Alternative Sigma Factors of RNA Polymerase as Master Regulators in the Pathogenic Spirochaete Leptospira interrogans
Abstract
1. Introduction
2. Diversity of Alternative σ Factors in Bacteria
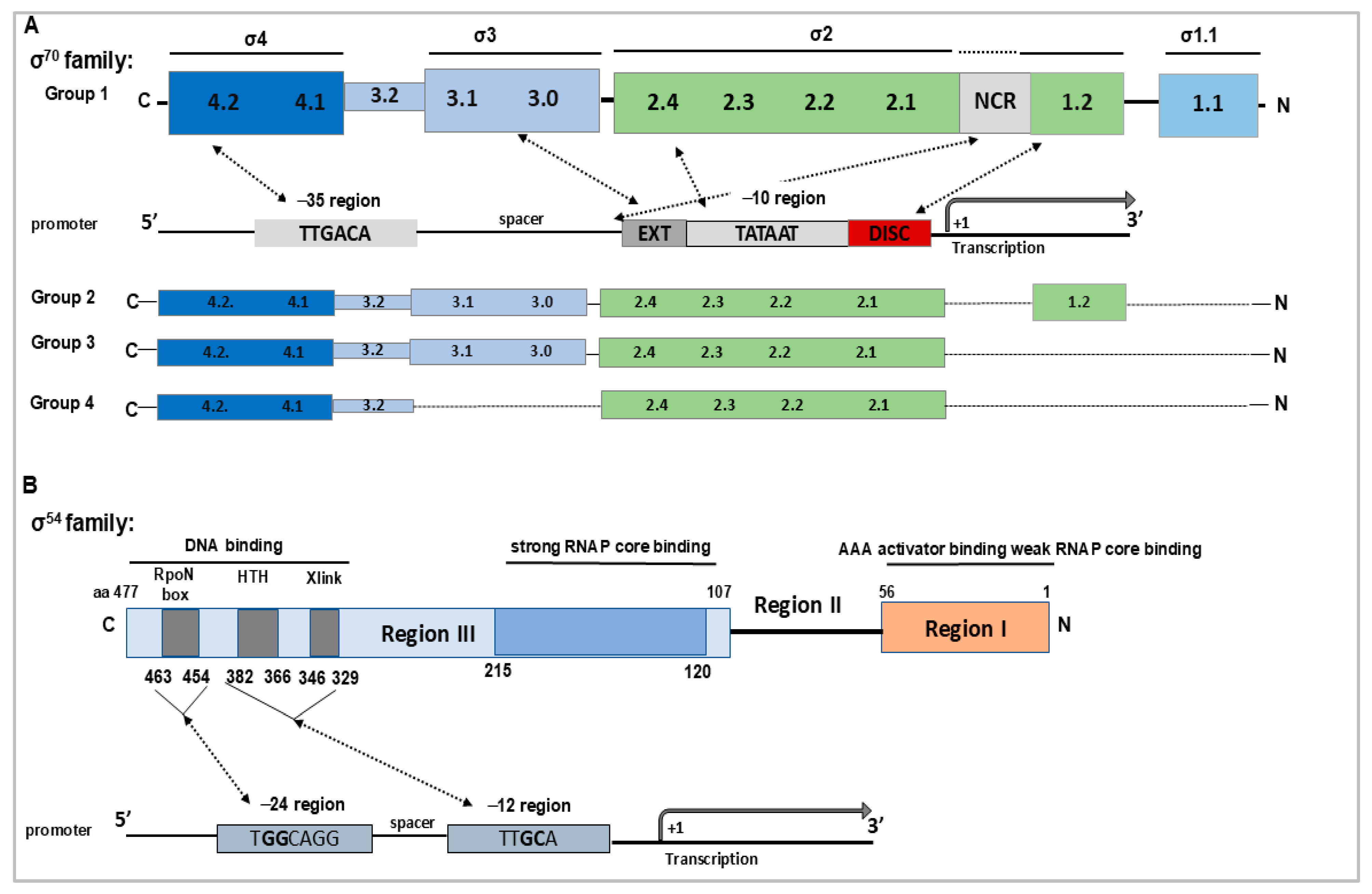
3. Alternative σ Factors in L. interrogans
3.1. σ54 (RpoN): Metabolic Adaptation and Virulence Potential
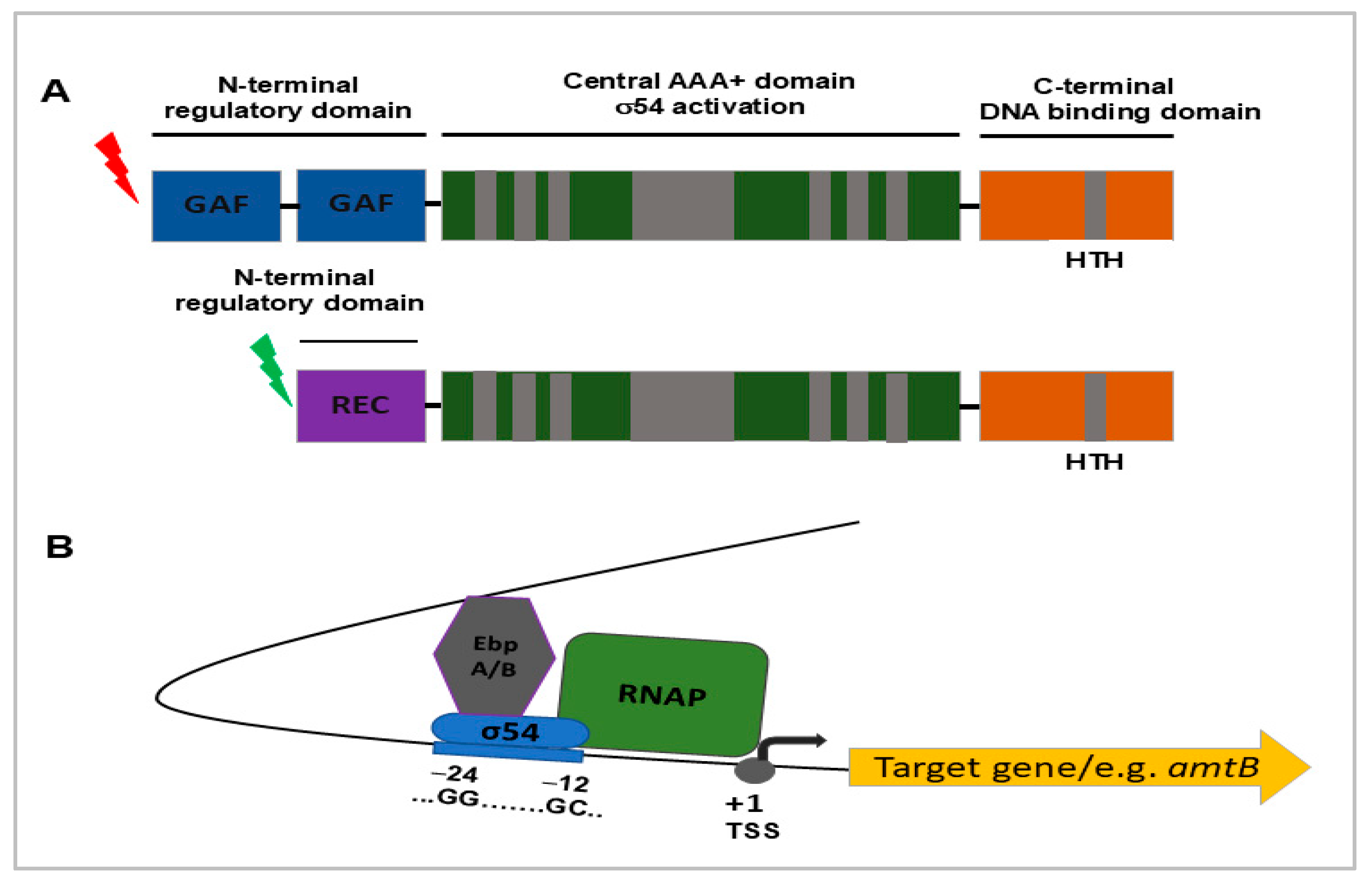
3.2. σ28 (FliA): Motility Control and Contribution to Virulence
3.3. ECF σ Factors: Diversity, Regulatory Mechanisms and Predicted Functions
| a Gene ID | ECF Group | ECF Subgroup | NCBI Reference Sequence (Protein ID) | Number of Amino Acid Residues | Calculated Molecular Weight |
|---|---|---|---|---|---|
| Chromosome I (large) | |||||
| LIC_10144 | ungrouped | unsubgrouped | WP_000777857.1 | 174 | 20,096 |
| LIC_10225 | unclassified | unclassified | WP_001054050.1 | 301 (21-194 σ70-ECF family region) | 35,314 |
| LIC_10386 | ECF242 | ECF242s1 | WP_000951509.1 | 182 | 20,970 |
| LIC_10559 | ECF208 | ECF208s1 | WP_000988152.1 | 181 | 21,087 |
| LIC_10644 | ECF229 | ECF229s2 | WP_000777857.1 | 174 | 20,321 |
| LIC_11817 | ECF16 (SigF-like) | ECF16s14 | WP_001274737.1 | 184 | 21,620 |
| b LIC_12490 | ungrouped | ECFs21 | WP_000378482.1 | 206 | 23,881 |
| LIC_12757 | ECF229 | ECF229s1 | WP_000081452.1 | 180 | 21,105 |
| LIC_13266 | ECF246 | ECF246s2 | WP_001209037.1 | 192 | 22,906 |
| LIC_13285 | ECF208 | ECF208s2 | WP_000435437.1 | 169 | 19,550 |
| Chromosome II (small) | |||||
| LIC_20115 | ECF242 | ECF242s2 | WP_001971886.1 | 166 | 19,377 |
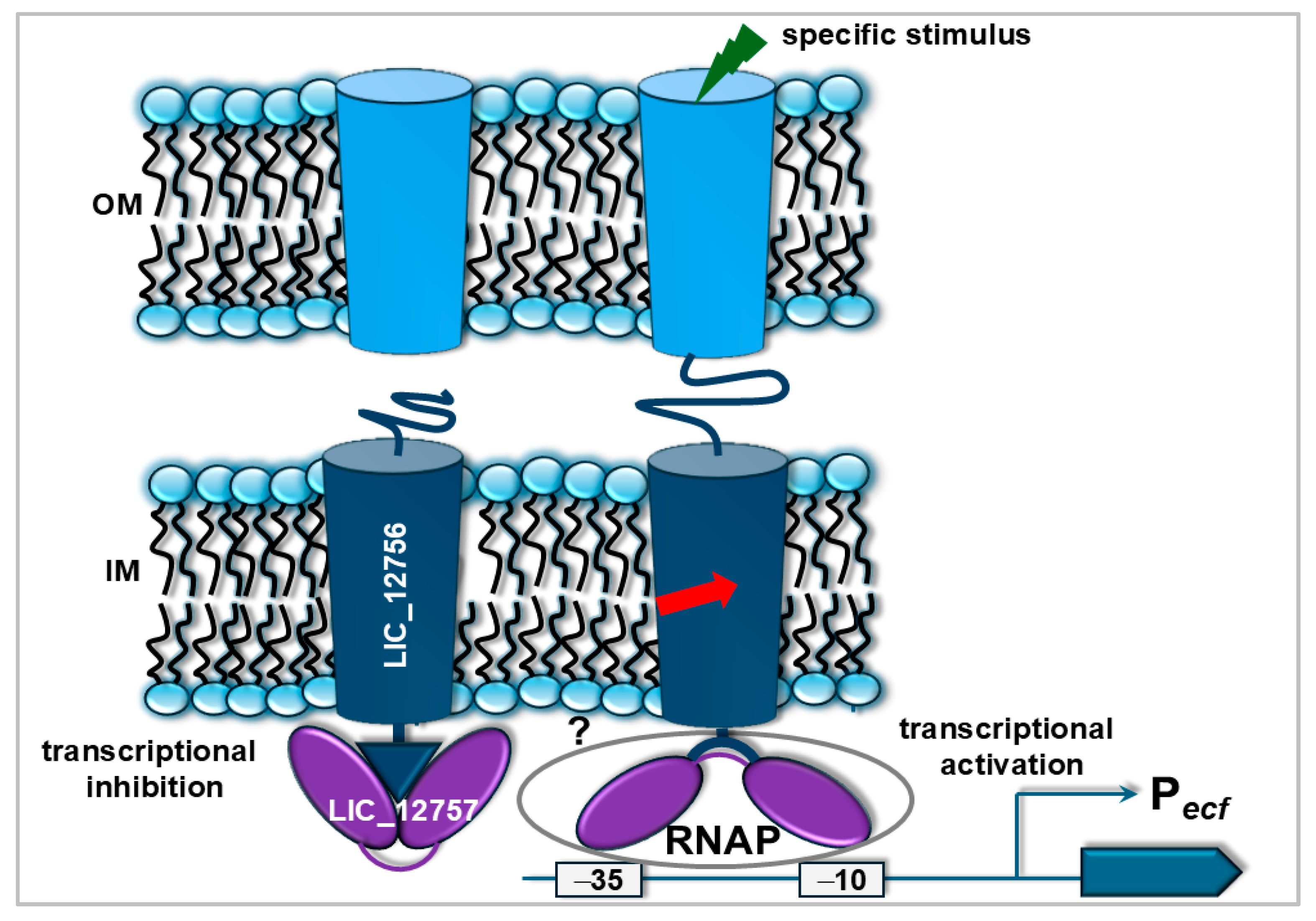
4. Concluding Remarks and Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| EBP | enhancer-binding protein |
| ECF σs | extracytoplasmic function σ factors |
| L. interrogans | Leptospira interrogans |
| RNAP | RNA polymerase core enzyme |
References
- Adler, B.; de la Peña Moctezuma, A. Leptospira and leptospirosis. Vet. Microbiol. 2010, 140, 287–296. [Google Scholar] [CrossRef]
- Vincent, A.T.; Schiettekatte, O.; Goarant, C.; Neela, V.K.; Bernet, E.; Thibeaux, R.; Ismail, N.; Mohd Khalid, M.K.; Amran, F.; Masuzawa, T.; et al. Revisiting the taxonomy and evolution of pathogenicity of the genus Leptospira through the prism of genomics. PLoS Negl. Trop. Dis. 2019, 13, e0007270. [Google Scholar] [CrossRef]
- Adler, B. History of Leptospira and leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 1–9. [Google Scholar] [CrossRef]
- Haake, D.A.; Levett, P.N. Human leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 65–97. [Google Scholar] [CrossRef]
- Pal, M.; Bulcha, M.R.; Bune, W.M. Leptospirosis and one health perspective. Am. J. Public Health Res. 2021, 9, 180–183. [Google Scholar] [CrossRef]
- Costa, F.; Hagan, J.E.; Calcagno, J.; Kane, M.; Torgerson, P.; Martinez-Silveira, M.S.; Stein, C.; Abela-Ridder, B.; Ko, A.I. Global morbidity and mortality of leptospirosis: A Systematic Review. PLoS Negl. Trop. Dis. 2015, 9, e0003898. [Google Scholar] [CrossRef] [PubMed]
- Rajapakse, S.; Fernando, N.; Dreyfus, A.; Smith, C.; Rodrigo, C. Leptospirosis. Nat. Rev. Dis. Primers 2025, 11, 32. [Google Scholar] [CrossRef] [PubMed]
- Ryan, E.G.; Nola, L.; O’Grady, L.; More, S.J.; Doherty, L.M. Seroprevalence of Leptospira Hardjo in the irish suckler cattle population. Ir. Vet. J. 2012, 65, 8. [Google Scholar] [CrossRef] [PubMed]
- Ellis, W.A. Animal leptospirosis. Curr. Top. Microbiol. Immunol. 2015, 387, 99–137. [Google Scholar] [CrossRef]
- Arent, Z.; Kędzierska-Mieszkowska, S. Seroprevalence study of leptospirosis in horses in northern Poland. Vet. Rec. 2013, 172, 269. [Google Scholar] [CrossRef]
- Orjuela, A.G.; Parra-Arango, J.L.; Sarmiento-Rubiano, L.A. Bovine leptospirosis: Effects on reproduction and an approach to research in Colombia. Trop. Anim. Health Prod. 2022, 54, 251. [Google Scholar] [CrossRef]
- Casanovas-Massana, A.; Pedra, G.G.; Wunder, E.A.; Diggle, P.J.; Begon, M.; Ko, A.I. Quantification of Leptospira interrogans survival in soil and water microcosms. Appl. Environ. Microbiol. 2018, 84, e00507-18. [Google Scholar] [CrossRef]
- Levett, P.N. Leptospirosis. Clin. Microbiol. Rev. 2001, 14, 296–326. [Google Scholar] [CrossRef]
- Fouts, D.E.; Matthias, M.A.; Adhikarla, H.; Adler, B.; Amorim-Santos, L.; Berg, D.E.; Bulach, D.; Buschiazzo, A.; Chang, Y.F.; Galloway, R.L.; et al. What makes a bacterial species pathogenic?: Comparative genomic analysis of the genus Leptospira. PLoS Negl. Trop. Dis. 2016, 10, e0004403. [Google Scholar] [CrossRef] [PubMed]
- Zhukova, A.; Fernandes, L.G.; Hugon, P.; Pappas, C.J.; Sismeiro, O.; Coppée, J.-Y.; Becavin, C.; Malabat, C.; Eshghi, A.; Zhang, J.J.; et al. Genome-wide transcriptional start site mapping and sRNA identification in the pathogen Leptospira interrogans. Front. Cell. Infect. Microbiol. 2017, 7, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Gruber, T.M.; Gross, C.A. Multiple sigma subunits and the partitioning of bacterial transcription space. Annu. Rev. Microbiol. 2003, 57, 441–466. [Google Scholar] [CrossRef]
- Feklístov, A.; Sharon, B.D.; Darst, S.A.; Gross, C.A. Bacterial sigma factors: A historical, structural, and genomic perspective. Annu. Rev. Microbiol. 2014, 68, 357–376. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S. Sigma factors of RNA polymerase in the pathogenic spirochaete Leptospira interrogans, the causative agent of leptospirosis. FASEB J. 2023, 37, e23163. [Google Scholar] [CrossRef]
- Kazmierczak, M.J.; Wiedmann, M.; Boor, K.J. Alternative sigma factors and their roles in bacterial virulence. Microbiol. Mol. Biol. Rev. 2005, 69, 527–543. [Google Scholar] [CrossRef]
- Paget, M.S. Bacterial sigma factors and anti-sigma factors: Structure, function and distribution. Biomolecules 2015, 5, 1245–1265. [Google Scholar] [CrossRef]
- Shingler, V. Signal sensory systems that impact σ54-dependent transcription. FEMS Microbiol. Rev. 2011, 35, 425–440. [Google Scholar] [CrossRef]
- Paget, M.S.; Helmann, J.D. The σ70family of sigma factors. Genome Biol. 2003, 4, 203. [Google Scholar] [CrossRef]
- Li, J.; Zhang, H.; Li, D.; Liu, Y.J.; Bayer, E.A.; Cui, Q.; Feng, Y.; Zhu, P. Structure of the transcription open complex of distinct σI factors. Nat. Commun. 2023, 14, 6455. [Google Scholar] [CrossRef]
- Sanchez- Vazquez, P.; Dewey, C.N.; Kitten, N.; Ross, W.; Gourse, R.L. Genome- wide effects on Escherichia coli transcription from ppGpp binding to its two sites on RNA polymerase. Proc. Natl. Acad. Sci. USA 2019, 116, 8310–8319. [Google Scholar] [CrossRef]
- Narayanan, A.; Vago, F.S.; Li, K.; Qayyum, M.Z.; Yernool, D.; Jiang, W.; Murakami, K.S. Cryo-EM structure of Escherichia coli σ70 RNA polymerase and promoter DNA complex revealed a role of σ non-conserved region during the open complex formation. J. Biol. Chem. 2018, 293, 7367–7375. [Google Scholar] [CrossRef]
- Zhang, N.; Buck, M.A. A perspective on the enhancer dependent bacterial RNA polymerase. Biomolecules 2015, 5, 1012–1019. [Google Scholar] [CrossRef]
- Southern, S.; Merrick, M. The role of region II in the RNA polymerase σ factor σN (σ54). Nucleic Acids Res. 2000, 28, 2563–2570. [Google Scholar] [CrossRef]
- Hu, W.L.; Pappas, C.J.; Zhang, J.J.; Yang, Y.Y.; Yan, J.; Picardeau, M.; Yang, X.F. The EbpA- RpoN regulatory pathway of the pathogen Leptospira interrogans is essential for survival in the environment. Appl. Environ. Microbiol. 2017, 83, e02377-16. [Google Scholar] [CrossRef]
- Zhang, J.-J.; Hu, W.L.; Yang, Y.; Li, H.; Picardeau, M.; Yan, J.; Yang, X.F. The sigma factor σ54 is required for the long- term survival of Leptospira biflexa in water. Mol. Microbiol. 2018, 109, 63–77. [Google Scholar] [CrossRef]
- Boardman, B.K.; He, M.; Ouyang, Z.; Xu, H.; Pang, X.; Yang, X.F. Essential role of the response regulator Rrp2 in the infectious cycle of Borrelia burgdorferi. Infect. Immun. 2008, 76, 3844–3853. [Google Scholar] [CrossRef]
- Dunham-Ems, S.M.; Caimano, M.J.; Eggers, C.H.; Radolf, J.D. Borrelia burgdorferi requires the alternative sigma factor RpoS for dissemination within the vector during tick-to-mammal transmission. PLoS Pathog. 2012, 8, e1002532. [Google Scholar] [CrossRef]
- Alanazi, F.; Raghunandanan, S.; Priya, R.; Yang, X.F. The Rrp2-RpoN-RpoS pathway plays an important role in the blood-brain barrier transmigration of the Lyme disease pathogen. Infect. Immun. 2023, 91, e0022723. [Google Scholar] [CrossRef]
- Yang, X.F.; Hübner, A.; Popova, T.G.; Hagman, K.E.; Norgard, M.V. Regulation of expression of the paralogous Mlp family in Borrelia burgdorferi. Infect. Immun. 2003, 71, 5012–5020. [Google Scholar] [CrossRef]
- Xu, H.; Caimano, M.J.; Lin, T.; He, M.; Radolf, J.D.; Norris, S.J.; Gheradini, F.; Wolfe, A.J.; Yang, X.F. Role of acetyl-phosphate in activation of the Rpr2-RpoN-RpoS pathway in Borrelia burgdorferi. PLoS Pathog. 2010, 6, e100104. [Google Scholar] [CrossRef]
- Nascimento, A.L.T.O.; Ko, A.I.; Martins, E.A.L.; Monteiro-Vitorello, C.B.; Ho, L.P.; Haake, D.A.; Verjovski-Almeida, S.; Hartskeerl, R.A.; Marques, M.D.; Oliveira, M.C.; et al. Comparative genomics of two Leptospira interrogans reveals novel insight into physiology and pathogenesis. J. Bcateriol. 2004, 186, 2164–2172. [Google Scholar] [CrossRef]
- Nakamura, S. Motility of the Zoonotic Spirochete Leptospira: Insight into Association with Pathogenicity. Int. J. Mol. Sci. 2022, 23, 1859. [Google Scholar] [CrossRef]
- Lambert, A.; Picardeau, M.; Haake, D.A.; Sermswan, R.W.; Srikram, A.; Adler, B.; Murray, G.A. FlaA proteins in Leptospira interrogans are essential for motility and virulence but are not required for formation of the flagellum sheath. Infect. Immun. 2012, 80, 2019–2025. [Google Scholar] [CrossRef]
- Schons-Fonseca, L.; da Silva, J.B.; Milanez, J.S.; Domingos, R.H.; Smith, J.L.; Nakaya, H.I.; Grossman, A.D.; Ho, P.L.; da Costa, R.M. Analysis of LexA binding sites and transcriptomics in response to genotoxic stress in Leptospira interrogans. Nucleic Acids Res. 2016, 44, 1179–1191. [Google Scholar] [CrossRef]
- Patarakul, K.; Lo, M.; Adler, B. Global transcriptomic response of Leptospira interrogans serovar Copenhageni upon exposure to serum. BMC Microbiol. 2010, 10, 31. [Google Scholar] [CrossRef]
- Kurniyati, K.; Chang, Y.; Guo, W.; Liu, J.; Malkowski, M.G.; Li, C. Anti-σ28 factor FlgM regulates flagellin gene expression and flagellar polarity of Treponema denticola. J. Bacteriol. 2023, 205, e00463-22. [Google Scholar] [CrossRef]
- Casas-Pastor, D.; Müller, R.R.; Jaenicke, S.; Brinkrolf, K.; Becker, A.; Buttner, M.J.; Gross, C.A.; Mascher, T.; Goesmann, A.; Fritz, G. Expansion and re-classification of the extracytoplasmic function (ECF) σ factor family. Nucleic Acids Res. 2021, 49, 986–1005. [Google Scholar] [CrossRef]
- Lo, M.; Bulach, D.M.; Powell, D.R.; Haake, D.A.; Matsunaga, J.; Paustian, M.L.; Zuerner, R.L.; Adler, B. Effects of temperature on gene expression patterns in Leptospira interrogans serowar Lai as assessed by whole-genome microarrays. Infect. Immun. 2006, 74, 5848–5859. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S.; Arent, Z. Immunoreactivity of a putative ECF σ factor, LIC_10559, from Leptospira interrogans with sera from Leptospira-infected animals. Pathogens 2023, 12, 512. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S.; Potrykus, K.; Arent, Z.; Krajewska, J. Identification of σE-dependent promoter upstream of clpB from the pathogenic spirochaete Leptospira interrogans by applying an E. coli two-plasmid system. Int. J. Mol. Sci. 2019, 20, 6325. [Google Scholar] [CrossRef]
- Krajewska, J.; Modrak-Wójcik, A.; Arent, Z.; Więckowski, D.; Zolkiewski, M.; Bzowska, A. Characterization of the molecular chaperone ClpB from the pathogenic spirochaete Leptospira interrogans. PLoS ONE 2017, 12, e0181118. [Google Scholar] [CrossRef]
- Lourdault, K.; Cerqueira, G.M.; Wunder Jr, E.A.; Picardeau, M. Inactivation of clpB in the pathogen Leptospira interrogans reduces virulence and resistance to stress conditions. Infect. Immun. 2011, 79, 3711–3717. [Google Scholar] [CrossRef]
- Kumar, S.; Lata, K.S.; Sharma, P.; Bhairappanavar, S.B.; Soni, S.; Das, J. Inferring pathogen-host interactions between Leptospira interrogans and Homo sapiens using network theory. Sci. Rep. 2019, 9, 1434. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S.; Kędzierska, B.; Potrykus, K. LIC_12757 from the pathogenic spirochaetes Leptospira interrogans encodes an autoregulated ECF σE-type factor. Vet. Microbiol. 2024, 293, 110092. [Google Scholar] [CrossRef]
- Kędzierska-Mieszkowska, S.; Kędzierska, B.; Pardyak, L.; Arent, Z. Evidence for a putative regulatory system consisting of an ECF σE-type factor, LIC_12757, and a FecR-like σ factor regulator, LIC_12756, in the pathogenic spirochaetes Leptospira interrogans. Int. J. Mol. Sci. 2025, 26, 4994. [Google Scholar] [CrossRef]
- Yokoyama, T.; Niinae, T.; Tsumagari, K.; Imami, K.; Ishihama, Y.; Hizukuri, Y.; Akiyama, Y. The Escherichia coli S2P intramembrane protease RseP regulates ferric citrate uptake by cleaving the sigma factor regulator FecR. J. Biol. Chem. 2021, 296, 100673. [Google Scholar] [CrossRef]
- Louvel, H.; Bommezzadri, S.; Zidane, N.; Boursaux-Eude, C.; Creno, S.; Magnier, A.; Rouy, Z.; Médigue, C.; Girons, I.S.; Bouchier, C.; et al. Comparative and functional genomic analyses of iron transport and regulation in Leptospira spp. J. Bacteriol. 2006, 188, 7893–7904. [Google Scholar] [CrossRef]
- Merien, F.; Baranton, G.; Perolat, P. Invasion of vero cells and induction of apoptosis in macrophages by pathogenic Leptospira interrogans are correlated with virulence. Infect. Immun. 1997, 65, 729–738. [Google Scholar] [CrossRef] [PubMed]
| σ Factor/Group/Gene Locus | Predicted Number | Proposed Function/Controlled Genes | Evidence/Characterization | Representative Homolog (E. coli) |
|---|---|---|---|---|
| σ70 family | ||||
| σ70/group 1 LIC_11701 | 1 | controls basal transcription; essential for growth and basic cellular functions/mainly housekeeping genes (>1000 genes) | supported by genomic and expression data | RpoD (σ70) |
| σ28/group 3 LIC_11380 | 1 | directs motility and flagellar gene regulation; contributes to virulence/genes encoding components of the endoflagellum (flaA1, flaB1, flaB4) and the flagellin-specific chaperone FliS (fliS) | predicted by comparative genomics | FliA (σ28) |
| ECF (σᴱ-type)/ group 4 (see Section 3.3) | 11 | mediates responses to extracytoplasmic and envelope stresses, contributing to adaptation and virulence/469 putative binding sites in the L. interrogans genome | predicted by genomic analyses; biochemically uncharacterized, except for LIC_12757 and LIC_10559 | RpoE (σ24/σᴱ) |
| σ54 family | ||||
| σ54 LIC_11545 | 1 | regulates nitrogen metabolism and complex cellular processes, contributing to environmental adaptation/genes encoding putative lipoproteins and the ammonium transporter AmtB; might be involved in glutamine synthesis (glnA), alanine racemization (alr), flagellar synthesis (fliO), sodium bile acid symport (ncP1), and lipid A biosynthesis (lpxC) | predicted by comparative genomics; studied in vitro | RpoN (σ54/σN) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kędzierska-Mieszkowska, S.; Arent, Z. Alternative Sigma Factors of RNA Polymerase as Master Regulators in the Pathogenic Spirochaete Leptospira interrogans. Pathogens 2025, 14, 1100. https://doi.org/10.3390/pathogens14111100
Kędzierska-Mieszkowska S, Arent Z. Alternative Sigma Factors of RNA Polymerase as Master Regulators in the Pathogenic Spirochaete Leptospira interrogans. Pathogens. 2025; 14(11):1100. https://doi.org/10.3390/pathogens14111100
Chicago/Turabian StyleKędzierska-Mieszkowska, Sabina, and Zbigniew Arent. 2025. "Alternative Sigma Factors of RNA Polymerase as Master Regulators in the Pathogenic Spirochaete Leptospira interrogans" Pathogens 14, no. 11: 1100. https://doi.org/10.3390/pathogens14111100
APA StyleKędzierska-Mieszkowska, S., & Arent, Z. (2025). Alternative Sigma Factors of RNA Polymerase as Master Regulators in the Pathogenic Spirochaete Leptospira interrogans. Pathogens, 14(11), 1100. https://doi.org/10.3390/pathogens14111100





