Synovial Fluid Analysis in Melioidosis: Experiences from the Darwin Prospective Melioidosis Study
Abstract
1. Introduction
2. Methods
2.1. Patient Cohort and Data Collection
2.2. Laboratory Measurements
2.3. Statistical Analysis and Figure Generation
2.4. Ethics Statement
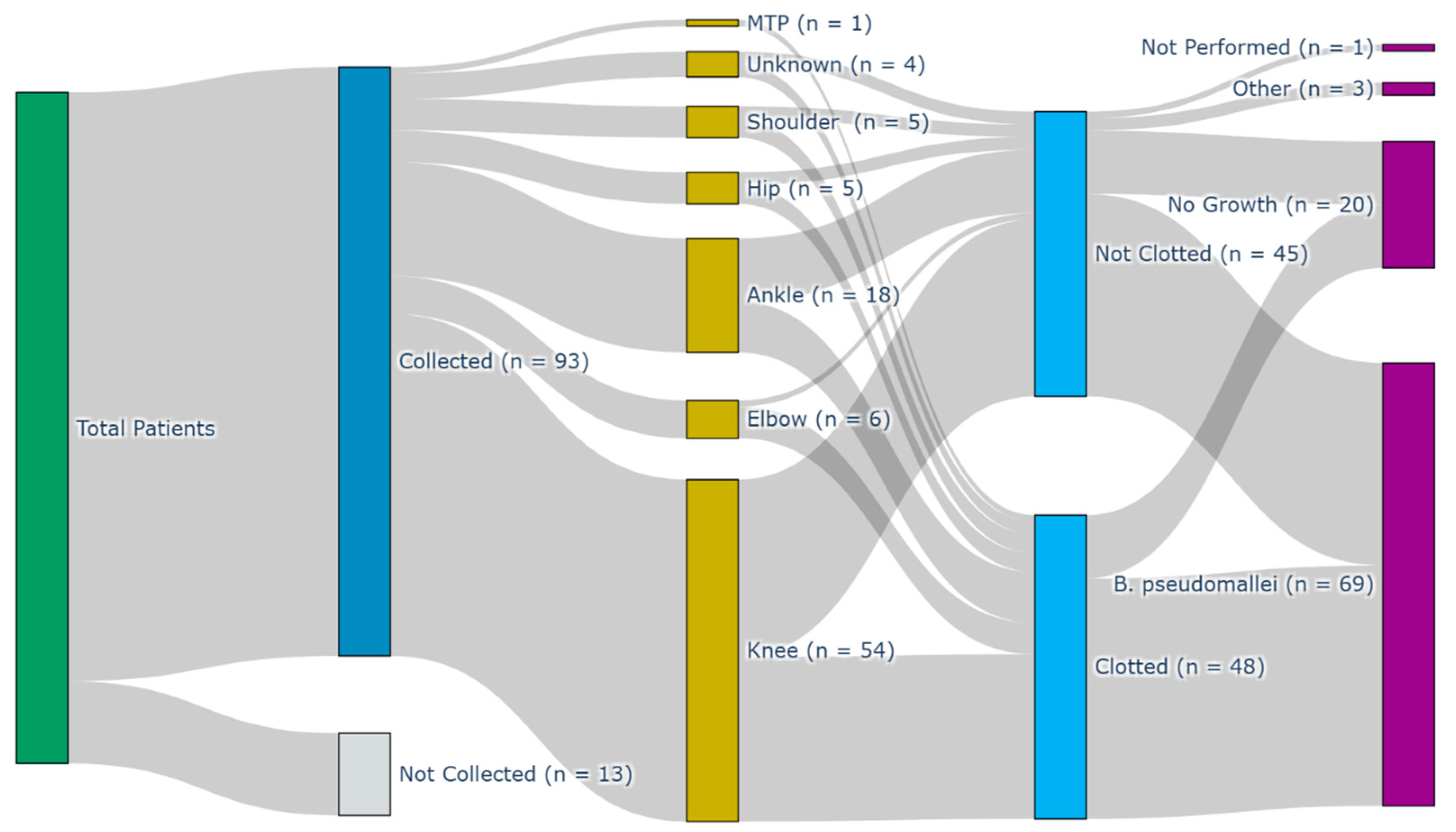
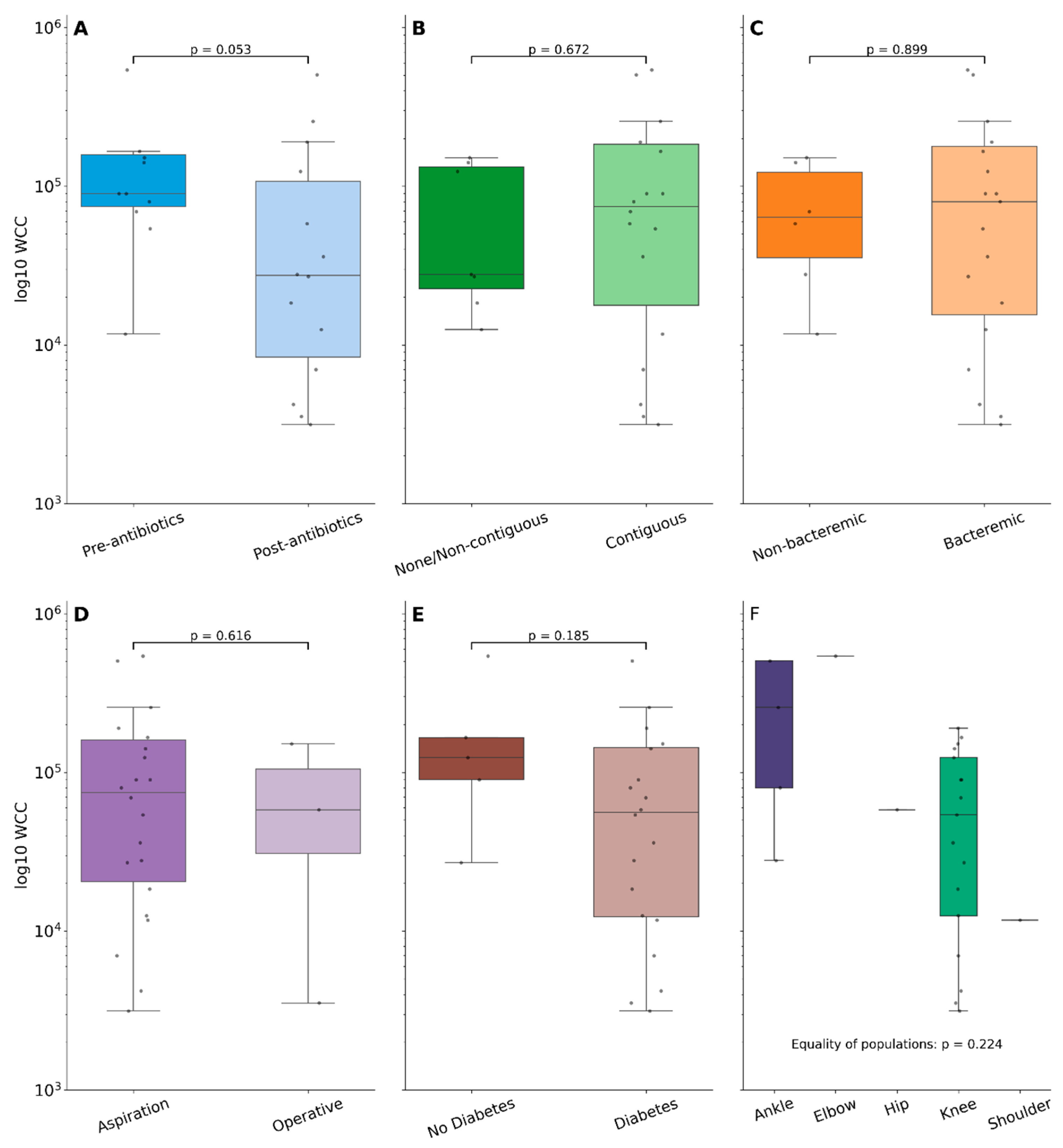
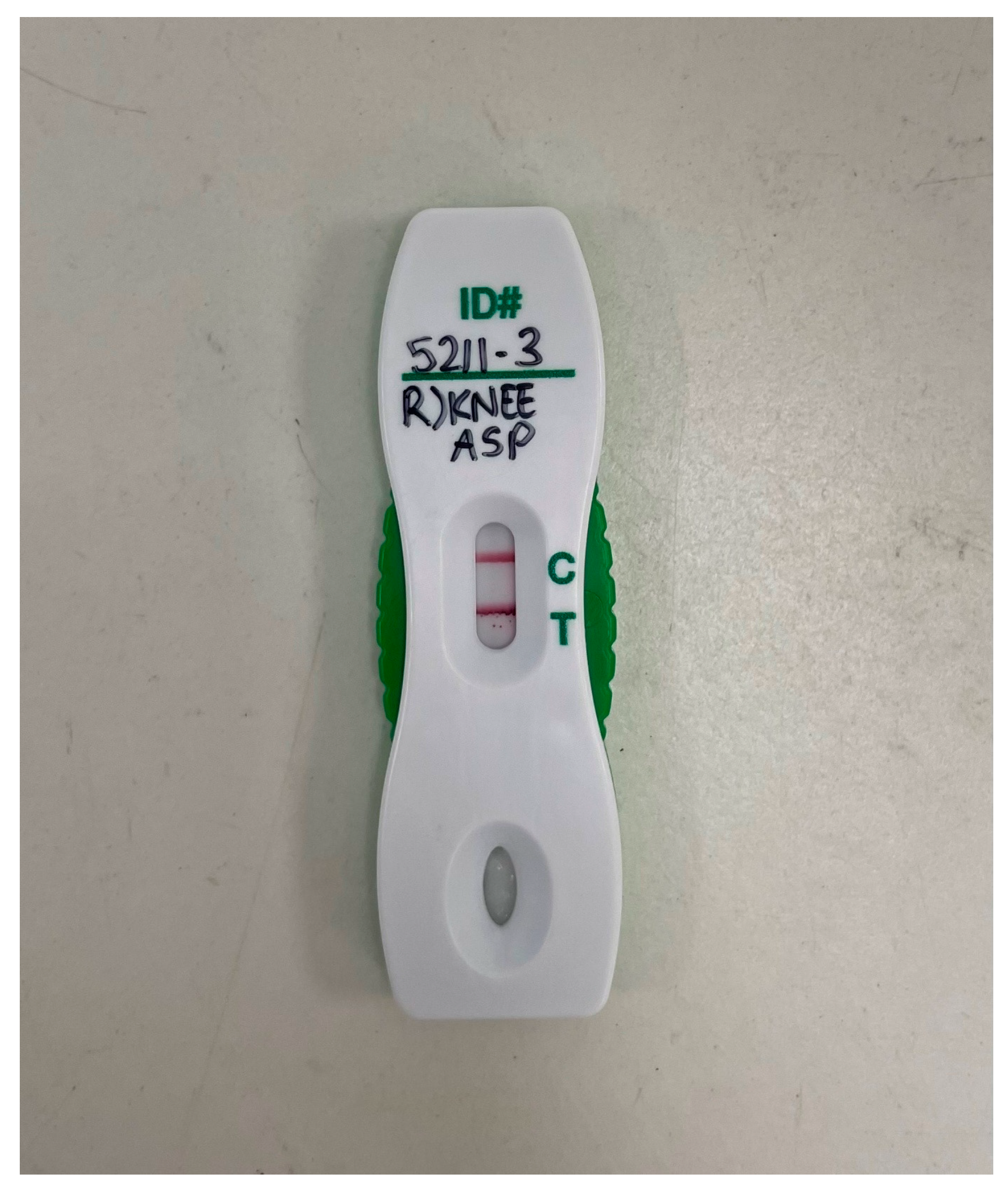
3. Results
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Meumann, E.M.; Limmathurotsakul, D.; Dunachie, S.J.; Wiersinga, W.J.; Currie, B.J. Burkholderia pseudomallei and melioidosis. Nat. Rev. Microbiol. 2024, 22, 155–169. [Google Scholar] [CrossRef] [PubMed]
- Chewapreecha, C.; Holden, M.T.G.; Vehkala, M.; Välimäki, N.; Yang, Z.; Harris, S.R.; Mather, A.E.; Tuanyok, A.; De Smet, B.; Le Hello, S.; et al. Global and regional dissemination and evolution of Burkholderia pseudomallei. Nat. Microbiol. 2017, 2, 16263. [Google Scholar] [CrossRef] [PubMed]
- Gee, J.E.; Bower, W.A.; Kunkel, A.; Petras, J.; Gettings, J.; Bye, M.; Firestone, M.; Elrod, M.G.; Liu, L.; Blaney, D.D.; et al. Multistate Outbreak of Melioidosis Associated with Imported Aromatherapy Spray. N. Engl. J. Med. 2022, 386, 861–868. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Mayo, M.; Ward, L.M.; Kaestli, M.; Meumann, E.M.; Webb, J.R.; Woerle, C.; Baird, R.W.; Price, R.N.; Marshall, C.S.; et al. The Darwin Prospective Melioidosis Study: A 30-year prospective, observational investigation. Lancet Infect. Dis. 2021, 21, 1737–1746. [Google Scholar] [CrossRef] [PubMed]
- Chantratita, N.; Phunpang, R.; Yarasai, A.; Dulsuk, A.; Yimthin, T.; Onofrey, L.A.; Coston, T.D.; Thiansukhon, E.; Chaisuksant, S.; Tanwisaid, K.; et al. Characteristics and one year outcomes of melioidosis patients in Northeastern Thailand: A prospective, multicenter cohort study. Lancet Reg. Health—Southeast Asia 2023, 9, 100118. [Google Scholar] [CrossRef] [PubMed]
- Campbell, S.; Hicks, D.; Shetty, R.P.; Currie, B.J. Osteomyelitis and Septic Arthritis in the Darwin Prospective Melioidosis Study. Open Forum Infect. Dis. 2024, 12, ofae741. [Google Scholar] [CrossRef] [PubMed]
- Dadwal, P.; Bonner, B.; Fraser, D.; Loveridge, J.; Withey, G.; Puri, A.; Smith, S.; Hanson, J. Bone and joint infections due to melioidosis; diagnostic and management strategies to optimise outcomes. PLoS Neglected Trop. Dis. 2024, 18, e0012317. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Janson, S.; Meumann, E.M.; Martin, G.E.; Ewin, T.; Marshall, C.S. The 2024 revised Darwin Melioidosis Treatment Guideline. N. Territ. Dis. Control Bull. 2023, 30, 3–11. [Google Scholar]
- Chittrakarn, S.; Siripaitoon, P.; Kositpantawong, N.; Kanchanasuwan, S.; Aiewruengsurat, D.; Bintachitt, P.; Charoenmak, B.; Jullangkoon, M.; Chusri, S. Outcomes of Adjunctive Therapy with Open Arthrotomy for Patients with Septic Arthritis Due to Melioidosis: A Retrospective Study. Am. J. Trop. Med. Hyg. 2024, 110, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Teparrukkul, P.; Nilsakul, J.; Dunachie, S.; Limmathurotsakul, D. Clinical Epidemiology of Septic Arthritis Caused by Burkholderia pseudomallei and Other Bacterial Pathogens in Northeast Thailand. Am. J. Trop. Med. Hyg. 2017, 97, 1695–1701. [Google Scholar] [CrossRef] [PubMed]
- Dunachie, S.J.; Jenjaroen, K.; Reynolds, C.J.; Quigley, K.J.; Sergeant, R.; Sumonwiriya, M.; Chaichana, P.; Chumseng, S.; Ariyaprasert, P.; Lassaux, P.; et al. Infection with Burkholderia pseudomallei-immune correlates of survival in acute melioidosis. Sci. Rep. 2017, 7, 12143. [Google Scholar] [CrossRef] [PubMed]
- Ravn, C.; Neyt, J.; Benito, N.; Abreu, M.A.; Achermann, Y.; Bozhkova, S.; Coorevits, L.; Ferrari, M.C.; Gammelsrud, K.W.; Gerlach, U.-J.; et al. Guideline for management of septic arthritis in native joints (SANJO). J. Bone Jt. Infect. 2023, 8, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Earwood, J.S.; Walker, T.R.; Sue, G.J.C. Septic Arthritis: Diagnosis and Treatment. Am. Fam. Physician 2021, 104, 589–597. [Google Scholar] [PubMed]
- Morgan, D.S.; Fisher, D.; Merianos, A.; Currie, B.J. An 18 year clinical review of septic arthritis from tropical Australia. Epidemiol. Infect. 1996, 117, 423–428. [Google Scholar] [CrossRef] [PubMed]
- Puzzitiello, R.N.; Lipson, E.S.; Michaud, R.G.; York, B.R.; Finch, D.J.; Menendez, E.M.; Ryan, S.P.; Wurcel, A.G.; Salzler, M.J. Effect of Antibiotic Administration Before Joint Aspiration on Synovial Fluid White Blood Cell Count in Native Joint Septic Arthritis. Open Forum Infect. Dis. 2024, 11, ofad600. [Google Scholar] [CrossRef] [PubMed]
- Meumann, E.M.; Novak, R.T.; Gal, D.; Kaestli, M.E.; Mayo, M.; Hanson, J.P.; Spencer, E.; Glass, M.B.; Gee, J.E.; Wilkins, P.P.; et al. Clinical evaluation of a type III secretion system real-time PCR assay for diagnosing melioidosis. J. Clin. Microbiol. 2006, 44, 3028–3030. [Google Scholar] [CrossRef] [PubMed]
- Schoenmakers, J.W.A.; De Boer, R.; Gard, L.; Kampinga, G.A.; Van Oosten, M.; Van Dijl, J.M.; Jutte, P.C.; Wouthuyzen-Bakker, M. First evaluation of a commercial multiplex PCR panel for rapid detection of pathogens associated with acute joint infections. J. Bone Jt. Infect. 2023, 8, 45–50. [Google Scholar] [CrossRef] [PubMed]
- Currie, B.J.; Woerle, C.; Mayo, M.; Meumann, E.M.; Baird, R.W. What is the Role of Lateral Flow Immunoassay for the Diagnosis of Melioidosis? Open Forum Infect. Dis. 2022, 9, ofac149. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campbell, S.; Lee, T.I.; Baird, R.W.; Meumann, E.M.; Currie, B.J. Synovial Fluid Analysis in Melioidosis: Experiences from the Darwin Prospective Melioidosis Study. Pathogens 2025, 14, 1120. https://doi.org/10.3390/pathogens14111120
Campbell S, Lee TI, Baird RW, Meumann EM, Currie BJ. Synovial Fluid Analysis in Melioidosis: Experiences from the Darwin Prospective Melioidosis Study. Pathogens. 2025; 14(11):1120. https://doi.org/10.3390/pathogens14111120
Chicago/Turabian StyleCampbell, Stuart, Tze I. Lee, Robert W. Baird, Ella M. Meumann, and Bart J. Currie. 2025. "Synovial Fluid Analysis in Melioidosis: Experiences from the Darwin Prospective Melioidosis Study" Pathogens 14, no. 11: 1120. https://doi.org/10.3390/pathogens14111120
APA StyleCampbell, S., Lee, T. I., Baird, R. W., Meumann, E. M., & Currie, B. J. (2025). Synovial Fluid Analysis in Melioidosis: Experiences from the Darwin Prospective Melioidosis Study. Pathogens, 14(11), 1120. https://doi.org/10.3390/pathogens14111120





