Optimizing Surgical Antibiotic Prophylaxis in the Era of Antimicrobial Resistance: A Position Paper from the Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections (SIMPIOS)
Abstract
1. Introduction
2. Materials and Methods
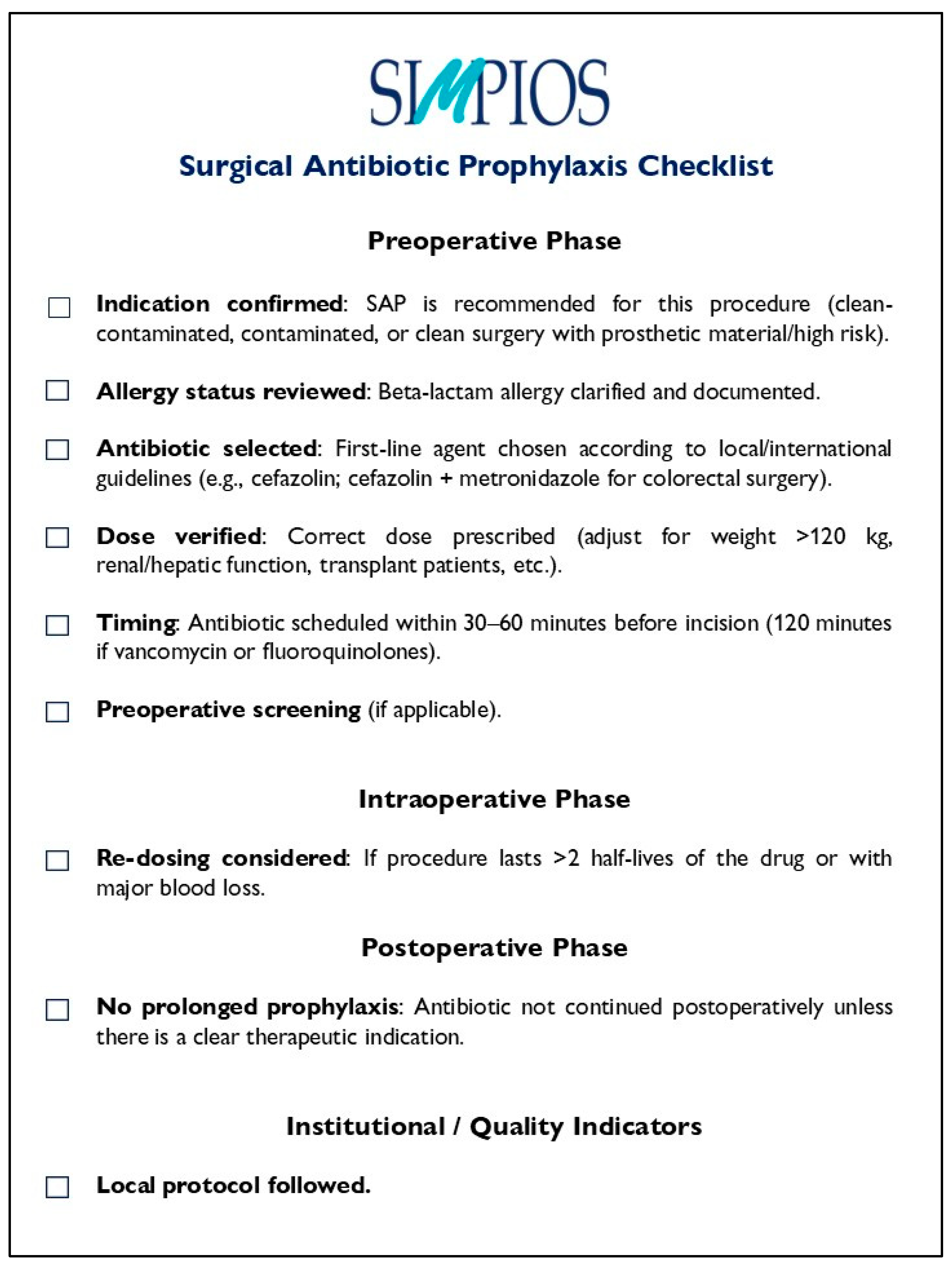
3. Core Principles for Appropriate Antibiotic Prophylaxis
Recommendations
4. Screening, Decolonization, and Targeted SAP in Patients Colonized with Multidrug-Resistant Bacteria
4.1. Gram-Positive Bacteria
Recommendations
4.2. Gram-Negative Bacteria
Recommendations
5. Behavioural Changes Across the Surgical Pathway
- Appropriateness of indication.
- Percentage of surgical procedures with SAP prescribed according to evidence-based guidelines (only when indicated).
- Choice of antibiotic.
- Percentage of patients receiving the recommended first-line antibiotic for the specific procedure (e.g., cefazolin for most clean/clean-contaminated surgeries).
- Percentage of patients unnecessarily prescribed broad-spectrum agents (should be as low as possible).
- Timing of administration.
- Percentage of patients receiving SAP within the optimal window before incision:
- within 30–60 min for most antibiotics (e.g., cefazolin).
- within 120 min for agents requiring longer infusion (e.g., vancomycin).
- Adequacy of dose.
- Percentage of patients receiving the correct dose based on guidelines.
- Intraoperative re-dosing.
- Percentage of prolonged procedures (>2 half-lives of antibiotic or significant blood loss) with correct re-dosing.
- Duration of prophylaxis.
- Percentage of cases where SAP is discontinued at wound closure.
- Percentage of cases with inappropriate postoperative continuation (>24 h).
- Screening and targeted prophylaxis (when indicated)
- Percentage of high-risk patients screened for S. aureus before orthopaedic/cardiac surgery.
- Percentage of MRSA carriers receiving vancomycin for SAP.
- Compliance with local protocols
- Overall adherence rate to hospital SAP protocols (combined indicator of indication, timing, agent, dosing, duration).
- Outcome indicators
- SSI rate per procedure type.
- SSI rate among patients receiving guideline-compliant SAP vs. non-compliant SAP.
Recommendations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AMR | Antimicrobial resistance |
| APIC | Association for Professionals in Infection Control and Epidemiology |
| ASP | Antimicrobial stewardship program |
| CDC | Centers for Disease Control and Prevention |
| CRE | Carbapenem-resistant Enterobacterales |
| ESBL | Extended-spectrum beta-lactamase |
| ESMID | European Society of Clinical Microbiology and Infectious Diseases |
| EUCIC | European Committee on Infection Control |
| GRADE | Grading of Recommendations Assessment, Development and Evaluation |
| IDSA | Infectious Diseases Society of America |
| KPI | Key performance indicator |
| MDRO | Multidrug-resistant organism |
| MRSA | Methicillin-resistant Staphylococcus aureus |
| MSSA | Methicillin-susceptible Staphylococcus aureus |
| RCT | Randomized controlled trial |
| SAP | Surgical antibiotic prophylaxis |
| SIMPIOS | Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections |
| SHEA | Society for Healthcare Epidemiology of America |
| SSI | Surgical site infection |
| VRE | Vancomycin-resistant enterococci |
| WHO | World Health Organization |
References
- Sartelli, M.; Boermeester, M.A.; Cainzos, M.; Coccolini, F.; de Jonge, S.W.; Rasa, K.; Dellinger, E.P.; McNamara, D.A.; Fry, D.E.; Cui, Y.; et al. Six Long-Standing Questions about Antibiotic Prophylaxis in Surgery. Antibiotics 2023, 12, 908. [Google Scholar] [CrossRef]
- Bratzler, D.W.; Dellinger, E.P.; Olsen, K.M.; Perl, T.M.; Auwaerter, P.G.; Bolon, M.K.; Fish, D.N.; Napolitano, L.M.; Sawyer, R.G.; Slain, D.; et al. Clinical practice guidelines for antimicrobial prophylaxis in surgery. Surg. Infect. 2013, 14, 73–156. [Google Scholar] [CrossRef] [PubMed]
- Sistema Nazionale Linee Guida. Antibioticoprofilassi Peri-Operatoria nell’Adulto. (SNLG 17). 2011. Available online: https://www.anmdo.org/wp-content/uploads/2016/10/Linee-guida-Antibioticoprofilassi-perioperatoria-nelladulto.pdf (accessed on 28 August 2025).
- Harbarth, S.; Samore, M.H.; Lichtenberg, D.; Carmeli, Y. Prolonged antibiotic prophylaxis after cardiovascular surgery and its effect on surgical site infections and antimicrobial resistance. Circulation 2000, 101, 2916–2921. [Google Scholar] [CrossRef]
- Tangden, T.; Giske, C.G. Global dissemination of extensively drug-resistant carbapenemases-producing Enterobacteriaceae: Clinical perspectives on detection, treatment and infection control. J. Int. Med. 2015, 277, 501–512. [Google Scholar] [CrossRef] [PubMed]
- Navarra, A.; Cicalini, S.; D’Arezzo, S.; Pica, F.; Selleri, M.; Nisii, C.; Venditti, C.; Cannas, A.; Mazzarelli, A.; Vulcano, A.; et al. Vancomycin-Resistant Enterococci: Screening Efficacy and the Risk of Bloodstream Infections in a Specialized Healthcare Setting. Antibiotics 2025, 14, 304. [Google Scholar] [CrossRef]
- Riedlinger, D.; Holert, F.; Gastmeier, P.; Kola, A.; Slagman, A.; Möckel, M. Risk factors for Staphylococcus aureus colonization in a general emergency department patient cohort—Results of an observational cohort study. Biomarkers 2025, 30, 97–103. [Google Scholar] [CrossRef]
- Iskandar, K.; Sartelli, M.; Tabbal, M.; Ansaloni, L.; Baiocchi, G.L.; Catena, F.; Coccolini, F.; Haque, M.; Labricciosa, F.M.; Moghabghab, A.; et al. Highlighting the gaps in quantifying the economic burden of surgical site infections associated with antimicrobial-resistant bacteria. World. J. Emerg. Surg. 2019, 14, 50. [Google Scholar] [CrossRef]
- Bowater, R.J.; Stirling, S.A.; Lilford, R.J. Is antibiotic prophylaxis in surgery a generally effective intervention? Testing a generic hypothesis over a set of meta-analyses. Ann. Surg. 2009, 249, 551–556. [Google Scholar] [CrossRef] [PubMed]
- Whitman, G.; Cowell, V.; Parris, K.; McCullough, P.; Howard, T.; Gaughan, J.; Karavite, D.; Kennedy, M.; McInerney, J.; Rose, C. Prophylactic antibiotic use: Hardwiring of physician behaviour, not education, leads to compliance. J. Am. Coll. Surg. 2008, 207, 88–94. [Google Scholar] [CrossRef]
- Global Guidelines for the Prevention of Surgical Site Infection; World Health Organization: Geneva, Switzerland, 2018. Available online: https://www.ncbi.nlm.nih.gov/books/NBK536404/ (accessed on 12 August 2025).
- Berríos-Torres, S.I.; Umscheid, C.A.; Bratzler, D.W.; Leas, B.; Stone, E.C.; Kelz, R.R.; Reinke, C.E.; Morgan, S.; Solomkin, J.S.; Mazuski, J.E.; et al. Centers for Disease Control and Prevention Guideline for the Prevention of Surgical Site Infection, 2017. JAMA Surg. 2017, 152, 784–791. [Google Scholar] [CrossRef]
- Calderwood, M.S.; Anderson, D.J.; Bratzler, D.W.; Dellinger, E.P.; Garcia-Houchins, S.; Maragakis, L.L.; Nyquist, A.C.; Perkins, K.M.; Preas, M.A.; Saiman, L.; et al. Strategies to prevent surgical site infections in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 695–720. [Google Scholar] [CrossRef]
- de Jonge, S.W.; Gans, S.L.; Atema, J.J.; Solomkin, J.S.; Dellinger, P.E.; Boermeester, M.A. Timing of preoperative antibiotic prophylaxis in 54,552 patients and the risk of surgical site infection: A systematic review and meta-analysis. Medicine 2017, 96, e6903. [Google Scholar] [CrossRef]
- Weber, W.P.; Mujagic, E.; Zwahlen, M.; Bundi, M.; Hoffmann, H.; Soysal, S.D.; Kraljević, M.; Delko, T.; von Strauss, M.; Iselin, L.; et al. Timing of surgical antimicrobial prophylaxis: A phase 3 randomised controlled trial. Lancet Infect. Dis. 2017, 17, 605–614. [Google Scholar] [CrossRef]
- Wolfhagen, N.; Boldingh, Q.J.J.; de Lange, M.; Boermeester, M.A.; de Jonge, S.W. Intraoperative redosing of surgical antibiotic prophylaxis in addition to preoperative prophylaxis versus single-dose prophylaxis for the prevention of surgical site infection: A meta-analysis and GRADE recommendation. Ann. Surg. 2022, 275, 1050–1057. [Google Scholar] [CrossRef] [PubMed]
- Branch-Elliman, W.; O’Brien, W.; Strymish, J.; Itani, K.; Wyatt, C.; Gupta, K. Association of Duration and Type of Surgical Prophylaxis With Antimicrobial-Associated Adverse Events. JAMA Surg. 2019, 154, 590–598. [Google Scholar] [CrossRef]
- de Jonge, S.W.; Boldingh, Q.J.J.; Solomkin, J.S.; Dellinger, E.P.; Egger, M.; Salanti, G.; Allegranzi, B.; Boermeester, M.A. Effect of postoperative continuation of antibiotic prophylaxis on the incidence of surgical site infection: A systematic review and meta-analysis. Lancet Infect. Dis. 2020, 20, 1182–1192. [Google Scholar] [CrossRef]
- Ljungqvist, O.; Scott, M.; Fearon, K.C. Enhanced recovery after surgery: A review. JAMA Surg. 2017, 152, 292–298. [Google Scholar] [CrossRef] [PubMed]
- Jalalzadeh, H.; Wolfhagen, N.; Harmsen, W.J.; Griekspoor, M.; Boermeester, M.A. A Network Meta-Analysis and GRADE Assessment of the Effect of Preoperative Oral Antibiotics with and Without Mechanical Bowel Preparation on Surgical Site Infection Rate in Colorectal Surgery. Ann. Surg. Open 2022, 3, e175. [Google Scholar] [CrossRef] [PubMed]
- Rollins, K.E.; Javanmard-Emamghissi, H.; Acheson, A.G.; Lobo, D.N. The Role of Oral Antibiotic Preparation in Elective Colorectal Surgery: A Meta-analysis. Ann. Surg. 2019, 270, 43–58. [Google Scholar] [CrossRef]
- Nelson, R.L.; Hassan, M.; Grant, M.D. Antibiotic prophylaxis in colorectal surgery: Are oral, intravenous or both best and is mechanical bowel preparation necessary? Tech. Coloproctol. 2020, 24, 1233–1246. [Google Scholar] [CrossRef]
- Koskenvuo, L.; Lunkka, P.; Varpe, P.; Hyöty, M.; Satokari, R.; Haapamäki, C.; Lepistö, A.; Sallinen, V. Morbidity After Mechanical Bowel Preparation and Oral Antibiotics Prior to Rectal Resection: The MOBILE2 Randomized Clinical Trial. JAMA Surg. 2024, 159, 606–614. [Google Scholar] [CrossRef]
- Yao, J.; Chen, L.; Liu, X.; Wang, J.; Zeng, J.; Cai, Y. Meta-analysis of efficacy of perioperative oral antibiotics in intestinal surgery with surgical site infection. J. Glob. Antimicrob. Resist. 2023, 35, 223–236. [Google Scholar] [CrossRef]
- Menz, B.D.; Charani, E.; Gordon, D.L.; Leather, A.J.M.; Moonesinghe, S.R.; Phillips, C.J. Surgical Antibiotic Prophylaxis in an Era of Antibiotic Resistance: Common Resistant Bacteria and Wider Considerations for Practice. Infect. Drug Resist. 2021, 14, 5235–5252. [Google Scholar] [CrossRef]
- Schweizer, M.; Perencevich, E.; McDanel, J.; Carson, J.; Formanek, M.; Hafner, J.; Braun, B.; Herwaldt, L. Effectiveness of a bundled intervention of decolonization and prophylaxis to decrease Gram positive surgical site infections after cardiac or orthopedic surgery: Systematic review and meta-analysis. BMJ 2013, 346, f2743. [Google Scholar] [CrossRef]
- Lin, L.; Ke, Z.Y.; Wang, Y.; Chen, X.L.; Zhong, D.; Cheng, S. Efficacy of preoperative screening and decolonization for staphylococcus aureus in total joint arthroplasty: A meta-analysis. Asian J. Surg. 2021, 44, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ribau, A.I.; Collins, J.E.; Chen, A.F.; Sousa, R.J. Is Preoperative Staphylococcus aureus Screening and Decolonization Effective at Reducing Surgical Site Infection in Patients Undergoing Orthopedic Surgery? A Systematic Review and Meta-Analysis With a Special Focus on Elective Total Joint Arthroplasty. J. Arthroplast. 2021, 36, 752–766.e6. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Mutters, N.T.; Guirao, X.; Del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Presterl, E.; Christaki, E.; Cross, E.L.A.; et al. European Society of Clinical Microbiology and Infectious Diseases/European Committee on infection control clinical guidelines on pre-operative decolonization and targeted prophylaxis in patients colonized by multidrug-resistant Gram-positive bacteria before surgery. Clin. Microbiol. Infect. 2024, 30, 1537–1550. [Google Scholar] [PubMed]
- Bode, L.G.; Kluytmans, J.A.; Wertheim, H.F.; Bogaers, D.; Vandenbroucke-Grauls, C.M.; Roosendaal, R.; Troelstra, A.; Box, A.T.; Voss, A.; van der Tweel, I.; et al. Preventing surgical-site infections in nasal carriers of Staphylococcus aureus. N. Engl. J. Med. 2010, 362, 9–17. [Google Scholar] [CrossRef]
- Lepelletier, D.; Maillard, J.Y.; Pozzetto, B.; Simon, A. Povidone Iodine: Properties, Mechanisms of Action, and Role in Infection Control and Staphylococcus aureus Decolonization. Antimicrob. Agents Chemother. 2020, 64, e00682-20. [Google Scholar] [CrossRef]
- Popovich, K.J.; Aureden, K.; Ham, D.C.; Harris, A.D.; Hessels, A.J.; Huang, S.S.; Maragakis, L.L.; Milstone, A.M.; Moody, J.; Yokoe, D.; et al. SHEA/IDSA/APIC Practice Recommendation: Strategies to prevent methicillin-resistant Staphylococcus aureus transmission and infection in acute-care hospitals: 2022 Update. Infect. Control Hosp. Epidemiol. 2023, 44, 1039–1067. [Google Scholar] [CrossRef] [PubMed]
- Righi, E.; Mutters, N.T.; Guirao, X.; Del Toro, M.D.; Eckmann, C.; Friedrich, A.W.; Giannella, M.; Kluytmans, J.; Presterl, E.; Christaki, E.; et al. ESCMID/EUCIC clinical practice guidelines on perioperative antibiotic prophylaxis in patients colonized by multidrug-resistant Gram-negative bacteria before surgery. Clin. Microbiol. Infect. 2023, 29, 463–479. [Google Scholar] [CrossRef] [PubMed]
- Dubinsky-Pertzov, B.; Temkin, E.; Harbarth, S.; Fankhauser-Rodriguez, C.; Carevic, B.; Radovanovic, I.; Ris, F.; Kariv, Y.; Buchs, N.C.; Schiffer, E.; et al. Carriage of Extended-spectrum Beta-lactamase-producing Enterobacteriaceae and the Risk of Surgical Site Infection After Colorectal Surgery: A Prospective Cohort Study. Clin. Infect. Dis. 2019, 68, 1699–1704. [Google Scholar] [CrossRef] [PubMed]
- Golzarri, M.F.; Silva-Sánchez, J.; Cornejo-Juárez, P.; Barrios-Camacho, H.; Chora-Hernández, L.D.; Velázquez-Acosta, C.; Vilar-Compte, D. Colonization by fecal extended-spectrum β-lactamase-producing Enterobacteriaceae and surgical site infections in patients with cancer undergoing gastrointestinal and gynecologic surgery. Am. J. Infect. Control 2019, 47, 916–921. [Google Scholar] [CrossRef]
- Apisarnthanarak, A.; Kondo, S.; Mingmalairak, C.; Mahawongkajit, P.; Juntong, J.; Limpavitayaporn, P.; Sriussadaporn, E.; Tongyoo, A.; Mundy, L.M. Outcomes of extended-spectrum beta-lactamases producing Enterobacteriaceae colonization among patients abdominal surgery patients. Infect. Control Hosp. Epidemiol. 2019, 40, 1290–1293. [Google Scholar] [CrossRef]
- Tumbarello, M.; Trecarichi, E.M.; Bassetti, M.; De Rosa, F.G.; Spanu, T.; Di Meco, E.; Losito, A.R.; Parisini, A.; Pagani, N.; Cauda, R. Identifying patients harboring extended-spectrum-beta-lactamase-producing Enterobacteriaceae on hospital admission: Derivation and validation of a scoring system. Antimicrob. Agents Chemother. 2011, 55, 3485–3490. [Google Scholar] [CrossRef]
- Nutman, A.; Temkin, E.; Harbarth, S.; Carevic, B.; Ris, F.; Fankhauser-Rodriguez, C.; Radovanovic, I.; Dubinsky-Pertzov, B.; Cohen-Percia, S.; Kariv, Y.; et al. Personalized Ertapenem Prophylaxis for Carriers of Extended-spectrum β-Lactamase-producing Enterobacteriaceae Undergoing Colorectal Surgery. Clin. Infect. Dis. 2020, 70, 1891–1897. [Google Scholar] [CrossRef]
- Solomkin, J.S. Is Personalized Colorectal Prophylaxis Ready for Prime Time? Clin. Infect. Dis. 2020, 70, 1898–1899. [Google Scholar] [CrossRef]
- Hoffman, T.; Lellouche, J.; Nutman, A.; Temkin, E.; Frenk, S.; Harbarth, S.; Carevic, B.; Cohen-Percia, S.; Kariv, Y.; Fallach, N.; et al. The effect of prophylaxis with ertapenem versus cefuroxime/metronidazole on intestinal carriage of carbapenem-resistant or third-generation-cephalosporin-resistant Enterobacterales after colorectal surgery. Clin. Microbiol. Infect. 2021, 27, 1481–1487. [Google Scholar]
- Isler, B.; Aslan, A.T.; Akova, M.; Harris, P.; Paterson, D.L. Treatment strategies for OXA-48-like and NDM producing Klebsiella pneumoniae infections. Expert Rev. Anti Infect. Ther. 2022, 20, 1389–1400. [Google Scholar] [CrossRef]
- Bonkat, G.; Pilatz, A.; Wagenlehner, F. Time to adapt our practice? The European commission has restricted the use of fluoroquinolones since March 2019. Eur. Urol. 2019, 76, 273–275. [Google Scholar] [CrossRef] [PubMed]
- Freitas, D.M.; Moreira, D.M. Fosfomycin trometamol vs ciprofloxacin for antibiotic prophylaxis before transrectal ultrasonography-guided prostate biopsy: A meta-analysis of clinical studies. Arab. J. Urol. 2019, 17, 114–119. [Google Scholar] [CrossRef]
- Charani, E.; Tarrant, C.; Moorthy, K.; Sevdalis, N.; Brennan, L.; Holmes, A.H. Understanding antibiotic decision making in surgery-a qualitative analysis. Clin. Microbiol. Infect. 2017, 23, 752–760. [Google Scholar] [CrossRef]
- Ripabelli, G.; Salzo, A.; Sammarco, M.L.; Guerrizio, G.; Cecere, G.; Tamburro, M. Infections and Colon Surgery: Preliminary Results from a Surveillance Program in an Italian Hospital. Hosp. Top. 2023, 101, 27–38. [Google Scholar] [CrossRef] [PubMed]
- Charani, E.; Ahmad, R.; Rawson, T.M.; Castro-Sanchèz, E.; Tarrant, C.; Holmes, A.H. The Differences in Antibiotic Decision-making Between Acute Surgical and Acute Medical Teams: An Ethnographic Study of Culture and Team Dynamics. Clin. Infect. Dis. 2019, 69, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Global Alliance for Infections in Surgery collaborators group. A Global Declaration on Appropriate Use of Antimicrobial Agents across the Surgical Pathway. Surg. Infect. 2017, 18, 846–853. [Google Scholar] [CrossRef]
- Brink, A.J.; Messina, A.P.; Feldman, C.; Richards, G.A.; van den Bergh, D.; Netcare Antimicrobial Stewardship Study Alliance. From guidelines to practice: A pharmacist-driven prospective audit and feedback improvement model for peri-operative antibiotic prophylaxis in 34 South African hospitals. J. Antimicrob. Chemother. 2017, 72, 1227–1234. [Google Scholar] [CrossRef]
- Elligsen, M.; Walker, S.A.; Simor, A.; Daneman, N. Prospective audit and feedback of antimicrobial stewardship in critical care: Program implementation, experience, and challenges. Can. J. Hosp. Pharm. 2012, 65, 31–36. [Google Scholar] [CrossRef] [PubMed]
- Chan, A.J.; Tsang, M.E.; Langford, B.J.; Nisenbaum, R.; Wan, M.; Downing, M.A. Evaluating a pilot, structured, face-to-face, antimicrobial stewardship, prospective audit-and-feedback program in emergency general surgery service in a community hospital. Antimicrob. Steward. Healthc. Epidemiol. 2023, 3, e96. [Google Scholar] [CrossRef]
| Antibiotic | Adult Dose | Pediatric Dose | Redosing Interval | Time of Administration |
|---|---|---|---|---|
| Cefazolin | 2 g IV (3 g if >120 kg) | 30 mg/kg (max 2 g) | q4h | Within 60 min before incision |
| Cefuroxime | 1.5 g IV | 50 mg/kg | q4h | Within 60 min before incision |
| Cefotetan | 2 g IV | 40 mg/kg | q6h | Within 60 min before incision |
| Cefoxitin | 2 g IV | 40 mg/kg | q2h | Within 60 min before incision |
| Metronidazole | 500 mg IV | 15 mg/kg | No redose needed | Within 60 min before incision |
| Clindamycin | 900 mg IV | 10 mg/kg | q6h | Within 60 min before incision |
| Vancomycin | 15 mg/kg IV (≈1–1.5 g) | 15 mg/kg | No redose needed (long half-life) | Begin infusion within 120 min before incision |
| Ertapenem | 1 g IV | 15 mg/kg (max 1 g) | No redose needed (long half-life) | Within 60 min before incision |
| Recommendation Statements | |
|---|---|
| General core principles |
|
| |
| |
| |
| |
| |
| |
| Screening, decolonization and targeted SAP |
|
| |
| |
| |
| |
| |
| Behaviours’ changes across the surgical pathway |
|
|
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sartelli, M.; Labricciosa, F.M.; Casini, B.; Cortese, F.; Cricca, M.; Facciolà, A.; Foghetti, D.; Moro, M.; Pan, A.; Pasero, D.; et al. Optimizing Surgical Antibiotic Prophylaxis in the Era of Antimicrobial Resistance: A Position Paper from the Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections (SIMPIOS). Pathogens 2025, 14, 1031. https://doi.org/10.3390/pathogens14101031
Sartelli M, Labricciosa FM, Casini B, Cortese F, Cricca M, Facciolà A, Foghetti D, Moro M, Pan A, Pasero D, et al. Optimizing Surgical Antibiotic Prophylaxis in the Era of Antimicrobial Resistance: A Position Paper from the Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections (SIMPIOS). Pathogens. 2025; 14(10):1031. https://doi.org/10.3390/pathogens14101031
Chicago/Turabian StyleSartelli, Massimo, Francesco M. Labricciosa, Beatrice Casini, Francesco Cortese, Monica Cricca, Alessio Facciolà, Domitilla Foghetti, Matteo Moro, Angelo Pan, Daniela Pasero, and et al. 2025. "Optimizing Surgical Antibiotic Prophylaxis in the Era of Antimicrobial Resistance: A Position Paper from the Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections (SIMPIOS)" Pathogens 14, no. 10: 1031. https://doi.org/10.3390/pathogens14101031
APA StyleSartelli, M., Labricciosa, F. M., Casini, B., Cortese, F., Cricca, M., Facciolà, A., Foghetti, D., Moro, M., Pan, A., Pasero, D., Pipitone, G., & Ripabelli, G. (2025). Optimizing Surgical Antibiotic Prophylaxis in the Era of Antimicrobial Resistance: A Position Paper from the Italian Multidisciplinary Society for the Prevention of Healthcare-Associated Infections (SIMPIOS). Pathogens, 14(10), 1031. https://doi.org/10.3390/pathogens14101031









