Exploratory Study on Efficacy and Safety of Minocycline-Based Dual Therapy for Helicobacter pylori Eradication
Abstract
1. Introduction
2. Materials and Methods
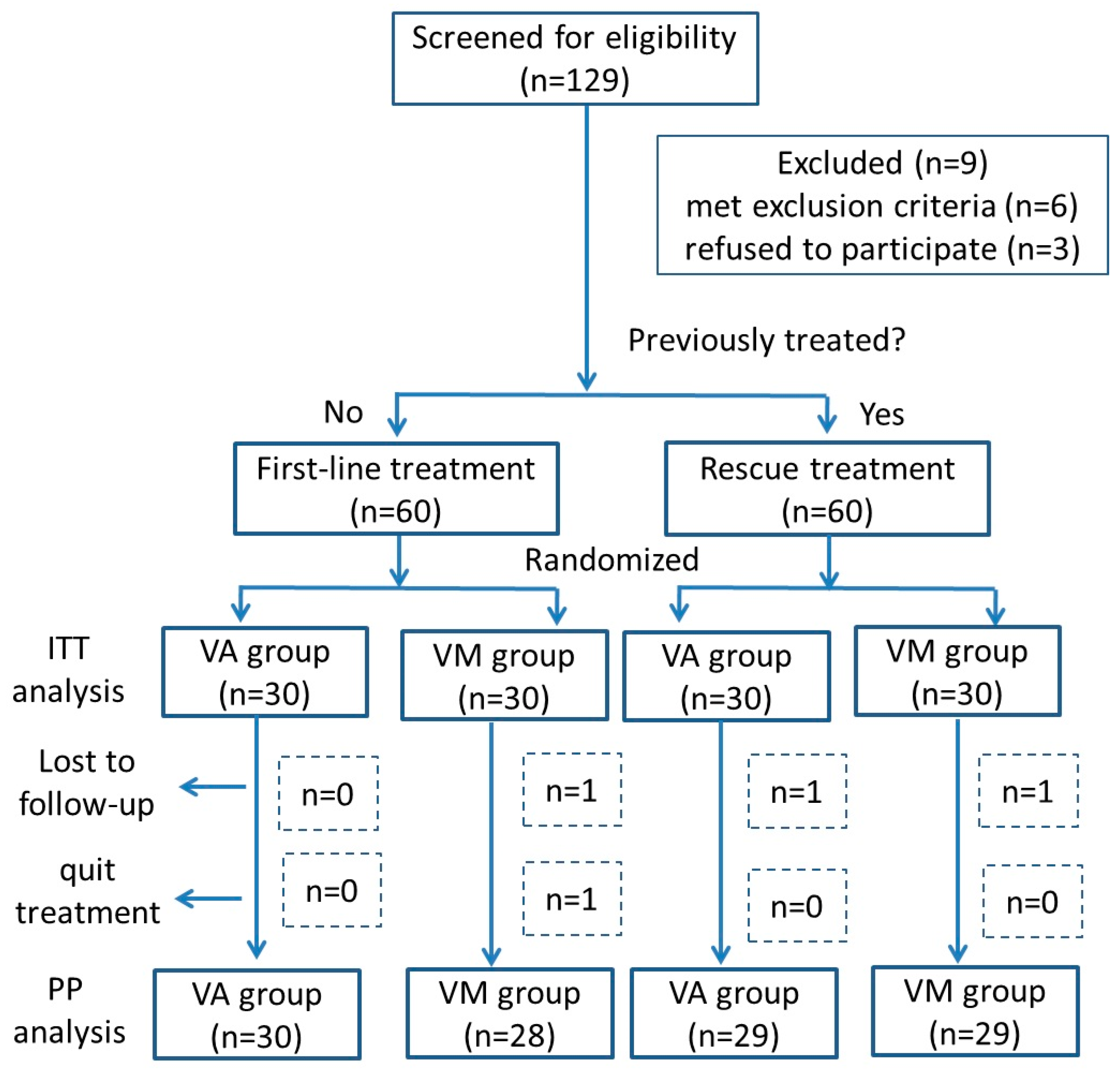
2.1. Study Design and Participants
2.2. Randomization and Interventions
2.3. Trial Assessments
2.4. Sample Size Calculation and Statistical Analysis
3. Results
3.1. Patients Enrolled and Baseline Characteristics
3.2. Eradication of H. pylori Infection
3.3. Adverse Events and Adherence
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Malfertheiner, P.; Megraud, F.; Rokkas, T.; Gisbert, J.P.; Liou, J.-M.; Schulz, C.; Gasbarrini, A.; Hunt, R.H.; Leja, M.; O’Morain, C.; et al. Management of Helicobacter pylori infection: The Maastricht VI/Florence consensus report. Gut 2022, 71, 1724–1762. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.; Laine, L.; Moayyedi, P.; Wu, J. AGA Clinical Practice Update on Integrating Potassium-Competitive Acid Blockers Into Clinical Practice: Expert Review. Gastroenterology 2024, 167, 1228–1238. [Google Scholar] [CrossRef] [PubMed]
- Rokkas, T.; Ekmektzoglou, K.; Niv, Y.; Graham, D.Y. Comparative efficacy and safety of Potassium-Competitive Acid Blocker (P-CAB) based dual, triple and quadruple regimens for first line H. pylori infection treatment: A systematic review and network meta-analysis. Am. J. Gastroenterol. 2024, 120, 787–798. [Google Scholar] [CrossRef] [PubMed]
- Hong, T.-C.; El-Omar, E.M.; Kuo, Y.-T.; Wu, J.-Y.; Chen, M.-J.; Chen, C.-C.; Fang, Y.-J.; Leow, A.H.R.; Lu, H.; Lin, J.-T.; et al. Primary antibiotic resistance of Helicobacter pylori in the Asia-Pacific region between 1990 and 2022: An updated systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 2023, 9, 56–67. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Liu, J.; Wang, X.; Li, J.; Zhang, X.; Ye, H.; Li, J.; Dong, X.; Liu, B.; Wang, C.; et al. Simplified Helicobacter pylori therapy for patients with penicillin allergy: A randomised controlled trial of vonoprazan-tetracycline dual therapy. Gut 2024, 73, 1414–1420. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Zhou, K.; Li, C.-L.; Zhang, H.; Suo, B.-J.; Zhang, Y.-X.; Ren, X.-L.; Wang, Y.-X.; Mi, C.-M.; Ma, L.-L.; Zhou, L.-Y.; et al. Minocycline in the eradication of Helicobacter pylori infection: A systematic review and meta-analysis. World J Gastroenterol. World J. Gastroenterol. 2024, 30, 2354–2368. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Suo, B.; Tian, X.; Zhang, H.; Lu, H.; Li, C.; Zhang, Y.; Ren, X.; Yao, X.; Zhou, L.; Song, Z. Bismuth, esomeprazole, metronidazole, and minocycline or tetracycline as a first-line regimen for Helicobacter pylori eradication: A randomized controlled trial. Chin. Med. J. 2023, 136, 933–940. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Huang, Y.; Chen, J.; Ding, Z.; Chen, X.; Liang, X.; Zeng, X.; Xu, F.; Han, Y.; Lu, H. Minocycline vs. tetracycline in bismuth-containing quadruple therapy for Helicobacter pylori rescue treatment: A multicentre, randomized controlled trial. J. Gastroenterol. 2023, 58, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Qiu, S.; Guo, Y.; Chen, J.; Li, M.; Ding, Z.; Zhang, W.; Liang, X.; Lu, H. Optimization of Minocycline-Containing Bismuth Quadruple Therapy for Helicobacter pylori Rescue Treatment: A Real-World Evidence Study. Helicobacter 2024, 29, e13138. [Google Scholar] [CrossRef] [PubMed]
- Savarino, V.; Vigneri, S.; Celle, G. The 13C urea breath test in the diagnosis of Helicobacter pylori infection. Gut 1999, 45 (Suppl. S1), i18–i22. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Choi, I.J.; Kim, C.G.; Lee, J.Y.; Kim, Y.-I.; Kook, M.-C.; Park, B.; Joo, J. Family History of Gastric Cancer and Helicobacter pylori Treatment. N. Engl. J. Med. 2020, 382, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Xu, Y.; Liu, J.; Wang, X.; Dong, X.; Teng, G.; Liu, B.; Dong, J.; Ge, C.; Ye, H.; et al. A real-world exploratory study on the feasibility of vonoprazan and tetracycline dual therapy for the treatment of Helicobacter pylori infection in special populations with penicillin allergy or failed in previous amoxicillin-containing therapies. Helicobacter 2023, 28, e12947. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, F.; Starosta, A.L.; Arenz, S.; Sohmen, D.; Dönhöfer, A.; Wilson, D.N. Tetracycline antibiotics and resistance mechanisms. Biol. Chem. 2014, 395, 559–575. [Google Scholar] [CrossRef] [PubMed]
- Smith, K.; Leyden, J.J. Safety of doxycycline and minocycline: A systematic review. Clin. Ther. 2005, 27, 1329–1342. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zou, Y.; Li, K.; Huang, X.; Niu, C.; Wang, Z.; Zhao, S.; Zhang, Y.; Song, C.; Xie, Y. Doxycycline and minocycline in Helicobacter pylori treatment: A systematic review and meta-analysis. Helicobacter 2021, 26, e12839. [Google Scholar] [CrossRef] [PubMed]
- You, S.; Tang, X.; Zhou, J.; Shen, Y.; Song, X.; Benghezal, M.; Marshall, B.J.; Tang, H.; Li, H. Minocycline/Amoxicillin-Based Bismuth Quadruple Therapy for Helicobacter pylori Eradication: A Pilot Study. Microorganisms 2024, 12, 429. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gao, W.; Zhu, M.; Yin, Y.; Zhang, X.; Wang, L. Efficacy and safety of minocycline quadruple therapy for Helicobacter pylori eradication: A meta-analysis of RCTs. Helicobacter 2023, 28, e13022. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Suo, B.; Zhang, L.; Zhou, L. Rabeprazole, Minocycline, Amoxicillin, and Bismuth as First-Line and Second-Line Regimens for Helicobacter pylori Eradication. Helicobacter 2016, 21, 462–470. [Google Scholar] [CrossRef] [PubMed]
- Shirley, M. Vonoprazan: A Review in Helicobacter pylori Infection. Drugs 2024, 84, 319–327, Erratum in Drugs 2024, 84, 621. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Fanning, W.L.; Gump, D.W.; Sofferman, R.A. Side effects of minocycline: A double-blind study. Antimicrob. Agents Chemother. 1977, 11, 712–717. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Gump, D.W.; Ashikaga, T.; Fink, T.J.; Radin, A.M. Side effects of minocycline: Different dosage regimens. Antimicrob. Agents Chemother. 1977, 12, 642–646. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Unge, P.; Gad, A.; Gnarpe, H.; Olsson, J. Does omeprazole improve antimicrobial therapy directed towards gastric Campylobacter pylori in patients with antral gastritis? A pilot study. Scand. J. Gastroenterol. 1989, 24, 49–54. [Google Scholar] [CrossRef] [PubMed]
- Sue, S.; Maeda, S. Is a Potassium-Competitive Acid Blocker Truly Superior to Proton Pump Inhibitors in Terms of Helicobacter pylori Eradication? Gut Liver 2021, 15, 799–810. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- al-Assi, M.T.; Genta, R.M.; Graham, D.Y. Short report: Omeprazole-tetracycline combinations are inadequate as therapy for Helicobacter pylori infection. Aliment. Pharmacol. Ther. 1994, 8, 259–262. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Shi, H.; Shi, Y.; Wang, A.; Guo, N.; Li, F.; Nahata, M.C. Vonoprazan-based therapies versus PPI-based therapies in patients with H. pylori infection: Systematic review and meta-analyses of randomized controlled trials. Helicobacter 2024, 29, e13094. [Google Scholar] [CrossRef] [PubMed]
- Chey, W.D.; Mégraud, F.; Laine, L.; López, L.J.; Hunt, B.J.; Howden, C.W. Vonoprazan Triple and Dual Therapy for Helicobacter pylori Infection in the United States and Europe: Randomized Clinical Trial. Gastroenterology 2022, 163, 608–619. [Google Scholar] [CrossRef] [PubMed]
- Graham, D.Y. Why the Vonoprazan Helicobacter pylori Therapies in the US-European Trial Produced Unacceptable Cure Rates. Dig. Dis. Sci. 2023, 68, 1691–1697. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | First-Line Treatment (n = 60) | Rescue Treatment (n = 60) | ||
|---|---|---|---|---|
| VA Group (n = 30) | VM Group (n = 30) | VA Group (n = 30) | VM Group (n = 30) | |
| Age, y, mean (SD) | 52.3 (4.2) | 47.1 (9.9) | 52.3 (6.4) | 47.9 (17.0) |
| Sex (M/F) | 15/15 | 15/15 | 16/14 | 10/20 |
| Body weight (mean, SD) kg | 65.5 (3.5) | 65.5 (2.1) | 64.7 (14.1) | 62.9 (8.5) |
| BMI (mean, SD) kg/m2 | 23.8 (0.5) | 23.8 (2.0) | 23.2 (3.6) | 22.9 (1.2) |
| Cigarette smoking | 4 (13.3%) | 1 (3.3%) | 2 (6.6%) | 1 (3.3%) |
| Alcohol drinking | 6 (20.0%) | 1 (3.3%) | 4 (13.3%) | 2 (6.6%) |
| Family history of gastric cancer [11] | 5 (16.7%) | 1 (3.3%) | 2 (6.6%) | 6 (20.0%) |
| Endoscopy diagnosis | ||||
| Gastritis | 21 (70.0%) | 25 (83.3%) | 25 (83.3%) | 25 (83.3%) |
| Peptic ulcers | 7 (23.4%) | 5 (16.7%) | 5 (16.7%) | 4 (13.3%) |
| Gastric cancer | 1 (3.3%) | 0 | 0 | 1 (3.3%) |
| MALToma | 1 (3.3%) | 0 | 0 | 0 |
| Times of treatment failure (mean) | - | - | 1.93 | 2 |
| Loss of follow-up | 0 | 0 | 1 (3.3%) | 0 |
| Quit treatment | 0 | 2 (6.7%) | 0 | 1 (3.3%) |
| Adherence, n/N (%) | 30/30 (100%) | 28/30 (93.3%) | 29/30 (96.7%) | 29/30 (96.7%) |
| Analysis | Total (n = 120) | First-Line Treatment (n = 60) | Rescue Treatment (n = 60) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| VA Group (n = 60) | VM Group (n = 60) | p Value | VA Group (n = 30) | VM Group (n = 30) | p Value | VA Group (n = 30) | VM Group (n = 30) | p Value | |
| ITT | 91.7% (55/60) | 83.3% (50/60) | 0.17 | 96.7% (29/30) | 90.0% (27/30) | 0.30 | 86.7% (26/30) | 76.7% (23/30) | 0.32 |
| 95% CI | 81.9–96.4% | 72.0–90.7% | 83.3–99.4% | 74.4–96.6% | 70.3–94.7% | 59.1–88.2% | |||
| PP | 93.2% (55/59) | 87.7% (50/57) | 0.31 | 96.7% (29/30) | 96.4% (27/28) | 0.96 | 89.7% (26/29) | 79.3% (23/29) | 0.28 |
| 95% CI | 83.8–97.3% | 76.8–93.9% | 83.3–99.4% | 82.3–99.4% | 73.6–96.4% | 61.6–90.2% | |||
| Group | Subgroup | No. | Sex | Age (y) | Body Wight (kg) | BMI kg/m2 | Adverse Events | Medication Days | Times of Previous Failure | DOB Before Treatment | DOB After Treatment | Rescue Treatment After Failed in Trial |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| VA group (n = 4) | First-line treatment (n = 1) | 18 | M | 34 | 95 | 31.74 | No | 14 | 0 | 59.1 | 19.8 | NA |
| Rescue treatment (n = 3) | 1 | F | 49 | 55 | 20.20 | No | 14 | 3 | 45.3 | 55.7 | NA | |
| 3 | F | 46 | 49 | 19.14 | No | 14 | 2 | 18.8 | 8.9 | NA | ||
| 14 | F | 47 | 54 | 19.83 | No | 14 | 1 | 36.7 | 26.8 | Successful in VM dual therapy | ||
| VM group (n = 10) | First-line treatment (n = 3) | 1 (quit) | F | 45 | 59 | 22.21 | Dizziness + nausea + fatigue + Decreased blood pressure + Decreased heart rate | 4 | 0 | 18 | 12.5 | Successful in VA dual therapy |
| 3 (quit) | F | 47 | 84 | 31.23 | Dizziness + nausea + vomiting + tenderness of breasts | 4 | 0 | 18 | NA | NA | ||
| 30 | M | 31 | 56 | 19.38 | No | 14 | 0 | 42.6 | 6.2 | NA | ||
| Rescue treatment (n = 7) | 2 | F | 38 | 57 | 22.31 | No | 14 | 2 | 17.8 | 15.6 | NA | |
| 5 | F | 36 | 57 | 20.20 | No | 14 | 2 | 52.4 | 35.4 | NA | ||
| 11 (quit) | F | 28 | 47 | 24.34 | Low fever + rash | 11 | 1 | 38.0 | NA | NA | ||
| 12 | F | 38 | 55 | 22.31 | No | 14 | 2 | 53.4 | 47.1 | NA | ||
| 20 | F | 57 | 59 | 19.27 | No | 14 | 1 | 10.2 | 7.4 | NA | ||
| 21 | M | 27 | 72 | 24.91 | No | 14 | 2 | 14.5 | 19.3 | NA | ||
| 25 | M | 61 | 60 | 22.04 | No | 14 | 4 | 38.9 | 15.3 | NA |
| Variables | VA Group (n = 60) | VM group (n = 60) | p Value |
|---|---|---|---|
| Total, n/N (%) | 6/60 (10.0%) | 18/60 (30.0%) | 0.006 |
| AEs variety | |||
| Dizziness | 0 | 11 (18.3%) | |
| Nausea | 0 | 4 (6.7%) | |
| Abdominal discomfort | 2 (3.3%) | 3 (5.0%) | |
| Rash | 1 (1.7%) | 1 (1.7%) | |
| Constipation | 1 (1.7%) | 1 (1.7%) | |
| Diarrhea | 1 (1.7%) | 0 | |
| Tenderness of breasts | 0 | 1 (1.7%) | |
| Low fever | 0 | 1 (1.7%) | |
| Knee joint redness and swelling | 1 (1.7%) | 0 | |
| Vomiting | 0 | 1 (1.7%) | |
| Abdominal pain | 0 | 1 (1.7%) | |
| Fatigue | 0 | 1 (1.7%) | |
| Decreased blood pressure+ Decreased heart rate | 0 | 1 (1.7%) | |
| Anxiety | 0 | 1 (1.7%) | |
| Manifestation of Cases who discontinued due to AEs (n = 3) | |||
| Dizziness +nausea + fatigue + Decreased blood pressure + Decreased heart rate | 0 | 1 | |
| Dizziness + nausea + vomiting + tenderness of breasts | 0 | 1 | |
| Low fever + rash | 0 | 1 | |
| Discontinued due to AEs | 0 | 3/60 (5.0%) | 0.08 |
| Adherence, n/N (%) | 59/60 (98.3%) | 57/60 (95.0%) | 0.31 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, W.; Li, J.; Tian, Y.; Ge, C.; Wang, C.; Liu, J.; Li, Y.; Cheng, H. Exploratory Study on Efficacy and Safety of Minocycline-Based Dual Therapy for Helicobacter pylori Eradication. Pathogens 2025, 14, 1121. https://doi.org/10.3390/pathogens14111121
Gao W, Li J, Tian Y, Ge C, Wang C, Liu J, Li Y, Cheng H. Exploratory Study on Efficacy and Safety of Minocycline-Based Dual Therapy for Helicobacter pylori Eradication. Pathogens. 2025; 14(11):1121. https://doi.org/10.3390/pathogens14111121
Chicago/Turabian StyleGao, Wen, Jingwen Li, Yuling Tian, Chaoyi Ge, Chi Wang, Jianxiang Liu, Yixuan Li, and Hong Cheng. 2025. "Exploratory Study on Efficacy and Safety of Minocycline-Based Dual Therapy for Helicobacter pylori Eradication" Pathogens 14, no. 11: 1121. https://doi.org/10.3390/pathogens14111121
APA StyleGao, W., Li, J., Tian, Y., Ge, C., Wang, C., Liu, J., Li, Y., & Cheng, H. (2025). Exploratory Study on Efficacy and Safety of Minocycline-Based Dual Therapy for Helicobacter pylori Eradication. Pathogens, 14(11), 1121. https://doi.org/10.3390/pathogens14111121





