This section is divided by subheadings. It aims to provide a concise and precise description of the experimental results, their interpretation, as well as the experimental conclusions that can be drawn.
3.2. Top Bacterial Diseases Requiring Improved Vaccines
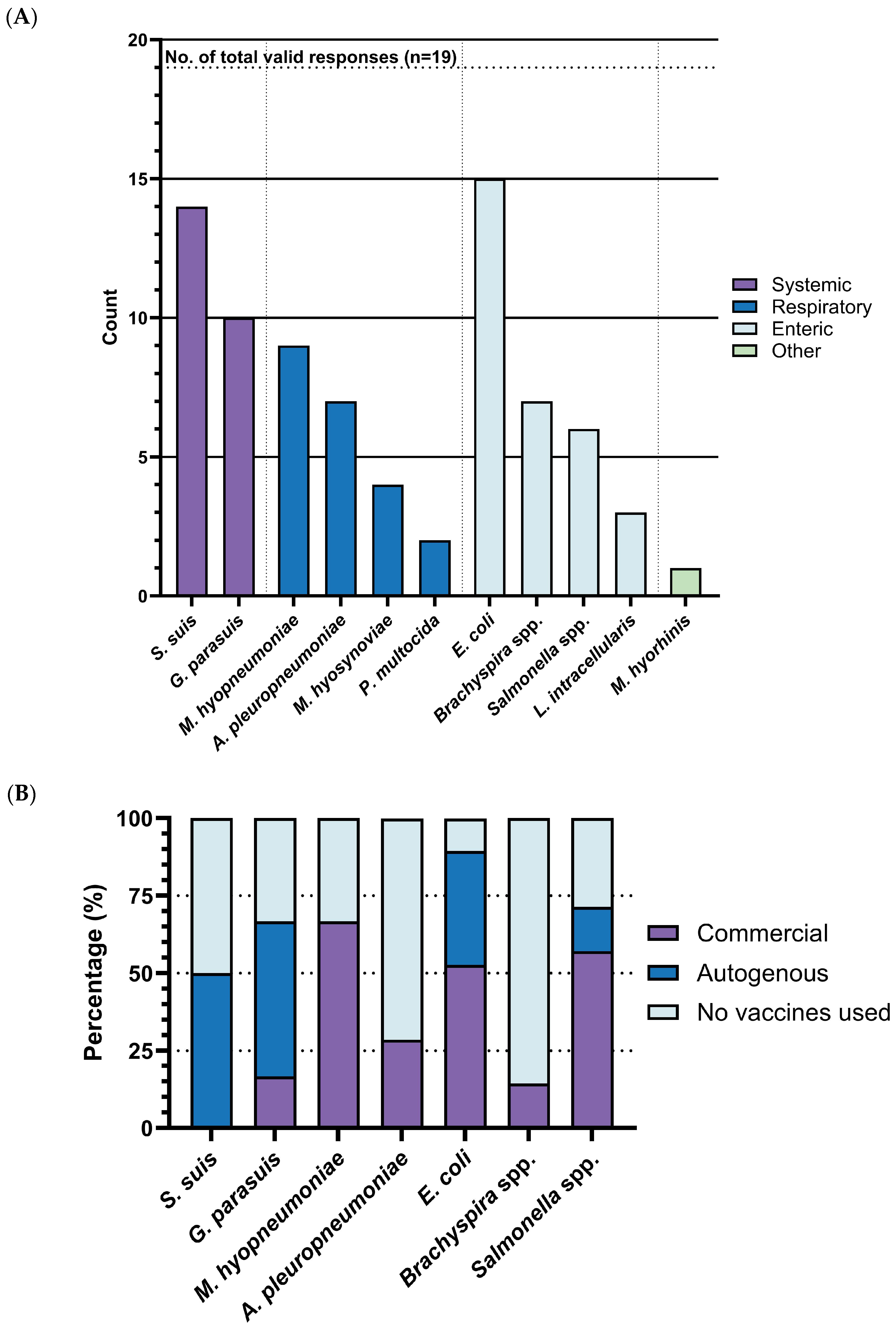
Our survey identified
Escherichia coli (
E. coli),
Streptococcus suis (S. suis), and
Glaesserella parasuis (
G. parasuis) as the top three bacterial diseases most urgently requiring improved vaccines. Over half of respondents selected these diseases, with rates of 78.9% (15/19), 73.7% (14/19), and 52.6% (10/19), respectively. These were followed by
Mycoplasma hyopneumoniae (
M. hyo) with rates of 47.4% (9/19),
Actinobacillus pleuropneumoniae with rates of 36.8% (7/19),
Brachyspira spp. with rates of 36.8% (7/19), and
Salmonella spp. with rates of 31.6% (6/19). In contrast,
Mycoplasma hyosynoviae with rates of 21.0% (4/19),
Lawsonia intracellularis with rates of 15.8% (3/19),
Pasteurella multocida with rates of 10.5% (2/19), and
Mycoplasma hyorhinis with rates of 5.3% (1/19) were selected by only a small number of respondents (
Figure 3A). Based on these response rates and statistical significance, our subsequent analysis was focused on the top four diseases:
E. coli,
S. suis,
G. parasuis, and
M. hyo. Notably, few respondents proposed additional bacterial diseases, further reinforcing the consensus around the importance of these identified bacterial diseases.
3.3. Vaccine Landscape, Expectations and Challenges for Urgent Bacterial Diseases in Swine
For the most urgently needed bacterial disease vaccines-
S. suis,
G. parasuis and
E. coli, notably rely on autogenous vaccines, with usage rates of 50% (7/14), 50% (6/12), and 36.8% (7/19), respectively (
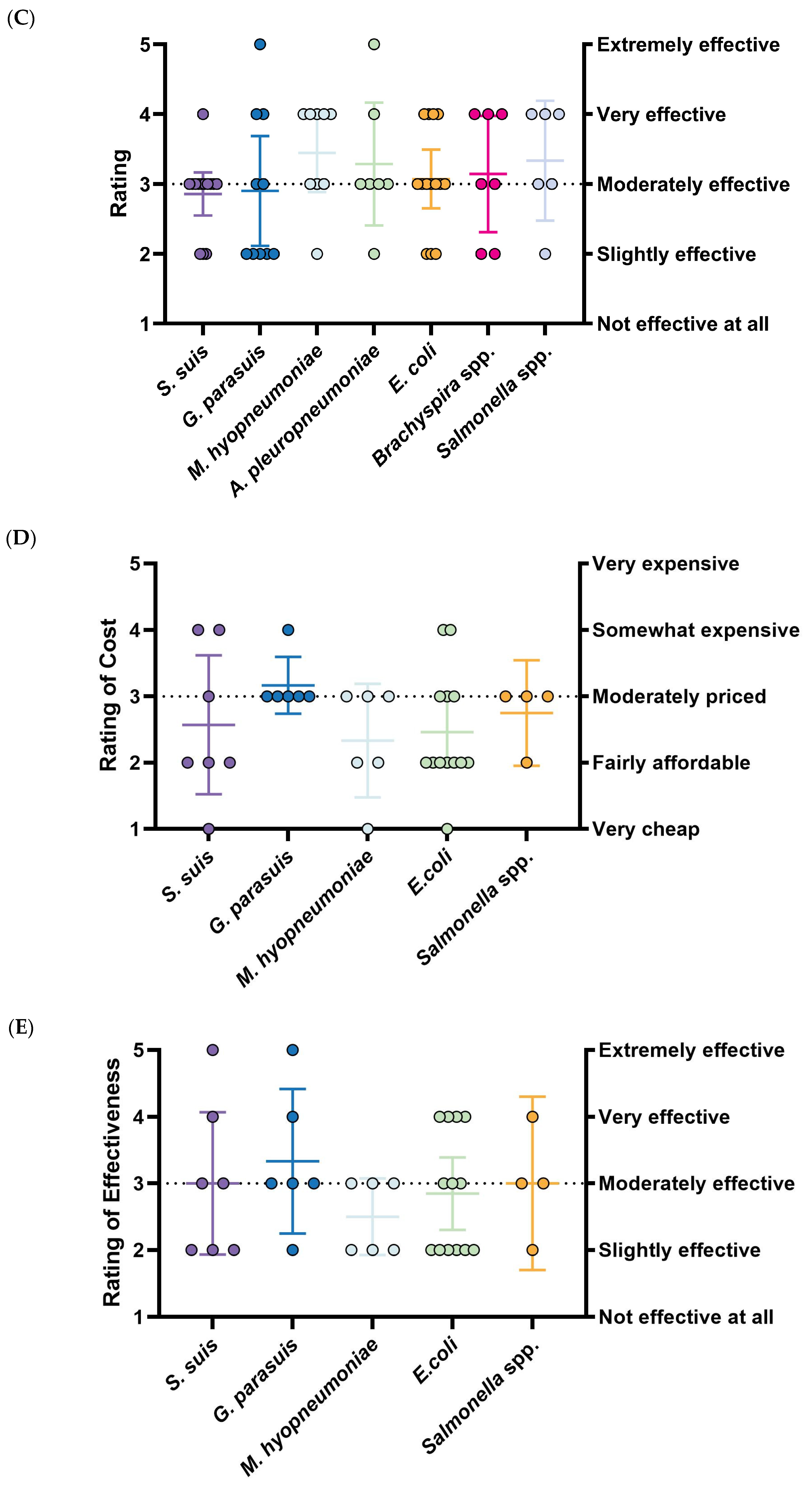
Figure 3B). In contrast, the percentages of commercial vaccines utilized for these diseases are 0% (0/14), 16.67% (2/12), and 52.6% (10/19). The lower perceived effectiveness of management for these diseases (
S. suis: 2.86, 95%CI: 2.55–3.17,
G. parasuis: 2.90, 95%CI: 2.11–3.69, and
E. coli: 3.07, 95%CI: 2.65–3.49) (
Figure 3C) likely contributes to the higher dependence on autogenous options.
The effectiveness rating of
S. suis vaccines is highly inconsistent with a wide distribution (3.00, 95%CI: 1.93–4.07).
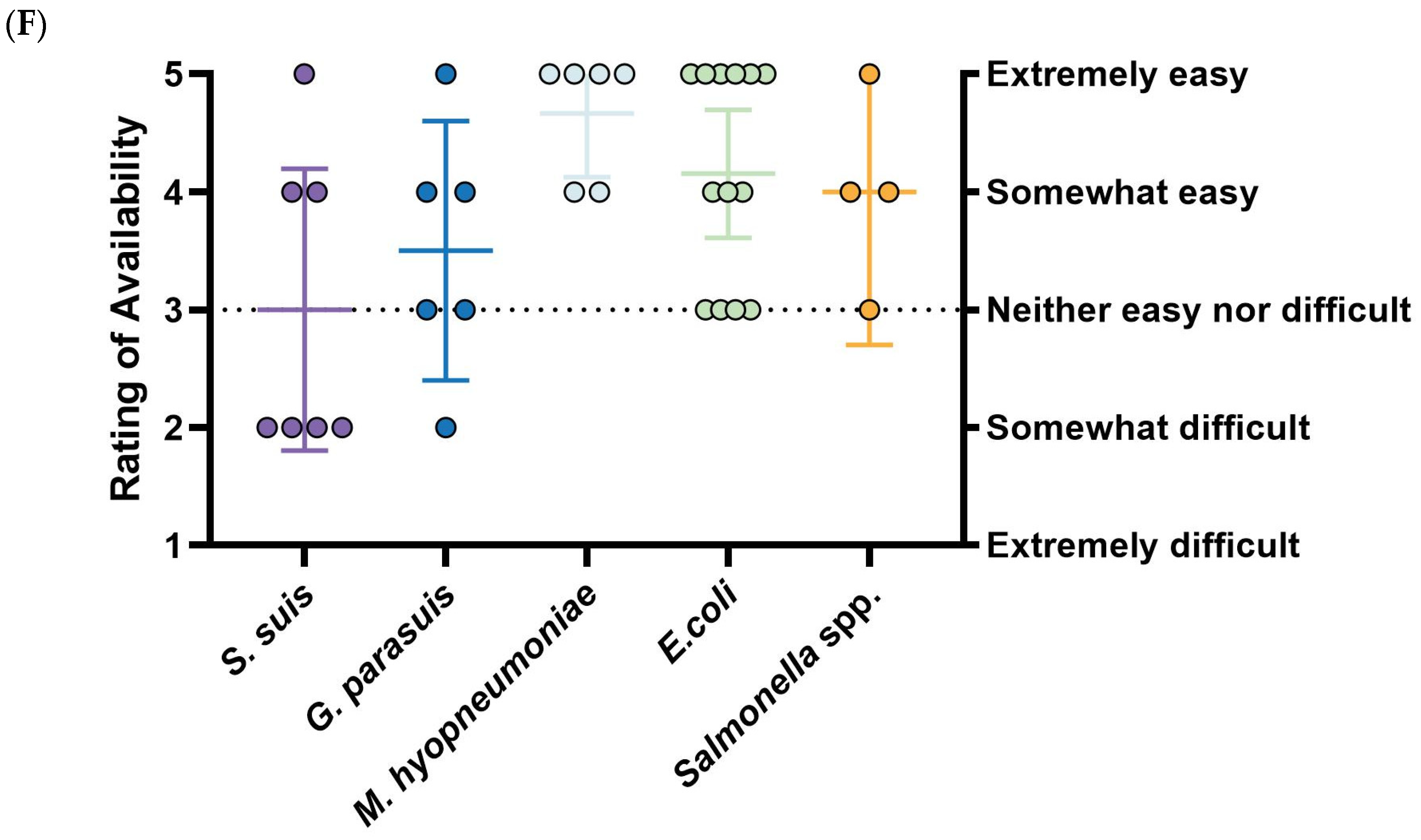
S. suis vaccines are considered the least accessible, with over half of respondents rating their availability as “somewhat difficult” (3.00, 95%CI: 1.80–4.19).
G. parasuis vaccines are regarded as the most effective among prioritized diseases (3.33, 95%CI: 2.25–4.42) and reasonably accessible (3.50, 95%CI: 2.40–4.60), but they are also the most expensive (3.17, 95%CI: 2.74–3.60).
E. coli vaccines are considered inexpensive (2.46, 95%CI: 1.93–2.99) and easy to obtain (4.15, 95%CI: 3.61–4.70), though their effectiveness is rated as below moderate (2.85, 95%CI: 2.30–3.39), mirroring trends seen with
M. hyo vaccines. The results of
M. hyo vaccines are quite distinct: they are exclusively commercial (66.6%, 6/9) and received the highest ratings for existing control measures (3.44, 95%CI: 2.89–4.00). While these vaccines are the cheapest (2.33, 95%CI: 1.48–3.19) and most accessible (4.67, 95%CI: 4.13–5.20), they are simultaneously rated as the least effective (2.50, 95%CI: 1.93–3.08) (
Figure 3D–F). Due to limited responses and a lack of significance,
A. pleuropneumoniae,
Brachyspira spp., and
Salmonella spp. were not analyzed further.
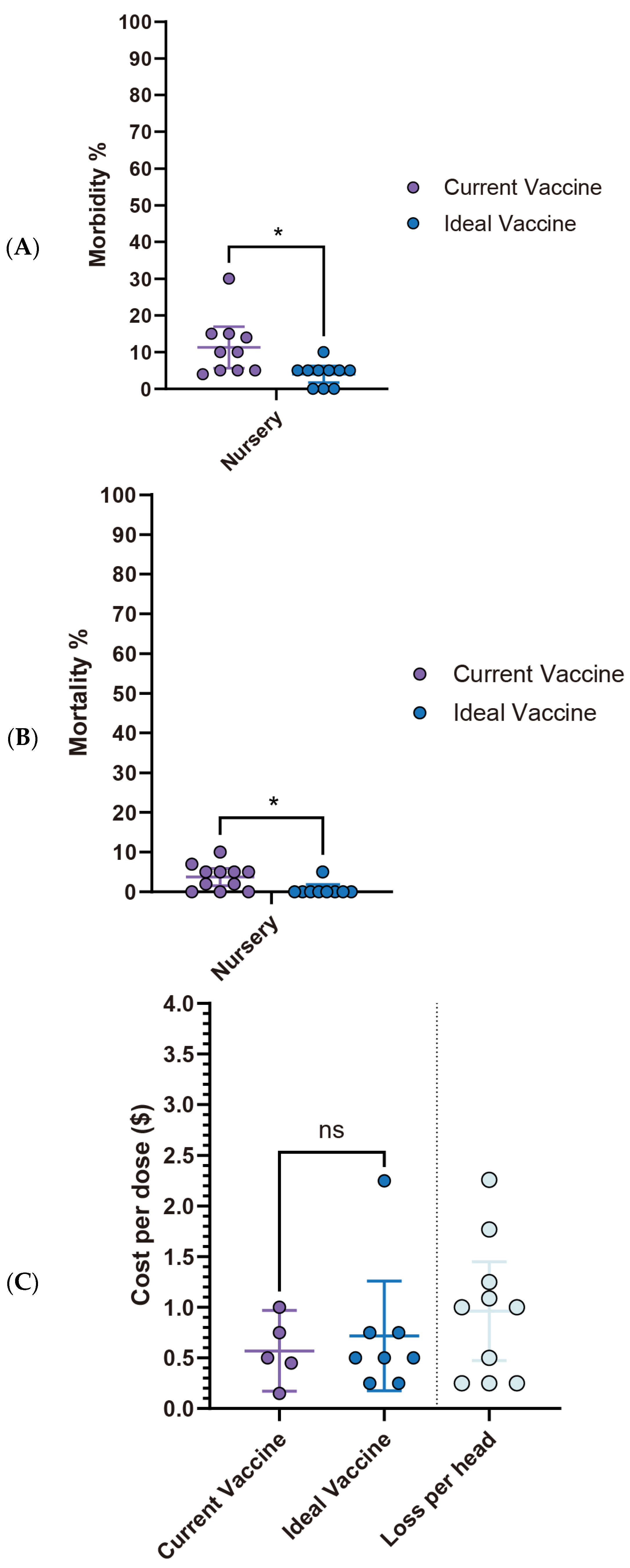
3.3.1. S. suis in Swine Production: Current Landscape, Vaccine Expectations and Challenges
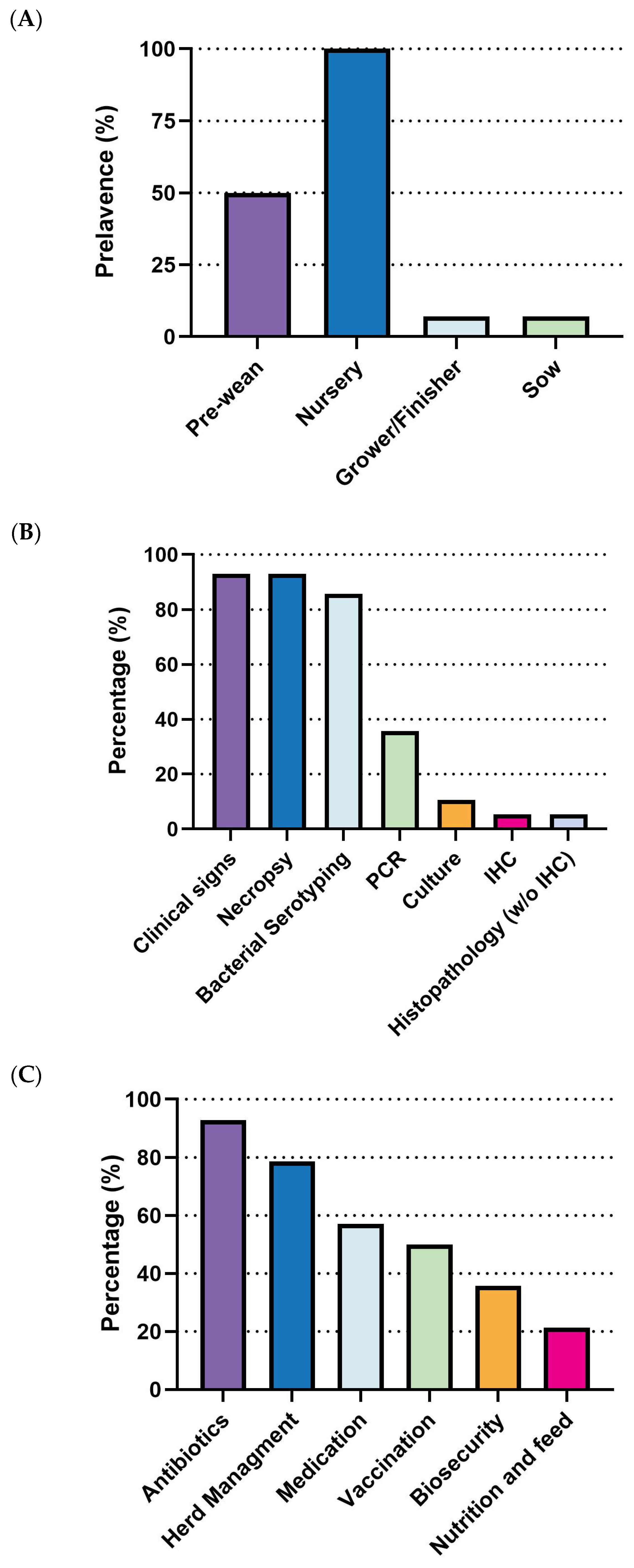
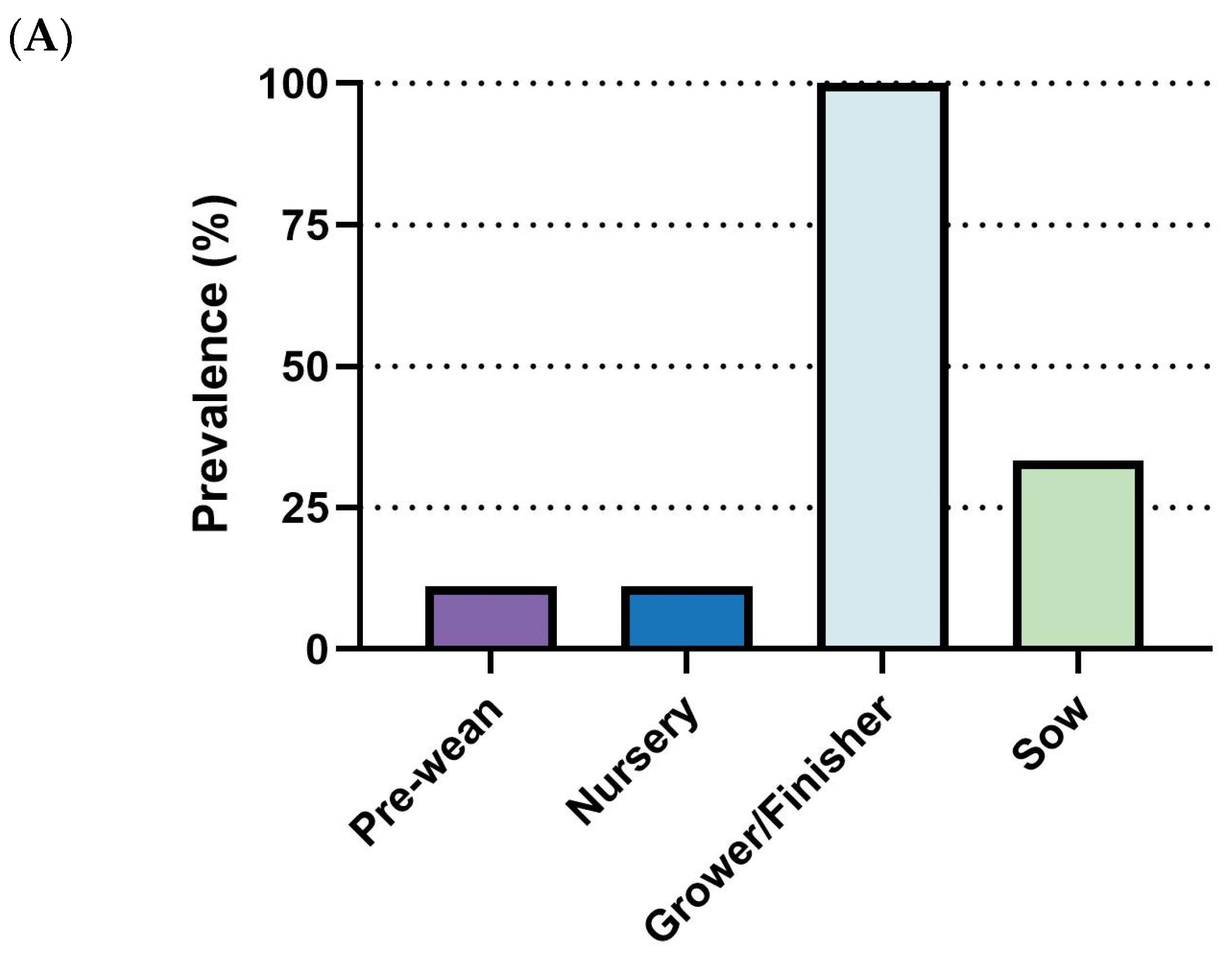
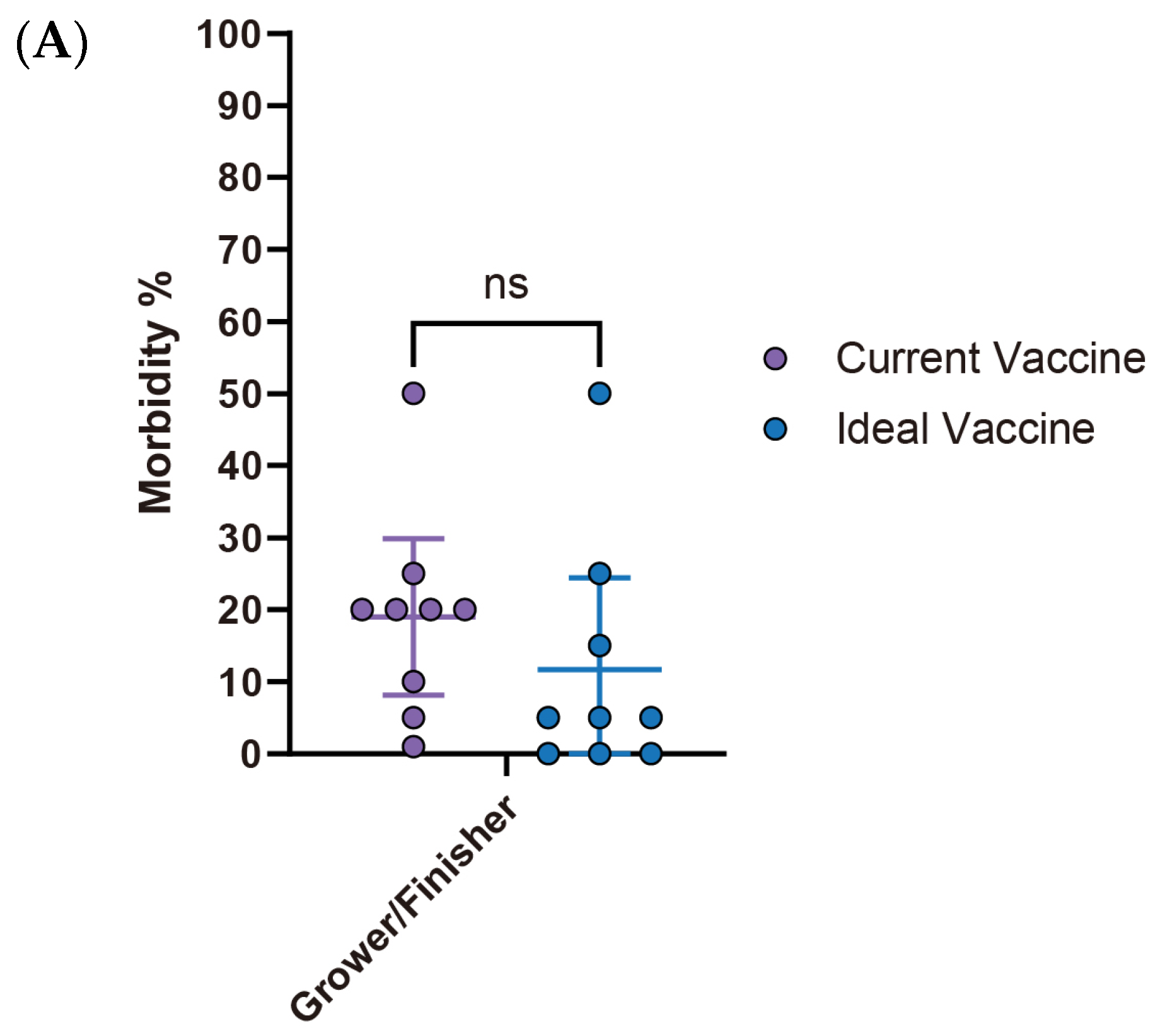
S. suis outbreaks primarily affect the early stages of swine production. All surveyed respondents (100%, 14/14) reported cases in the nursery stage, making it the highest incidence rate. The pre-weaning stage followed with a 50% incidence rate (7/14), while both grower/finisher and sow stages reported significantly lower rates (1/14) (
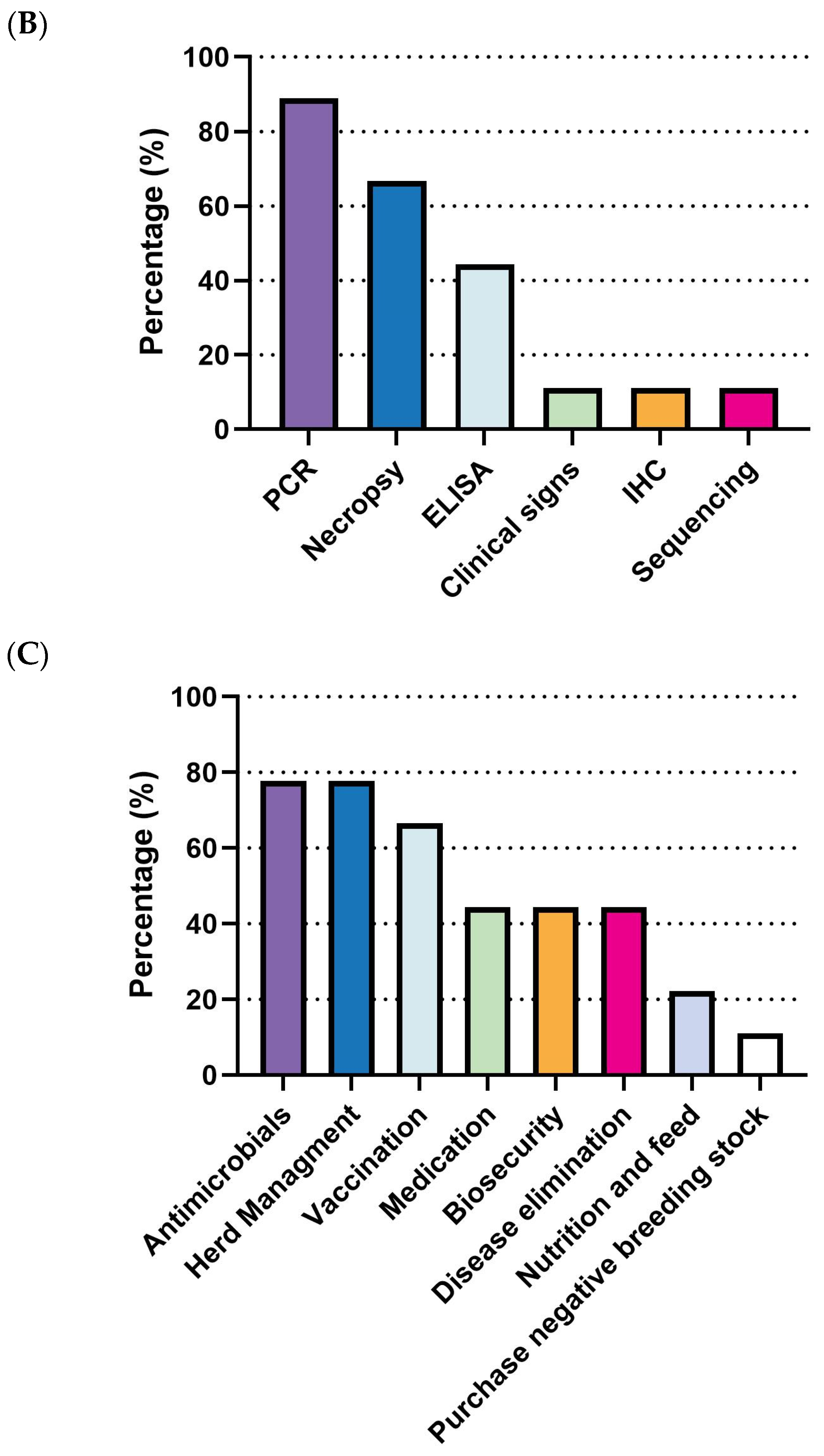
Figure 4A). Diagnostic efforts largely rely on clinical signs and necropsy (92.8%, 13/14) and bacterial serotyping (85.7%, 12/14). Less frequent methods include PCR (35.7%, 5/14), culture, immunohistochemistry (IHC), and histopathology (without IHC) (
Figure 4B). Regarding prevention and control strategies, there is a clear prioritization of antibiotics, with 92.9% (13/14) of respondents using them, followed by herd management (78.6%, 11/14). Medication (57.1%, 8/14) and vaccination (50.0%, 7/14) are moderately utilized, while biosecurity (35.7%, 5/14) and nutrition and feed (21.4%, 3/14) are the least adopted strategies (
Figure 4C).
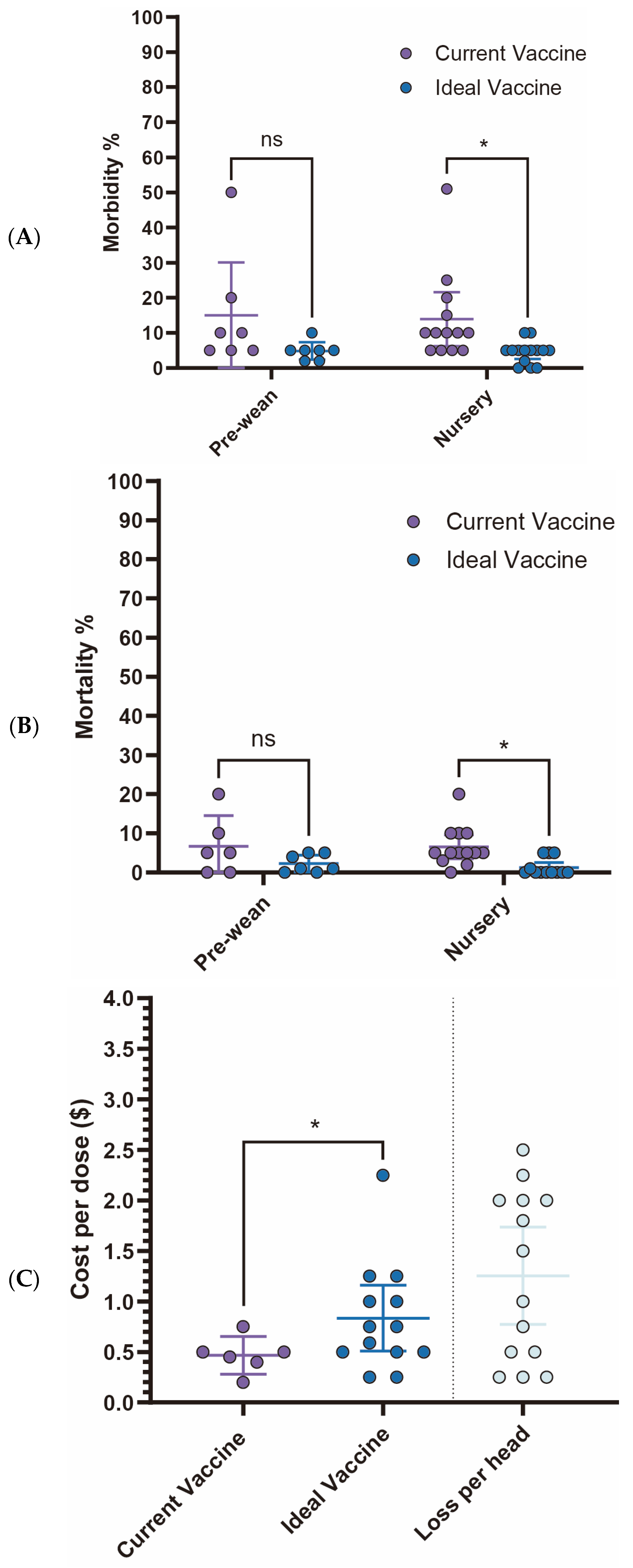
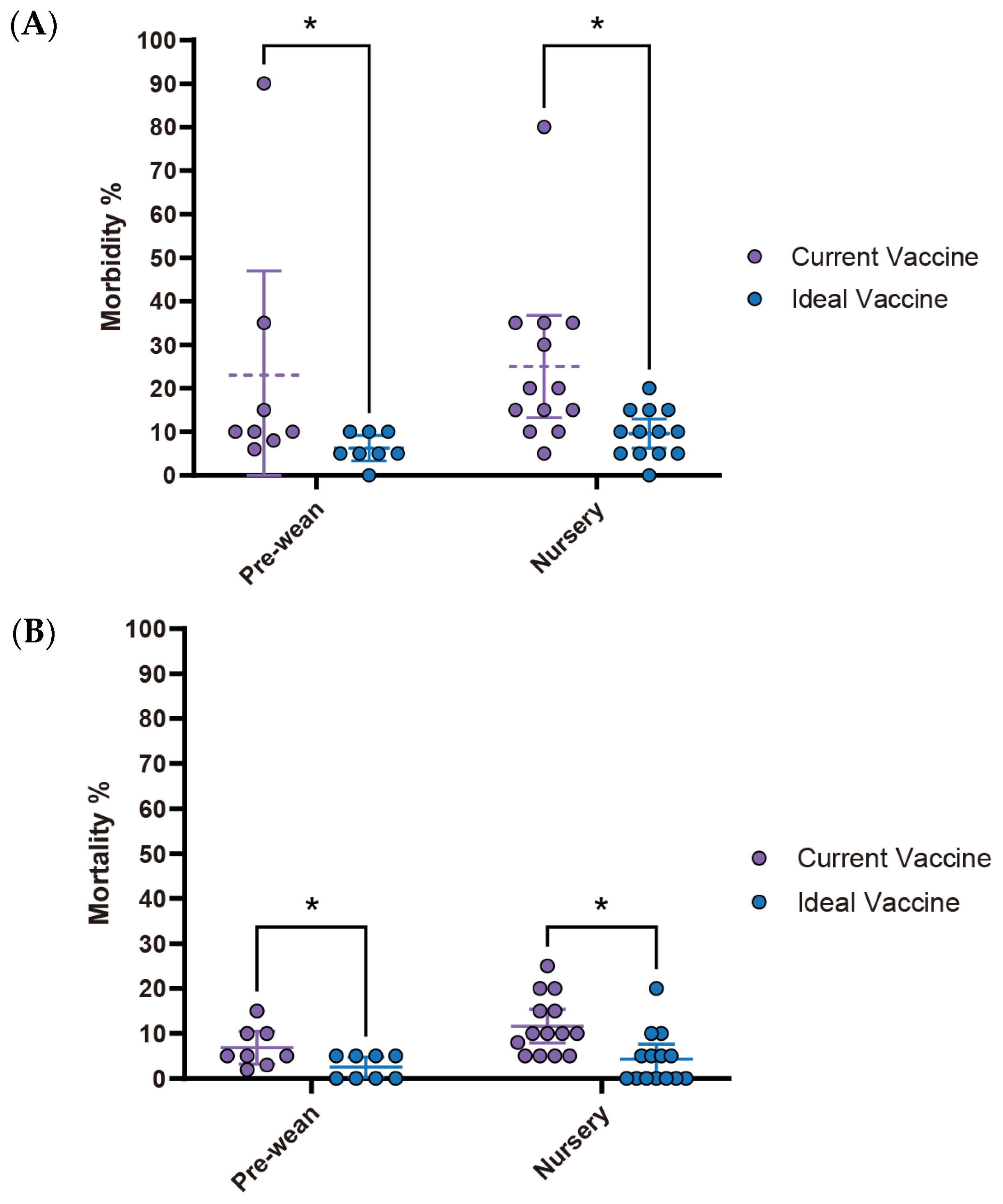
Current
S. suis vaccine fails to meet veterinarians’ expectations, particularly concerning morbidity and mortality rates in the nursery stage. While no statistically significant differences are observed in the pre-wean stage, the nursery stage showed a significant reduction in morbidity from 13.9% (95% CI: 6.26–21.60) with current vaccines to 4.4% (95% CI: 2.14–6.75) with an ideal vaccine (
p < 0.05). Similarly, nursery mortality rates significantly declined from 6.5% (95% CI: 3.48–9.60) to 1.2% (95% CI: −0.07–2.54) (
Figure 5A,B). Economically, current
S. suis vaccines are priced at an average of
$0.47 per dose (95% CI:
$0.28–
$0.65). However, survey participants expressed a willingness to pay an average of
$0.83 per dose (95% CI:
$0.51–
$1.16) for an ideal vaccine that significantly reduces morbidity and mortality (
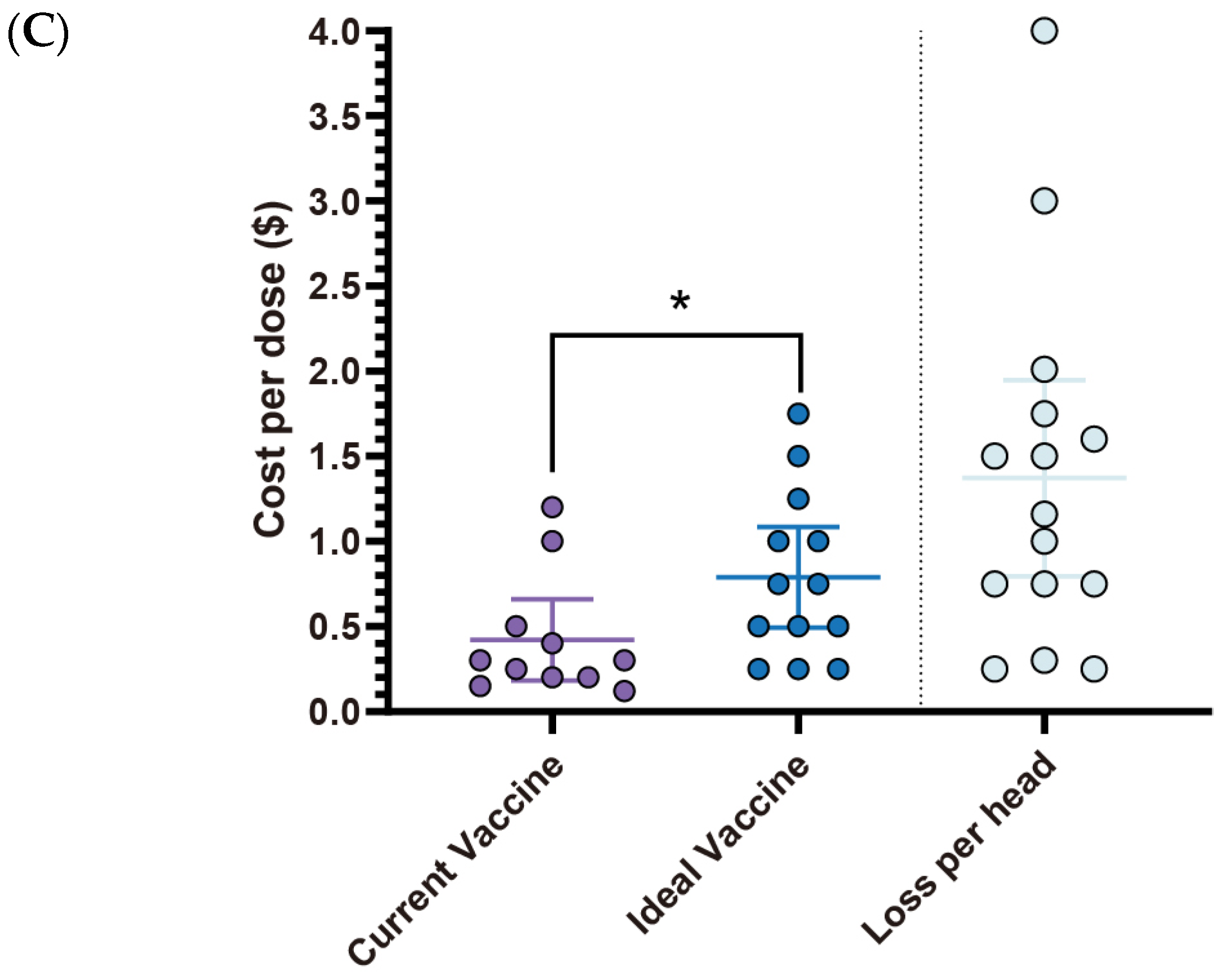
Figure 5C,
Table 1). With an estimated loss
$1.254 per head (95% CI:
$0.77–
$1.74), this suggests a promising opportunity for a 1.786-fold increase in price for a more effective vaccine. The primary challenges associated with current
S. suis vaccines, as summarized in
Table 2, include limitations in efficacy: lack of cross-protection against new variants (85.7%) and broader-spectrum protection (71.4%), availability issues delay in vaccine distribution (71.4%), and adverse effects like local reactions (42.9%).
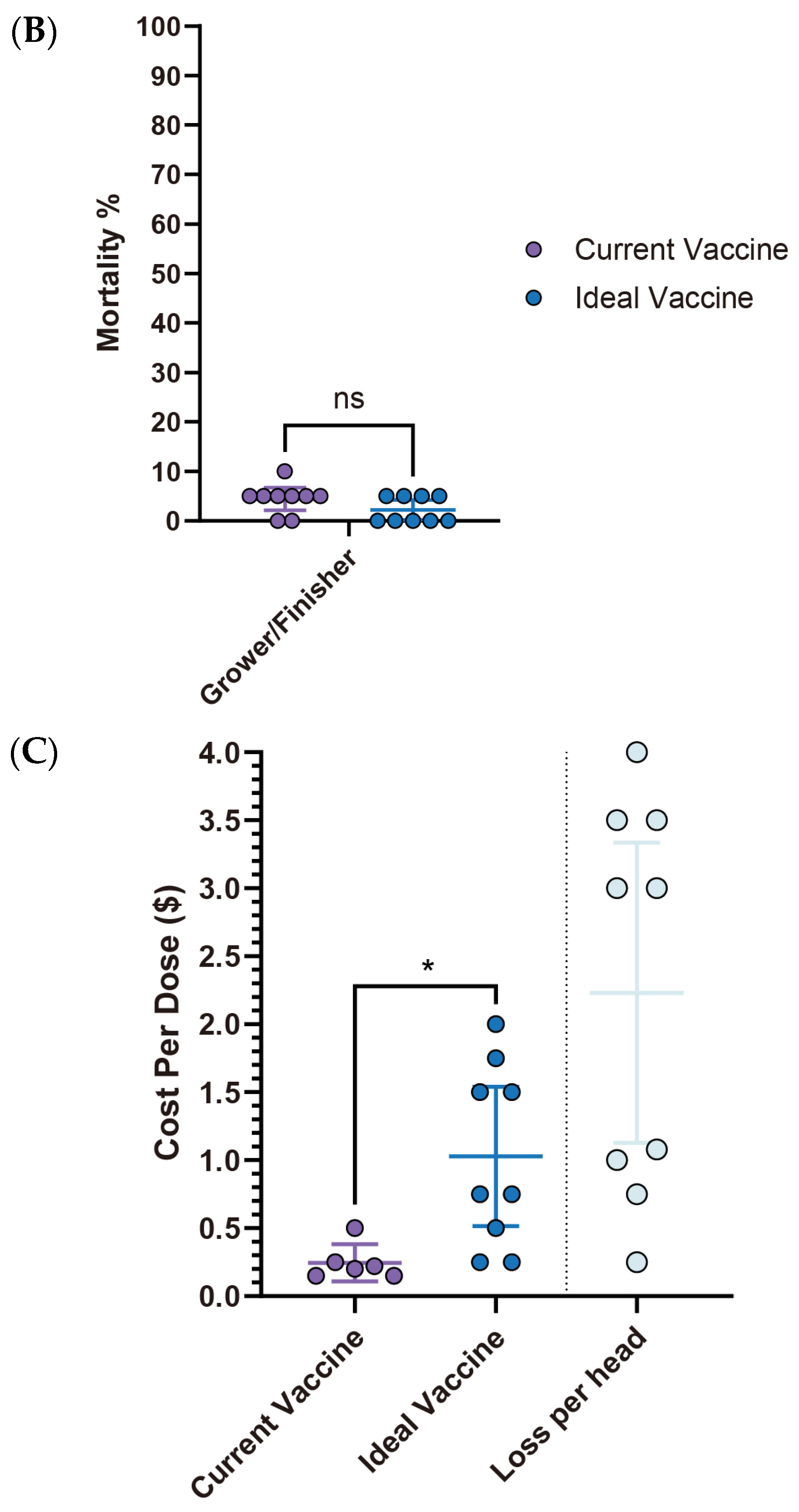
3.3.2. Escherichia coli (E. coli) in Swine Production: Current Landscape, Vaccine Expectations and Challenges
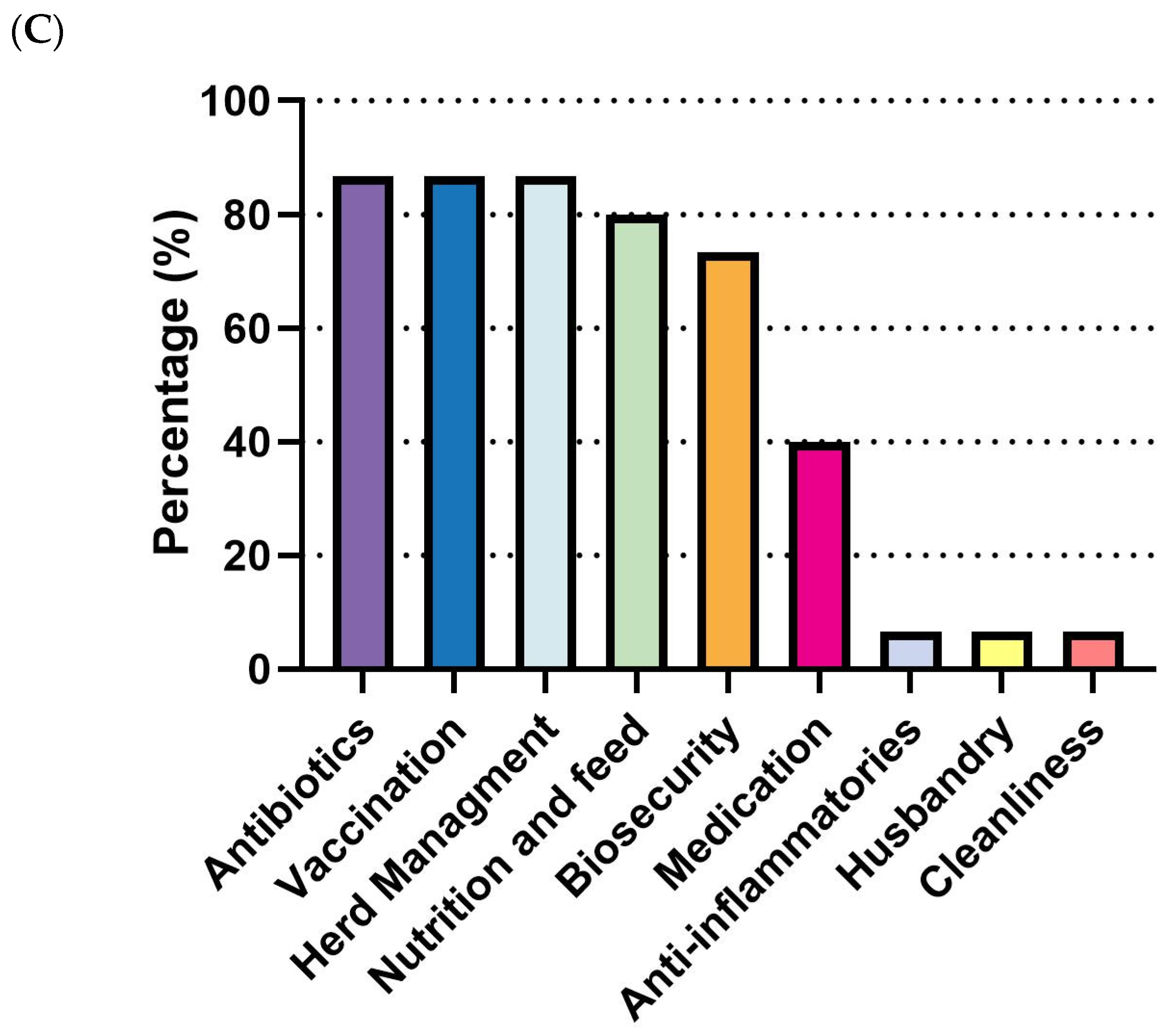
E. coli outbreaks in swine production are most common in nursery-stage pigs, showing the highest incidence (93.3%, 14/15), followed by the pre-wean stage (53.3%, 8/15). The grower/finisher and sow stages were not reported as significant outbreak periods (
Figure 6A). For diagnosis, clinical signs (100%, 15/15) were universally used for disease identification, followed by necropsy (86.7%, 13/15) and bacterial serotyping (86.7%, 13/15). PCR was moderately utilized (26.7%, 4/15), while culture was rarely employed (6.7%, 1/15) (
Figure 6B). As for management, antibiotics (86.7%, 13/15), vaccination (86.7%, 13/15), and herd management (86.7%, 13/15) were the most commonly employed measures. Nutrition and feed interventions (80%, 12/15) and biosecurity (73.3%, 11/15) were also frequently adopted. Medication (40%, 6/15) and other measures, including anti-inflammatories, husbandry, and cleanliness (6.7%, 1/15 each), were less commonly reported (
Figure 6C).
Respondents expressed significantly higher expectations for improvements in
E. coli vaccines, noting substantial differences between current and ideal vaccine performance in both pre-wean and nursery stages. In the pre-wean stage, morbidity with an ideal vaccine is anticipated to decrease from 23% to 6.3%, while in the nursery stage, it’s expected to drop from 25% to 9.6%. Similarly, mortality rates are projected to fall from 6.9% to 2.5% in pre-wean piglets and from 11.6% to 4.2% in the nursery, highlighting the desired impact of improved vaccines (
Figure 7A,B).
The average price of the current
E. coli vaccine is
$0.42 (95% CI:
$0.18–
$0.66), while the ideal vaccine price is able to reach
$0.79 (95% CI:
$0.49–
$1.08), reflecting a fold change of 1.88. This perceived value aligns with the estimated loss of
$1.37 (95% CI:
$0.79–
$1.95) per affected pig (
Figure 7C,
Table 3), suggesting a willingness to invest more for better protection. Current
E. coli vaccines face several challenges (
Table 4). The most frequently reported issue was the short duration of protection (54.5%), followed by lack of broader-spectrum protection, interference from maternal antibodies, and timing of administration, each reported by 36.4% of respondents. As for availability, difficulty in administration (45.5%) was the most significant concern, which is closely related to issues such as timing of administration and transient duration. Adverse effects are not a major concern (
Table 4).
3.3.3. Mycoplasma hyopneumoniae (M. hyo) in Swine Production: Current Landscape, Vaccine Expectations and Challenges
Outbreaks of
M. hyo primarily affect the grower/finisher phase, with 100% (9/9) of respondents identifying it as the main stage for outbreaks. The sow stage followed with a lower prevalence rate (33.3%, 3/9), while pre-wean and nursery stages showed minimal reported incidence (
Figure 8A). For diagnosis, PCR was the most frequently employed diagnostic tool (88.9%, 8/9), followed by necropsy (66.7%, 6/9) and ELISA (44.4%, 4/9). Other diagnostic approaches, including clinical signs, immunohistochemistry (IHC), and sequencing, were rarely utilized (11.1%, 1/9 each) (
Figure 8B).
Regarding management strategies, antimicrobials and herd management were the most commonly adopted measures (77.8%, 7/9), followed by vaccination (66.7%, 6/9). Medication, biosecurity, and disease elimination each accounted for 44.4% (4/9). Nutrition and feed interventions and the purchase of negative breeding stock were reported at lower frequencies (
Figure 8C).
Interestingly, despite its high economic impact (estimated loss of
$2.23 per affected pig),
M. hyo vaccine improvements showed the lowest expectations among the four diseases analyzed. There were no significant differences (ns) observed in both morbidity and mortality rates between the current and ideal vaccines. Specifically, morbidity decreased from 19% (95% CI: 8.12–29.88) with the current vaccine to 11.7% (95% CI: −1.08–24.41) with the ideal vaccine, while mortality declined from 4.4% (95% CI: 2.14–6.75) to 2.2% (95% CI: 0.20–4.25), with neither showing statistical significance (
Figure 9A,B). However, a notable observation lies in the vaccine price. The ideal vaccine’s price not only exhibited the most significant fold change (4.2 times) compared to the current vaccine, rising from
$0.25 (95% CI:
$0.11–
$0.38) to
$1.03 (95% CI:
$0.52–
$1.54), but also had the highest average cost per dose at
$1.03 (95% CI:
$0.52–
$1.54). Moreover, the estimated economic loss per affected pig was also the highest among the four diseases, averaging
$2.23 (95% CI:
$1.13–
$3.34) (
Figure 9C,
Table 5). The primary challenge with current
M. hyo vaccines is their poor quality (66.7%, 4/6). This primary concern is accompanied by the short duration of protection (33.3%, 2/6) and shortage of vaccine storage (33.3%, 2/6). Adverse effects are not a major concern (
Table 6).
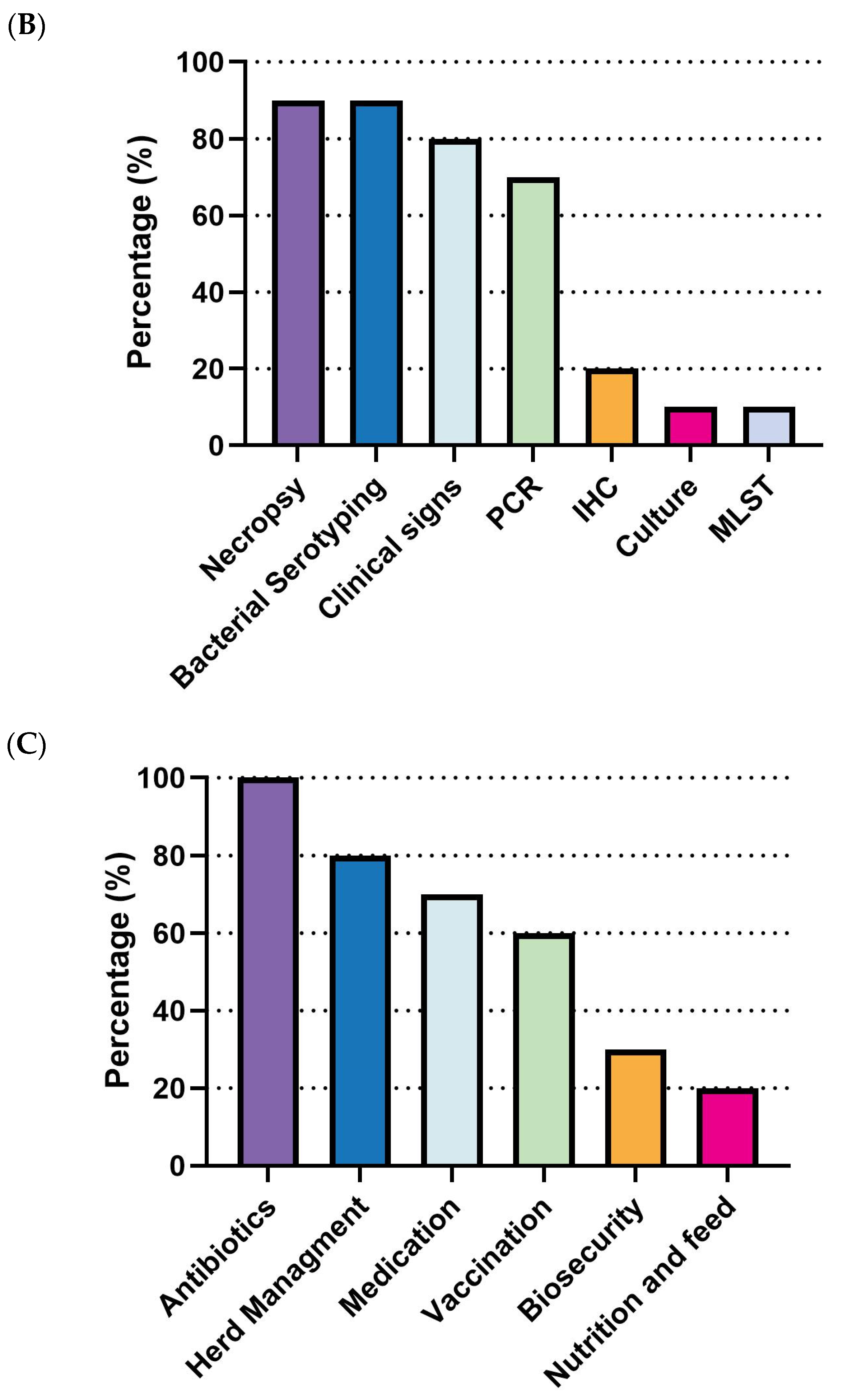
3.3.4. Glaesserella parasuis (G. parasuis) in Swine Production: Current Landscape, Vaccine Expectations and Challenges
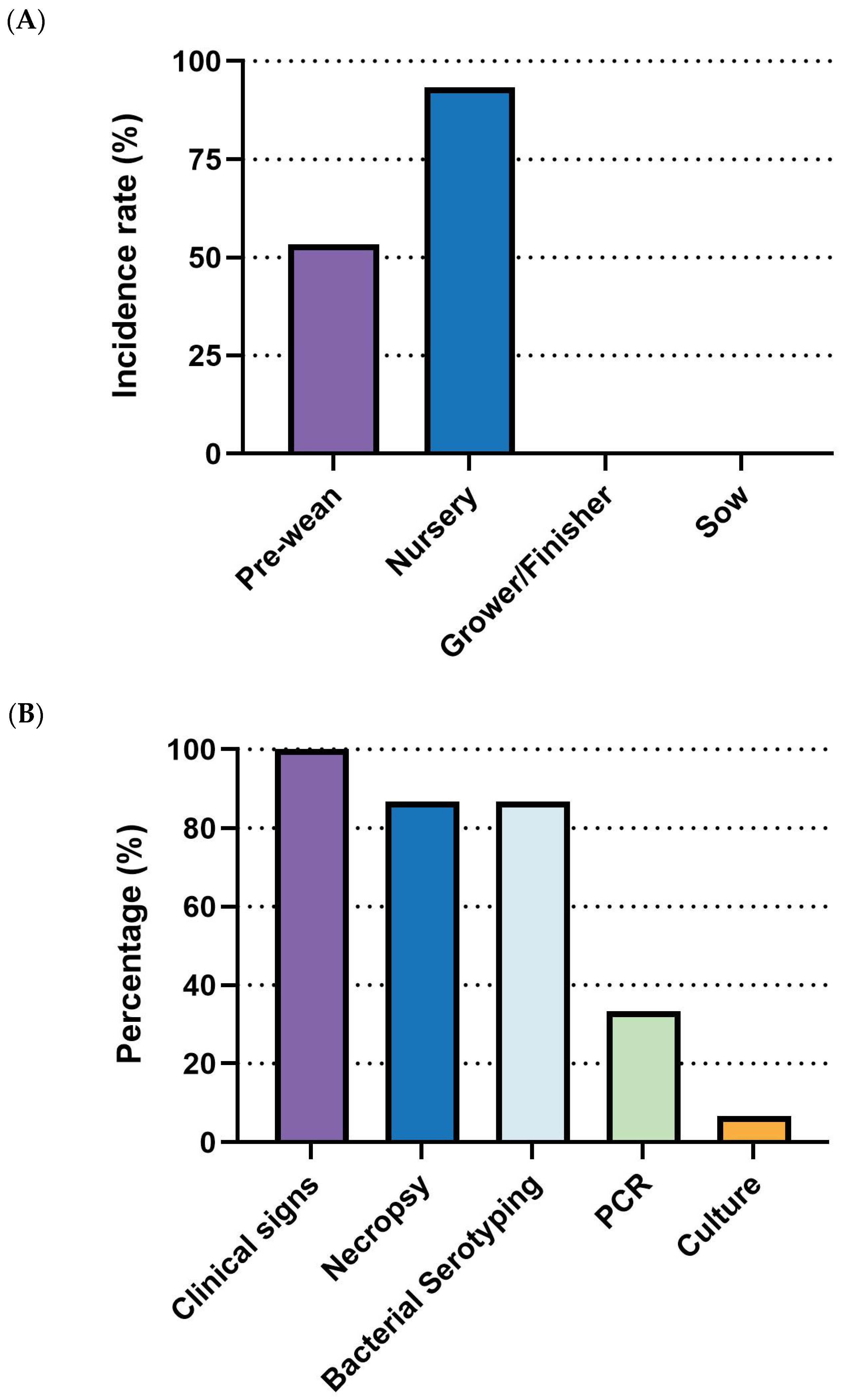
G. parasuis outbreaks are most prevalent in the nursery stage, with all respondents (100%, 10/10) identifying it as the primary phase for disease occurrence. Other stages showed significantly lower prevalence, remaining under 20% (
Figure 10A). For diagnostic methods, necropsy (90%, 9/10), bacterial serotyping (90%, 9/10), clinical signs (80%, 8/10), and PCR (70%, 7/10) were the most commonly employed approaches. Culture (10%, 1/10), immunohistochemistry (IHC, 20%, 2/10), and Multi-Locus Sequence Typing (MLST) were rarely applied (
Figure 10B). Regarding management strategies, antibiotics were universally used by all respondents (100%, 10/10), followed by herd management (80%, 8/10) and medication (70%, 7/10). Vaccination was employed by 60% (6/10) of the respondents. Lastly, biosecurity (30%, 3/10) and nutrition/feed strategies (20%, 2/10) were less frequently adopted (
Figure 10C).
The gap between current and ideal
G. parasuis vaccines remains significant. In the nursery stage, an ideal vaccine is projected to reduce morbidity from 11.3% (95% CI: 5.66–16.94) to 4.0% (95% CI: 1.74–6.26) and mortality from 4.1% (95% CI: 1.85–6.35) to 0.5% (95% CI: −0.63–1.63), demonstrating substantial expected improvements in efficacy (
Figure 11A,B). The estimated economic loss per affected pig was approximately
$0.96 (95% CI:
$0.47–
$1.45), while the cost per dose, the mean price of the current vaccine, was
$0.57 (95% CI:
$0.17–
$0.97), and respondents only willing to pay a price slightly higher than that for an ideal vaccine, at
$0.72 (95% CI:
$0.18–
$1.26). This suggests that while improvements are desired, there’s a limited acceptable price increase for enhanced performance (
Figure 11C,
Table 7). Current
G. parasuis vaccines do not exhibit particularly prominent shortcomings (
Table 8), but key concerns include a lack of cross-protection against new variants (33.3%), limited broader-spectrum protection (33.3%), and efficacy interference by maternal antibodies (33.3%). Additionally, delays in vaccine availability (33.3%) and high costs (33.3%) were noted as issues, alongside reported adverse effects like local reactions (50.0%) and temporary decreases in productivity (33.3%) (
Table 8).
3.4. Interview Insights
We conducted interviews with three highly experienced veterinarians from the respondent pool, each possessing an average of 35.3 years of professional experience. Their work (or past roles) spans academic institutions, industry, and associations. Hereafter, they will be referred to as Dr. A, Dr. B, and Dr. C.
3.4.1. Relationship Between Vaccines and Antibiotics
When asked about the relationship between vaccines and antibiotics, the veterinarians shared varied and thoughtful insights.
Insights from Dr. A: “It’s a matter of do vaccines give us an opportunity of more effectively, more judiciously use of our antimicrobials, but we are never going to completely eliminate the use of antimicrobials. There are some animals that don’t respond to the vaccine or some difficulties in administering it. Certainly, could make great opportunities to use less of it, right? Certainly, could make great opportunities to make them more effective when we do use them. For example, we know when we have PRRS-infected pigs that change the pharmacokinetics of antimicrobials. So, I think they are an additional tool, and every additional tool we get, the more judicious we can use the one we have available.”
Insights from Dr. B: “Maybe there are situations when vaccines would be an alternative. But generally, when I think about that, it would be an alternative to mass treatments. What we want to do is to decrease the routine use of feed-grade antibiotics in water, soluble antibiotics. But still, because of animal care, we want to be able to still do individual animal treatments. A great example is Streptococcus suis. If you look at the amount of Amoxicillin that is administered in the water across our entire system, it is quite high. Because it is relatively low-cost and the only alternative we have at this time. So there, if we had an effective vaccine, I would anticipate we would not have to use the Amoxicillin, but we still would use one of the injectables for the individual pig. So that’s kind of a good way to think about it.”
Insights from Dr. C: “I’m not sure about the term ‘alternative’. I don’t think it’s vaccines or antibiotics. I think there will always be a balance of the use of both. If we had an effective PRRS vaccine, as an example. Antibiotic use across the industry would go down for sure. But I don’t see vaccines replacing antibiotics entirely. See the balance changing.”
3.4.2. Potential for Disease Elimination
For the question “Which disease do you believe we can potentially eliminate first?”, the veterinarians highlighted those that compromise the pig’s immune system or have well-defined targets.
Insights from Dr. A: “Well, I think by far the most important would be the primary infectious agents that would compromise the pigs’ immune system. Because that creates more opportunities for bacteria, so PRRS is definitely on that list, right? Mycoplasma hyopneumoniae would be on that list because they would impact the mucociliary defense to protect the lung. Influenza would certainly be on that list. Circovirus type 2, although we have a reasonably effective vaccine, there is always room for improvement on that one. I think any time we could take one element out of the equation, we start to skew the odds in favor of the pigs. And that’s what we really want to do. So, yeah, any of those diseases would simplify the picture, and the reason I say those diseases have an impact on the immune system is because I think then we will get a lot of secondary benefits, right? A lot of bacteria that kill pigs are just opportunistic because the pigs’ defenses are down. So if the pigs’ defenses are re-established or protected, then, you know, potentially will have an entirely different outcome. And we narrow the list of things they are going to be susceptible to.”
Insights from Dr. B: “Mycoplasma hyopneumoniae, we’re in the industry being very successful. Or if we had a more effective vaccine, you can look at it the other way. The Mycoplasma hyopneumoniae vaccine only cost us about 10 cents now a pig, a dose, but they’re not very effective. So, if there was technology that would give us a more effective vaccine. You would answer the question differently. Certainly, we know what PIC (Pig Improvement Company, a major global swine genetics company) did, they were able to eliminate Streptococcus suis, Actinobacillus suis and Mycoplasma hyorhinis. Now, you can’t do that on a commercial farm. But it still says the opportunities there to eliminate those bacteria. And we can successfully eliminate swine influenza today in the sow herds. We do it all the time. It takes us about six weeks. But the problem is they become reinfected, different strain comes in and affect the pig flow. We can eliminate it from the sow herds. So the pigs are negative at weaning to the virus, but most of them get contaminated in the growing period.”
Insights from Dr. C: “Yes. For example, M.hyo causes pneumonia and slow growth. We don’t deal with single infections a lot. Focused elimination is a good choice. All the pathogens alone, you know, Mycoplasma alone in a naive herd is very problematic. Mycoplasma alone and endemically infected herd as a drag on production. But we don’t see an increase in mortality now. We just see pneumonia and slow growth. More and more successful elimination of mycoplasma. So I’m not sure focusing on that one. As you look at viruses, especially the viruses in that complex situation, PRRS virus, influenza, our influence is difficult. We have vaccines that do an okay job reducing disease, but they’re very specific, and it’s a constantly changing virus, as is PRRS. So yeah, our commercial vaccines are never a perfect match for these viral diseases. So right, those are probably the bigger opportunities in my mind and where we’re lacking good products today. I would say influenza, PRRS, enteric viruses, PEDV, Rotavirus. Then on the bacterial side, Streptococcus suis. I mean, I have friends and colleagues working on that for the last three decades, and we still don’t have a great option for Streptococcus suis.”
3.4.3. Support Necessary for Antimicrobial Reduction
For the question “What kind of support (e.g., financial, educational, regulatory) do you think is necessary to facilitate the reduction in the use of antimicrobials.”, the veterinarians pointed to a mix of education, effective vaccines, and regulatory measures.
Insights from Dr. A: “I think a much better understanding about antimicrobial stewardship, and how things contribute to stewardship. I think farms are going to be expected in the future to establish formal stewardship plans. Most of human health in U.S. has required to have a formal stewardship plan. Based on the core 5 principle that the AVMA has adopted to vet med in order to get their federal funding, we don’t have that sort of reward on veterinarians’ side. The owner pays the cost. So, I think we can expect some sort of regulatory legislation to force that issue. So, the sooner farms start thinking about that, the better.”
Insights from Dr. B: “It is more effective vaccines against these primarily bacterial pathogens and viral pathogens. If we’re Mycoplasma negative and we’re PRRS negative, our growing pigs do not have any antibiotics other than an individual pig treatment, and we would have mortality in, say, on the top 10% between 3 and 3.5%. And that mortality would be just for a number of reasons: lame, fighting. So the goal is that is to raise pigs without antibiotics. To do that, we’ve still got some gaps in our vaccines. And a big thing to put on your list would be, that still frustrates me, even in our autogenous and prescription vaccines, it tends to take us 12 to 14 weeks from the time we start to get a product. And I argue with our vets because in production, for 14 weeks is almost a third of a year. So, I have to tell my producer and myself, well, that’s, we’ll have something in four months. And yeah, anything you can do to shorten that would improve the opportunity for market.”
Insights from Dr. C: “Antibiotic use. Education is always important. Within the industry, we’re constantly monitoring antimicrobials resistance target by antibiotics stewardship. So I think the education is there and hopefully the industry, we’re trending with less and less antibiotic use. From a regulatory perspective, we’re limited in our antibiotic choices today. Hopefully don’t foresee more stringent regulatory use in antibiotics. We’re fairly limited in what we have to use today for animal welfare treatment.”