Therapeutic Applications of Aggregatibacter actinomycetemcomitans Leukotoxin
Abstract
1. Introduction
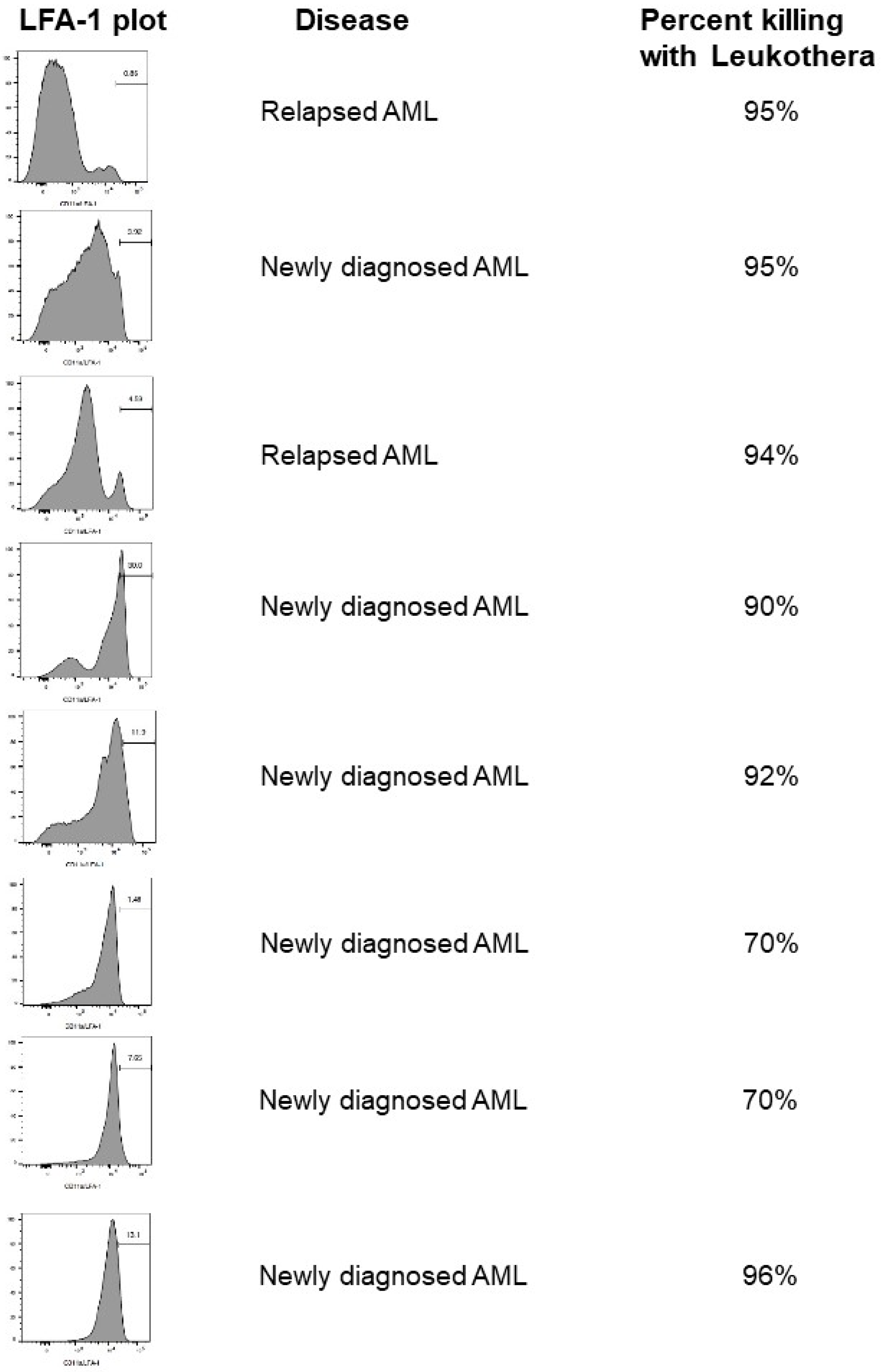
2. Hematologic Malignancies
3. Psoriasis
4. Allergic Asthma
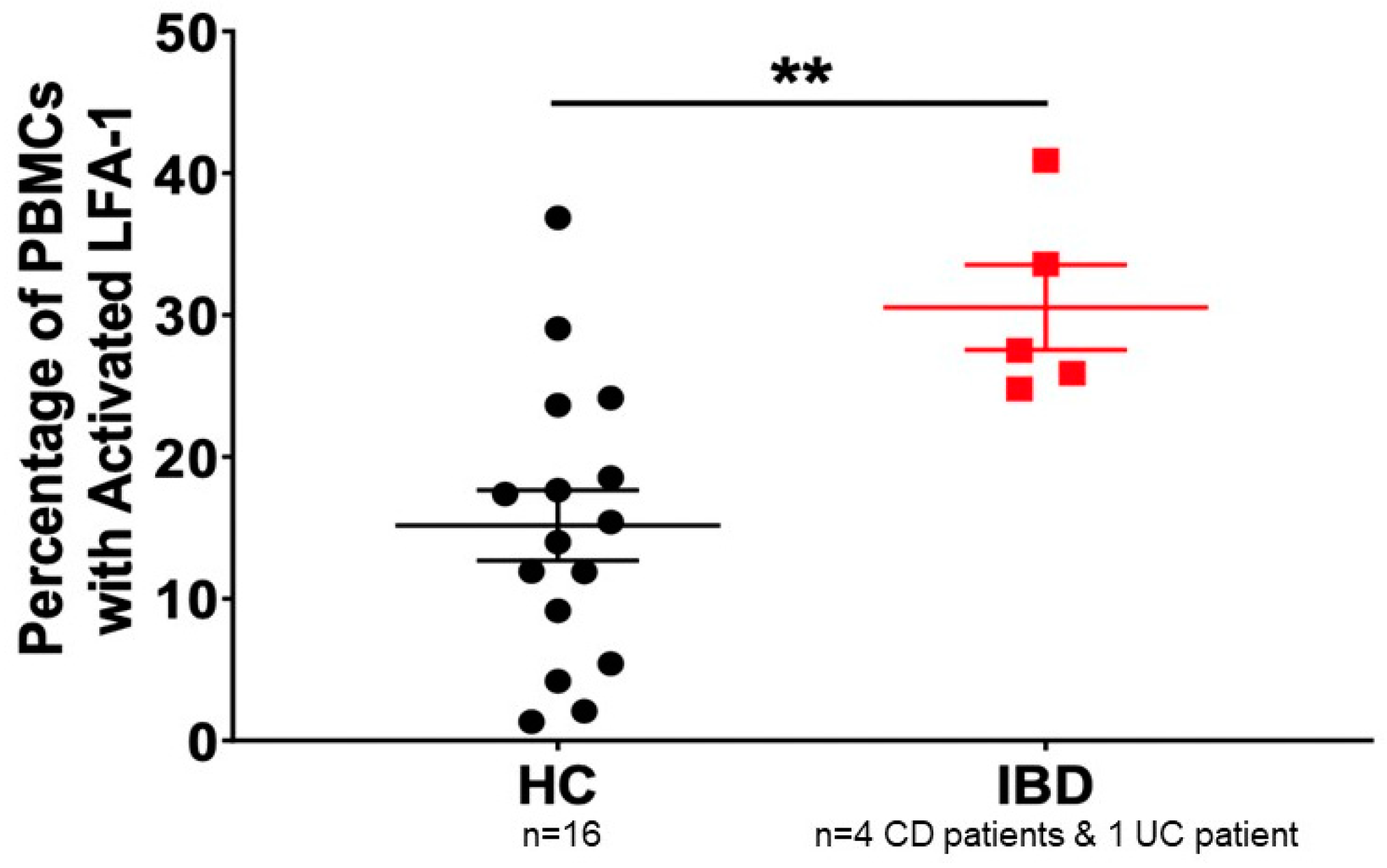
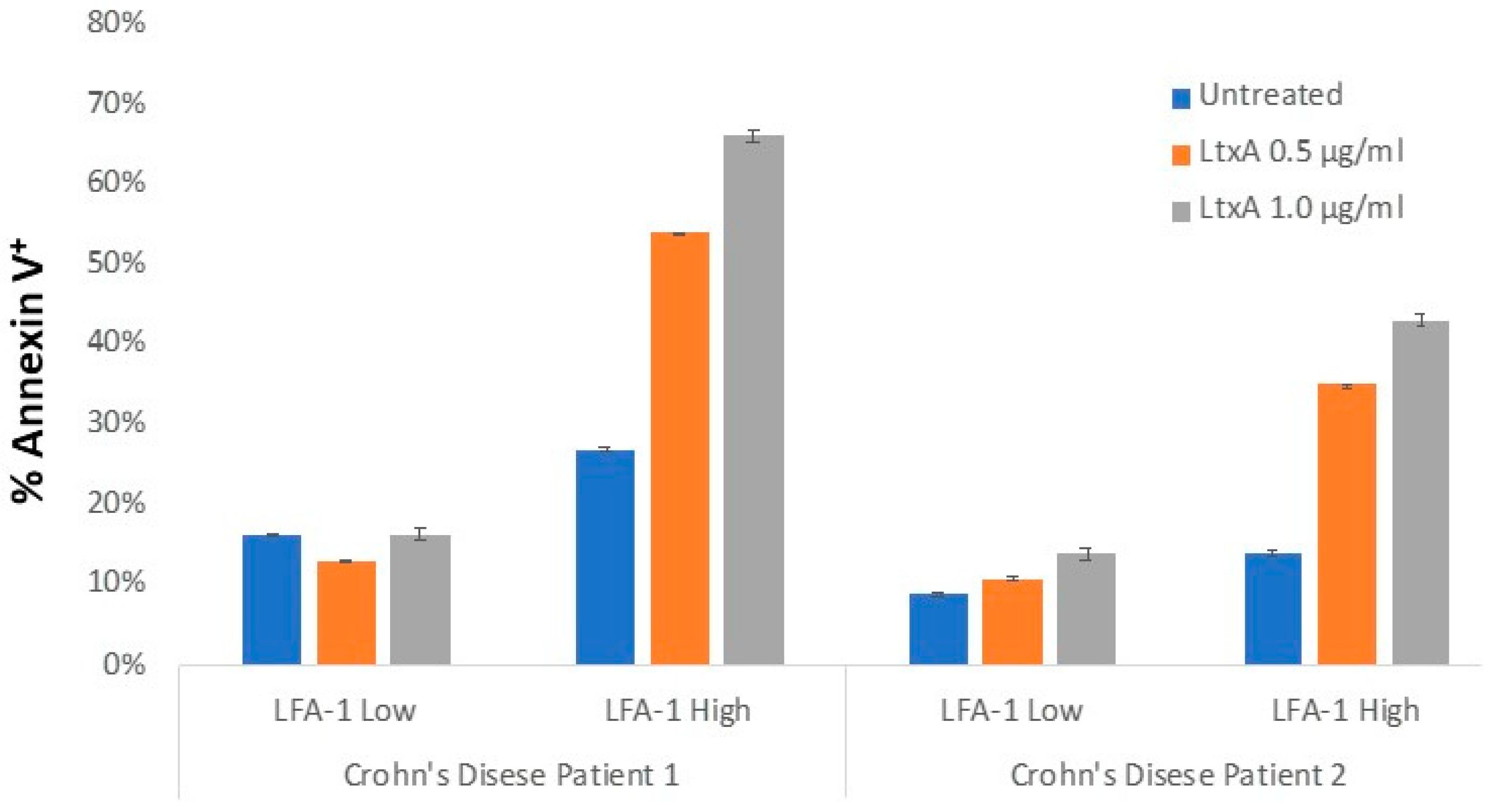
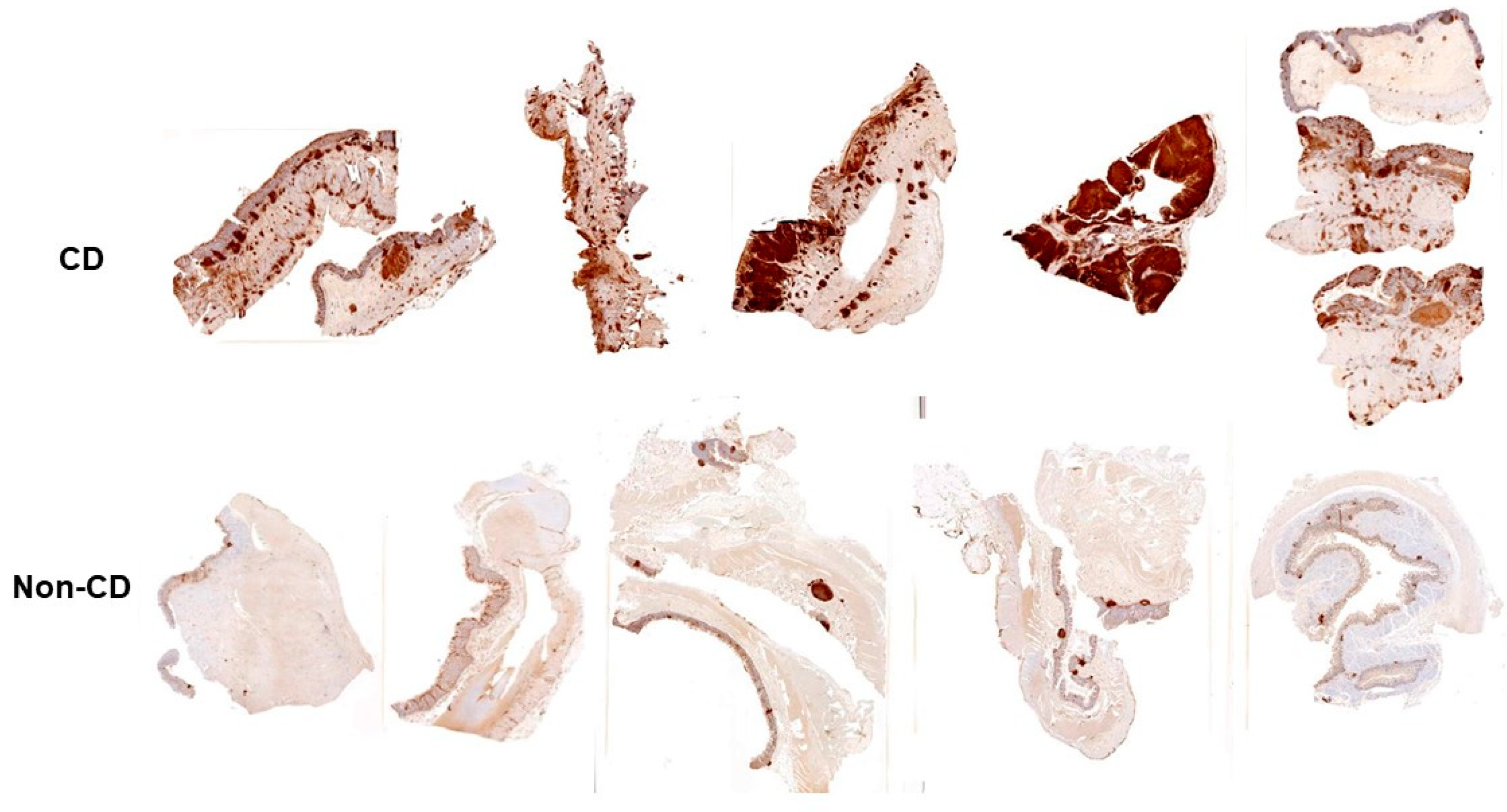
5. Inflammatory Bowel Disease
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Haubek, D.; Ennibi, O.K.; Poulsen, K.; Vaeth, M.; Poulsen, S.; Kilian, M. Risk of aggressive periodontitis in adolescent carriers of the JP2 clone of Aggregatibacter (Actinobacillus) actinomycetemcomitans in Morocco: A prospective longitudinal cohort study. Lancet 2008, 371, 237–242. [Google Scholar] [CrossRef]
- Lally, E.T.; Kieba, I.R.; Demuth, D.R.; Rosenbloom, J.; Golub, E.E.; Taichman, N.S.; Gibson, C.W. Identification and expression of the Actinobacillus actinomycetemcomitans leukotoxin gene. Biochem. Biophys. Res. Commun. 1989, 159, 256–262. [Google Scholar] [CrossRef]
- Lally, E.T.; Kieba, I.R.; Sato, A.; Green, C.L.; Rosenbloom, J.; Korostoff, J.; Wang, J.F.; Shenker, B.J.; Ortlepp, S.; Robinson, M.K.; et al. RTX toxins recognize a beta2 integrin on the surface of human target cells. J. Biol. Chem. 1997, 272, 30463–30469. [Google Scholar] [CrossRef]
- Evans, R.; Patzak, I.; Svensson, L.; De Filippo, K.; Jones, K.; McDowall, A.; Hogg, N. Integrins in immunity. J. Cell Sci. 2009, 122, 215–225. [Google Scholar] [CrossRef]
- Hogg, N.; Harvey, J.; Cabanas, C.; Landis, R.C. Control of leukocyte integrin activation. Am. Rev. Respir. Dis. 1993, 148, S55–S59. [Google Scholar] [CrossRef]
- Smith, A.; Stanley, P.; Jones, K.; Svensson, L.; McDowall, A.; Hogg, N. The role of the integrin LFA-1 in T-lymphocyte migration. Immunol. Rev. 2007, 218, 135–146. [Google Scholar] [CrossRef]
- Tsai, C.C.; McArthur, W.P.; Baehni, P.C.; Hammond, B.F.; Taichman, N.S. Extraction and partial characterization of a leukotoxin from a plaque-derived Gram-negative microorganism. Infect. Immun. 1979, 25, 427–439. [Google Scholar] [CrossRef]
- Tsai, C.C.; Shenker, B.J.; DiRienzo, J.M.; Malamud, D.; Taichman, N.S. Extraction and isolation of a leukotoxin from Actinobacillus actinomycetemcomitans with polymyxin B. Infect. Immun. 1984, 43, 700–705. [Google Scholar] [CrossRef]
- DiRienzo, J.M.; Tsai, C.C.; Shenker, B.J.; Taichman, N.S.; Lally, E.T. Monoclonal antibodies to leukotoxin of Actinobacillus actinomycetemcomitans. Infect. Immun. 1985, 47, 31–36. [Google Scholar] [CrossRef]
- Lally, E.T.; Golub, E.E.; Kieba, I.R.; Taichman, N.S.; Decker, S.; Berthold, P.; Gibson, C.W.; Demuth, D.R.; Rosenbloom, J. Structure and function of the B and D genes of the Actinobacillus actinomycetemcomitans leukotoxin complex. Microb. Pathog. 1991, 11, 111–121. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Secretion of RTX leukotoxin by Actinobacillus actinomycetemcomitans. Infect. Immun. 2000, 68, 6094–6100. [Google Scholar] [CrossRef]
- Kachlany, S.C.; Fine, D.H.; Figurski, D.H. Purification of secreted leukotoxin (LtxA) from Actinobacillus actinomycetemcomitans. Protein Expr. Purif. 2002, 25, 465–471. [Google Scholar] [CrossRef]
- Berthold, P.; Forti, D.; Kieba, I.R.; Rosenbloom, J.; Taichman, N.S.; Lally, E.T. Electron immunocytochemical localization of Actinobacillus actinomycetemcomitans leukotoxin. Oral Microbiol. Immunol. 1992, 7, 24–27. [Google Scholar] [CrossRef]
- Kato, S.; Kowashi, Y.; Demuth, D.R. Outer membrane-like vesicles secreted by Actinobacillus actinomycetemcomitans are enriched in leukotoxin. Microb. Pathog. 2002, 32, 1–13. [Google Scholar] [CrossRef]
- Ohta, H.; Kato, K.; Kokeguchi, S.; Hara, H.; Fukui, K.; Murayama, Y. Nuclease-sensitive binding of an Actinobacillus actinomycetemcomitans leukotoxin to the bacterial cell surface. Infect. Immun. 1991, 59, 4599–4605. [Google Scholar] [CrossRef]
- Hogg, N.; Smith, A.; McDowall, A.; Giles, K.; Stanley, P.; Laschinger, M.; Henderson, R. How T cells use LFA-1 to attach and migrate. Immunol. Lett. 2004, 92, 51–54. [Google Scholar] [CrossRef]
- Giblin, P.A.; Lemieux, R.M. LFA-1 as a key regulator of immune function: Approaches toward the development of LFA-1-based therapeutics. Curr. Pharm. Des. 2006, 12, 2771–2795. [Google Scholar] [CrossRef]
- Ma, Q.; Shimaoka, M.; Lu, C.; Jing, H.; Carman, C.V.; Springer, T.A. Activation-induced conformational changes in the I domain region of lymphocyte function-associated antigen 1. J. Biol. Chem. 2002, 277, 10638–10641. [Google Scholar] [CrossRef]
- Dransfield, I.; Cabanas, C.; Barrett, J.; Hogg, N. Interaction of leukocyte integrins with ligand is necessary but not sufficient for function. J. Cell Biol. 1992, 116, 1527–1535. [Google Scholar] [CrossRef] [PubMed]
- Dransfield, I.; Cabanas, C.; Craig, A.; Hogg, N. Divalent cation regulation of the function of the leukocyte integrin LFA-1. J. Cell Biol. 1992, 116, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Porter, J.C.; Bracke, M.; Smith, A.; Davies, D.; Hogg, N. Signaling through integrin LFA-1 leads to filamentous actin polymerization and remodeling, resulting in enhanced T cell adhesion. J. Immunol. 2002, 168, 6330–6335. [Google Scholar] [CrossRef] [PubMed]
- Hioe, C.E.; Tuen, M.; Vasiliver-Shamis, G.; Alvarez, Y.; Prins, K.C.; Banerjee, S.; Nadas, A.; Cho, M.W.; Dustin, M.L.; Kachlany, S.C. HIV envelope gp120 activates LFA-1 on CD4 T-lymphocytes and increases cell susceptibility to LFA-1-targeting leukotoxin (LtxA). PLoS ONE 2011, 6, e23202. [Google Scholar] [CrossRef] [PubMed]
- Kachlany, S.C.; Schwartz, A.B.; Balashova, N.V.; Hioe, C.E.; Tuen, M.; Le, A.; Kaur, M.; Mei, Y.; Rao, J. Anti-leukemia activity of a bacterial toxin with natural specificity for LFA-1 on white blood cells. Leuk. Res. 2010, 34, 777–785. [Google Scholar] [CrossRef] [PubMed]
- DiFranco, K.M.; Gupta, A.; Galusha, L.E.; Perez, J.; Nguyen, T.V.; Fineza, C.D.; Kachlany, S.C. Leukotoxin (Leukothera(R)) targets active leukocyte function antigen-1 (LFA-1) protein and triggers a lysosomal mediated cell death pathway. J. Biol. Chem. 2012, 287, 17618–17627. [Google Scholar] [CrossRef] [PubMed]
- DiFranco, K.M.; Kaswala, R.H.; Patel, C.; Kasinathan, C.; Kachlany, S.C. Leukotoxin kills rodent WBC by targeting leukocyte function associated antigen 1. Comp. Med. 2013, 63, 331–337. [Google Scholar] [PubMed]
- Kaur, M.; Kachlany, S.C. Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera) induces cofilin dephosphorylation and actin depolymerization during killing of malignant monocytes. Microbiology 2014, 160, 2443–2452. [Google Scholar] [CrossRef] [PubMed]
- DiFranco, K.M.; Johnson-Farley, N.; Bertino, J.R.; Elson, D.; Vega, B.A.; Belinka, B.A., Jr.; Kachlany, S.C. LFA-1-targeting Leukotoxin (LtxA; Leukothera(R)) causes lymphoma tumor regression in a humanized mouse model and requires caspase-8 and Fas to kill malignant lymphocytes. Leuk. Res. 2015, 39, 649–656. [Google Scholar] [CrossRef] [PubMed]
- Vega, B.A.; Schober, L.T.; Kim, T.; Belinka, B.A., Jr.; Kachlany, S.C. Aggregatibacter actinomycetemcomitans leukotoxin (LtxA; Leukothera(R)) requires death receptor Fas, in addition to LFA-1, to trigger cell death in T lymphocytes. Infect. Immun. 2019, 87, 10–1128. [Google Scholar] [CrossRef] [PubMed]
- Prince, D.J.; Patel, D.; Kachlany, S.C. Leukotoxin (LtxA/Leukothera) induces ATP expulsion via pannexin-1 channels and subsequent cell death in malignant lymphocytes. Sci. Rep. 2021, 11, 18086. [Google Scholar] [CrossRef]
- Hasegawa, Y.; Yokono, K.; Taki, T.; Amano, K.; Tominaga, Y.; Yoneda, R.; Yagi, N.; Maeda, S.; Yagita, H.; Okumura, K.; et al. Prevention of autoimmune insulin-dependent diabetes in non-obese diabetic mice by anti-LFA-1 and anti-ICAM-1 mAb. Int. Immunol. 1994, 6, 831–838. [Google Scholar] [CrossRef]
- McMurray, R.W. Adhesion molecules in autoimmune disease. Semin. Arthritis Rheum. 1996, 25, 215–233. [Google Scholar] [CrossRef] [PubMed]
- Yokomori, H.; Oda, M.; Yoshimura, K.; Nomura, M.; Ogi, M.; Wakabayashi, G.; Kitajima, M.; Ishii, H. Expression of intercellular adhesion molecule-1 and lymphocyte function-associated antigen-1 protein and messenger RNA in primary biliary cirrhosis. Intern. Med. 2003, 42, 947–954. [Google Scholar] [CrossRef] [PubMed]
- Connolly, M.K.; Kitchens, E.A.; Chan, B.; Jardieu, P.; Wofsy, D. Treatment of murine lupus with monoclonal antibodies to lymphocyte function-associated antigen-1: Dose-dependent inhibition of autoantibody production and blockade of the immune response to therapy. Clin. Immunol. Immunopathol. 1994, 72, 198–203. [Google Scholar] [CrossRef] [PubMed]
- Elovaara, I.; Lalla, M.; Spare, E.; Lehtimaki, T.; Dastidar, P. Methylprednisolone reduces adhesion molecules in blood and cerebrospinal fluid in patients with MS. Neurology 1998, 51, 1703–1708. [Google Scholar] [CrossRef]
- Engelhardt, B. Molecular mechanisms involved in T cell migration across the blood-brain barrier. J. Neural Transm. 2006, 113, 477–485. [Google Scholar] [CrossRef] [PubMed]
- Thomson, A.W.; Satoh, S.; Nussler, A.K.; Tamura, K.; Woo, J.; Gavaler, J.; van Thiel, D.H. Circulating intercellular adhesion molecule-1 (ICAM-1) in autoimmune liver disease and evidence for the production of ICAM-1 by cytokine-stimulated human hepatocytes. Clin. Exp. Immunol. 1994, 95, 83–90. [Google Scholar] [CrossRef] [PubMed]
- Yusuf-Makagiansar, H.; Anderson, M.E.; Yakovleva, T.V.; Murray, J.S.; Siahaan, T.J. Inhibition of LFA-1/ICAM-1 and VLA-4/VCAM-1 as a therapeutic approach to inflammation and autoimmune diseases. Med. Res. Rev. 2002, 22, 146–167. [Google Scholar] [CrossRef]
- Aureli, A.; Marziani, B.; Venditti, A.; Sconocchia, T.; Sconocchia, G. Acute Lymphoblastic Leukemia Immunotherapy Treatment: Now, Next, and Beyond. Cancers 2023, 15, 3346. [Google Scholar] [CrossRef]
- Eisfeld, A.K. Disparities in acute myeloid leukemia treatments and outcomes. Curr. Opin. Hematol. 2024, 31, 58–63. [Google Scholar] [CrossRef]
- Mikhael, J.; Cichewicz, A.; Mearns, E.S.; Girvan, A.; Pierre, V.; Rawashdh, N.A.; Yellow-Duke, A.; Cornell, R.F.; Nixon, M. Overall Survival in Patients with Multiple Myeloma in the U.S.: A Systematic Literature Review of Racial Disparities. Clin. Lymphoma Myeloma Leuk. 2024, 24, e1–e12. [Google Scholar] [CrossRef]
- Schimmoeller, C.J.; Bastian, C.; Fleming, J.; Morales, J. A Review of Hodgkin Lymphoma in the Era of Checkpoint Inhibitors. Cureus 2023, 15, e41660. [Google Scholar] [CrossRef] [PubMed]
- Afvari, S.; Beck, T.C.; Kazlouskaya, M.; Afrahim, R.; Valdebran, M. Diet, sleep, and exercise in inflammatory skin diseases. Our Dermatol. Online 2023, 14, 430–435. [Google Scholar] [CrossRef]
- Bhagwat, A.P.; Madke, B. The Current Advancement in Psoriasis. Cureus 2023, 15, e47006. [Google Scholar] [CrossRef]
- Lie, E.; Choi, M.; Wang, S.P.; Eichenfield, L.F. Topical Management of Pediatric Psoriasis: A Review of New Developments and Existing Therapies. Paediatr. Drugs 2024, 26, 9–18. [Google Scholar] [CrossRef] [PubMed]
- Reali, E.; Ferrari, D. From the Skin to Distant Sites: T Cells in Psoriatic Disease. Int. J. Mol. Sci. 2023, 24, 15707. [Google Scholar] [CrossRef]
- Stenderup, K.; Rosada, C.; Dam, T.N.; Salerno, E.; Belinka, B.A.; Kachlany, S.C. Resolution of psoriasis by a leukocyte-targeting bacterial protein in a humanized mouse model. J. Investig. Dermatol. 2011, 131, 2033–2039. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.L.M.; Rothenberg, M.E. 2021 year in review: Spotlight on eosinophils. J. Allergy Clin. Immunol. 2022, 149, 517–524. [Google Scholar] [CrossRef]
- Kim, Y.M.; Kim, Y.S.; Jeon, S.G.; Kim, Y.K. Immunopathogenesis of allergic asthma: More than the th2 hypothesis. Allergy Asthma Immunol. Res. 2013, 5, 189–196. [Google Scholar] [CrossRef]
- Matos-Semedo, F.; Cruz, C.; Inacio, F.; Gama, J.M.R.; Nwaru, B.I.; Taborda-Barata, L. House dust mite (HDM) and storage mite (SM) molecular sensitisation profiles and association with clinical outcomes in allergic asthma and rhinitis: Protocol for a systematic review. BMJ Open 2021, 11, e046519. [Google Scholar] [CrossRef]
- Medeleanu, M.V.; Qian, Y.C.; Moraes, T.J.; Subbarao, P. Early-immune development in asthma: A review of the literature. Cell. Immunol. 2023, 393, 104770. [Google Scholar] [CrossRef]
- Woloski, J.R.; Heston, S.; Escobedo Calderon, S.P. Respiratory Allergic Disorders. Prim. Care 2016, 43, 401–415. [Google Scholar] [CrossRef] [PubMed]
- Gupta, A.; Espinosa, V.; Galusha, L.E.; Rahimian, V.; Miro, K.L.; Rivera-Medina, A.; Kasinathan, C.; Capitle, E.; Aguila, H.A.; Kachlany, S.C. Expression and targeting of lymphocyte function-associated antigen 1 (LFA-1) on white blood cells for treatment of allergic asthma. J. Leukoc. Biol. 2015, 97, 439–446. [Google Scholar] [CrossRef]
- Abdulla, M.; Mohammed, N. A Review on Inflammatory Bowel Diseases: Recent Molecular Pathophysiology Advances. Biol. Targets Ther. 2022, 16, 129–140. [Google Scholar] [CrossRef]
- Imbrizi, M.; Magro, F.; Coy, C.S.R. Pharmacological Therapy in Inflammatory Bowel Diseases: A Narrative Review of the Past 90 Years. Pharmaceuticals 2023, 16, 1272. [Google Scholar] [CrossRef]
- Khan, S.; Sebastian, S.A.; Parmar, M.P.; Ghadge, N.; Padda, I.; Keshta, A.S.; Minhaz, N.; Patel, A. Factors influencing the quality of life in inflammatory bowel disease: A comprehensive review. Dis. Mon. 2023, 70, 101672. [Google Scholar] [CrossRef]
- Mihai, I.R.; Burlui, A.M.; Rezus, I.I.; Mihai, C.; Macovei, L.A.; Cardoneanu, A.; Gavrilescu, O.; Dranga, M.; Rezus, E. Inflammatory Bowel Disease as a Paradoxical Reaction to Anti-TNF-alpha Treatment-A Review. Life 2023, 13, 1779. [Google Scholar] [CrossRef]
- Sousa, P.; Bertani, L.; Rodrigues, C. Management of inflammatory bowel disease in the elderly: A review. Dig. Liver Dis. 2023, 55, 1001–1009. [Google Scholar] [CrossRef] [PubMed]
- Ostanin, D.V.; Furr, K.L.; Pavlick, K.P.; Gray, L.; Kevil, C.G.; Shukla, D.; D’Souza, D.; Hoffman, J.M.; Grisham, M.B. T cell-associated CD18 but not CD62L, ICAM-1, or PSGL-1 is required for the induction of chronic colitis. Am. J. Physiol. Gastrointest. Liver Physiol. 2007, 292, G1706–G1714. [Google Scholar] [CrossRef] [PubMed]
- Pavlick, K.P.; Ostanin, D.V.; Furr, K.L.; Laroux, F.S.; Brown, C.M.; Gray, L.; Kevil, C.G.; Grisham, M.B. Role of T-cell-associated lymphocyte function-associated antigen-1 in the pathogenesis of experimental colitis. Int. Immunol. 2006, 18, 389–398. [Google Scholar] [CrossRef]
- Abdelbaqi, M.; Chidlow, J.H.; Matthews, K.M.; Pavlick, K.P.; Barlow, S.C.; Linscott, A.J.; Grisham, M.B.; Fowler, M.R.; Kevil, C.G. Regulation of dextran sodium sulfate induced colitis by leukocyte beta 2 integrins. Lab. Investig. A J. Tech. Methods Pathol. 2006, 86, 380–390. [Google Scholar] [CrossRef]
- James, D.G.; Seo, D.H.; Chen, J.; Vemulapalli, C.; Stone, C.D. Efalizumab, a human monoclonal anti-CD11a antibody, in the treatment of moderate to severe Crohn’s Disease: An open-label pilot study. Dig. Dis. Sci. 2011, 56, 1806–1810. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kachlany, S.C.; Vega, B.A. Therapeutic Applications of Aggregatibacter actinomycetemcomitans Leukotoxin. Pathogens 2024, 13, 354. https://doi.org/10.3390/pathogens13050354
Kachlany SC, Vega BA. Therapeutic Applications of Aggregatibacter actinomycetemcomitans Leukotoxin. Pathogens. 2024; 13(5):354. https://doi.org/10.3390/pathogens13050354
Chicago/Turabian StyleKachlany, Scott C., and Brian A. Vega. 2024. "Therapeutic Applications of Aggregatibacter actinomycetemcomitans Leukotoxin" Pathogens 13, no. 5: 354. https://doi.org/10.3390/pathogens13050354
APA StyleKachlany, S. C., & Vega, B. A. (2024). Therapeutic Applications of Aggregatibacter actinomycetemcomitans Leukotoxin. Pathogens, 13(5), 354. https://doi.org/10.3390/pathogens13050354





