Candida Susceptibility to Antifungals in Amniotic Fluid: A Preliminary Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Sample Collection
2.2. Inclusion and Exclusion Criteria
2.3. Culture and Preliminary Identification
2.4. Automated Identification and Antifungal Susceptibility Testing
2.5. Molecular Confirmation by RT-PCR
2.5.1. DNA Extraction
2.5.2. PCR Assay
2.6. Statistical Analysis
2.7. Ethical Considerations
3. Results
3.1. Species Distribution by Month, Age, Parity, Gravidity, and Associated Pathologies
3.2. Antifungal Susceptibility Profiles of Candida Species
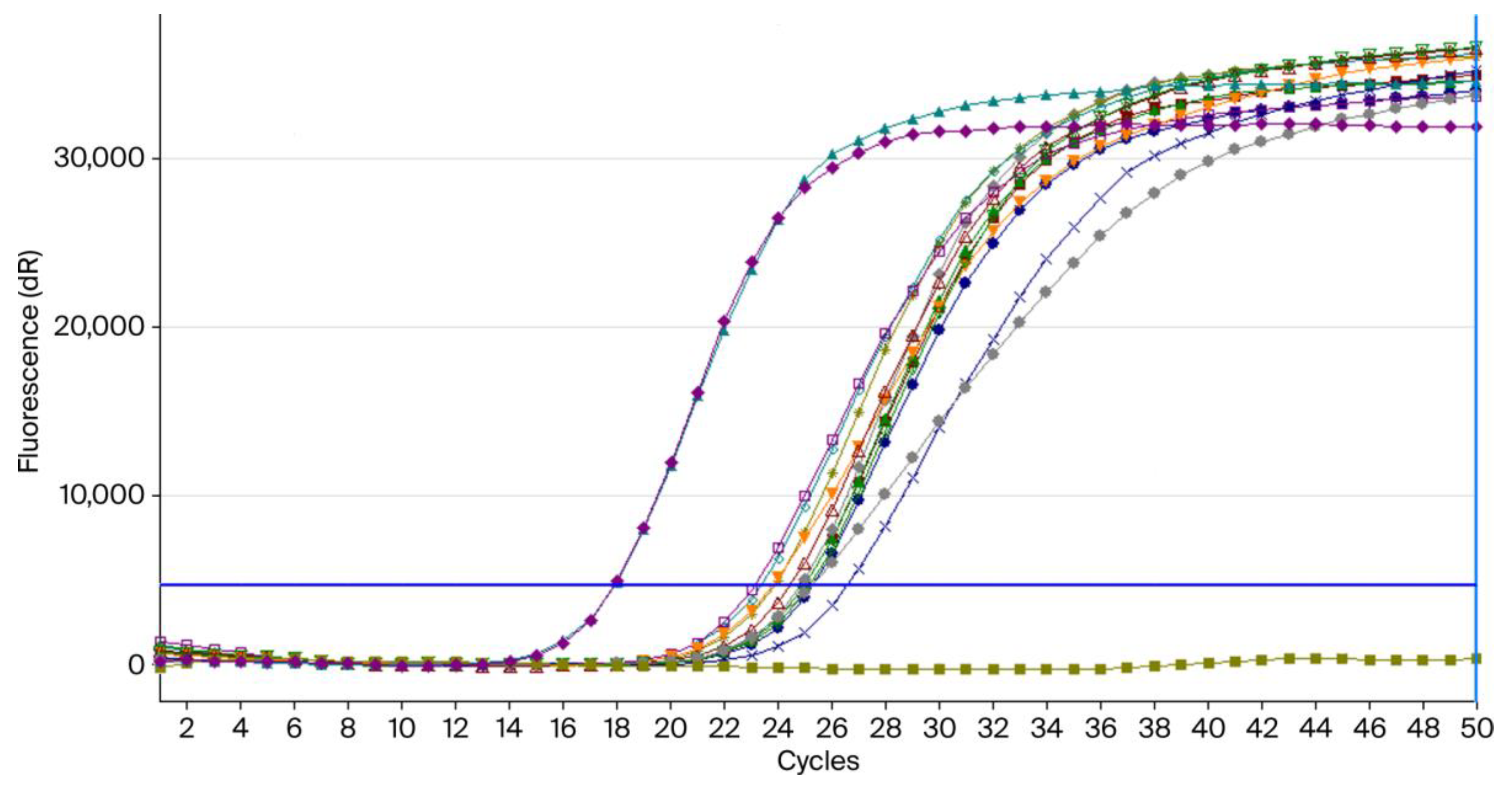
3.3. Confirmation of C. albicans by RT-PCR
4. Discussion
4.1. Main Findings and Comparison with the Literature
4.2. Clinical Implications: Management Challenges in Obstetrics, Need for Stewardship
4.3. Distinguishing True Infection from Contamination
4.4. Study Limitations and Future Directions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO Fungal Priority Pathogens List to Guide Research, Development and Public Health Action, 1st ed.; World Health Organization: Geneva, Switzerland, 2022; ISBN 978-92-4-006024-1.
- Gutiérrez, N.U.; López, M.J.V.; Bustos, C.Á.; Vidal, C.C.; Carvajal, J.A.; Severino, N.; Giordano, A.; Baquedano, S.U.; Feuerhake, T.; Rabagliati, R.; et al. Intra-Amniotic Candida albicans Infection Treated with Liposomal Amphotericin B with a Successful Neonatal Outcome. Open Forum Infect. Dis. 2024, 11, ofae047. [Google Scholar] [CrossRef]
- Chon, A.H.; Monson, M.A.; Gomez, N.G.; Butler-Wu, S.M.; Chmait, R.H. Multimodal Management of Cervical Insufficiency Complicated by Intra-Amniotic Candida albicans Infection. Am. J. Perinatol. 2023, 41, e1463–e1469. [Google Scholar] [CrossRef]
- Okumura, T.; Horiba, K.; Tetsuka, N.; Sato, Y.; Sugiyama, Y.; Haruta, K.; Yamaguchi, M.; Suzuki, T.; Torii, Y.; Kawada, J.-I.; et al. Next-Generation Sequencing-Based Detection of Ureaplasma in the Gastric Fluid of Neonates with Respiratory Distress and Chorioamnionitis. J. Matern. Neonatal Med. 2023, 36, 2207113. [Google Scholar] [CrossRef]
- Shiro, M.; Yamamoto, R.; Moriuchi, K.; Ishii, K. Candida glabrata Infection of the Amniotic Fluid with Chorioamnionitis and Maternal Candidemia and a Negative 1,3-β-D-Glucan Test: A Case Report. Case Rep. Women’s Health 2022, 36, e00462. [Google Scholar] [CrossRef]
- Kusanovic, J.P.; Jung, E.; Romero, R.; Green, P.M.; Nhan-Chang, C.-L.; Vaisbuch, E.; Erez, O.; Kim, C.J.; Gonçalves, L.F.; Espinoza, J.; et al. Characterization of Amniotic Fluid Sludge in Preterm and Term Gestations. J. Matern. Neonatal Med. 2022, 35, 9770–9779. [Google Scholar] [CrossRef]
- Yu, Y.; Huang, Q.; Liu, A. Analysis of Pathogens, Drug Resistance, Sensitive Antibiotic Treatment and Risk Factors of Early-Onset Sepsis in Very Low Birth Weight Infants. J. Pediatr. Neonatal Med. 2021, 13, 12939–12948. [Google Scholar]
- Maraki, S.; Stafylaki, D.; Karageorgos, S.A.; Koutroumpakis, F.; Hamilos, G.; Makrigiannakis, A.; Samonis, G. Antifungal Activity of Amniotic Fluid against Different Candida Species. Rev. Iberoam. Micol. 2021, 38, 148–150. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, S.; Luchian, I.; Damian, C.; Goriuc, A.; Porumb-Andrese, E.; Popa, C.G.; Cobzaru, R.G.; Ripa, C.; Ursu, R.G. Candida auris Updates: Outbreak Evaluation through Molecular Assays and Antifungal Stewardship—A Narrative Review. Curr. Issues Mol. Biol. 2024, 46, 6069–6084. [Google Scholar] [CrossRef]
- Hakobyan, M.; Manrikyan, G.; Markaryan, M.; Vardanyan, I.; Manrikyan, M. The Influence of Dental Status and Blood Parameters Characterizing Endogenous Intoxication on the Timing of Childbirth. Medicina. 2024, 60, 1176. [Google Scholar] [CrossRef] [PubMed]
- Chaim, W.; Mazor, M.; Wiznitzer, A. The Prevalence and Clinical Significance of Intraamniotic Infection with Candida Species in Women with Preterm Labor. Arch. Gynecol. Obstet. 1992, 251, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Mudenda, S. Global Burden of Fungal Infections and Antifungal Resistance from 1961 to 2024: Findings and Future Implications. Pharmacol. Pharm. 2024, 15, 81–112. [Google Scholar] [CrossRef]
- Moraitaki, E.; Kyriakidis, I.; Pelagiadis, I.; Katzilakis, N.; Stratigaki, M.; Chamilos, G.; Tragiannidis, A.; Stiakaki, E. Epidemiology of Invasive Fungal Diseases: A 10-Year Experience in a Tertiary Pediatric Hematology–Oncology Department in Greece. J. Fungi. 2024, 10, 498. [Google Scholar] [CrossRef]
- Pinho, S.; Miranda, I.M.; Costa-De-Oliveira, S. Global Epidemiology of Invasive Infections by Uncommon Candida Species: A Systematic Review. J. Fungi. 2024, 10, 558. [Google Scholar] [CrossRef]
- Popova, M.; Rogacheva, Y. Epidemiology of Invasive Fungal Diseases in Patients with Hematological Malignancies and Haematopoietic Cell Transplantation Recipients: Systematic Review and Meta-Analysis of Trends over Time. J. Infect. Public Heal. 2025, 18, 102804. [Google Scholar] [CrossRef] [PubMed]
- Innings, A.; Ullberg, M.; Johansson, A.; Rubin, C.J.; Noreus, N.; Isaksson, M.; Herrmann, B. Multiplex Real-Time PCR Targeting the RNase P RNA Gene for Detection and Identification of Candida Species in Blood. J. Clin. Microbiol. 2007, 45, 874–880. [Google Scholar] [CrossRef] [PubMed]
- Bové, B.; Barragán, I.; Pratcorona, L.; Porta, R.; de Diego, R.; Comas, M.C.; Méndez, M.; de Liria, C.R.G. Conservative Approach for Intra-Amniotic Candida albicans Colonisation: Case Report and Review of Current Evidence. Case Rep. Perinat. Med. 2025, 14, 20240047. [Google Scholar] [CrossRef] [PubMed]
- Nishino, C.; Kawamura, H.; Hattori, Y.; Orisaka, M.; Yoshida, Y. Asymptomatic Vaginal Candidiasis Complicated by Chorioamnionitis and Pelvic Abscess at Full-Term Delivery: A Case Report. Cureus. 2025, 17, e79906. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Lim, Y.K.; Choe, K.W.; Choi, Y.H.; Lee, M.-K. Seasonality and Epidemiological Trends in Species Distribution and Antifungal Susceptibility of Candida Species Isolated from Various Clinical Specimens Conducted during 2011–2022, Korea: A Retrospective Surveillance Study. Ann. Clin. Microbiol. 2024, 27, 185–196. [Google Scholar] [CrossRef]
- Berezowsky, A.; Romano, A.; Hochberg, A.; Krispin, E.; Danieli, H.Z.; Krencel, A.; Hadar, E. The Correlation between Placental Histology and Microbiologic Infection in the Diagnosis of Chorioamnionitis in Preterm Delivery. Placenta. 2022, 128, 18–22. [Google Scholar] [CrossRef]
- Polat, I.H.; Marin, S.; Ríos, J.; Larroya, M.; Sánchez-García, A.B.; Murillo, C.; Rueda, C.; Cascante, M.; Gratacós, E.; Cobo, T. Exploratory and Confirmatory Analysis to Investigate the Presence of Vaginal Metabolome Expression of Microbial Invasion of the Amniotic Cavity in Women with Preterm Labor Using High-Performance Liquid Chromatography. Am. J. Obstet. Gynecol. 2021, 224, 90.e1–90.e9. [Google Scholar] [CrossRef]
- Maftei, N.-M.; Arbune, M.; Georgescu, C.V.; Elisei, A.M.; Iancu, A.V.; Tatu, A.L. Vulvovaginal Candidiasis in Pregnancy—Between Sensitivity and Resistance to Antimycotics. J. Xenobiotics. 2023, 13, 312–322. [Google Scholar] [CrossRef]
- Passera, M.; Corbellini, S.; Cavalli, G.; Vailati, F.; Frigerio, L.; Farina, C. Candida Nivariensis Isolates from a Pregnant Patient: Molecular Identification Using Sequencing and MALDI-TOF. Int. J. Clin. Exp. Pathol. 2016, 9, 10774–10777. [Google Scholar]
- Purnamasari, I.; Ervianti, E.; Damayanti, D.; Prasetyo, B.; Astari, L.; Endraswari, P.D.; Listiawan, M.Y.; Prakoeswa, C.R. Pattern of Candida Species Isolated from Patient with Vulvovaginal Candidiasis in Pregnancy. Berk. Ilmu Kesehat. Kulit Kelamin. 2022, 34, 178–183. [Google Scholar] [CrossRef]
- Kim, H.J.; Choi, J.; Oh, E.J.; Lee, K.-N.; Park, J.Y.; Oh, K.J. Semi-Quantitative Metalloproteinase-8 Rapid Test for the Prediction of Adverse Pregnancy Outcomes in Patients with Preterm Premature Rupture of Membranes. Eur. J. Obstet. Gynecol. Reprod. Biol. 2024, 297, 65–71. [Google Scholar] [CrossRef]
- Chougule, S.; Patil, S.; Gavandi, T.; Basrani, S.; Jadhav, A.K.; Karuppayil, S.M. Alpha-Bisabolol Inhibits Yeast to Hyphal form Transition and Biofilm Development in Candida albicans: In Vitro and In Silico Studies. Silico Pharmacol. 2025, 13, 53. [Google Scholar] [CrossRef] [PubMed]
- The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs for Antifungal Agents, 11th ed.; EUCAST: Växjö, Sweden, 2024. [Google Scholar]
- Clinical and Laboratory Standards Institute. Performance Standards for Antifungal Susceptibility Testing of Yeasts, 3rd ed.; CLSI Supplement M27M44S; CLSI: Wayne, PA, USA, 2022. [Google Scholar]
- Righi, E.; Mutters, N.T.; Guirao, X.; del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Presterl, E.; Christaki, E.; Cross, E.L.; et al. European Society of Clinical Microbiology and Infectious Diseases/European Committee on Infection Control Clinical Guidelines on Pre-Operative Decolonization and Targeted Prophylaxis in Patients Colonized by Multidrug-Resistant Gram-Positive Bacteria before Surgery. Clin. Microbiol. Infect. 2024, 30, 1537–1550. [Google Scholar] [CrossRef] [PubMed]
- Bodilsen, J.; D’Alessandris, Q.G.; Humphreys, H.; Iro, M.A.; Klein, M.; Last, K.; Montesinos, I.L.; Pagliano, P.; Sipahi, O.R.; San-Juan, R.; et al. European Society of Clinical Microbiology and Infectious Diseases Guidelines on Diagnosis and Treatment of Brain Abscess in Children and Adults. Clin. Microbiol. Infect. 2023, 30, 66–89. [Google Scholar] [CrossRef]
- Giannella, M.; Lanternier, F.; Dellière, S.; Groll, A.H.; Mueller, N.J.; Alastruey-Izquierdo, A.; Slavin, M.A. Invasive Fungal Disease in the Immunocompromised Host: Changing Epidemiology, New Antifungal Therapies, and Management Challenges. Clin. Microbiol. Infect. 2024, 31, 29–36. [Google Scholar] [CrossRef]
- Gedefie, A.; Shimeles, G.; Motbainor, H.; Kassanew, B.; Genet, C. Vaginal Colonization and Vertical Transmission of Candida Species: Prevalence and Associated Factors among Pregnant Women and Their Neonates at Public Health Facilities of Northeast Ethiopia. BMC Pregnancy Childbirth. 2025, 25, 22. [Google Scholar] [CrossRef]
- Pavlovska, O.M.; Pavlovska, K.M.; Heryak, S.M.; Khmil, S.V.; Gorban, N.Y. Intestinal Dysbiosis as a Possible Predictor of Very Early Preterm Labor in Pregnant Women with Metabolic Syndrome. J. Med. Life. 2020, 13, 200–205. [Google Scholar] [CrossRef]
- Geremia, N.; Bragato, B.; Giovagnorio, F.; Zuglian, G.; Brugnaro, P.; Solinas, M.; Stano, P.; Panese, S.; Parisi, S.G. Distribution and Prevalence of Fungemia: A Five-Year Retrospective Multicentric Survey in Venetian Region, Italy. JAC-Antimicrob. Resist. 2025, 7, dlaf044. [Google Scholar] [CrossRef]
- Bal, A.M.; Pana, Z.D.; Carlesse, F.; Marek, A.; Seidel, D.; Mehler, K.; Butzer, S.; Sprute, R.; Stemler, J.; Ludwig-Bettin, D.; et al. The Paediatric European Confederation of Medical Mycology (ECMM) Quality (Paed-EQUAL) Candida Score for the Management of Candidemia in Children and Neonates. Mycoses 2025, 68, e70041. [Google Scholar] [CrossRef]
- Scott, N.E.; Wash, E.; Zajac, C.; Erayil, S.E.; Kline, S.E.; Selmecki, A. Heterogeneity of Candida Bloodstream Isolates in an Academic Medical Center and Affiliated Hospitals. Microbiol. Spectr. 2025, 13, e0046425. [Google Scholar] [CrossRef]
- Cornely, O.A.; Sprute, R.; Bassetti, M.; Chen, S.C.-A.; Groll, A.H.; Kurzai, O.; Lass-Flörl, C.; Ostrosky-Zeichner, L.; Rautemaa-Richardson, R.; Revathi, G.; et al. Global Guideline for the Diagnosis and Management of Candidiasis: An Initiative of the ECMM in Cooperation with ISHAM and ASM. Lancet Infect. Dis. 2025, 25, e280–e293. [Google Scholar] [CrossRef]
- Best, K.A.; McKinney, J.; Alkhasawneh, A.; McCullough, D.C.; Sanchez-Ramos, L. Amniotic Fluid “Sludge” Due to Intra-Amniotic Infection/Inflammation with Candida albicans. Am. J. Obstet. Gynecol. 2022, 227, 329–330. [Google Scholar] [CrossRef] [PubMed]
- Nagamizu, M.; Hotta, Y.; Noda, M.; Nakamura, D.; Hori, M.; Otsuka, Y.; Takemoto, R.; Horita, Y.; Wakita, E.; Morishita, N.; et al. Association of Doses Based on Body Constitutional Parameters with the Efficacy of Micafungin in Candidemia. J. Infect. Chemother. 2025, 31, 102654. [Google Scholar] [CrossRef]
- Kaufman, D.A.; Mukhopadhyay, S. Neonatal Invasive Fungal Infections. Clin. Perinatol. 2024, 52, 47–66. [Google Scholar] [CrossRef] [PubMed]
- Cuesta, I.; Bielza, C.; Cuenca-Estrella, M.; Larrañaga, P.; Rodríguez-Tudela, J.L. Evaluation by Data Mining Techniques of Fluconazole Breakpoints Established by the Clinical and Laboratory Standards Institute (CLSI) and Comparison with Those of the European Committee on Antimicrobial Susceptibility Testing (EUCAST). Antimicrob. Agents Chemother. 2010, 54, 1541–1546. [Google Scholar] [CrossRef] [PubMed]
- Pfaller, M.; Andes, D.; Diekema, D.; Espinel-Ingroff, A.; Sheehan, D. Wild-Type MIC Distributions, Epidemiological Cutoff Values and Species-Specific Clinical Breakpoints for Fluconazole and Candida: Time for Harmonization of CLSI and EUCAST Broth Microdilution Methods. Drug Resist. Updat. 2010, 13, 180–195. [Google Scholar] [CrossRef]
- van Hal, S.J.; Chen, S.C.A.; Sorrell, T.C.; Ellis, D.H.; Slavin, M.; Marriott, D.M. Support for the EUCAST and Revised CLSI Fluconazole Clinical Breakpoints by Sensititre® YeastOne® for Candida albicans: A Prospective Observational Cohort Study. J. Antimicrob. Chemother. 2014, 69, 2210–2214. [Google Scholar] [CrossRef]
- Espinel-Ingroff, A. Commercial Methods for Antifungal Susceptibility Testing of Yeasts: Strengths and Limitations as Predictors of Resistance. J. Fungi. 2022, 8, 309. [Google Scholar] [CrossRef] [PubMed]
- Lass-Flörl, C. Beyond Guidelines: What Do I Need to Know When Dealing with Fungal Diagnostics? Clin. Microbiol. Infect. 2025. [Google Scholar] [CrossRef] [PubMed]
| Characteristic | Total n (%) | C. albicans n (%) | N. glabratus n (%) | C. parapsilosis n (%) | C. dubliniensis n (%) | P. kudriavzevii n (%) |
|---|---|---|---|---|---|---|
| Distribution by month | ||||||
| February | 8 (20.0%) | 6 (15.0%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| March | 10 (25.0%) | 7 (17.5%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | 2 (5.0%) |
| April | 3 (7.5%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| May | 5 (12.5%) | 4 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| June | 14 (35.0%) | 8 (20.0%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 4 (10.0%) |
| Distribution by age group (5-year intervals) | ||||||
| 17–21 years | 7 (17.5%) | 7 (17.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| 22–26 years | 9 (22.5%) | 7 (17.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) |
| 27–31 years | 10 (25.0%) | 5 (12.5%) | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) | 3 (7.5%) |
| 32–36 years | 6 (15.0%) | 3 (7.5%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) | 2 (5.0%) |
| 37–41 years | 8 (20.0%) | 5 (12.5%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 1 (2.5%) |
| Parity | ||||||
| I | 20 (50.0%) | 13 (32.5%) | 2 (5.0%) | 0 (0.0%) | 1 (2.5%) | 4 (10.0%) |
| II | 16 (40.0%) | 12 (30.0%) | 0 (0.0%) | 1 (2.5%) | 0 (0.0%) | 3 (7.5%) |
| III | 2 (5.0%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IV | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| VII | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Gravidity | ||||||
| I | 14 (35.0%) | 9 (22.5%) | 1 (2.5%) | 0 (0.0%) | 1 (2.5%) | 3 (7.5%) |
| II | 18 (45.0%) | 12 (30.0%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 4 (10.0%) |
| III | 4 (10.0%) | 4 (10.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| IV | 2 (5.0%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| V | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| VII | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Associated maternal–fetal pathologies | ||||||
| Threatened preterm birth | 14 (35.0%) | 9 (22.5%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 3 (7.5%) |
| Nuchal cord | 8 (20.0%) | 5 (12.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) |
| Morbid obesity | 3 (7.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 2 (5.0%) |
| Gestational diabetes | 3 (7.5%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| Rh incompatibility | 2 (5.0%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Type 2 diabetes mellitus | 2 (5.0%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Threatened miscarriage | 2 (5.0%) | 2 (5.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Marginal placenta praevia | 2 (5.0%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| Cervico-isthmic incompetence | 2 (5.0%) | 0 (0.0%) | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) |
| PIH * | 2 (5.0%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| Subchorionic hematoma | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Vaginitis | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Autoimmune thrombocytopenia | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Genital bleeding | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Fetal ultrasound anomalies | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Large for gestational age | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| IUGR ** | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Reversed Doppler indices | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Type 1 IDDM *** | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 1 (2.5%) |
| Autoimmune thyroiditis | 1 (2.5%) | 1 (2.5%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) | 0 (0.0%) |
| Species/Antifungal | Fluconazole | Voriconazole | Micafungin | Amphotericin B | ||||
|---|---|---|---|---|---|---|---|---|
| Susceptible n (%) | Resistant n (%) | Susceptible n (%) | Susceptible, IP * n (%) | Susceptible n (%) | Resistant n (%) | Susceptible n (%) | Resistant n (%) | |
| Candida albicans | ≤2 mg/L | >4 mg/L | ≤0.06 mg/L | >0.06–≤0.25 mg/L | ≤0.03 mg/L | >0.03 mg/L | ≤1 mg/L | >1 mg/L |
| 25/27 (92.6%) | 2/27 (7.4%) | 0 (0.0%) | 15/27 (55.6%) | 2/27 (7.4%) | 15/27 (55.6%) | 23/27 (85.3%) | 2/27 (7.4%) | |
| Candida dubliniensis | ≤2 mg/L | >4 mg/L | ≤0.06 mg/L | >0.06–≤0.25 mg/L | ≤0.06 mg/L | >0.06 mg/L | ≤1 mg/L | >1 mg/L |
| 0/1 (0.0%) | 1/1 (100%) | 0/1 (0.0%) | 1/1 (100%) | 0/1 (0.0%) | 1/1 (100%) | 1/1 (100%) | 0/1 (0.0%) | |
| Candida parapsilosis | ≤2 mg/L | >4 mg/L | ≤0.125 mg/L | >0.125–≤0.25 mg/L | ≤4 mg/L | >4 mg/L | ≤1 mg/L | >1 mg/L |
| 1/1 (100%) | 0/1 (0.0%) | 1/1 (100%) | 0/1(0.0%) | 1/1 (100%) | 0/1 (0.0%) | 1/1 (100%) | 0/1 (0.0%) | |
| Nakaseomyces glabratus | ≤0.001 mg/L | >16 mg/L | - | - | ≤0.03 mg/L | >0.03 mg/L | ≤1 mg/L | >1 mg/L |
| 0/3 (0.0%) | 0/3 (0.0%) | 0/3 (0.0%) | 0/3 (0.0%) | 0/3 (0.0%) | 2/3 (66.7%) | 3/3 (100%) | 0/3 (0.0%) | |
| Pichia kudriavzevii | - | - | - | - | - | - | ≤1 mg/L | >1 mg/L |
| 0/8 (0.0%) | 8/8 (100%) | 0/8 (0.0%) | 0/8 (0.0%) | 0/8 (0.0%) | 0/8 (0.0%) | 0/8 (0.0%) | 7/8 (87.5%) | |
| Total | 26 (65.0%) | 11 (27.5%) | 1 (2.5%) | 16 (40.0%) | 3 (7.5%) | 18 (45.0%) | 28 (70.0%) | 9 (22.5%) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ionescu, S.G.; Dimitriu, C.D.; Socolov, D.G.; Grigore, M.; Iancu, L.S.; Damian, C.; Cobzaru, R.G.; Ripa, C.V.; Costin, D.; Popa, R.-F.; et al. Candida Susceptibility to Antifungals in Amniotic Fluid: A Preliminary Study. Pathogens 2025, 14, 972. https://doi.org/10.3390/pathogens14100972
Ionescu SG, Dimitriu CD, Socolov DG, Grigore M, Iancu LS, Damian C, Cobzaru RG, Ripa CV, Costin D, Popa R-F, et al. Candida Susceptibility to Antifungals in Amniotic Fluid: A Preliminary Study. Pathogens. 2025; 14(10):972. https://doi.org/10.3390/pathogens14100972
Chicago/Turabian StyleIonescu, Silvia Gabriela, Cristina Daniela Dimitriu, Demetra Gabriela Socolov, Mihaela Grigore, Luminita Smaranda Iancu, Costin Damian, Roxana Gabriela Cobzaru, Carmen Valerica Ripa, Diana Costin, Radu-Florin Popa, and et al. 2025. "Candida Susceptibility to Antifungals in Amniotic Fluid: A Preliminary Study" Pathogens 14, no. 10: 972. https://doi.org/10.3390/pathogens14100972
APA StyleIonescu, S. G., Dimitriu, C. D., Socolov, D. G., Grigore, M., Iancu, L. S., Damian, C., Cobzaru, R. G., Ripa, C. V., Costin, D., Popa, R.-F., Copacianu, B., & Ursu, R. G. (2025). Candida Susceptibility to Antifungals in Amniotic Fluid: A Preliminary Study. Pathogens, 14(10), 972. https://doi.org/10.3390/pathogens14100972








