Abstract
Phlebotomine-borne diseases, transmitted by sand flies, cause significant public health burdens worldwide. In Morocco, Phlebotomus papatasi is a primary vector for Leishmania major and phleboviruses. Despite extensive research in other countries, entomopathogenic parasite investigations in P. papatasi have not been conducted in Morocco until now. This study performed proteomic analysis of female P. papatasi collected from four Moroccan localities using liquid chromatography-tandem mass spectrometry (LC-MS/MS). Our analysis revealed that Phlebotomus papatasi peptides were the most abundant, with 884 peptides identified. Additionally, we detected 732 peptides from nematodes, 86 from Leishmania major, 79 from L. infantum, eight from L. tropica, and two peptides associated with phleboviruses. Microscopic examination of 1752 sand flies confirmed P. sergenti female infected with Tetranematidae, Didilia spp. in Imintanout (Z2). This study provides the first report of nematodes in sand flies in Africa and represents the first application of proteomics to identify pathogens carried by P. papatasi. These findings highlight remarkable proteomic differences among localities and generate critical data for understanding parasite-vector interactions.
1. Introduction
Phlebotomine sandflies (Diptera: Psychodidae, Phlebotominae) are small, fragile, blood-sucking insects with a wide range of hosts, facilitating pathogen transmission to humans and other animals [1,2]. These insects are recognized as vectors of various diseases, including canine and human leishmaniasis, bartonellosis, and several arboviruses [3]. Leishmaniasis is a significant public health concern in Morocco, with a broad geographical distribution of sand fly species [4]. Moroccan sand fly populations act as vectors not only for protozoa but also for viruses [5].
Phlebotomus papatasi is widely distributed around the Mediterranean basin [6]. This species is quite common and has a significant ecological plasticity [7,8]. The wide distribution of P. papatasi extends across North Africa, through Eurasia, and into India [9]. It is the vector responsible for transmitting cutaneous leishmaniasis (CL) and sandfly fever that occurs in the Mediterranean regions [10]. Many sandfly-borne phleboviruses such as the Toscana virus, Sicilian and Naples viruses are also transmitted to humans by P. papatasi in countries around the Mediterranean Sea and eastwards to central Asia and India [11]. Aside from its vectorial role in human and animal diseases, P. papatasi has also been observed to have an entomophilic nematode infestation in Pondicherry, India [12]. In Morocco, P. papatasi is the primary vector of zoonotic leishmaniasis, the most prevalent form of the disease in terms of case numbers [13]. This species is associated with all L. major-endemic cutaneous leishmaniasis (CL) foci across the country, particularly in arid regions [8]. P. papatasi exhibits peak activity during the hot, dry season and is most abundant when ambient temperatures range between 32 and 36 °C [13]. It has demonstrated a strong adaptation to arid climatic conditions [14,15].
Given the complex role P. papatasi plays in pathogen transmission, advanced molecular techniques such as metabarcoding and metagenomics are essential for further understanding the pathogens circulating in these sandfly populations. These techniques are particularly effective for determining the whole genome sequences of pathogens [16,17]. In parallel, liquid chromatography coupled with tandem mass spectrometry (LC-MS/MS) has become a powerful approach for studying host–pathogen interactions at the protein level [18]. Label-free LC-MS/MS allows the identification and quantification of thousands of proteins across multiple samples in a single run, providing an unprecedented opportunity to explore proteomic profiles and their changes in response to biological challenges [19].
In the present study, we analyzed female P. papatasi from four Moroccan populations, using LC-MS/MS to identify circulating pathogens. This proteomic analysis provided rich data, revealing peptides and their corresponding proteins, contributing valuable information to the understanding of this species in Morocco.
2. Materials and Methods
2.1. Sand Fly Collection and Species Identification
Sandflies were collected using miniature CDC light traps (John W. Hook Co., Gainesville, FL, USA) between June 2018 and June 2019 from four localities in Morocco; three of them are endemic foci of leishmaniasis (Z1: Errachidia (31°56′52.6″ N 4°25′47.7″ W), Z2: Imintanout (31°10′18.1″ N 8°51′02.4″ W), Z3: Zagora (30°20′52.5″ N 5°50′13.1″ W)) and one is a non-endemic foci from NE: Marrakech (31°39′11.6″ N 8°01′30.2″ W) [18]. A total of 1752 sandflies were collected from the four localities investigated. Male and female sandflies were separated using a cold table in the laboratory and stored in pools of 50 specimens at −80 °C in the Microbial Biotechnologies, Agrosciences and Environment Laboratory in Marrakech, Morocco, until use.
Since the type of trap can substantially affect the quality of protein spectra in collected sandflies, we used specimens trapped using CDC techniques to provide a more accurate proteomic analysis, contrary to sticky trap collection techniques which could have compromised the samples and their proteomic analysis accuracy [20].
Females were washed carefully using PBS solution and then dissected and identified individually at the species level according to morphological characteristics. The genitalia and head of the female sandflies were mounted on a slide and species identification was made using identification keys [21]. The thorax and abdomen of female P. papatasi were preserved in RNAlater solution for use in the proteomic assay.
2.2. Sample Preparation and Liquid Chromatography–Ms/Ms
Proteomic analysis by LC-MS/MS was performed at the proteomic facility of the Research Institute of the McGill University Health Centre (RI-MUHC; Montréal, QC, Canada). From each locality, fifteen P. papatasi females (arranged in three pools of five specimens) were analyzed to detect proteins linked to medically important pathogens, including Leishmania spp., phleboviruses, and nematodes.
The sandflies collected were preserved in 70% ethanol and transported to the RI-MUHC. The remaining sandflies were examined under a binocular microscope for the presence of potential nematodes. The infected specimens were dissected to remove the nematodes from the sand fly body and identified morphologically according to the Moroccan sand fly key [21]. Nematodes were identified by comparing the body size, egg diameter, and morphological characteristics with those in previous studies of the Tetradonematid nematodes [22,23,24]. The sandflies collected were preserved in 70% ethanol and transported to the RI-MUHC.
A standard TCA protein precipitation was first performed to remove ethanol from P. papatasi specimens. Protein extracts were then re-solubilized in 10 µL of a 6M urea buffer. Proteins were reduced by adding 2.5 µL of the reduction buffer (45 mM DTT, 100 mM ammonium bicarbonate) for 30 min at 37 °C and then alkylated by adding 2.5 µL of the alkylation buffer (100 mM iodoacetamide, 100 mM ammonium bicarbonate) for 20 min at 24 °C in the dark. Prior to trypsin digestion, 20 µL of de-ionized distilled water was added to reduce the urea concentration to 2M. A total of 10 µL of the trypsin solution (5 ng/µL of trypsin sequencing grade from Promega, 50 mM ammonium bicarbonate) was added to each sample. Protein digestion was performed at 37 °C for 18 h and stopped with 5 µL of 5% formic acid. Protein digests were dried in vacuum centrifuge and stored at −20 °C until LC-MS/MS analysis.
Liquid chromatography-tandem mass spectrometry (LC-MS/MS) was performed at the RI-MUHC proteomic facility as described by Atayde et al. [18]. Sample proteins were precipitated with 15% trichloroacetic acid (TCA)/acetone and digested with trypsin at a final concentration of 2 ng/mL. After an 18 h incubation at 37 °C, the reactions were quenched by the addition of formic acid to a final concentration of 1% prior to the LC-MS/MS analysis. The LC column was a PicoFrit fused silica capillary column (New Objective, MA, USA) self-packed with C-18 reverse-phase material (Phenomenex, CA, USA). This column was installed on the Easy-nLC II system (Proxeon Biosystems, Odense, Denmark) and coupled to the Q Exactive mass spectrometer (Thermo Fisher Scientific, MA, USA) equipped with a Proxeon nanoelectrospray Flex ion source. The buffers used for chromatography were 0.2% formic acid (buffer A) and 100% acetonitrile/0.2% formic acid (buffer B). Peptides were loaded onto a column at a flow rate of 600 nL/min and eluted using a two-slope gradient at 250 nL/min. Solvent B first increased from 2% to 40% in 85 min and then from 40% to 80% in 25 min. LC-MS/MS data were acquired using a data-dependent top-15 method, with standard settings applied for all mass spectrometer parameters [18].
2.3. Protein Database Search
The peak list files were generated using Proteome Discoverer (version 2.1) using the following parameters: minimum mass set to 500 Da, maximum mass set to 6000 Da, no grouping of MS/MS spectra, precursor charge set to auto, and minimum number of fragment ions set to five. Protein database searching was performed with Mascot 2.6 (Matrix Science, IL, USA) against the Moroccan Leishmania species (L. major, L. infantum and L. tropica), Phleboviruses, Nematode protein databases. The mass tolerances for precursor and fragment ions were set to 10 ppm and 0.1 Da, respectively. Trypsin was used as an enzyme allowing for up to 1 missed cleavage. Cysteine carbamidomethylation was specified as a fixed modification and methionine oxidation as variable modifications. Data analysis was performed using Scaffold (version 4.10.0). Only proteins with a minimum of three peptides and peptides scoring higher than 20 were considered. To assess differential protein expression in Phlebotomus papatasi, we established a synthetic control based on sandfly-specific proteins, which served as a baseline reference. In standard proteomic analyses, volcano plots are used to compare the same set of proteins across two conditions, with log2; fold change representing the magnitude of change and p-values indicating statistical significance. In contrast, our study examined different protein sets, including pathogen-related proteins (from Leishmania, nematodes, and Phlebovirus) as well as intrinsic sandfly proteins. The use of synthetic control relies on the assumption that sandfly-specific proteins remain relatively stable across samples, since their expression is not expected to be substantially affected by infection.
3. Results
The analysis of three pools of five P. papatasi specimens from each of the four localities enabled differentiation of biomarker peptides among the sandfly populations, including those associated with Leishmania spp., phleboviruses, and nematodes. We compared the list of identified proteins across the localities and observed that most protein hits were consistent across all locations. Detailed spectra, peptide reports, and sample replicates from each locality are available in Supplementary Material File S1. We identified varying levels of abundance for P. papatasi proteins, including spot number, UniProt identifier, protein name, and function, as annotated through Blast2GO (version 6.0.3) similarity searches (refer to Supplementary Material File S1).
Our initial analysis clearly showed that P. papatasi peptides were the most abundant, with 884 peptides identified, which was expected. This was followed by 732 peptides from nematodes, 86 from Leishmania major, 79 from L. infantum, eight peptides from L. tropica, and two peptides associated with phleboviruses (Figure 1A). The Venn diagram illustrates the distribution of shared and unique proteins among P. papatasi populations from the four studied localities, revealing a core set of 206 peptides common to all specimens. This shared peptide set suggests a conserved protein profile across the different geographical populations. Additionally, we observed unique proteins specific to each region, indicating potential location-dependent variations. These unique peptides may reflect regional environmental influences, genetic adaptations, or differences in pathogen exposure among sandfly populations (Figure 1B).
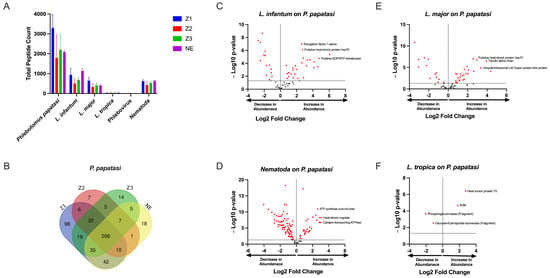
Figure 1.
Protein Content Analysis of P. papatasi from Four Moroccan Localities. (A) Total number of identified peptides in P. papatasi samples across four Moroccan localities. (B) Venn diagram illustrates the shared and unique peptide among P. papatasi populations from the studied localities. (C–F) Volcano plots displaying the differential abundance of identified pathogen-associated peptides compared to P. papatasi proteins. Locality abbreviations: Z1, Errachidia; Z2, Imintanout; Z3, Zagora; NE, Marrakech.
By comparing spectrum or peptide counts of different samples against this synthetic control, we identified peptides whose expression levels were significantly altered in response to infection. This approach effectively distinguishes infection-driven variations from the inherent expression of sandfly proteins. The results highlight key proteins associated with pathogen presence, providing insights into host–pathogen interactions within different sandflies (Figure 1C–F).
Leishmania spp. peptides were present in different localities including the non-endemic locality (NE) (Figure 1A). Comparing spectral counts for Leishmania spp. peptides from the different localities studied, our results show a significant difference for L. tropica proteins, but not for other Leishmania spp. by peptide group-based spectral count differentiation (Figure 2). We describe Leishmania species determination on entomological samples based on partial sequencing of Leishmania spp. proteins such as the heat-shock protein 70 gene (Hsp70), Alpha tubulin protein (Tuba1) and Ubiquitin (Ubi) (Figure 2). These proteins involve a variety of cellular functions for Leishmania spp. We observed the presence of Leishmania spp. biomarker proteins in P. papatasi at all sampled sites, including the non-endemic area (NE) (Figure 2). A noteworthy protein detected was the viscerotropic leishmaniasis protein specific to L. tropica, which is responsible for anthroponotic cutaneous leishmaniasis in Morocco [18]. The clustering of differentially identified proteins for L. tropica, L. major, and L. infantum is presented in Figure 2. We also observed a shared abundance of Hsp70, Tuba1, and Ubi peptides across all three Leishmania species, while other peptides showed differential abundance or were absent in each species. Mitochondrial isocitrate dehydrogenase, partial aconitase, and vacuolar ATP synthase catalytic subunit A (putative) were the main peptides highlighting Leishmania tropica specifically in this analysis, whereas hypothetical protein (conserved) and elongation factor 1-alpha were not detected in this species but were shared between L. infantum and L. major. In addition, the putative calmodulin peptide appears to be specific for L. major. The heat-shock 70-related protein 1 (mitochondrial precursor, putative) was highly detected in L. major, but not in the other Leishmania species analyzed.
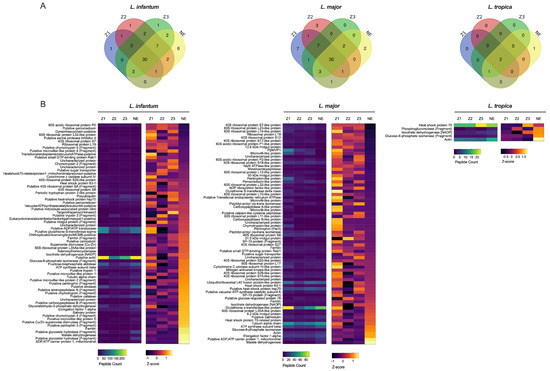
Figure 2.
Quantitative Proteomic Profiles of P. papatasi for Leishmania Across Different Moroccan Localities. (A) Relative expression levels of Leishmania species proteins obtained from mass spectrometry analysis (L. major, L. infantum, L. tropica databases) using biological triplicates for each locality (n = 3) and analyzed with Mascot (version 2.6) and Scaffold (version 4.8) software. (B) Heat map representing quantitative proteomic profiles, generated using Z-scores without normalization and clustered based on protein sets with positive Z-scores. Locality abbreviations: Z1, Errachidia; Z2, Imintanout; Z3, Zagora; NE, Marrakech.
Regarding phleboviruses, the number of specific proteins according to locality is lower, as these did not show in abundance. We recorded only two phlebovirus peptides that refer to Sand fly fever virus (SFSV) and Severe fever with Syndrome Virus (SFTSV) (Figure 1A).
Interestingly, nematode proteins were consistently found to be highly abundant in sandflies across all localities, as indicated by the high number of unique peptides detected (Figure 1A and Figure 3, Supplementary Material File S2). This observation suggests the potential value of conducting direct microscopic examinations of sandflies collected from different localities to further explore the presence of nematodes.
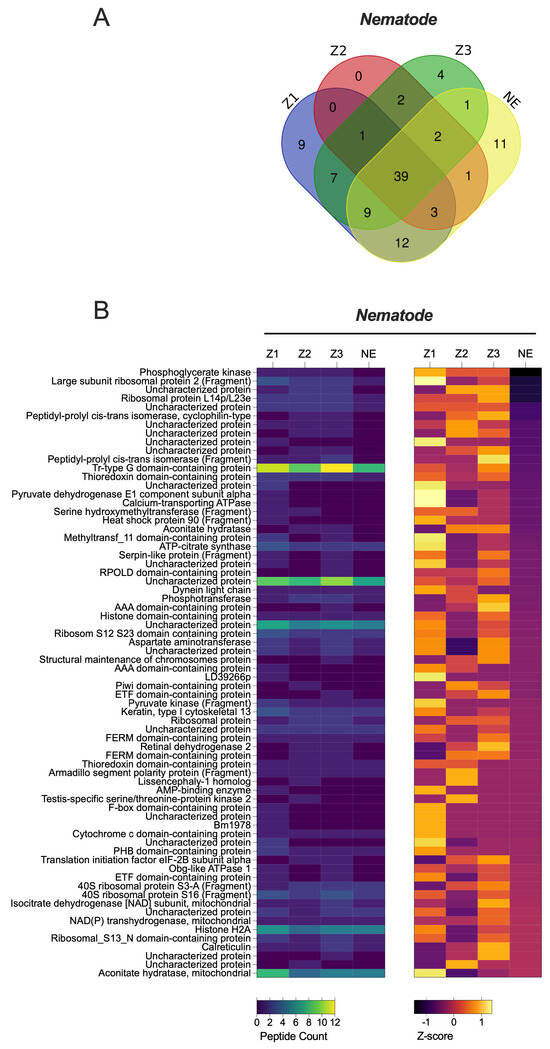
Figure 3.
Quantitative Proteomic Profiles of P. papatasi for Nematodes Across Different Moroccan Localities. (A) Relative expression levels of Nematodes proteins obtained from mass spectrometry analysis using biological triplicates for each locality (n = 3), analyzed with Mascot and Scaffold Software. (B) Heat map representing quantitative proteomic profiles, generated using Z-scores without normalization, and clustered based on protein sets with positive Z-scores. abbreviations: Z1, Errachidia; Z2, Imintanout; Z3, Zagora; NE, Marrakech.
Direct microscopic examination of 1752 sand fly specimens collected from multiple localities revealed nematode infection in only one specimen, which was found in the Imintanout locality (Z2) in central Morocco. Morphological analysis identified this specimen as a female Phlebotomus sergenti. Based on measurements of the nematodes’ body length (approximately 3400 μm), body width (around 200 μm), and egg diameter (26.4 ± 2.2 μm), the nematodes were identified as Didilia species belonging to the family Tetradonematidae (Figure 4).
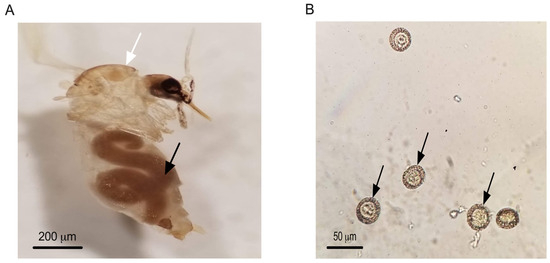
Figure 4.
Female of P. sergenti from Imintanout locality infected with a single female. Tetranematidae. Black arrow pointing to Tetranematidae, white arrow pointing to P. sergenti (A) Eggs of a Tetradonematidae isolated from P. sergenti in Morocco. Arrows pointing to Tetradonematidae eggs (B).
4. Discussion
To better understand the potential pathogens circulating in Phlebotomus papatasi populations in Morocco, we conducted a proteomic analysis to detect Leishmania spp., phleboviruses, and entomopathogenic parasites across different study localities. The application of proteomics in the surveillance of vector-borne pathogens in natural sandfly populations remains relatively unexplored, making this study one of the few to leverage this approach for pathogen detection in sandflies. Among the 22 recorded sandfly species in Morocco, five species of the genus Phlebotomus have been identified as vectors of the three nosogeographic forms of leishmaniasis present in the country. Specifically, Phlebotomus papatasi is a proven vector of L. major, the causative agent of zoonotic cutaneous leishmaniasis [25]. Our findings contribute to a better understanding of the natural circulation of pathogens within sandfly populations and highlight the potential of proteomic tools in vector-borne disease surveillance. P. ariasi, P. longicuspis, and P. perniciosus are vectors of L. infantum [26], while P. sergenti is a proven vector of L. tropica [27]. Proteomics, particularly LC-MS/MS, may improve our understanding of parasite biology and pathogenesis and has also paved the way for the screening of pathogens proteins in Phlebotomus papatasi. Our results revealed that some specific biomarker proteins such as Hsp-70, alpha-tubulin and ubiquitin of L. infantum and L. tropica proteins were present in P. papatasi. Our emphasis on Hsp70, Tuba1, and Ubi arises from their well-documented roles as central regulators of Leishmania biology that directly influence the parasite’s capacity to persist within the sandfly and establish infection in the mammalian host. Heat shock proteins such as Hsp70 have been shown to form stage-specific phosphorylation-dependent complexes that buffer the parasite against thermal and oxidative stresses encountered during vector colonization, while simultaneously facilitating the differentiation of promastigotes into the infectious metacyclic stage [28]. The parasite cytoskeleton, in turn, undergoes significant remodeling during development in the vector, and recent work has demonstrated that post-translational modifications of alpha-tubulin (Tuba1), including detyrosination, are critical for shaping cell morphology, maintaining flagellar function, and promoting efficient motility and virulence [29]. Complementing these processes, ubiquitin-mediated protein conjugation has emerged as a key mechanism controlling proteome plasticity, with UBC2-UEV1-dependent ubiquitination shown to be essential for differentiation between life-cycle stages [30]. Together, these proteins highlight the interconnected pathways of stress adaptation, cytoskeletal remodeling, and regulated protein turnover that underlie parasite development within the sandfly midgut, reinforcing their biological relevance in the context of host–pathogen interactions. Among significant proteins which are different in our analysis for Leishmania species, we could mention hypothetical protein-conserved, elongation factor 1-alpha and Vacuolar ATP synthase catalytic subunit A-putative. Those peptides play a crucial role in oxidative stress defense and protein synthesis [31]. We report in our analysis the abundance of these proteins, or L. major and L. infantum, and the absence of L. tropica. The Calmodulin protein is a calcium-binding protein and one of the cell-signaling molecules in Leishmania species [32,33]; however, it was detected only in L. infantum and not in the other Leishmania species. Among the specific mitochondrial proteins identified that highlight species specificity, mitochondrial isocitrate dehydrogenase and aconitase were found in L. tropica but absent in other Leishmania species. These proteins are essential for parasite proliferation and participate in critical cellular functions, including transport, stress response, and signaling [34]. These proteins have been investigated to permit the discrimination of medically important Leishmania species worldwide without the need for parasite isolation [19,35,36,37,38]. Among them, some proteins that are highly conserved along the eukaryotic evolutionary tree are available on the genome sequences for several Leishmania species and are important in genome organization of Leishmania species [39,40]. The presence of all Moroccan Leishmania nosogeographic species proteins in P. papatasi could be explained by the overlapping of three Leishmania spp. in Morocco, which renders the Moroccan leishmaniasis epidemiological profile as inaccurate. Effectively, L. tropica has been recorded in endemic L. major foci [41,42], and visceral (VL) leishmaniasis cases have been found in established foci of zoonotic cutaneous leishmaniasis ZCL in Morocco [43]. The presence of viscerotropic L. tropica antigen protein could stem from the presence of zymodeme (L. tropica-279) which is responsible for canine VL in Morocco [44]. Since there is no evidence that P. papatasi is involved in the transmission of any Leishmania species other than L. major [44], several experimental studies demonstrated that P. papatasi feeding on lesions or through a membrane will support the full growth and development of L. major, but not of any other Leishmania species [45,46]. They noted a high resistance of sand-fly vectors to various species of Leishmania with an increase in ingested parasites [45,47].
Moroccan populations of sandflies are not only vectors of protozoa, but also viruses. Es-Sette et al. [27] identified the Toscana virus and its distribution in our country [5]. In central Morocco, antibodies against the Naples virus and Sicilian virus have been observed in human populations living in this part of Morocco [11]. Interestingly, antibodies of these two viruses were detected in areas where P. papatasi is present and abundant [48], raising the question of the potential incrimination of P. papatasi in the transmission of those viruses. Seemingly, our proteomic analysis for phlebovirus detection revealed a low number of identified peptides; for example, the polymerase SFSV protein was recorded in Zagora (Z3) and Marrakech (NE). Also, some emerging tick-borne viruses [49,50,51] such as SFTSV glycoprotein detected in Imintanout (Z2), Zagora (Z3) and Marrakech (NE) and Errachidia (Z1), Zagora (Z3) and Marrakech (NE) for UUKV nucleocapsid protein were identified. Lower numbers of identified peptides could be due to RNA degradation, while P. papatasi morphological identification was performed prior to proteomics analysis [52].
Unlike our findings in the phlebovirus proteomic screening, a substantial number of identified proteins indicated the presence of nematodes (Figure 1). This important discovery prompted us to conduct microscopic examinations of Phlebotomus papatasi and other sand fly species collected from various localities. Entomoparasitic nematodes of phlebotomine sand flies have been reported in several regions, including Pakistan, Saudi Arabia, Afghanistan, and Portugal [22,53,54,55,56,57,58], but until now, they have not been observed in Africa. Notably, a Tetradonematidae nematode was reported infecting Lutzomyia longipalpis in a laboratory colony maintained at the National Institute of Health in Bogotá, Colombia, and was also detected in P. sergenti and P. ariasi in Portugal [55,56]. In our study, we examined 1752 sand fly specimens from four localities for nematode presence. One female P. sergenti specimen was found infected at the Imintanout locality, a known endemic focus for cutaneous leishmaniasis caused by Leishmania tropica in Morocco. Morphological analysis identified the nematodes as Didilia spp. (Tetradonematidae) [22,23,24]. This finding represents the first report of Didilia spp. in sand flies from North Africa and indeed from the African continent. A limitation of our study is the lack of molecular identification of the nematodes detected in P. sergenti.
While proteomic and microscopic analyses provided strong evidence for the presence of nematodes, molecular confirmation would be necessary to validate species identity and to further explore their epidemiological significance.
5. Conclusions
This study represents the first application of LC-MS/MS-based proteomics to identify pathogen-related proteins in wild-caught Phlebotomus papatasi from Morocco. Proteins associated with Leishmania, phleboviruses, and nematodes were detected, providing molecular evidence of co-circulating pathogens in sand fly populations. Additionally, microscopic analysis revealed the presence of Tetranematidae, Didilia spp. in the same region endemic for leishmaniasis, marking the first report of nematode infections in sand flies from the African continent.
These findings highlight the complex pathogen diversity harbored by Moroccan sand fly populations and underscore the need for further investigation into their potential role as vectors of multiple parasites beyond Leishmania.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/pathogens14101012/s1, File S1: Detailed spectra, peptide reports, and sample replicates from each locality; File S2: UniProt analysis results.
Author Contributions
M.O., A.B., S.B. and M.D. were conceived the project, M.D., G.D., M.N. and S.B. were performing the experiment. M.D., M.B., G.D., M.N., S.B., M.H., M.O. and A.B. were involved in data analysis writing and reviewing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by M.O. and is supported by the Canadian Institute of Health Research (CIHR; Grant PJT308 159765) and the Natural Sciences and Engineering Research Council of Canada (NSERC; Discovery Grant RGPIN-2018-03849).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All required data are available in the manuscript. Any additional data can be provided upon request.
Acknowledgments
The authors want to thank Denis Faubert from the Insitut de Recherche Clinique de Montréal for the access to the proteomic facility.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Galati, E.A.B.; de Andrade, A.J.; Perveen, F.; Loyer, M.; Vongphayloth, K.; Randrianambinintsoa, F.J.; Prudhomme, J.; Rahola, N.; Akhoundi, M.; Shimabukuro, P.H.F.; et al. Phlebotomine sand flies (Diptera: Psychodidae) of the world. Parasites Vectors 2025, 18, 220. [Google Scholar] [CrossRef]
- Tsirigotakis, N.; Pavlou, C.; Christodoulou, V.; Dokianakis, E.; Kourouniotis, C.; Alten, B.; Antoniou, M. Phlebotomine sand flies (Diptera: Psychodidae) in the Greek Aegean Islands: Ecological approaches. Parasites Vectors 2018, 11, 97. [Google Scholar] [CrossRef]
- Cecílio, P.; Cordeiro-da-Silva, A.; Oliveira, F. Sand flies: Basic information on the vectors of leishmaniasis and their interactions with Leishmania parasites. Commun. Biol. 2022, 5, 305. [Google Scholar] [CrossRef]
- Daoudi, M.; Boussaa, S.; Hafidi, M.; Boumezzough, A. Potential distributions of phlebotomine sand fly vectors of human visceral leishmaniasis caused by Leishmania infantum in Morocco. Med. Vet. Entomol. 2020, 34, 385–393. [Google Scholar] [CrossRef]
- Daoudi, M.; Calzolari, M.; Boussaa, S.; Bonilauri, P.; Torri, D.; Romeo, G.; Lelli, D.; Lavazza, A.; Hafidi, M.; Dottori, M.; et al. Identification of Toscana virus in natural population of sand flies (Diptera: Psychodidae) from Moroccan leishmaniasis foci. J. Infect. Public Health 2022, 15, 406–411. [Google Scholar] [CrossRef] [PubMed]
- Hamarsheh, O.; Guernaoui, S.; Karakus, M.; Yaghoobi-Ershadi, M.R.; Kruger, A.; Amro, A.; Kenawy, M.A.; Dokhan, M.R.; Shoue, D.A.; McDowell, M.A. Population structure analysis of Phlebotomus papatasi populations using transcriptome microsatellites: Possible implications for leishmaniasis control and vaccine development. Parasites Vectors 2024, 17, 410. [Google Scholar] [CrossRef] [PubMed]
- Boussaa, S.; Neffa, M.; Pesson, B.; Boumezzough, A. Phlebotomine sandflies (Diptera: Psychodidae) of southern Morocco: Results of entomological surveys along the Marrakech-Ouarzazat and Marrakech-Azilal roads. Ann. Trop. Med. Parasitol. 2010, 104, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Boussaa, S.; Kahime, K.; Samy, A.M.; Salem, A.B.; Boumezzough, A. Species composition of sand flies and bionomics of Phlebotomus papatasi and P. sergenti (Diptera: Psychodidae) in cutaneous leishmaniasis endemic foci, Morocco. Parasites Vectors 2016, 9, 60. [Google Scholar] [CrossRef]
- Karmaoui, A.; Ben Salem, A.; Sereno, D.; El Jaafari, S.; Hajji, L. Geographic distribution of Meriones shawi, Psammomys obesus, and Phlebotomus papatasi, the main reservoirs and principal vector of zoonotic cutaneous leishmaniasis in the Middle East and North Africa. Parasite Epidemiol. Control 2022, 17, e00247. [Google Scholar] [CrossRef]
- Ayhan, N.; Charrel, R.N. Emergent Sand Fly–Borne Phleboviruses in the Balkan Region. Emerg. Infect. Dis. 2018, 24, 2324–2330. [Google Scholar] [CrossRef]
- Sellali, S.; Lafri, I.; Garni, R.; Manseur, H.; Besbaci, M.; Lafri, M.; Bitam, I. Epidemiology of sandfly-borne phleboviruses in North Africa: An overview. Insects 2024, 15, 846. [Google Scholar] [CrossRef] [PubMed]
- Srinvasan, R.; Panicker, K.N.; Dhanda, V. Occurrence of entomophilic nematode infestation among phlebotomid sandfly, Phlebotomus papatasi—A preliminary report. J. Commun. Dis. 1992, 24, 8–11. [Google Scholar] [PubMed]
- Karmaoui, A. Seasonal Distribution of Phlebotomus papatasi, Vector of Zoonotic Cutaneous Leishmaniasis. Acta Parasitol. 2020, 65, 585–598. [Google Scholar] [CrossRef] [PubMed]
- Iguermia, S.; Harmouche, T.; Mikou, O.; Amarti, A.; Mernissi, F.Z. Mucocutaneous leishmaniasis in Morocco, evidence of the parasite’s ecological evolution? Med. Mal. Infect. 2011, 41, 47–48. [Google Scholar] [CrossRef]
- Boussaa, S.; Guernaoui, S.; Pesson, B.; Boumezzough, A. Seasonal fluctuations of phlebotomine sand fly populations (Diptera: Psychodidae) in the urban area of Marrakech, Morocco. Acta Trop. 2005, 95, 86–91. [Google Scholar] [CrossRef]
- Lema, N.K.; Gemeda, M.T.; Woldesemayat, A.A. Recent Advances in Metagenomic Approaches, Applications, and Challenge. Curr. Microbiol. 2023, 80, 347. [Google Scholar] [CrossRef]
- Kocher, A.; Gantier, J.C.; Gaborit, P.; Zinger, L.; Holota, H.; Valiere, S.; Dusfour, I.; Girod, R.; Bañuls, A.L.; Murienne, J. Vector soup: High-throughput identification of Neotropical phlebotomine sand flies using metabarcoding. Mol. Ecol. Resour. 2017, 17, 172–182. [Google Scholar] [CrossRef]
- Atayde, V.D.; da Silva Lira Filho, A.; Chaparro, V.; Zimmermann, A.; Martel, C.; Jaramillo, M.; Olivier, M. Exploitation of the Leishmania exosomal pathway by Leishmania RNA virus 1. Nat. Microbiol. 2019, 4, 714–723. [Google Scholar] [CrossRef]
- Arike, L.; Peil, L. Spectral Counting Label-Free Proteomics. In Shotgun Proteomics; Martins-de-Souza, D., Ed.; Humana Press: New York, NY, USA, 2014; Volume 1156. [Google Scholar]
- Pratlong, F.; Rioux, J.A.; Dereure, J.; Mahjour, J.; Gallego, M.; Guilvard, E.; Lanotte, G.; Perieres, J.; Martini, A.; Saddiki, A. Leishmania tropica in Morocco. IV–Intrafocal enzyme diversity. Ann. Parasitol. Hum. Comp. 1991, 66, 100–104. [Google Scholar] [CrossRef]
- Boussaa, S. Épidémiologie des leishmanioses dans la région de Marrakech, Maroc: Effet de l’urbanisation sur la répartition spatio-temporelle des Phlébotomes et caractérisation moléculaire de leurs populations. Ph.D. Thesis, Cadi Ayad University, Marrakech, Morocco, 2008. [Google Scholar]
- Killick-Kendrick, R.; Killick-Kendrick, M.; I Nawi, N.A.Q.; Ashford, R.W.; Tang, Y. Preliminary Observations on a Tetradonematid Nematode of Phlebotomine Sandflies of Afghanistan. Ann. Parasitol. Hum. Comp. 1989, 64, 332–339. [Google Scholar] [CrossRef]
- Tang, Y.; Hominick, W.M.; Killick-Kendrick, R.; Killick-Kendrick, M.; Page, A.M. Didilia ooglypta n. gen., n. sp. (Tetradonematidae: Mermithoidea: Nematoda), a Parasite of Phlebotomine Sandflies in Afghanistan. Fundam. Appl. Nematol. 1993, 16, 325–331. [Google Scholar]
- Tang, Y.; Killick-Kendrick, R.; Hominick, W.M. Life Cycle of Didilia ooglypta (Nematoda: Tetradonematidae), a Parasite of Phlebotomine Sandflies of Afghanistan. Nematologica 1997, 43, 491–503. [Google Scholar] [CrossRef]
- Al-Koleeby, Z.; El Aboudi, A.; Aboulfadl, S.; Faraj, C. Diversity and bionomics of sandflies (Diptera: Psychodidae) of an endemic focus of cutaneous leishmaniasis in Zagora Province, southeast of Morocco. J. Parasitol. Res. 2021, 2021, 8812691. [Google Scholar] [CrossRef] [PubMed]
- Mhaidi, I.; El Kacem, S.; Ait Kbaich, M.; El Hamouchi, A.; Sarih, M.; Akrid, K.; Lemrani, M. Molecular identification of Leishmania infection in the most relevant sand fly species and in-patient samples from a cutaneous leishmaniasis focus in Morocco. PLoS Negl. Trop. Dis. 2018, 12, e0006315. [Google Scholar] [CrossRef]
- Es-Sette, N.; Ajaoud, M.; Laamrani-Idrissi, A.; Mellouki, F.; Lemrani, M. Molecular detection and identification of Leishmania infection in naturally infected flies in a focus of cutaneous leishmaniasis in northern Morocco. Parasite Vectors 2014, 7, 305. [Google Scholar] [CrossRef]
- Morales, M.A.; Watanabe, R.; Dacher, M.; Chafey, P.; Osorio y Fortéa, J.; Scott, D.A.; Beverley, S.M.; Ommen, G.; Clos, J.; Hem, S.; et al. Phosphoproteome dynamics reveal heat-shock protein complexes specific to the Leishmania donovani infectious stage. Proc. Natl. Acad. Sci. USA 2010, 107, 8381–8386. [Google Scholar] [CrossRef]
- Corrales, R.M.; Vincent, J.; Crobu, L.; Neish, R.; Nepal, B.; Espeut, J.; Pasquier, G.; Gillard, G.; Cazevieille, C.; Mottram, J.C.; et al. Tubulin detyrosination shapes Leishmania cytoskeletal architecture and virulence. Proc. Natl. Acad. Sci. USA 2025, 122, e2415296122. [Google Scholar] [CrossRef]
- Burge, R.J.; Damianou, A.; Wilkinson, A.J.; Rodenko, B.; Mottram, J.C. Leishmania differentiation requires ubiquitin conjugation mediated by a UBC2-UEV1 E2 complex. PLoS Pathog. 2020, 16, e1008784. [Google Scholar] [CrossRef]
- Torrecilhas, A.C.; Schumacher, R.I.; Alves, M.J.M.; Colli, W. Vesicles as carriers of virulence factors in parasitic protozoan diseases. Microbes Infect. 2012, 14, 1465–1474. [Google Scholar] [CrossRef]
- Chin, D.; Means, A.R. Calmodulin: A prototypical calcium sensor. Trends Cell Biol. 2000, 10, 322–328. [Google Scholar] [CrossRef]
- Zhang, M.; Abrams, C.; Wang, L.; Gizzi, A.; He, L.; Lin, R.; Chen, Y.; Loll, P.J.; Pascal, J.M.; Zhang, J.-F. Structural basis for calmodulin as a dynamic calcium sensor. Structure 2012, 20, 911–923. [Google Scholar] [CrossRef] [PubMed]
- Tasbihi, M.; Shekari, F.; Hajjaran, H.; Masoori, L.; Hadighi, R. Mitochondrial proteome profiling of Leishmania tropica. Microb. Pathog. 2019, 133, 103542. [Google Scholar] [CrossRef] [PubMed]
- Fraga, J.; Montalvo, A.M.; De Doncker, S.; Dujardin, J.C.; Van der Auwera, G. Phylogeny of Leishmania species based on the heat shock protein 70 gene. Infect. Genet. Evol. 2010, 10, 238–245. [Google Scholar] [CrossRef] [PubMed]
- Nugent, P.G.; Karsani, S.A.; Wait, R.; Tempero, J.; Smith, D.F. Proteomic analysis of Leishmania mexicana differentiation. Mol. Biochem. Parasitol. 2004, 136, 51–62. [Google Scholar] [CrossRef]
- Pinho, N.; Wiśniewski, J.R.; Dias-Lopes, G.; Saboia-Vahia, L.; Bombaça, A.C.S.; Mesquita-Rodrigues, C.; Menna-Barreto, R.; Cupolillo, E.; de Jesus, J.B.; Padrón, G.; et al. In-depth quantitative proteomics uncovers species-specific metabolic programs in Leishmania (Viannia) species. PLoS Negl. Trop. Dis. 2020, 14, e0008509. [Google Scholar] [CrossRef]
- Yao, C.; Li, Y.; Donelson, J.E.; Wilson, M.E. Proteomic examination of Leishmania chagasi plasma membrane proteins: Contrast between avirulent and virulent (metacyclic) parasite forms. Proteom. Clin. Appl. 2010, 4, 4–16. [Google Scholar] [CrossRef]
- Menezes, J.P.; Almeida, T.F.; Petersen, A.L.; Guedes, C.E.; Mota, M.S.; Lima, J.G.; Palma, L.C.; Buck, G.A.; Krieger, M.A.; Probst, C.M.; et al. Proteomic analysis reveals differentially expressed proteins in macrophages infected with Leishmania amazonensis or Leishmania major. Microbes Infect. 2013, 15, 579–591. [Google Scholar] [CrossRef]
- Chiheb, S.; Slaoui, W.; Mouttaqui, T.; Riyad, M.; Benchikhi, H. Les leishmanioses cutanées à Leishmania major et à Leishmania tropica au Maroc: Aspects épidémio-cliniques comparatifs de 268 cas [Cutaneous leishmaniasis by Leishmania major and Leishmania tropica in Morocco: Comparative epidemioclinical aspects of 268 cases]. Pan Afr. Med. J. 2014, 19, 160. [Google Scholar]
- Kbaich, M.A.; Mhaidi, I.; Ezzahidi, A.; Dersi, N.; El Hamouchi, A.; Riyad, M.; Akarid, K.; Lemrani, M. New epidemiological pattern of cutaneous leishmaniasis in two pre-Saharan arid provinces, southern Morocco. Acta Trop. 2017, 173, 11–16. [Google Scholar] [CrossRef]
- Dereure, J.; Velez, I.D.; Pratlong, F.; Denial, M.; Lardi, M.; Moreno, G.; Serres, E.; Lanotte, G.; Rioux, J.P. La leishmaniose viscérale autochtone au Maroc méridional: Présence de Leishmania infantum MON-1 chez le Chien en zone présaharienne. Leishmania Taxonomie et phylogenèse Application éco-épidémiologique (Coll int CNRS/INSERM, 1984); IMEEE: Montpellier, France, 1986; pp. 421–425. [Google Scholar]
- Rhajaoui, M.; Fellah, H.; Pratlong, F.; Dedet, J.; Lyagoubi, M. Leishmaniasis due to Leishmania tropica MON-102 in a new Moroccan focus. Trans. R. Soc. Trop. Med. Hyg. 2004, 98, 299–301. [Google Scholar] [CrossRef]
- Pimenta, P.F.; Saraiva, E.M.; Rowton, E.; Modi, G.B.; Garraway, L.A.; Beverley, S.M.; Turco, S.J.; Sacks, D.L. Evidence that the vectorial competence of phlebotomine sand flies for different species of Leishmania is controlled by structural polymorphisms in the surface lipophosphoglycan. Proc. Natl. Acad. Sci. USA 1994, 91, 9155–9159. [Google Scholar] [CrossRef]
- Dobson, D.E.; Kamhawi, S.; Lawyer, P.; Turco, S.J.; Beverley, S.M.; Sacks, D.L. Leishmania major survival in selective Phlebotomus papatasi sand fly vector requires a specific SCG-encoded lipophosphoglycan galactosylation pattern. PLoS Pathog. 2010, 6, e1001185. [Google Scholar] [CrossRef]
- Vojtková, B.; Bečvář, T.; Pacáková, L.; Frynta, D.; Mekarnia, N.; Benallal, K.E.; Volf, P.; Sádlová, J. Infectiousness of Leishmania major to Phlebotomus papatasi: Differences between natural reservoir host Meriones shawi and laboratory model BALB/c mice. PLoS Negl. Trop. Dis. 2025, 19, e0013183. [Google Scholar] [CrossRef]
- Volf, P.; Myskova, J. Sand flies and Leishmania: Specific versus permissive vectors. Trends Parasitol. 2007, 23, 91–92. [Google Scholar] [CrossRef] [PubMed]
- Daoudi, M.; Outammassine, A.; Olivier, D.; Amane, M.; Beaulieu, M.; Akarid, A.; Ndao, M.; Hafidi, M.; Boussaa, S.; Boumezzough, A. Modeling the Impact of Climate Change for the Potential Distribution of the Main Vector and Reservoirs of Zoonotic Cutaneous Leishmaniasis due to Leishmania major in Morocco. Front. Trop. Dis. 2025, 6, 1629454. [Google Scholar] [CrossRef]
- Yu, X.J.; Liang, M.F.; Zhang, S.Y.; Liu, Y.; Li, J.D.; Sun, Y.L.; Zhang, L.; Zhang, Q.-F.; Popov, V.L.; Li, C.; et al. Fever with thrombocytopenia associated with a novel bunyavirus in China. N. Engl. J. Med. 2011, 364, 1523–1532. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.H.; Yi, J.; Kim, G.; Choi, S.J.; Jun, K.I.; Kim, N.H.; Choe, P.G.; Kim, N.-J.; Lee, J.-K.; Oh, M.-D. Severe fever with thrombocytopenia syndrome, South Korea. Emerg. Infect. Dis. 2012, 19, 1892–1894. [Google Scholar]
- Takahashi, T.; Maeda, K.; Suzuki, T.; Ishido, A.; Shigeoka, T.; Tominaga, T.; Kamei, T.; Honda, M.; Ninomiya, D.; Sakai, T.; et al. The first identification and retrospective study of severe fever with thrombocytopenia syndrome in Japan. J. Infect. Dis. 2014, 209, 816–827. [Google Scholar] [CrossRef]
- Ayhan, N.; Baklouti, A.; Prudhomme, J.; Walder, G.; Amaro, F.; Alten, B.; Moutailler, S.; Ergunay, K.; Charrel, R.N.; Huemer, H. Practical guidelines for studies on sandfly-borne phleboviruses: Part I—Important points to consider ante field work. Vector-Borne Zoonotic Dis. 2017, 17, 73–80. [Google Scholar] [CrossRef]
- Poinar, G.O. Entomogenous Nematodes; E.J. Brill: Leiden, The Netherlands, 1975; p. 317. [Google Scholar]
- Lutz, A.; Neiva, A. Contribuição para o conhecimento das espécies do gênero Phlebotomus existentes no Brasil. Mem. Inst. Oswaldo Cruz 1912, 4, 84–95. [Google Scholar] [CrossRef]
- Ribeiro, H.; Fernandes, T.; Candeias, C. Primeiro registo da ocorrência de um nematode endoparasita em flebotomos de Portugal. Bol. Soc. Port. Entomol. 1994, 18, 377–382. [Google Scholar]
- Pires, C.A.; Tang, Y.; Killick-Kendrick, R. Didilia sp. (Tetradonematidae: Mermithoidea: Nematoda) a parasite of Phlebotomus sergenti in Portugal. Parasite 1997, 4, 191–192. [Google Scholar] [CrossRef][Green Version]
- Secundino, N.F.; Araújo, M.S.; Oliveira, G.H.; Massara, C.L.; Carvalho, O.S.; Lanfredi, R.M.; Pimenta, P.F. Preliminary description of a new entomoparasitic nematode infecting Lutzomyia longipalpis sand fly, the vector of visceral leishmaniasis in the New World. J. Invertebr. Pathol. 2002, 80, 35–40. [Google Scholar] [CrossRef]
- Sor-Suwan, S.; Jariyapan, N.; Mano, C.; Apiwathnasorn, C.; Sriwichai, P.; Samung, Y.; Siriyasatien, P.; Bates, P.A.; Somboon, P. Didilia sp. Infecting Phlebotomus stantoni in Thailand. Trop. Biomed. 2017, 34, 956–962. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).