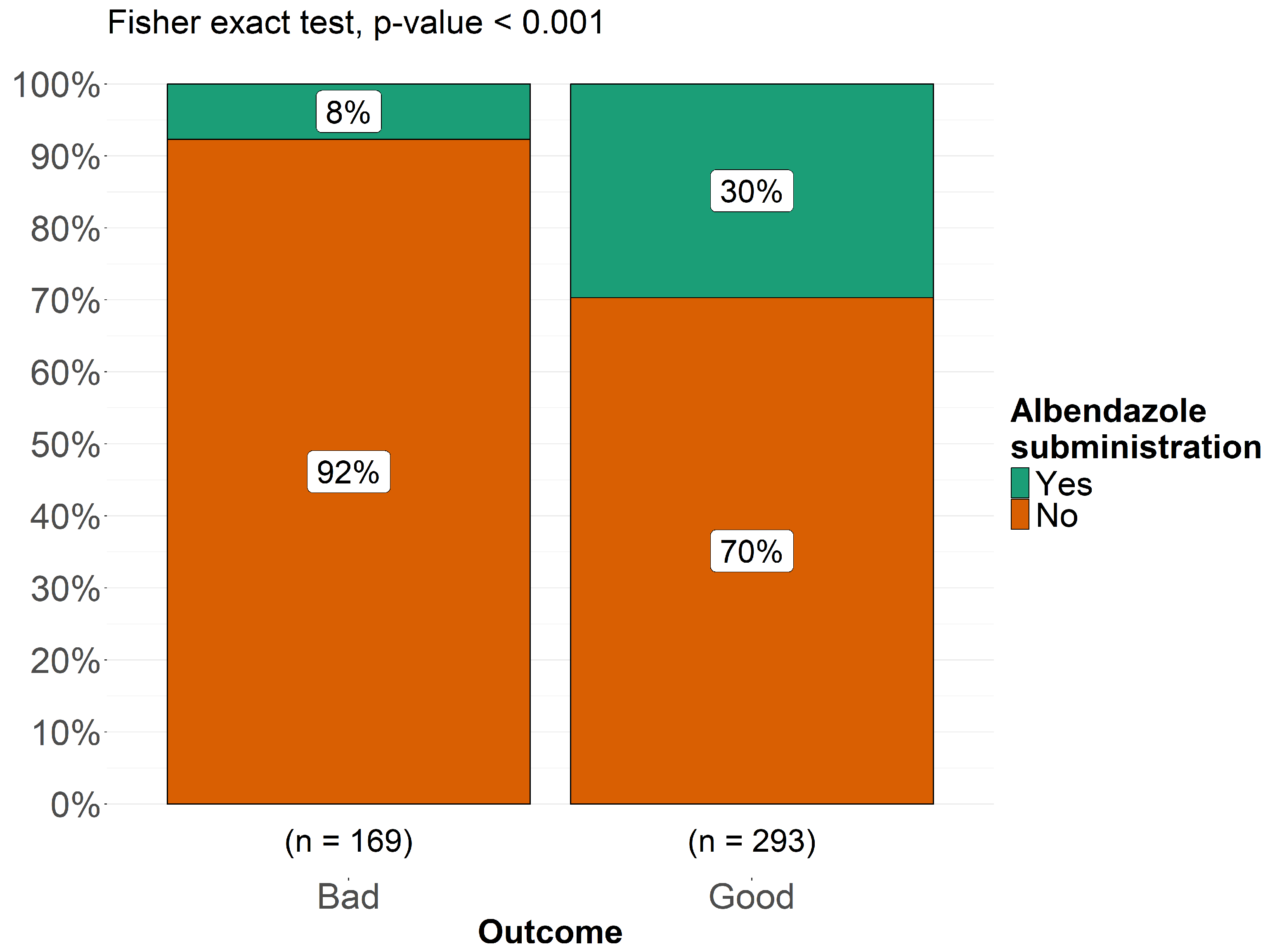
| Neurological Sign/Symptom | Present n (%) | Absent n (%) | Total Cases Described |
|---|---|---|---|
| Headache | 323 (97%) | 9 (3%) | 332 |
| Raised intracranial pressure | 205 (96%) | 8 (4%) | 213 |
| Vomiting | 242 (96%) | 10 (4%) | 252 |
| Papilledema | 191 (95%) | 10 (5%) | 201 |
| Hemiparesis | 141 (94%) | 9 (6%) | 150 |
| Weakness of lower limbs | 36 (88%) | 5 (12%) | 41 |
| Seizures | 92 (91%) | 9 (9%) | 101 |
| Aphasia | 7 (50%) | 7 (50%) | 14 |
| Ataxia | 41 (85%) | 7 (15%) | 48 |
| Cerebellar signs | 13 (57%) | 10 (43%) | 23 |
| Pyramidal signs | 120 (92%) | 10 (8%) | 130 |
| Ptosis | 6 (43%) | 8 (57%) | 14 |
| Optic atrophy | 38 (83%) | 8 (17%) | 46 |
| Cranial nerve palsies | 31 (79%) | 8 (21%) | 39 |
| Neuropsychiatric symptoms | 61 (88%) | 8 (12%) | 69 |
| Visual problems | 107 (91%) | 10 (9%) | 117 |
| Altered consciousness | 52 (69%) | 23 (31%) | 75 |
| Coma | 7 (100%) | — | 7 |
| Hemichorea | 3 (30%) | 7 (70%) | 10 |
| Cranial asymmetry | 42 (84%) | 8 (16%) | 50 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leonardi, R.; Curatolo, A.; Lo Bianco, M.; Migliore, A.; Privitera, G.F.; Pulvirenti, A.; Nunnari, G.; Marino, A.; Spampinato, S.; Maniaci, A.; et al. Clinical Presentation, Management and Outcome of Cerebral Echinococcosis in Children: A Systematic Review and Meta-Analysis. Pathogens 2025, 14, 1144. https://doi.org/10.3390/pathogens14111144
Leonardi R, Curatolo A, Lo Bianco M, Migliore A, Privitera GF, Pulvirenti A, Nunnari G, Marino A, Spampinato S, Maniaci A, et al. Clinical Presentation, Management and Outcome of Cerebral Echinococcosis in Children: A Systematic Review and Meta-Analysis. Pathogens. 2025; 14(11):1144. https://doi.org/10.3390/pathogens14111144
Chicago/Turabian StyleLeonardi, Roberta, Alessandra Curatolo, Manuela Lo Bianco, Alessia Migliore, Grete Francesca Privitera, Alfredo Pulvirenti, Giuseppe Nunnari, Andrea Marino, Serena Spampinato, Antonino Maniaci, and et al. 2025. "Clinical Presentation, Management and Outcome of Cerebral Echinococcosis in Children: A Systematic Review and Meta-Analysis" Pathogens 14, no. 11: 1144. https://doi.org/10.3390/pathogens14111144
APA StyleLeonardi, R., Curatolo, A., Lo Bianco, M., Migliore, A., Privitera, G. F., Pulvirenti, A., Nunnari, G., Marino, A., Spampinato, S., Maniaci, A., Betta, P., Ruggieri, M., Polizzi, A., & Pavone, P. (2025). Clinical Presentation, Management and Outcome of Cerebral Echinococcosis in Children: A Systematic Review and Meta-Analysis. Pathogens, 14(11), 1144. https://doi.org/10.3390/pathogens14111144











