Phage to ESKAPE: Personalizing Therapy for MDR Infections—A Comprehensive Clinical Review
Abstract
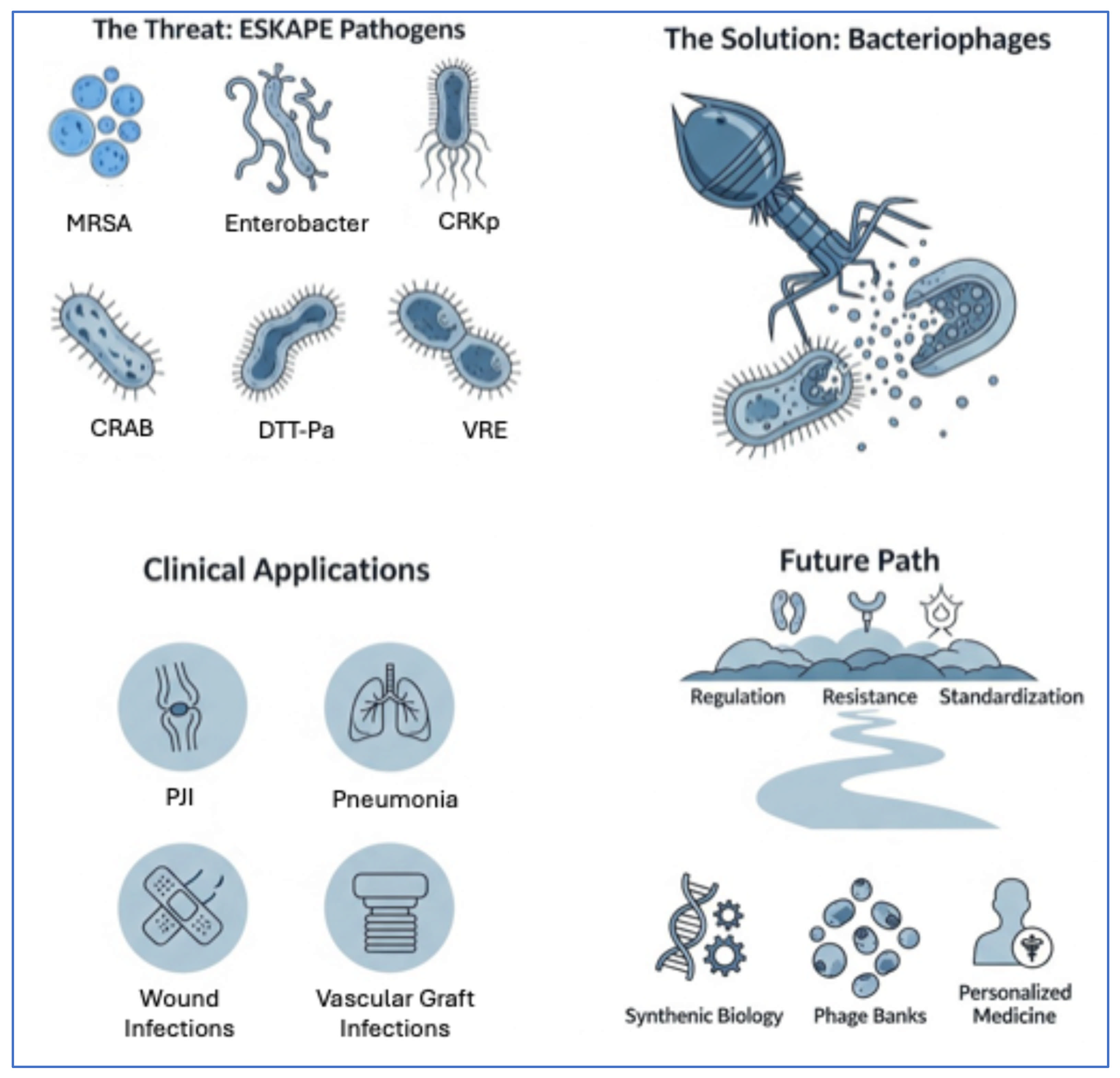
1. Introduction
2. Mechanisms of Phage Action and Therapeutic Potential
2.1. Phage Isolation and Characterization
2.2. Phage Cocktails
2.3. Pharmacokinetics, Pharmacodynamics, and Synergy
2.4. Clinical Trials and Animal Models
2.5. Regulatory Landscape
2.6. Future Perspectives
3. Phage Therapy Against Individual ESKAPE Pathogens (Preclinical and Clinical Trials/Experiences)
3.1. Enterococcus faecium
3.2. Staphylococcus aureus
3.3. Klebsiella pneumoniae
3.4. Acinetobacter baumannii
3.5. Pseudomonas aeruginosa
3.6. Enterobacter spp.
4. Challenges and Future Perspectives
5. Conclusions
6. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Marino, A.; Maniaci, A.; Lentini, M.; Ronsivalle, S.; Nunnari, G.; Cocuzza, S.; Parisi, F.M.; Cacopardo, B.; Lavalle, S.; La Via, L. The Global Burden of Multidrug-Resistant Bacteria. Epidemiologia 2025, 6, 21. [Google Scholar] [CrossRef]
- Marino, A.; Augello, E.; Bellanca, C.M.; Cosentino, F.; Stracquadanio, S.; La Via, L.; Maniaci, A.; Spampinato, S.; Fadda, P.; Cantarella, G.; et al. Antibiotic Therapy Duration for Multidrug-Resistant Gram-Negative Bacterial Infections: An Evidence-Based Review. Int. J. Mol. Sci. 2025, 26, 6905. [Google Scholar] [CrossRef]
- Murray, C.J.L.; Ikuta, K.S.; Sharara, F.; Swetschinski, L.; Aguilar, G.R.; Gray, A.; Han, C.; Bisignano, C.; Rao, P.; Wool, E.; et al. Global Burden of Bacterial Antimicrobial Resistance in 2019: A Systematic Analysis. Lancet 2022, 399, 629–655. [Google Scholar] [CrossRef]
- La Via, L.; Sangiorgio, G.; Stefani, S.; Marino, A.; Nunnari, G.; Cocuzza, S.; La Mantia, I.; Cacopardo, B.; Stracquadanio, S.; Spampinato, S.; et al. The Global Burden of Sepsis and Septic Shock. Epidemiologia 2024, 5, 456–478. [Google Scholar] [CrossRef]
- Naghavi, M.; Vollset, S.E.; Ikuta, K.S.; Swetschinski, L.R.; Gray, A.P.; Wool, E.E.; Aguilar, G.R.; Mestrovic, T.; Smith, G.; Han, C.; et al. Global Burden of Bacterial Antimicrobial Resistance 1990–2021: A Systematic Analysis with Forecasts to 2050. Lancet 2024, 404, 1199–1226. [Google Scholar] [CrossRef]
- Rice, L.B. Federal Funding for the Study of Antimicrobial Resistance in Nosocomial Pathogens: No ESKAPE. J. Infect. Dis. 2008, 197, 1079–1081. [Google Scholar] [CrossRef] [PubMed]
- Boucher, H.W.; Talbot, G.H.; Bradley, J.S.; Edwards, J.E.; Gilbert, D.; Rice, L.B.; Scheld, M.; Spellberg, B.; Bartlett, J. Bad Bugs, No Drugs: No ESKAPE! An Update from the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 48, 1–12. [Google Scholar] [CrossRef]
- Santajit, S.; Indrawattana, N. Mechanisms of Antimicrobial Resistance in ESKAPE Pathogens. BioMed. Res. Int. 2016, 2016, 2475067. [Google Scholar] [CrossRef]
- Miller, W.R.; Arias, C.A. ESKAPE Pathogens: Antimicrobial Resistance, Epidemiology, Clinical Impact and Therapeutics. Nat. Rev. Microbiol. 2024, 22, 598–616. [Google Scholar] [CrossRef]
- Laxminarayan, R.; Duse, A.; Wattal, C.; Zaidi, A.K.M.; Wertheim, H.F.L.; Sumpradit, N.; Vlieghe, E.; Hara, G.L.; Gould, I.M.; Goossens, H.; et al. Antibiotic Resistance-the Need for Global Solutions. Lancet Infect. Dis. 2013, 13, 1057–1098. [Google Scholar] [CrossRef]
- Theuretzbacher, U.; Outterson, K.; Engel, A.; Karlén, A. The Global Preclinical Antibacterial Pipeline. Nat. Rev. Microbiol. 2020, 18, 275–285. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Guerrero-Bustamante, C.A.; Garlena, R.A.; Russell, D.A.; Ford, K.; Harris, K.; Gilmour, K.C.; Soothill, J.; Jacobs-Sera, D.; Schooley, R.T.; et al. Engineered Bacteriophages for Treatment of a Patient with a Disseminated Drug-Resistant Mycobacterium Abscessus. Nat. Med. 2019, 25, 730–733. [Google Scholar] [CrossRef] [PubMed]
- Schooley, R.T.; Biswas, B.; Gill, J.J.; Hernandez-Morales, A.; Lancaster, J.; Lessor, L.; Barr, J.J.; Reed, S.L.; Rohwer, F.; Benler, S.; et al. Development and Use of Personalized Bacteriophage-Based Therapeutic Cocktails To Treat a Patient with a Disseminated Resistant Acinetobacter baumannii Infection. Antimicrob. Agents Chemother. 2017, 61, e00954-17. [Google Scholar] [CrossRef] [PubMed]
- Brives, C.; Pourraz, J. Phage Therapy as a Potential Solution in the Fight against AMR: Obstacles and Possible Futures. Palgrave Commun. 2020, 6, 100. [Google Scholar] [CrossRef]
- Chanishvili, N. Phage Therapy-History from Twort and d’Herelle through Soviet Experience to Current Approaches. Adv. Virus Res. 2012, 83, 3–40. [Google Scholar] [CrossRef]
- Abedon, S.T.; Kuhl, S.J.; Blasdel, B.G.; Kutter, E.M. Phage Treatment of Human Infections. Bacteriophage 2011, 1, 66–85. [Google Scholar] [CrossRef]
- Kortright, K.E.; Chan, B.K.; Koff, J.L.; Turner, P.E. Phage Therapy: A Renewed Approach to Combat Antibiotic-Resistant Bacteria. Cell Host Microbe 2019, 25, 219–232. [Google Scholar] [CrossRef]
- Jault, P.; Leclerc, T.; Jennes, S.; Pirnay, J.P.; Que, Y.-A.; Resch, G.; Rousseau, A.F.; Ravat, F.; Carsin, H.; Floch, R.L.; et al. Efficacy and Tolerability of a Cocktail of Bacteriophages to Treat Burn Wounds Infected by Pseudomonas aeruginosa (PhagoBurn): A Randomised, Controlled, Double-Blind Phase 1/2 Trial. Lancet Infect. Dis. 2019, 19, 35–45. [Google Scholar] [CrossRef]
- Kim, M.K.; Suh, G.A.; Cullen, G.D.; Rodriguez, S.P.; Dharmaraj, T.; Chang, T.H.W.; Li, Z.; Chen, Q.; Green, S.I.; Lavigne, R.; et al. Bacteriophage Therapy for Multidrug-Resistant Infections: Current Technologies and Therapeutic Approaches. J. Clin. Invest. 2025, 135, e187996. [Google Scholar] [CrossRef]
- Drulis-Kawa, Z.; Majkowska-Skrobek, G.; Maciejewska, B.; Delattre, A.-S.; Lavigne, R. Learning from Bacteriophages—Advantages and Limitations of Phage and Phage-Encoded Protein Applications. Curr. Protein Pept. Sci. 2012, 13, 699–722. [Google Scholar] [CrossRef]
- Pires, D.P.; Costa, A.R.; Pinto, G.; Meneses, L.; Azeredo, J. Current Challenges and Future Opportunities of Phage Therapy. FEMS Microbiol. Rev. 2020, 44, 684–700. [Google Scholar] [CrossRef] [PubMed]
- Springer Nature Experiments. Rapid Bench to Bedside Therapeutic Bacteriophage Production. 2023. Available online: https://experiments.springernature.com/articles/10.1007/978-1-0716-3523-0_5 (accessed on 26 August 2025).
- Pirnay, J.-P.; Verbeken, G.; Rose, T.; Jennes, S.; Zizi, M.; Huys, I.; Lavigne, R.; Merabishvili, M.; Vaneechoutte, M.; Buckling, A.; et al. Introducing Yesterday‘s Phage Therapy in Today‘s Medicine. Future Virol. 2012, 7, 379–390. [Google Scholar] [CrossRef]
- Yoo, S.; Lee, K.-M.; Kim, N.; Vu, T.N.; Abadie, R.; Yong, D. Designing Phage Cocktails to Combat the Emergence of Bacteriophage-Resistant Mutants in Multidrug-Resistant Klebsiella pneumoniae. Microbiol. Spectr. 2023, 12, e01258-23. [Google Scholar] [CrossRef] [PubMed]
- Hesse, S.; Adhya, S. Phage Therapy in the Twenty-First Century: Facing the Decline of the Antibiotic Era; Is It Finally Time for the Age of the Phage? Annu. Rev. Microbiol. 2019, 73, 155–174. [Google Scholar] [CrossRef]
- Kulshrestha, M.; Tiwari, M.; Tiwari, V. Bacteriophage Therapy against ESKAPE Bacterial Pathogens: Current Status, Strategies, Challenges, and Future Scope. Microb. Pathog. 2024, 186, 106467. [Google Scholar] [CrossRef]
- Clokie, M.R.J.; Millard, A.D.; Letarov, A.V.; Heaphy, S. Phages in Nature. Bacteriophage 2011, 1, 31–45. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, S.; Zhang, J.; Cai, X.; Sun, Y.; Zhan, C.; Zhou, X. Characterization of Lytic Bacteriophage vB_EhoP_ZX13 and Its Therapeutic Potential against Enterobacter Hormaechei Infection. Res. Vet. Sci. 2025, 195, 105864. [Google Scholar] [CrossRef]
- Mapes, A.C.; Trautner, B.W.; Liao, K.S.; Ramig, R.F. Development of Expanded Host Range Phage Active on Biofilms of Multi-Drug Resistant Pseudomonas aeruginosa. Bacteriophage 2016, 6, e1096995. [Google Scholar] [CrossRef]
- Chan, B.K.; Abedon, S.T.; Loc-Carrillo, C. Phage Cocktails and the Future of Phage Therapy. Future Microbiol. 2013, 8, 769–783. [Google Scholar] [CrossRef]
- Pirnay, J.-P.; Verbeken, G.; Ceyssens, P.-J.; Huys, I.; De Vos, D.; Ameloot, C.; Fauconnier, A. The Magistral Phage. Viruses 2018, 10, 64. [Google Scholar] [CrossRef]
- Bosco, K.; Lynch, S.; Sandaradura, I.; Khatami, A. Therapeutic Phage Monitoring: A Review. Clin. Infect. Dis. 2023, 77, S384–S394. [Google Scholar] [CrossRef]
- Nang, S.C.; Lin, Y.-W.; Petrovic Fabijan, A.; Chang, R.Y.K.; Rao, G.G.; Iredell, J.; Chan, H.-K.; Li, J. Pharmacokinetics/Pharmacodynamics of Phage Therapy: A Major Hurdle to Clinical Translation. Clin. Microbiol. Infect. 2023, 29, 702–709. [Google Scholar] [CrossRef]
- Międzybrodzki, R.; Kasprzak, H.; Letkiewicz, S.; Rogóż, P.; Żaczek, M.; Thomas, J.; Górski, A. Pharmacokinetic and Pharmacodynamic Obstacles for Phage Therapy From the Perspective of Clinical Practice. Clin. Infect. Dis. 2023, 77, S395–S400. [Google Scholar] [CrossRef] [PubMed]
- Peez, C.; Chen, B.; Henssler, L.; Chittò, M.; Onsea, J.; Verhofstad, M.H.J.; Arens, D.; Constant, C.; Zeiter, S.; Obremskey, W.; et al. Evaluating the Safety, Pharmacokinetics and Efficacy of Phage Therapy in Treating Fracture-Related Infections with Multidrug-Resistant Staphylococcus aureus: Intravenous versus Local Application in Sheep. Front. Cell. Infect. Microbiol. 2025, 15, 1547250. [Google Scholar] [CrossRef] [PubMed]
- Washizaki, A.; Sakiyama, A.; Ando, H. Phage-Specific Antibodies: Are They a Hurdle for the Success of Phage Therapy? Essays Biochem. 2024, 68, 633–644. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.-C.; Tsai, Y.-C.; Lin, N.-T. Phage–Antibiotic Synergy Enhances Biofilm Eradication and Survival in a Zebrafish Model of Pseudomonas aeruginosa Infection. Int. J. Mol. Sci. 2025, 26, 5337. [Google Scholar] [CrossRef]
- Łusiak-Szelachowska, M.; Międzybrodzki, R.; Drulis-Kawa, Z.; Cater, K.; Knežević, P.; Winogradow, C.; Amaro, K.; Jończyk-Matysiak, E.; Weber-Dąbrowska, B.; Rękas, J.; et al. Bacteriophages and Antibiotic Interactions in Clinical Practice: What We Have Learned so Far. J. Biomed. Sci. 2022, 29, 23. [Google Scholar] [CrossRef]
- Uyttebroek, S.; Chen, B.; Onsea, J.; Ruythooren, F.; Debaveye, Y.; Devolder, D.; Spriet, I.; Depypere, M.; Wagemans, J.; Lavigne, R.; et al. Safety and Efficacy of Phage Therapy in Difficult-to-Treat Infections: A Systematic Review. Lancet Infect. Dis. 2022, 22, e208–e220. [Google Scholar] [CrossRef]
- Duyvejonck, H.; Merabishvili, M.; Vaneechoutte, M.; de Soir, S.; Wright, R.; Friman, V.-P.; Verbeken, G.; De Vos, D.; Pirnay, J.-P.; Van Mechelen, E.; et al. Evaluation of the Stability of Bacteriophages in Different Solutions Suitable for the Production of Magistral Preparations in Belgium. Viruses 2021, 13, 865. [Google Scholar] [CrossRef]
- Faltus, T. The Medicinal Phage—Regulatory Roadmap for Phage Therapy under EU Pharmaceutical Legislation. Viruses 2024, 16, 443. [Google Scholar] [CrossRef]
- Quality, Safety and Efficacy of Bacteriophages as Veterinary Medicines—Scientific Guideline|European Medicines Agency (EMA). Available online: https://www.ema.europa.eu/en/quality-safety-and-efficacy-bacteriophages-veterinary-medicines-scientific-guideline (accessed on 25 September 2025).
- Pirnay, J.-P.; Verbeken, G. Magistral Phage Preparations: Is This the Model for Everyone? Clin. Infect. Dis. 2023, 77, S360–S369. [Google Scholar] [CrossRef]
- Gill, J.J.; Hyman, P. Phage Choice, Isolation, and Preparation for Phage Therapy. Curr. Pharm. Biotechnol. 2010, 11, 2–14. [Google Scholar] [CrossRef] [PubMed]
- Merabishvili, M.; Pirnay, J.-P.; Verbeken, G.; Chanishvili, N.; Tediashvili, M.; Lashkhi, N.; Glonti, T.; Krylov, V.; Mast, J.; Parys, L.V.; et al. Quality-Controlled Small-Scale Production of a Well-Defined Bacteriophage Cocktail for Use in Human Clinical Trials. PLoS ONE 2009, 4, e4944. [Google Scholar] [CrossRef] [PubMed]
- Cocorullo, M.; Stelitano, G.; Chiarelli, L.R. Phage Therapy: An Alternative Approach to Combating Multidrug-Resistant Bacterial Infections in Cystic Fibrosis. Int. J. Mol. Sci. 2024, 25, 8321. [Google Scholar] [CrossRef]
- Lenneman, B.R.; Fernbach, J.; Loessner, M.J.; Lu, T.K.; Kilcher, S. Enhancing Phage Therapy through Synthetic Biology and Genome Engineering. Curr. Opin. Biotechnol. 2021, 68, 151–159. [Google Scholar] [CrossRef]
- Cui, L.; Watanabe, S.; Miyanaga, K.; Kiga, K.; Sasahara, T.; Aiba, Y.; Tan, X.-E.; Veeranarayanan, S.; Thitiananpakorn, K.; Nguyen, H.M.; et al. A Comprehensive Review on Phage Therapy and Phage-Based Drug Development. Antibiotics 2024, 13, 870. [Google Scholar] [CrossRef]
- Doud, M.B.; Robertson, J.M.; Strathdee, S.A. Optimizing Phage Therapy with Artificial Intelligence: A Perspective. Front. Cell. Infect. Microbiol. 2025, 15, 1611857. [Google Scholar] [CrossRef]
- Verbeken, G.; Pirnay, J.-P.; Lavigne, R.; Jennes, S.; De Vos, D.; Casteels, M.; Huys, I. Call for a Dedicated European Legal Framework for Bacteriophage Therapy. Arch. Immunol. Ther. Exp. 2014, 62, 117–129. [Google Scholar] [CrossRef]
- Wei, Y.; Palacios Araya, D.; Palmer, K.L. Enterococcus faecium: Evolution, Adaptation, Pathogenesis and Emerging Therapeutics. Nat. Rev. Microbiol. 2024, 22, 705–721. [Google Scholar] [CrossRef]
- Zhou, X.; Willems, R.J.L.; Friedrich, A.W.; Rossen, J.W.A.; Bathoorn, E. Enterococcus faecium: From Microbiological Insights to Practical Recommendations for Infection Control and Diagnostics. Antimicrob. Resist. Infect. Control 2020, 9, 130. [Google Scholar] [CrossRef]
- Miller, W.R.; Munita, J.M.; Arias, C.A. Mechanisms of Antibiotic Resistance in Enterococci. Expert Rev. Anti-Infect. Ther. 2014, 12, 1221–1236. [Google Scholar] [CrossRef]
- Hollenbeck, B.L.; Rice, L.B. Intrinsic and Acquired Resistance Mechanisms in Enterococcus. Virulence 2012, 3, 421–569. [Google Scholar] [CrossRef]
- Huang, C.; Moradi, S.; Sholeh, M.; Tabaei, F.M.; Lai, T.; Tan, B.; Meng, J.; Azizian, K. Global Trends in Antimicrobial Resistance of Enterococcus faecium: A Systematic Review and Meta-Analysis of Clinical Isolates. Front. Pharmacol. 2025, 16, 1505674. [Google Scholar] [CrossRef]
- Radford-Smith, D.E.; Anthony, D.C. Vancomycin-Resistant E. faecium: Addressing Global and Clinical Challenges. Antibiotics 2025, 14, 522. [Google Scholar] [CrossRef] [PubMed]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, Research, and Development of New Antibiotics: The WHO Priority List of Antibiotic-Resistant Bacteria and Tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Khazani Asforooshani, M.; Elikaei, A.; Abed, S.; Shafiei, M.; Barzi, S.M.; Solgi, H.; Badmasti, F.; Sohrabi, A. A Novel Enterococcus faecium Phage EF-M80: Unveiling the Effects of Hydrogel-Encapsulated Phage on Wound Infection Healing. Front. Microbiol. 2024, 15, 1416971. [Google Scholar] [CrossRef] [PubMed]
- Wandro, S.; Oliver, A.; Gallagher, T.; Weihe, C.; England, W.; Martiny, J.B.H.; Whiteson, K. Predictable Molecular Adaptation of Coevolving Enterococcus faecium and Lytic Phage EfV12-Phi1. Front. Microbiol. 2019, 9, 3192. [Google Scholar] [CrossRef]
- Raza, T.; Andleeb, S.; Ullah, S.R.; Jamal, M.; Mehmood, K.; Ali, M. Isolation and Characterization of a Phage to Control Vancomycin Resistant Enterococcus faecium. Open Life Sci. 2018, 13, 553–560. [Google Scholar] [CrossRef]
- Chatterjee, A.; Johnson, C.N.; Luong, P.; Hullahalli, K.; McBride, S.W.; Schubert, A.M.; Palmer, K.L.; Carlson, P.E.; Duerkop, B.A. Bacteriophage Resistance Alters Antibiotic-Mediated Intestinal Expansion of Enterococci. Infect. Immun. 2019, 87, e00085-19. [Google Scholar] [CrossRef]
- Pradal, I.; Casado, A.; del Rio, B.; Rodriguez-Lucas, C.; Fernandez, M.; Alvarez, M.A.; Ladero, V. Enterococcus faecium Bacteriophage vB_EfaH_163, a New Member of the Herelleviridae Family, Reduces the Mortality Associated with an E. faecium vanR Clinical Isolate in a Galleria mellonella Animal Model. Viruses 2023, 15, 179. [Google Scholar] [CrossRef]
- Lossouarn, J.; Beurrier, E.; Bouteau, A.; Moncaut, E.; Sir Silmane, M.; Portalier, H.; Zouari, A.; Cattoir, V.; Serror, P.; Petit, M.-A. The Virtue of Training: Extending Phage Host Spectra against Vancomycin-Resistant Enterococcus faecium Strains Using the Appelmans Method. Antimicrob. Agents Chemother. 2024, 68, e01439-23. [Google Scholar] [CrossRef]
- Ghatbale, P.; Sah, G.P.; Dunham, S.; Khong, E.; Blanc, A.; Monsibais, A.; Garcia, A.; Schooley, R.T.; Cobián Güemes, A.G.; Whiteson, K.; et al. In Vitro Resensitization of Multidrug-Resistant Clinical Isolates of Enterococcus faecium and E. faecalis through Phage-Antibiotic Synergy. Antimicrob. Agents Chemother. 2025, 69, e00740-24. [Google Scholar] [CrossRef]
- Paul, K.; Merabishvili, M.; Hazan, R.; Christner, M.; Herden, U.; Gelman, D.; Khalifa, L.; Yerushalmy, O.; Coppenhagen-Glazer, S.; Harbauer, T.; et al. Bacteriophage Rescue Therapy of a Vancomycin-Resistant Enterococcus faecium Infection in a One-Year-Old Child Following a Third Liver Transplantation. Viruses 2021, 13, 1785. [Google Scholar] [CrossRef]
- Stellfox, M.E.; Fernandes, C.; Shields, R.K.; Haidar, G.; Hughes Kramer, K.; Dembinski, E.; Mangalea, M.R.; Arya, G.; Canfield, G.S.; Duerkop, B.A.; et al. Bacteriophage and Antibiotic Combination Therapy for Recurrent Enterococcus faecium Bacteremia. mBio 2024, 15, e03396-23. [Google Scholar] [CrossRef] [PubMed]
- Leprince, A.; Mahillon, J. Phage Adsorption to Gram-Positive Bacteria. Viruses 2023, 15, 196. [Google Scholar] [CrossRef] [PubMed]
- Canfield, G.S.; Chatterjee, A.; Espinosa, J.; Mangalea, M.R.; Sheriff, E.K.; Keidan, M.; McBride, S.W.; McCollister, B.D.; Hang, H.C.; Duerkop, B.A. Lytic Bacteriophages Facilitate Antibiotic Sensitization of Enterococcus faecium. Antimicrob. Agents Chemother. 2021, 65, e00143-21. [Google Scholar] [CrossRef] [PubMed]
- Topka-Bielecka, G.; Dydecka, A.; Necel, A.; Bloch, S.; Nejman-Faleńczyk, B.; Węgrzyn, G.; Węgrzyn, A. Bacteriophage-Derived Depolymerases against Bacterial Biofilm. Antibiotics 2021, 10, 175. [Google Scholar] [CrossRef]
- Plumet, L.; Ahmad-Mansour, N.; Dunyach-Remy, C.; Kissa, K.; Sotto, A.; Lavigne, J.-P.; Costechareyre, D.; Molle, V. Bacteriophage Therapy for Staphylococcus aureus Infections: A Review of Animal Models, Treatments, and Clinical Trials. Front. Cell. Infect. Microbiol. 2022, 12, 907314. [Google Scholar] [CrossRef]
- Liu, K.; Wang, C.; Zhou, X.; Guo, X.; Yang, Y.; Liu, W.; Zhao, R.; Song, H. Bacteriophage Therapy for Drug-Resistant Staphylococcus aureus Infections. Front. Cell. Infect. Microbiol. 2024, 14, 1336821. [Google Scholar] [CrossRef]
- Ferry, T.; Kolenda, C.; Batailler, C.; Gustave, C.-A.; Lustig, S.; Malatray, M.; Fevre, C.; Josse, J.; Petitjean, C.; Chidiac, C.; et al. Phage Therapy as Adjuvant to Conservative Surgery and Antibiotics to Salvage Patients With Relapsing S. aureus Prosthetic Knee Infection. Front. Med. 2020, 7, 570572. [Google Scholar] [CrossRef]
- Ramirez-Sanchez, C.; Gonzales, F.; Buckley, M.; Biswas, B.; Henry, M.; Deschenes, M.V.; Horne, B.; Fackler, J.; Brownstein, M.J.; Schooley, R.T.; et al. Successful Treatment of Staphylococcus aureus Prosthetic Joint Infection with Bacteriophage Therapy. Viruses 2021, 13, 1182. [Google Scholar] [CrossRef]
- Doub, J.B.; Ng, V.Y.; Johnson, A.J.; Slomka, M.; Fackler, J.; Horne, B.; Brownstein, M.J.; Henry, M.; Malagon, F.; Biswas, B. Salvage Bacteriophage Therapy for a Chronic MRSA Prosthetic Joint Infection. Antibiotics 2020, 9, 241. [Google Scholar] [CrossRef] [PubMed]
- Onsea, J.; Post, V.; Buchholz, T.; Schwegler, H.; Zeiter, S.; Wagemans, J.; Pirnay, J.-P.; Merabishvili, M.; D’Este, M.; Rotman, S.G.; et al. Bacteriophage Therapy for the Prevention and Treatment of Fracture-Related Infection Caused by Staphylococcus aureus: A Preclinical Study. Microbiol. Spectr. 2021, 9, e01736-21. [Google Scholar] [CrossRef] [PubMed]
- Rubalskii, E.; Ruemke, S.; Salmoukas, C.; Boyle, E.C.; Warnecke, G.; Tudorache, I.; Shrestha, M.; Schmitto, J.D.; Martens, A.; Rojas, S.V.; et al. Bacteriophage Therapy for Critical Infections Related to Cardiothoracic Surgery. Antibiotics 2020, 9, 232. [Google Scholar] [CrossRef] [PubMed]
- Bleibtreu, A.; Fevre, C.; Robert, J.; Haddad, E.; Caumes, E.; Lantieri, L.; Peyre, M. Combining Bacteriophages and Dalbavancin for Salvage Therapy of Complex Staphylococcus aureus Extradural Empyema. Med. Mal. Infect. 2020, 50, 458–459. [Google Scholar] [CrossRef]
- Rodriguez, J.M.; Woodworth, B.A.; Horne, B.; Fackler, J.; Brownstein, M.J. Case Report: Successful Use of Phage Therapy in Refractory MRSA Chronic Rhinosinusitis. Int. J. Infect. Dis. 2022, 121, 14–16. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Balcaen, M.; Chatzis, O.; Haenecour, A.; Derycke, E.; Detaille, T.; Clément de Cléty, S.; Boulanger, C.; Belkhir, L.; Yombi, J.-C.; et al. Case Report: Personalized Triple Phage-Antibiotic Combination Therapy to Rescue Necrotizing Fasciitis Caused by Panton-Valentine Leukocidin-Producing MRSA in a 12-Year-Old Boy. Front. Cell. Infect. Microbiol. 2024, 14, 1354681. [Google Scholar] [CrossRef]
- Doub, J.B.; Tran, J.; Smith, R.; Pease, T.; Koh, E.; Ludwig, S.; Lee, A.; Chan, B. Feasibility of Using Bacteriophage Therapy to Reduce Morbidity and Mortality Associated with Spinal Epidural Abscesses. Infect. Chemother. 2023, 55, 257–263. [Google Scholar] [CrossRef]
- Van Nieuwenhuyse, B.; Galant, C.; Brichard, B.; Docquier, P.-L.; Djebara, S.; Pirnay, J.-P.; Van der Linden, D.; Merabishvili, M.; Chatzis, O. A Case of In Situ Phage Therapy against Staphylococcus aureus in a Bone Allograft Polymicrobial Biofilm Infection: Outcomes and Phage-Antibiotic Interactions. Viruses 2021, 13, 1898. [Google Scholar] [CrossRef]
- Loganathan, A.; Bozdogan, B.; Manohar, P.; Nachimuthu, R. Phage-Antibiotic Combinations in Various Treatment Modalities to Manage MRSA Infections. Front. Pharmacol. 2024, 15, 1356179. [Google Scholar] [CrossRef]
- Petrovic Fabijan, A.; Lin, R.C.Y.; Ho, J.; Maddocks, S.; Ben Zakour, N.L.; Iredell, J.R. Safety of Bacteriophage Therapy in Severe Staphylococcus aureus Infection. Nat. Microbiol. 2020, 5, 465–472. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Saffari, M.; Siadat, S.D. Phage Therapy of Antibiotic-Resistant Strains of Klebsiella pneumoniae, Opportunities and Challenges from the Past to the Future. Folia Microbiol 2023, 68, 357–368. [Google Scholar] [CrossRef] [PubMed]
- Kou, X.; Yang, X.; Zheng, R. Challenges and Opportunities of Phage Therapy for Klebsiella pneumoniae Infections. Appl. Environ. Microbiol. 2024, 90, e01353-24. [Google Scholar] [CrossRef]
- Singh, A.N.; Singh, A.; Singh, S.K.; Nath, G. Klebsiella pneumoniae Infections and Phage Therapy. Indian. J. Med. Microbiol. 2024, 52, 100736. [Google Scholar] [CrossRef]
- Geremia, N.; Zuglian, G.; Salvador, A.; Solinas, M.; Boraso, S.; Tini, L.; Fullin, G.; Vanin, M.; Lazzari, F.; Panese, S. Impact of Infection Control Measures and Antibiotic Stewardship Programs on Multidrug-Resistant Klebsiella pneumoniae Prevalence in Intensive Care Unit. Infect. Dis. Trop. Med. 2024, 10, E1413. [Google Scholar]
- Colombo, F.; Waheed, A.; Panese, S.; Scarparo, C.; Solinas, M.; Parisi, S.G.; Geremia, N. Treatment with Cefiderocol in K. pneumoniae KPC Nosocomial External Ventricular Drainage Meningitis: A Brief Report. Infez. Med. 2022, 30, 454–458. [Google Scholar] [CrossRef]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32, e00001-19. [Google Scholar] [CrossRef]
- Giacobbe, D.R.; Labate, L.; Russo Artimagnella, C.; Marelli, C.; Signori, A.; Di Pilato, V.; Aldieri, C.; Bandera, A.; Briano, F.; Cacopardo, B.; et al. Use of Cefiderocol in Adult Patients: Descriptive Analysis from a Prospective, Multicenter, Cohort Study. Infect. Dis. Ther. 2024, 13, 1929–1948. [Google Scholar] [CrossRef]
- Le Bris, J.; Chen, N.; Supandy, A.; Rendueles, O.; Van Tyne, D. Phage Therapy for Klebsiella pneumoniae: Understanding Bacteria-Phage Interactions for Therapeutic Innovations. PLoS Pathog. 2025, 21, e1012971. [Google Scholar] [CrossRef]
- Cano, E.J.; Caflisch, K.M.; Bollyky, P.L.; Van Belleghem, J.D.; Patel, R.; Fackler, J.; Brownstein, M.J.; Horne, B.; Biswas, B.; Henry, M.; et al. Phage Therapy for Limb-Threatening Prosthetic Knee Klebsiella pneumoniae Infection: Case Report and In Vitro Characterization of Anti-Biofilm Activity. Clin. Infect. Dis. 2021, 73, e144–e151. [Google Scholar] [CrossRef]
- Bao, J.; Wu, N.; Zeng, Y.; Chen, L.; Li, L.; Yang, L.; Zhang, Y.; Guo, M.; Li, L.; Li, J.; et al. Non-Active Antibiotic and Bacteriophage Synergism to Successfully Treat Recurrent Urinary Tract Infection Caused by Extensively Drug-Resistant Klebsiella pneumoniae. Emerg. Microbes Infect. 2020, 9, 771–774. [Google Scholar] [CrossRef]
- Le, T.; Nang, S.C.; Zhao, J.; Yu, H.H.; Li, J.; Gill, J.J.; Liu, M.; Aslam, S. Therapeutic Potential of Intravenous Phage as Standalone Therapy for Recurrent Drug-Resistant Urinary Tract Infections. Antimicrob. Agents Chemother. 2023, 67, e0003723. [Google Scholar] [CrossRef]
- Tang, M.; Yao, Z.; Liu, Y.; Ma, Z.; Zhao, D.; Mao, Z.; Wang, Y.; Chen, L.; Zhou, T. Host Immunity Involvement in the Outcome of Phage Therapy against Hypervirulent Klebsiella pneumoniae Infections. Antimicrob. Agents Chemother. 2024, 68, e0142923. [Google Scholar] [CrossRef]
- Rahimi, S.; Bakht, M.; Javadi, A.; Foroughi, F.; Marashi, S.M.A.; Nikkhahi, F. Characterization of Novel Bacteriophage PSKP16 and Its Therapeutic Potential against β-Lactamase and Biofilm Producer Strain of K2-Hypervirulent Klebsiella pneumoniae Pneumonia Infection in Mice Model. BMC Microbiol. 2023, 23, 233. [Google Scholar] [CrossRef]
- Kelishomi, F.Z.; Nikkhahi, F.; Amereh, S.; Ghayyaz, F.; Marashi, S.M.A.; Javadi, A.; Shahbazi, G.; Khakpour, M. Evaluation of the Therapeutic Effect of a Novel Bacteriophage in the Healing Process of Infected Wounds with Klebsiella pneumoniae in Mice. J. Glob. Antimicrob. Resist. 2024, 36, 371–378. [Google Scholar] [CrossRef]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef]
- Patro, L.P.P.; Rathinavelan, T. Targeting the Sugary Armor of Klebsiella Species. Front. Cell. Infect. Microbiol. 2019, 9, 367. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yan, B.; He, B.; Li, L.; Zhou, X.; Wu, N.; Wang, Q.; Guo, X.; Zhu, T.; Qin, J. Development of Phage Resistance in Multidrug-Resistant Klebsiella pneumoniae Is Associated with Reduced Virulence: A Case Report of a Personalised Phage Therapy. Clin. Microbiol. Infect. 2023, 29, 1601.e1–1601.e7. [Google Scholar] [CrossRef] [PubMed]
- Uddin, M.J.; Kim, B.; Dawan, J.; Ding, T.; Kim, J.-C.; Ahn, J. Assessment of Antibiotic Resistance in Bacteriophage-Insensitive Klebsiella pneumoniae. Microb. Pathog. 2019, 135, 103625. [Google Scholar] [CrossRef]
- Hesse, S.; Rajaure, M.; Wall, E.; Johnson, J.; Bliskovsky, V.; Gottesman, S.; Adhya, S. Phage Resistance in Multidrug-Resistant Klebsiella pneumoniae ST258 Evolves via Diverse Mutations That Culminate in Impaired Adsorption. mBio 2020, 11, e02530-19. [Google Scholar] [CrossRef]
- D’Angelo, F.; Rocha, E.P.C.; Rendueles, O. The Capsule Increases Susceptibility to Last-Resort Polymyxins, but Not to Other Antibiotics, in Klebsiella pneumoniae. Antimicrob. Agents Chemother. 2023, 67, e00127-23. [Google Scholar] [CrossRef]
- Tu, Q.; Pu, M.; Li, Y.; Wang, Y.; Li, M.; Song, L.; Li, M.; An, X.; Fan, H.; Tong, Y. Acinetobacter baumannii Phages: Past, Present and Future. Viruses 2023, 15, 673. [Google Scholar] [CrossRef]
- Jesudason, T. WHO Publishes Updated List of Bacterial Priority Pathogens. Lancet Microbe 2024, 5, 100940. [Google Scholar] [CrossRef] [PubMed]
- Malik, M.A.; Manzoor, S.; Ashraf, J. Bayesian Evaluation of Phage Therapy Efficacy against Multidrug-Resistant Acinetobacter baumannii. Int. J. Antimicrob. Agents 2025, 66, 107508. [Google Scholar] [CrossRef] [PubMed]
- Wienhold, S.-M.; Brack, M.C.; Nouailles, G.; Krishnamoorthy, G.; Korf, I.H.E.; Seitz, C.; Wienecke, S.; Dietert, K.; Gurtner, C.; Kershaw, O.; et al. Preclinical Assessment of Bacteriophage Therapy against Experimental Acinetobacter baumannii Lung Infection. Viruses 2021, 14, 33. [Google Scholar] [CrossRef]
- Sitthisak, S.; Manrueang, S.; Khongfak, S.; Leungtongkam, U.; Thummeepak, R.; Thanwisai, A.; Burton, N.; Dhanoa, G.K.; Tsapras, P.; Sagona, A.P. Antibacterial Activity of vB_AbaM_PhT2 Phage Hydrophobic Amino Acid Fusion Endolysin, Combined with Colistin against Acinetobacter baumannii. Sci. Rep. 2023, 13, 7470. [Google Scholar] [CrossRef]
- Liu, Y.; Leung, S.S.Y.; Guo, Y.; Zhao, L.; Jiang, N.; Mi, L.; Li, P.; Wang, C.; Qin, Y.; Mi, Z.; et al. The Capsule Depolymerase Dpo48 Rescues Galleria mellonella and Mice From Acinetobacter baumannii Systemic Infections. Front. Microbiol. 2019, 10, 545. [Google Scholar] [CrossRef]
- Tan, X.; Chen, H.; Zhang, M.; Zhao, Y.; Jiang, Y.; Liu, X.; Huang, W.; Ma, Y. Clinical Experience of Personalized Phage Therapy Against Carbapenem-Resistant Acinetobacter baumannii Lung Infection in a Patient With Chronic Obstructive Pulmonary Disease. Front. Cell. Infect. Microbiol. 2021, 11, 631585. [Google Scholar] [CrossRef]
- Tan, Y.; Su, J.; Fu, M.; Zhang, H.; Zeng, H. Recent Advances in Phage-Based Therapeutics for Multi-Drug Resistant Acinetobacter baumannii. Bioengineering 2023, 10, 35. [Google Scholar] [CrossRef]
- Moradali, M.F.; Ghods, S.; Rehm, B.H.A. Pseudomonas aeruginosa Lifestyle: A Paradigm for Adaptation, Survival, and Persistence. Front. Cell. Infect. Microbiol. 2017, 7, 39. [Google Scholar] [CrossRef]
- Chegini, Z.; Khoshbayan, A.; Taati Moghadam, M.; Farahani, I.; Jazireian, P.; Shariati, A. Bacteriophage Therapy against Pseudomonas aeruginosa Biofilms: A Review. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 45. [Google Scholar] [CrossRef] [PubMed]
- Eiselt, V.A.; Bereswill, S.; Heimesaat, M.M. Phage Therapy in Lung Infections Caused by Multidrug-Resistant Pseudomonas aeruginosa—A Literature Review. Eur. J. Microbiol. Immunol. 2024, 14, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Chang, R.Y.K.; Das, T.; Manos, J.; Kutter, E.; Morales, S.; Chan, H.-K. Bacteriophage PEV20 and Ciprofloxacin Combination Treatment Enhances Removal of Pseudomonas aeruginosa Biofilm Isolated from Cystic Fibrosis and Wound Patients. AAPS J. 2019, 21, 49. [Google Scholar] [CrossRef] [PubMed]
- Olszak, T.; Zarnowiec, P.; Kaca, W.; Danis-Wlodarczyk, K.; Augustyniak, D.; Drevinek, P.; de Soyza, A.; McClean, S.; Drulis-Kawa, Z. In Vitro and in Vivo Antibacterial Activity of Environmental Bacteriophages against Pseudomonas aeruginosa Strains from Cystic Fibrosis Patients. Appl. Microbiol. Biotechnol. 2015, 99, 6021–6033. [Google Scholar] [CrossRef]
- Alemayehu, D.; Casey, P.G.; McAuliffe, O.; Guinane, C.M.; Martin, J.G.; Shanahan, F.; Coffey, A.; Ross, R.P.; Hill, C. Bacteriophages ϕMR299-2 and ϕNH-4 Can Eliminate Pseudomonas aeruginosa in the Murine Lung and on Cystic Fibrosis Lung Airway Cells. mBio 2012, 3, e00029-12. [Google Scholar] [CrossRef]
- Pires, D.P.; Vilas Boas, D.; Sillankorva, S.; Azeredo, J. Phage Therapy: A Step Forward in the Treatment of Pseudomonas aeruginosa Infections. J. Virol. 2015, 89, 7449–7456. [Google Scholar] [CrossRef]
- Yang, X.; Haque, A.; Matsuzaki, S.; Matsumoto, T.; Nakamura, S. The Efficacy of Phage Therapy in a Murine Model of Pseudomonas aeruginosa Pneumonia and Sepsis. Front. Microbiol. 2021, 12, 682255. [Google Scholar] [CrossRef]
- Morris, T.C.; Reyneke, B.; Khan, S.; Khan, W. Phage-Antibiotic Synergy to Combat Multidrug Resistant Strains of Gram-Negative ESKAPE Pathogens. Sci. Rep. 2025, 15, 17235. [Google Scholar] [CrossRef]
- Holger, D.; Kebriaei, R.; Morrisette, T.; Lev, K.; Alexander, J.; Rybak, M. Clinical Pharmacology of Bacteriophage Therapy: A Focus on Multidrug-Resistant Pseudomonas aeruginosa Infections. Antibiotics 2021, 10, 556. [Google Scholar] [CrossRef]
- Chan, B.K.; Stanley, G.; Modak, M.; Koff, J.L.; Turner, P.E. Bacteriophage Therapy for Infections in CF. Pediatr. Pulmonol. 2021, 56 (Suppl. S1), S4–S9. [Google Scholar] [CrossRef]
- Khosravi, A.; Chen, Q.; Echterhof, A.; Koff, J.L.; Bollyky, P.L. Phage Therapy for Respiratory Infections: Opportunities and Challenges. Lung 2024, 202, 223–232. [Google Scholar] [CrossRef]
- Wright, A.; Hawkins, C.H.; Anggård, E.E.; Harper, D.R. A Controlled Clinical Trial of a Therapeutic Bacteriophage Preparation in Chronic Otitis Due to Antibiotic-Resistant Pseudomonas aeruginosa; a Preliminary Report of Efficacy. Clin. Otolaryngol. 2009, 34, 349–357. [Google Scholar] [CrossRef] [PubMed]
- Weiner, I.; Kahan-Hanum, M.; Buchstab, N.; Zelcbuch, L.; Navok, S.; Sherman, I.; Nicenboim, J.; Axelrod, T.; Berko-Ashur, D.; Olshina, M.; et al. Phage Therapy with Nebulized Cocktail BX004-A for Chronic Pseudomonas aeruginosa Infections in Cystic Fibrosis: A Randomized First-in-Human Trial. Nat. Commun. 2025, 16, 5579. [Google Scholar] [CrossRef] [PubMed]
- Pirnay, J.-P.; Djebara, S.; Steurs, G.; Griselain, J.; Cochez, C.; De Soir, S.; Glonti, T.; Spiessens, A.; Vanden Berghe, E.; Green, S.; et al. Personalized Bacteriophage Therapy Outcomes for 100 Consecutive Cases: A Multicentre, Multinational, Retrospective Observational Study. Nat. Microbiol. 2024, 9, 1434–1453. [Google Scholar] [CrossRef] [PubMed]
- Ferry, T.; Bouar, M.L.; Briot, T.; Roussel-Gaillard, T.; Perpoint, T.; Roux, S.; Ader, F.; Valour, F.; Kassai, B.; Boussaha, I.; et al. Access to Phage Therapy at Hospices Civils de Lyon in 2022: Implementation of the PHAGEinLYON Clinic Programme. Int. J. Antimicrob. Agents 2024, 64, 107372. [Google Scholar] [CrossRef]
- Cesta, N.; Pini, M.; Mulas, T.; Materazzi, A.; Ippolito, E.; Wagemans, J.; Kutateladze, M.; Fontana, C.; Sarmati, L.; Tavanti, A.; et al. Application of Phage Therapy in a Case of a Chronic Hip-Prosthetic Joint Infection Due to Pseudomonas aeruginosa: An Italian Real-Life Experience and In Vitro Analysis. Open Forum Infect. Dis. 2023, 10, ofad051. [Google Scholar] [CrossRef]
- Eiferman, V.; Vion, P.-A.; Bleibtreu, A. Phage Therapy as a Rescue Treatment for Recurrent Pseudomonas aeruginosa Bentall Infection. Viruses 2025, 17, 123. [Google Scholar] [CrossRef]
- Aslam, S.; Roach, D.; Nikolich, M.P.; Biswas, B.; Schooley, R.T.; Lilly-Bishop, K.A.; Rice, G.K.; Cer, R.Z.; Hamilton, T.; Henry, M.; et al. Pseudomonas aeruginosa Ventricular Assist Device Infections: Findings from Ineffective Phage Therapies in Five Cases. Antimicrob. Agents Chemother. 2024, 68, e0172823. [Google Scholar] [CrossRef]
- Fu, S.-Y.; Chen, X.-Z.; Yi, P.-C.; Gao, J.; Wang, W.-X.; Gu, S.-L.; Gao, J.-H.; Liu, D.-X.; Xu, H.-F.; Zeng, Y.; et al. Optimizing Phage Therapy for Carbapenem-Resistant Enterobacter cloacae Bacteremia: Insights into Dose and Timing. Antimicrob. Agents Chemother. 2025, 69, e01683-24. [Google Scholar] [CrossRef]
- Ali, S.F.; Teh, S.-H.; Yang, H.-H.; Tsai, Y.-C.; Chao, H.-J.; Peng, S.-S.; Chen, S.-C.; Lin, L.-C.; Lin, N.-T. Therapeutic Potential of a Novel Lytic Phage, vB_EclM_ECLFM1, against Carbapenem-Resistant Enterobacter cloacae. Int. J. Mol. Sci. 2024, 25, 854. [Google Scholar] [CrossRef]
- Pintor-Cora, A.; Carpintero, A.; Alegría, Á.; Giannis, A.; Lopez-Díaz, T.-M.; Santos, J.A.; Rodríguez-Calleja, J.M. A Novel Bacteriophage Targeting Mcr-9 Enterobacter Kobei with Potential Application in Fresh Leafy Greens. Appl. Microbiol. 2025, 5, 25. [Google Scholar] [CrossRef]
- González-Gómez, J.P.; Rodríguez-Arellano, S.N.; Gomez-Gil, B.; de Jesus Vergara-Jiménez, M.; Chaidez, C. Genomic and Biological Characterization of Bacteriophages against Enterobacter cloacae, a High-Priority Pathogen. Virology 2024, 595, 110100. [Google Scholar] [CrossRef]
- Cieślik, M.; Wójcicki, M.; Migdał, P.; Grygiel, I.; Bajrak, O.; Orwat, F.; Górski, A.; Jończyk-Matysiak, E. Fighting Biofilm: Bacteriophages Eliminate Biofilm Formed by Multidrug-Resistant Enterobacter Hormaechei on Urological Catheters. Med. Microbiol. Immunol. 2025, 214, 33. [Google Scholar] [CrossRef] [PubMed]
- Adaptive Phage Therapeutics, Inc. A Phase I/II Study of Bacteriophage Therapy to Evaluate Safety, Tolerability, and Efficacy of Targeted “Personalized” Bacteriophage Treatments in Patients With Bacterial Infection of the Urinary Tract; NCT04287478. Available online: https://www.clinicaltrials.gov/study/NCT04287478?term=bacteriophage%20AND%20bacteriophage%20therapy&aggFilters=studyType:int&viewType=Table&rank=5 (accessed on 8 September 2025).
- Green, S.I.; Clark, J.R.; Santos, H.H.; Weesner, K.E.; Salazar, K.C.; Aslam, S.; Campbell, J.W.; Doernberg, S.B.; Blodget, E.; Morris, M.I.; et al. A Retrospective, Observational Study of 12 Cases of Expanded-Access Customized Phage Therapy: Production, Characteristics, and Clinical Outcomes. Clin. Infect. Dis. 2023, 77, 1079–1091. [Google Scholar] [CrossRef] [PubMed]
- Subedi, D.; Altamirano, F.G.; Deehan, R.; Perera, A.; Patwa, R.; Kostoulias, X.; Korneev, D.; Blakeway, L.; Macesic, N.; Peleg, A.Y.; et al. Rational Design of Frontline Institutional Phage Cocktail for the Treatment of Nosocomial Enterobacter cloacae Complex Infections. Nat. Microbiol. 2025. [Google Scholar] [CrossRef]
- Almosuli, M.; Kirtava, A.; Chkhotua, A.; Tsveniashvili, L.; Chanishvili, N.; Irfan, S.S.; Ng, E.; McIntyre, H.; Hockenberry, A.J.; Araujo, R.P.; et al. Urinary Bacteriophage Cooperation with Bacterial Pathogens during Human Urinary Tract Infections Supports Lysogenic Phage Therapy. Commun. Biol. 2025, 8, 175. [Google Scholar] [CrossRef] [PubMed]
- Ibrahim, R.; Aranjani, J.M.; Kalikot Valappil, V.; Nair, G. Unveiling the Potential Bacteriophage Therapy: A Systematic Review. Future Sci. OA 2025, 11, 2468114. [Google Scholar] [CrossRef]
- Sawa, T.; Moriyama, K.; Kinoshita, M. Current Status of Bacteriophage Therapy for Severe Bacterial Infections. J. Intensive Care 2024, 12, 44. [Google Scholar] [CrossRef]
- Cesta, N.; Di Luca, M.; Corbellino, M.; Tavio, M.; Galli, M.; Andreoni, M. Bacteriophage Therapy: An Overview and the Position of Italian Society of Infectious and Tropical Diseases. Infez. Med. 2020, 28, 322–331. [Google Scholar]
- Egido, J.E.; Costa, A.R.; Aparicio-Maldonado, C.; Haas, P.-J.; Brouns, S.J.J. Mechanisms and Clinical Importance of Bacteriophage Resistance. FEMS Microbiol. Rev. 2022, 46, fuab048. [Google Scholar] [CrossRef]
- Mahler, M.; Costa, A.R.; van Beljouw, S.P.B.; Fineran, P.C.; Brouns, S.J.J. Approaches for Bacteriophage Genome Engineering. Trends Biotechnol. 2023, 41, 669–685. [Google Scholar] [CrossRef]
- Dedrick, R.M.; Smith, B.E.; Cristinziano, M.; Freeman, K.G.; Jacobs-Sera, D.; Belessis, Y.; Whitney Brown, A.; Cohen, K.A.; Davidson, R.M.; van Duin, D.; et al. Phage Therapy of Mycobacterium Infections: Compassionate Use of Phages in 20 Patients With Drug-Resistant Mycobacterial Disease. Clin. Infect. Dis. 2023, 76, 103–112. [Google Scholar] [CrossRef]
- Bernabéu-Gimeno, M.; Pardo-Freire, M.; Chan, B.K.; Turner, P.E.; Gil-Brusola, A.; Pérez-Tarazona, S.; Carrasco-Hernández, L.; Quintana-Gallego, E.; Domingo-Calap, P. Neutralizing Antibodies after Nebulized Phage Therapy in Cystic Fibrosis Patients. Med 2024, 5, 1096–1111.e6. [Google Scholar] [CrossRef]
- Frontiers. Regulations of Phage Therapy Across the World. Available online: https://www.frontiersin.org/journals/microbiology/articles/10.3389/fmicb.2023.1250848/full (accessed on 30 August 2025).
- New General Chapter on Phage Therapy Medicinal Products (5.31) Adopted and Pre-Published on the EDQM Website. Available online: https://www.edqm.eu/en/w/new-general-chapter-on-phage-therapy-medicinal-products-5.31-adopted-and-pre-published-on-the-edqm-website (accessed on 30 August 2025).
- Romero-Calle, D.; Guimarães Benevides, R.; Góes-Neto, A.; Billington, C. Bacteriophages as Alternatives to Antibiotics in Clinical Care. Antibiotics 2019, 8, 138. [Google Scholar] [CrossRef]
- Tkhilaishvili, T.; Potapov, E.; Starck, C.; Mulzer, J.; Falk, V.; Trampuz, A.; Schoenrath, F. Bacteriophage Therapy as a Treatment Option for Complex Cardiovascular Implant Infection: The German Heart Center Berlin Experience. J. Heart Lung Transpl. 2022, 41, 551–555. [Google Scholar] [CrossRef]
- Corbellino, M.; Kieffer, N.; Kutateladze, M.; Balarjishvili, N.; Leshkasheli, L.; Askilashvili, L.; Tsertsvadze, G.; Rimoldi, S.G.; Nizharadze, D.; Hoyle, N.; et al. Eradication of a Multidrug-Resistant, Carbapenemase-Producing Klebsiella pneumoniae Isolate Following Oral and Intra-Rectal Therapy With a Custom Made, Lytic Bacteriophage Preparation. Clin. Infect. Dis. 2020, 70, 1998–2001. [Google Scholar] [CrossRef]
| Gap | Why It Matters | Recommended Experiment/Plan |
|---|---|---|
| Human PK by route (IV, nebulized, local) | Dosing remains empirical; clearance and tissue penetration are poorly defined | Embed PK substudies in early-phase trials: serial blood and site sampling (PFU + qPCR) to model clearance and local persistence |
| MOI/exposure targets at infection site | Required titers for effective kill are undefined, especially in biofilm | Dose-escalation with local sampling (e.g., BAL, synovial fluid) to link input dose → site PFU → bacterial kill |
| Biofilm penetration and PAS sequencing | Order of phage–antibiotic administration may alter outcomes | Factorial preclinical/early clinical studies comparing phage→antibiotic vs. antibiotic→phage sequences with quantitative microbiology |
| Immunogenicity and neutralization kinetics | Neutralizing antibodies may reduce efficacy | Prospective neutralization assays (baseline, day 7, day 14, day 28) with prespecified cocktail switch criteria |
| Resistance emergence and management | On-therapy resistance can derail efficacy | Adaptive protocols enabling sequential cocktail switching upon phenotypic resistance; genomic analysis of resistant isolates |
| Syndrome-specific endpoints | Heterogeneous outcomes obscure efficacy signals | Core outcome sets: ventilator-free days + culture negativity for VAP; revision-free survival for PJI; symptom + culture clearance for otitis/burns |
| Domain | Minimum Recommendation |
|---|---|
| Phage characterization | Whole-genome sequencing; exclude lysogeny, toxins, AMR genes |
| Quality control | Sterility testing (bacteria, fungi, mycoplasma); endotoxin quantification; stability testing at intended storage conditions |
| Identity assays | Host-range determination (efficiency of plating), sequence-based ID, qPCR |
| Potency assays | PFU titers per batch; in vitro lytic activity against patient isolate |
| Documentation for compassionate use | Informed consent (investigational status, risks, uncertainty); clinical isolate susceptibility records; batch QC data retained for inspection |
| Regulatory filings | eIND or Expanded Access (US), magistral preparation (Belgium), or local equivalents |
| Manufacturing standards | GMP-like or magistral-level oversight; validated sterility and stability protocols |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Paul et al., 2021 [65] | VREfm infection post–liver transplant (pediatric) | Case report | E. faecium (VRE) | Personalized phage EFgrKN IV + vancomycin | Reduced intestinal colonization; shift toward vancomycin susceptibility; fewer hospitalizations; death from unrelated pneumonia |
| Stellfox et al., 2024 [66] | Recurrent VREfm bacteremia | Case report | E. faecium (VRE) | IV phage + antibiotics | Rapid bacteremia clearance; infection-free for months before recurrence likely due to adaptation and anti-phage antibodies |
| Khazani Asforooshani et al., 2024 [58] | Wound infection model | Preclinical (in vivo) | E. faecium | Hydrogel-encapsulated phage EF-M80 (topical) | Broad host range; biofilm degradation; accelerated wound healing features |
| Pradal et al., 2023 [62] | Sepsis model (Galleria) | Preclinical (in vivo) | E. faecium (vanR clinical isolate) | vB_EfaH_163 | Reduced mortality vs. control |
| Wandro et al., 2019 [59] | Coevolution/host-range | Preclinical (in vitro) | E. faecium | Lytic phage EfV12-phi1 | Predictable molecular adaptation; informs cocktail design |
| Lossouarn et al., 2024 [63] | Host-range expansion | Preclinical (in vitro) | E. faecium (VRE) | Appelmans training method | Extended host spectra against VREfm strains |
| Ghatbale et al., 2025 [64] | PAS/resensitization | Preclinical (in vitro) | E. faecium/E. faecalis | Phage + antibiotic combinations | Resensitized MDR isolates; supports PAS in treatment design |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Ferry et al. (2020) [72] | Prosthetic Knee Infection (PKI) | Case Series (n = 3) | S. aureus | Local intra-articular injection of phage cocktail post-DAIR surgery (“PhagoDAIR”). | Favorable outcomes with significant clinical improvement; potential as a salvage therapy. |
| Bleibtreu et al. (2020) [77] | Extradural Empyema | Case Report | S. aureus (MSSA) | Local administration of two phages via fistula, combined with IV dalbavancin. | Successful salvage therapy, stopping purulent flow and allowing for reconstructive surgery; culture-negative samples post-treatment. |
| Ramirez-Sanchez et al. (2021) [73] | Prosthetic Knee Infection (PJI) | Case Report | S. aureus (MSSA) | Two cycles: 1st (cocktail, 2 weeks) failed; 2nd (single phage, 6 weeks, intravenous and intra-articular) with surgery succeeded. | Highlights the importance of surgical debridement and sufficient duration. Success was achieved despite the development of serum neutralization. |
| Rubalskii et al. (2020) [76] | Cardiothoracic Surgery-Related Infections | Case Series (n = 8) | S. aureus, P. aeruginosa, E. faecium, K. pneumoniae, E. coli | Individualized phage preparations via local, oral, or inhalation routes. Three cases used a fibrin glue delivery system. | Eradication of target bacteria in 7 of 8 patients as a last resort therapy. |
| Onsea et al. (2021) [75] | Fracture-Related Infection (FRI) | Preclinical (Rabbit) | S. aureus | Prevention: Single intraoperative phage in saline. Treatment: Daily phage in saline vs. single phage-loaded hydrogel. | Highly effective in prevention. In treatment, hydrogel showed a trend but no statistical significance. The hydrogel avoided the antibody response seen with saline application. |
| Van Nieuwenhuyse et al. (2021) [81] | Polymicrobial Bone Allograft Infection | Case Report | S. aureus, C. hathewayi, P. mirabilis, F. magna | Local, in situ anti- | S. aureus phage therapy for 14 days. |
| Doub et al. (2023) [80] | Spinal Epidural Abscess (SEA) | Feasibility Study | S. aureus | N/A (in vitro testing only). | More than 50% of the patients either succumbed within three months, experienced a recurrence of their infection, required additional debridement, or suffered long-term sequelae. |
| Doub et al. (2020) [74] | Prosthetic Joint Infection (PJI) | Case Report | S. aureus (MRSA) | Intra-articular and 3 days of IV phage therapy. | Successful eradication of chronic infection despite a short course, which was stopped due to reversible transaminitis. |
| Petrovic Fabijan et al. (2020) [83] | Severe Bacteremia and Endocarditis | Single-Arm Safety Trial (n = 13) | S. aureus (MSSA & MRSA) | Intravenous administration of Good Manufacturing Practices-quality phage cocktail AB-SA01 twice daily for 14 days. | Primary outcome met: Therapy was safe and well-tolerated in critically ill patients, with no reported adverse reactions. |
| Van Nieuwenhuyse et al. (2024) [79] | Necrotizing Fasciitis (Pediatric) | Case Report | PVL-producing MRSA, P. aeruginosa, S. maltophilia | Personalized, multi-route (intravenous, topical, intra-pleural, etc.) therapy targeting all three pathogens, combined with antibiotics. | Successful rescue of a life-threatening infection failing standard care. Phage immune neutralization was observed. |
| Rodriguez et al. (2022) [78] | Chronic Rhinosinusitis (CRS) | Case Report | S. aureus (MRSA) | Two courses of IV and topical phage. Dramatic improvement seen only with frequent, daily topical (intranasal/ear) application. | Successful treatment of a multi-year refractory infection, highlighting the importance of achieving high local phage concentrations. |
| Loganathan et al. (2024) [82] | MRSA Biofilm Infection | In vitro & In vivo (Larvae) | S. aureus (MRSA) | Tested three different treatment sequences: phages before, with, or after antibiotics. | Confirmed synergy with multiple antibiotics. Phages before antibiotics (PRE) were most effective against biofilms; antibiotics before phages (POS) were most effective against planktonic cells. |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Cano et al., 2021 [92] | Prosthetic knee infection (biofilm) | Case report + in vitro | K. pneumoniae | Personalized phage therapy adjunctive to DAIR; local delivery | Limb-threatening PJI salvaged; anti-biofilm activity shown in vitro |
| Bao et al., 2020 [93] | Recurrent UTI (XDR) | Case report | K. pneumoniae (XDR) | Phage + “non-active” antibiotic | Successful eradication via synergism |
| Le et al., 2023 [94] | Recurrent drug-resistant UTI | Prospective series | Enterobacterales incl. K. pneumoniae | Intravenous phage as a standalone | Demonstrated safety/therapeutic potential for recurrent UTIs |
| Tang et al., 2024 [95] | Hypervirulent infection (hvKP) | Preclinical | Hv K. pneumoniae | Phage therapy with immune analyses | Host immunity engagement influenced outcomes |
| Rahimi et al., 2023 [5] | Pneumonia (murine model) | Preclinical (in vivo) | K2 hv K. pneumoniae | Phage PSKP16 | Therapeutic efficacy in mice |
| Kelishomi et al., 2024 [97] | Infected wounds (murine) | Preclinical (in vivo) | K. pneumoniae | Novel phage topical/application | Improved wound healing parameters |
| Li et al., 2023 [100] | MDR infection under personalized therapy | Case report | K. pneumoniae (MDR) | Personalized phage | Phage resistance emerged with reduced virulence; clinical improvement despite resistance |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Malik et al., 2025 [106] | Various animal infections | Meta-analysis (preclinical) | A. baumannii (MDR) | Multiple | Survival 60–90% with phage vs. 20–50% controls; >96% mean survival in murine models |
| Wienhold et al., 2021 [107] | Experimental lung infection | Preclinical (murine) | A. baumannii | Myovirus vB_AbaM_Acibel004 | Reduced lung pathology/inflammation; preserved architecture |
| Sitthisak et al., 2023 [108] | Respiratory infection model | Preclinical | A. baumannii | vB_AbaM_PhT2 endolysin ± colistin | Complete bacterial clearance/100% survival in immediate dosing; strong anti-inflammatory effects (model) |
| Liu et al., 2019 [109] | Systemic infection models | Preclinical (Galleria & mice) | A. baumannii | Depolymerase Dpo48 | Improved survival; no detectable toxicity in mice |
| Schooley et al., 2017 [13] | Disseminated, pan-resistant infection | Case report | A. baumannii | Personalized cocktail IV → intraperitoneal; sequential adaptation | Resolution after the second cocktail; showcases an adaptive phage strategy |
| Tan et al., 2021 [110] | COPD patient, CRAB VAP | Case report | A. baumannii (Carbapenem resistant) | Nebulized phages | Bacterial clearance after 16 days; respiratory improvement; no adverse effects |
| Tan et al., 2023 (review) [111] | COVID-19 secondary VAP (n = 4) | Case series (summarized) | A. baumannii | Nebulized via a mesh nebulizer | Clearance in 3/4; clinical improvement in 2; resistance observed in some |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Wright et al., 2009 [124] | Chronic otitis | Randomized, placebo-controlled | P. aeruginosa | Topical phage cocktail | Symptom and bacterial-count improvement over 42 days; no AEs |
| Jault et al., 2019 [18] | Burn wounds (PhagoBurn) | Randomized, double-blind Phase 1/2 | P. aeruginosa | Topical phage cocktail | Mixed efficacy; issues with low titers highlighted |
| Cesta et al., 2023 [128] | Hip prosthetic joint infection | Case report + in vitro | P. aeruginosa | Intra-articular phage Pa53 via drain + meropenem | Two-year relapse-free clearance; phage–meropenem synergy vs. biofilm |
| Eiferman et al., 2025 [129] | Chronic endocarditis (post-Bentall) | Case report | P. aeruginosa | Personalized phage therapy | Infection resolution; 12-month relapse-free survival |
| Weiner et al., 2025 [125] | CF chronic lung infection | Randomized first-in-human trial | P. aeruginosa | Nebulized cocktail BX004-A | Significant bacterial clearance; safety/tolerability; progressed development |
| Aslam et al., 2024 [130] | VAD-associated infections (n = 5) | Case series (negative) | P. aeruginosa | Various | Ineffective outcomes; underscores the need for dosing/PK optimization |
| Study (First Author, Year) | Infection Type | Study Design | Key Pathogen(s) | Phage Administration Details | Key Outcomes & Findings |
|---|---|---|---|---|---|
| Fu et al., 2025 [131] | Carbapenem-resistant E. cloacae bacteremia | Clinical study (dose/timing optimization) | E. cloacae complex | Targeted cocktail; early, high MOI dosing | Dosing & early administration shape outcomes; practical guidance for compassionate use |
| Ali et al., 2024 [132] | CR-E. cloacae | Preclinical (genomics + in vitro) | E. cloacae | vB_EclM_ECLFM1 (strictly lytic) | Genomically vetted; therapeutic potential against CR strains |
| González-Gómez et al., 2024 [134] | — | Preclinical (genomic/biological) | E. cloacae | Multiple novel phages | Characterized phages vs. high-priority pathogen |
| Pintor-Cora et al., 2025 [133] | Food/environment relevance | Preclinical | E. kobei (mcr-9) | Novel phage | Potential application; broadens ECC coverage |
| Cieślik et al., 2025 [135] | Urological catheter biofilm | Preclinical (device model) | E. hormaechei | Lytic phages on catheter | Marked reduction/elimination of established biofilm |
| Adaptive Phage Therapeutics, 2023 [136] | Urinary tract infections | Phase 1/2 (enrolling) | Enterobacterales incl. Enterobacter | Expanding PhageBank™; personalized matching | Will generate safety/efficacy data applicable to Enterobacter UTIs |
| Green et al., 2023 [137] | Various Gram-negatives | Retrospective expanded-access series (n = 12) | Mixed (incl. Enterobacterales) | Customized phages; multi-route | Feasibility & clinical responses; species-resolved data for Enterobacter are still limited |
| Advantages | Challenges |
|---|---|
| Highly present in nature and easy to isolate (i.e., from soil, wastewater) | Only lytic bacteriophages can be used in therapy |
| Self-replicating and self-limiting | Bacteriophages need to be isolated and checked for activity before therapeutic use |
| Low production costs | Narrow spectrum of action |
| Highly specific against the bacterial host | Possibility of phage resistance in the host |
| Rapid bacterial killing | Development of neutralizing antibodies |
| Activity against MDR pathogens | Unpredictable PK/PD parameters |
| Anti-biofilm activity | Uncertainty in dosages and duration of therapy |
| Synergism with antibiotics | Safety issues (especially if IV) |
| No activity against human microbiota | Regulatory heterogeneity among countries |
| No activity against eukaryotic cells | |
| Multiple ways of administration (local, aerosol, oral, intravenous) |
| Pathogen | Study (First Author, Year) | Design | N (Patients) | Route(s) of Administration | Primary Endpoint(s) | Key Outcome | Level of Evidence * |
|---|---|---|---|---|---|---|---|
| E. faecium | Paul, 2021 [65] | Case report (compassionate use) | 1 | IV + antibiotics | Safety, clearance | Clinical improvement, shift to vancomycin susceptibility | Level 4 |
| E. faecium | Stellfox, 2024 [66] | Case report | 1 | IV + antibiotics | Safety, clearance | Rapid bacteremia clearance, relapse-free interval | Level 4 |
| S. aureus | Petrovic Fabijan, 2020 [83] | Single-arm safety study | 13 | IV phage cocktail | Safety | Well tolerated, no adverse reactions | Level 2b |
| S. aureus | Ferry, 2020 [72] | Case series | 3 | Intra-articular | Clinical resolution | All improved, no relapse at follow-up | Level 4 |
| K. pneumoniae | Cano, 2021 [92] | Case report | 1 | IV phage + DAIR | Infection clearance | Sustained resolution at 34 weeks | Level 4 |
| K. pneumoniae | Bao, 2020 [93] | Case report | 1 | IV phage + ‘inactive’ antibiotic | Clearance | Successful eradication | Level 4 |
| A. baumannii | Schooley, 2017 [13] | Case report (adaptive cocktail) | 1 | IV → intraperitoneal | Survival | Resolution after second cocktail | Level 4 |
| A. baumannii | Tan, 2021 [110] | Case report | 1 | Nebulized | Clearance | Bacterial clearance, clinical improvement | Level 4 |
| P. aeruginosa | Wright, 2009 [124] | RCT (placebo-controlled) | 24 | Topical | Safety, bacterial counts | Improved symptoms and clearance | Level 1b |
| P. aeruginosa | Jault, 2019 [18] | RCT (Phase 1/2) | 27 | Topical | Safety, efficacy | Safe but efficacy limited (low titers) | Level 1b |
| P. aeruginosa | Cesta, 2023 [128] | Case report | 1 | Intra-articular + meropenem | Clearance | 2-year relapse-free cure | Level 4 |
| Enterobacter spp. | Fu, 2025 [131] | Clinical study (dose/timing optimization) | Not specified | IV cocktail | Survival, clearance | Favorable with early/high dosing | Level 3b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marino, A.; Stracquadanio, S.; Cosentino, F.; Maraolo, A.E.; Colpani, A.; De Vito, A.; Geremia, N.; Nicolosi, A.; Oliva, A.; Cacopardo, B.; et al. Phage to ESKAPE: Personalizing Therapy for MDR Infections—A Comprehensive Clinical Review. Pathogens 2025, 14, 1011. https://doi.org/10.3390/pathogens14101011
Marino A, Stracquadanio S, Cosentino F, Maraolo AE, Colpani A, De Vito A, Geremia N, Nicolosi A, Oliva A, Cacopardo B, et al. Phage to ESKAPE: Personalizing Therapy for MDR Infections—A Comprehensive Clinical Review. Pathogens. 2025; 14(10):1011. https://doi.org/10.3390/pathogens14101011
Chicago/Turabian StyleMarino, Andrea, Stefano Stracquadanio, Federica Cosentino, Alberto Enrico Maraolo, Agnese Colpani, Andrea De Vito, Nicholas Geremia, Alice Nicolosi, Alessandra Oliva, Bruno Cacopardo, and et al. 2025. "Phage to ESKAPE: Personalizing Therapy for MDR Infections—A Comprehensive Clinical Review" Pathogens 14, no. 10: 1011. https://doi.org/10.3390/pathogens14101011
APA StyleMarino, A., Stracquadanio, S., Cosentino, F., Maraolo, A. E., Colpani, A., De Vito, A., Geremia, N., Nicolosi, A., Oliva, A., Cacopardo, B., & Nunnari, G. (2025). Phage to ESKAPE: Personalizing Therapy for MDR Infections—A Comprehensive Clinical Review. Pathogens, 14(10), 1011. https://doi.org/10.3390/pathogens14101011










