Parasitic Contamination of Fresh Leafy Green Vegetables Sold in Northern Lebanon
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design and Area
2.2. Sample Collection
2.3. Sample Processing
2.4. Statistical Analysis
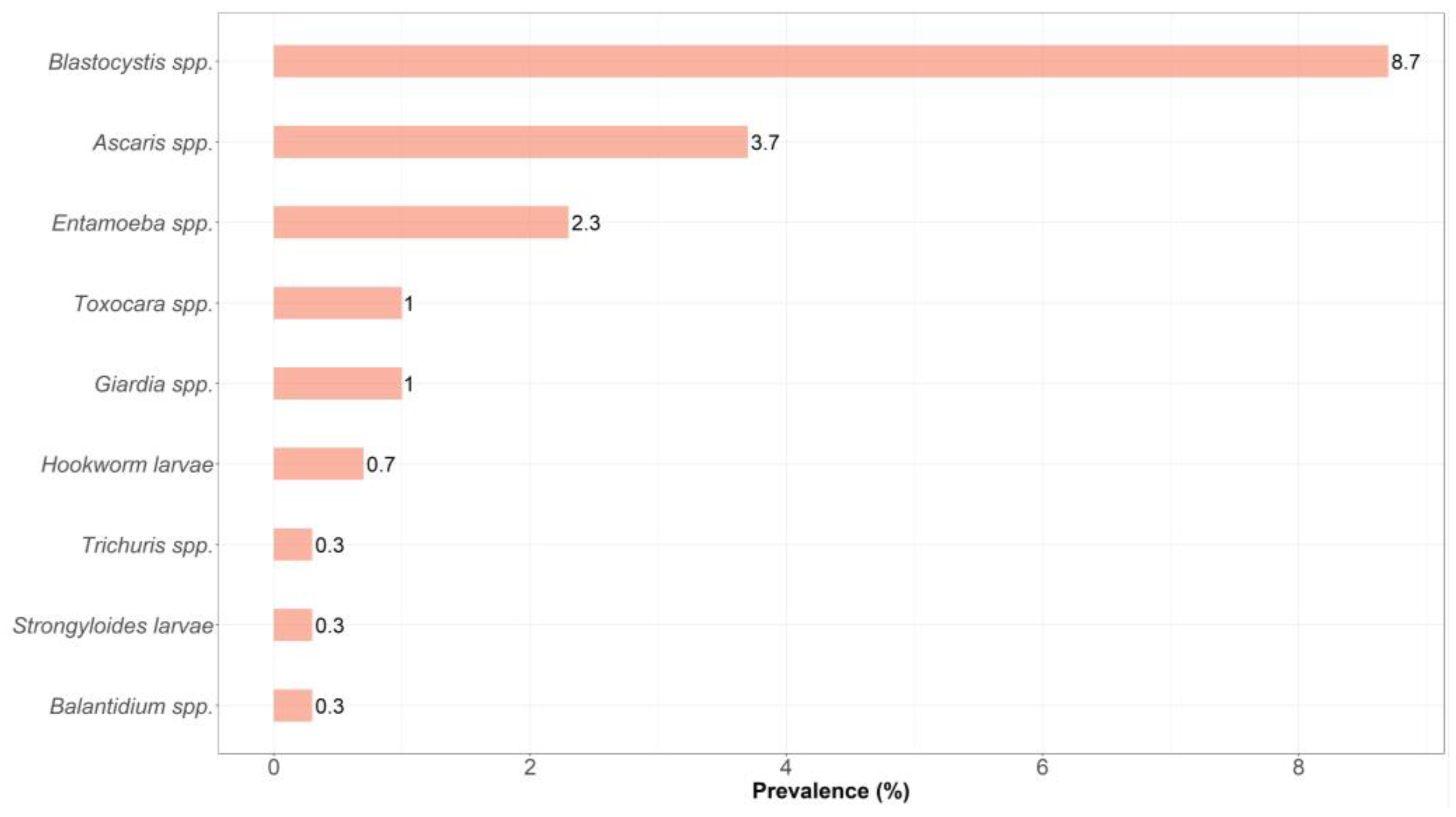
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Davis, C.; Bryan, J.; Hodgson, J.; Murphy, K. Definition of the Mediterranean Diet; a Literature Review. Nutrients 2015, 7, 9139–9153. [Google Scholar] [CrossRef] [PubMed]
- Paparella, A. Food safety: Definitions and aspects. In Food Safety Hazards; Al-Rub, F., Shibhab, P., Al-Rub, S., Pittia, P., Paparella, A., Eds.; Gavin Ebooks: Lisle, IL, USA, 2020; pp. 1–5. [Google Scholar]
- CDC. Lettuce, Other Leafy Greens, and Food Safety. 2023. Available online: https://www.cdc.gov/foodsafety/communication/leafy-greens.html (accessed on 24 July 2023).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global Estimates and Regional Comparisons of the Burden of Foodborne Disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef] [PubMed]
- Alegbeleye, O.O.; Singleton, I.; Sant’Ana, A.S. Sources and contamination routes of microbial pathogens to fresh produce during field cultivation: A review. Food Microbiol. 2018, 73, 177–208. [Google Scholar] [CrossRef]
- Iwu, C.D.; Okoh, A.I. Preharvest Transmission Routes of Fresh Produce Associated Bacterial Pathogens with Outbreak Potentials: A Review. Int. J. Environ. Res. Public Health 2019, 16, 4407. [Google Scholar] [CrossRef]
- Torrence, M.E. Introduction to Preharvest Food Safety. Microbiol. Spectr. 2016, 4. [Google Scholar] [CrossRef] [PubMed]
- Halablab, M.A.; Sheet, I.H.; Holail, H.M. Microbiological Quality of Raw Vegetables Grown in Bekaa Valley Lebanon. Am. J. Food Technol. 2011, 6, 129–139. [Google Scholar] [CrossRef]
- Murray, K.; Wu, F.; Shi, J.; Jun Xue, S.; Warriner, K. Challenges in the microbiological food safety of fresh produce: Limitations of post-harvest washing and the need for alternative interventions. Food Qual. Saf. 2017, 1, 289–301. [Google Scholar] [CrossRef]
- CDC. National Outbreak Reporting System (NORS). 2022. Available online: https://wwwn.cdc.gov/norsdashboard/ (accessed on 24 July 2023).
- Faour-Klingbeil, D. The Microbiological Safety of Fresh Produce in Lebanon—A Holistic “Farm-to Fork Chain” Approach to Evaluate Food Safety, Compliance Levels and Underlying Risk Factors. Ph.D. Thesis, University of Plymouth, Plymouth, UK, 2016. Available online: https://core.ac.uk/download/pdf/80690128.pdf (accessed on 6 March 2023).
- Kamleh, R.; Jurdi, M.; Annous, B.A. Management of microbial food safety in Arab countries. J. Food Prot. 2012, 75, 2082–2090. [Google Scholar] [CrossRef]
- Sourenian, T.; Mann, D.; Li, S.; Deng, X.; Jaafar, H.; Kassem, I.I. Dissemination of multidrug-resistant Escherichia coli harboring the mobile colistin resistance gene mcr-1.1 on transmissible plasmids in the Mediterranean Sea. J. Glob. Antimicrob. Resist. 2020, 22, 84–86. [Google Scholar] [CrossRef]
- Hmede, Z.; Sulaiman, A.A.A.; Jaafar, H.; Kassem, I.I. Emergence of plasmid-borne colistin resistance gene mcr-1 in multidrug-resistant Escherichia coli isolated from irrigation water in Lebanon. Int. J. Antimicrob. Agents 2019, 54, 102–104. [Google Scholar] [CrossRef]
- Kharroubi, S.; Nasser, N.A.; El-Harakeh, M.D.; Sulaiman, A.A.; Kassem, I.I. First Nation-Wide Analysis of Food Safety and Acceptability Data in Lebanon. Foods 2020, 9, 1717. [Google Scholar] [CrossRef]
- Kassem, I.I.; Nasser, N.A.; Salibi, J. Prevalence and Loads of Fecal Pollution Indicators and the Antibiotic Resistance Phenotypes of Escherichia coli in Raw Minced Beef in Lebanon. Foods 2020, 9, 1543. [Google Scholar] [CrossRef] [PubMed]
- Hussein, N.D.; Hassan, J.W.; Osman, M.; El-Omari, K.; Kharroubi, S.A.; Toufeili, I.; Kassem, I.I. Assessment of the Microbiological Acceptability of White Cheese (Akkawi) in Lebanon and the Antimicrobial Resistance Profiles of Associated Escherichia coli. Antibiotics 2023, 12, 610. [Google Scholar] [CrossRef]
- Khatib, A.; Olama, Z.; Khawaja, G. Shiga toxin-producing E. coli (STEC) associated with lebanese fresh produce. Int. J. Curr. Microbiol. Appl. Sci. 2015, 4, 481–496. [Google Scholar]
- Dagher, L.A.; Hassan, J.; Kharroubi, S.; Jaafar, H.; Kassem, I.I. Nationwide Assessment of Water Quality in Rivers across Lebanon by Quantifying Fecal Indicators Densities and Profiling Antibiotic Resistance of Escherichia coli. Antibiotics 2021, 10, 883. [Google Scholar] [CrossRef]
- Osman, M.; Al Mir, H.; Rafei, R.; Dabboussi, F.; Madec, J.Y.; Haenni, M.; Hamze, M. Epidemiology of antimicrobial resistance in Lebanese extra-hospital settings: An overview. J. Glob. Antimicrob. Resist. 2019, 17, 123–129. [Google Scholar] [CrossRef]
- Kassem, I.I.; Jaafar, H. The potential impact of water quality on the spread and control of COVID-19 in Syrian refugee camps in Lebanon. Water Int. 2020, 45, 423–429. [Google Scholar] [CrossRef]
- Sulaiman, A.A.A.; Kassem, I.I. First report on the detection of the plasmid-borne colistin resistance gene mcr-1 in multi-drug resistant E. coli isolated from domestic and sewer waters in Syrian refugee camps in Lebanon. Travel Med. Infect. Dis. 2019, 30, 117–120. [Google Scholar] [CrossRef]
- Osman, M.; Kassem, I.I.; Dabboussi, F.; Cummings, K.J.; Hamze, M. The indelible toll of enteric pathogens: Prevalence, clinical characterization, and seasonal trends in patients with acute community-acquired diarrhea in disenfranchised communities. PLoS ONE 2023, 18, e0282844. [Google Scholar] [CrossRef] [PubMed]
- El Safadi, D.; Meloni, D.; Poirier, P.; Osman, M.; Cian, A.; Gaayeb, L.; Wawrzyniak, I.; Delbac, F.; El Alaoui, H.; Delhaes, L.; et al. Molecular epidemiology of Blastocystis in Lebanon and correlation between subtype 1 and gastrointestinal symptoms. Am. J. Trop. Med. Hyg. 2013, 88, 1203–1206. [Google Scholar] [CrossRef]
- Osman, M.; El Safadi, D.; Cian, A.; Benamrouz, S.; Nourrisson, C.; Poirier, P.; Pereira, B.; Razakandrainibe, R.; Pinon, A.; Lambert, C.; et al. Prevalence and Risk Factors for Intestinal Protozoan Infections with Cryptosporidium, Giardia, Blastocystis and Dientamoeba among Schoolchildren in Tripoli, Lebanon. PLoS Negl. Trop. Dis. 2016, 10, e0004496. [Google Scholar] [CrossRef]
- Hamze, M.; Dabboussi, F.; Al-Ali, K.; Ourabi, L. Prevalence of infection by intestinal parasites in north Lebanon: 1997–2001. East. Mediterr. Health J. 2004, 10, 343–348. [Google Scholar] [CrossRef] [PubMed]
- Osman, M.; El Safadi, D.; Benamrouz, S.; Guyot, K.; Dei-Cas, E.; Aliouat, E.M.; Creusy, C.; Mallat, H.; Hamze, M.; Dabboussi, F.; et al. Initial data on the molecular epidemiology of cryptosporidiosis in Lebanon. PLoS ONE 2015, 10, e0125129. [Google Scholar] [CrossRef][Green Version]
- Koutsoumanis, K.; Allende, A.; Alvarez-Ordóñez, A.; Bolton, D.; Bover-Cid, S.; Chemaly, M.; Davies, R.; De Cesare, A.; Herman, L.; Hilbert, F.; et al. Public health risks associated with food-borne parasites. EFSA J. 2018, 16, e05495. [Google Scholar] [CrossRef]
- Ruijs, M. Value Chain Analysis of (Greenhouse) Vegetables in Lebanon: Strengthening Lebanese Water and Agriculture Sector; Component 5: Adaptive Greenhouse; Work Package; Wageningen Economic Research: Wageningen, The Netherlands, 2017; 27p. [Google Scholar]
- Mohamed, M.A.; Siddig, E.E.; Elaagip, A.H.; Edris, A.M.; Nasr, A.A. Parasitic contamination of fresh vegetables sold at central markets in Khartoum state, Sudan. Ann. Clin. Microbiol. Antimicrob. 2016, 15, 17. [Google Scholar] [CrossRef]
- Khan, W.; Rafiq, N.; Nawaz, M.A.; Kabir, M.; Farooqi, Z.U.R.; Romman, M.; Parvez, R.; Alfarraj, S.; Noor, A.; Ujjan, A.A. Parasitic contamination of fresh vegetables sold in open markets: A public health threat. Braz. J. Biol. 2021, 82, e242614. [Google Scholar] [CrossRef]
- Soulsby, E. Helminths, Arthropods and Protozoa of Domesticated Animals; Baillere Tindall: London, UK, 1982. [Google Scholar]
- Koo, J. Microbial Safety of Fresh and Processed Vegetables. In Handbook of Vegetables and Vegetable Processing, 2nd ed.; Siddiq, M., Uebersax, M., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2018; pp. 941–968. [Google Scholar]
- Abi Saab, M.T.; Jomaa, I.; El Hage, R.; Skaf, S.; Fahed, S.; Rizk, Z.; Massaad, R.; Romanos, D.; Khairallah, Y.; Azzi, V.; et al. Are Fresh Water and Reclaimed Water Safe for Vegetable Irrigation? Empirical Evidence from Lebanon. Water 2022, 14, 1437. [Google Scholar] [CrossRef]
- FAO. The Formulation of Official Standards for Agricultural Water Reuse in Lebanon, IWMI-ReWater MENA. 2022. Available online: https://www.fao.org/platforms/water-scarcity/Knowledge/partners-contributions/detail/the-formulation-of-official-standards-for-agricultural-water-reuse-in-lebanon-iwmi-rewater-mena/en (accessed on 12 April 2023).
- McHeik, A.; Awad, A.; Fadel, A.; Mounzer, C.; Nasreddine, S. Effect of Irrigation Water Quality on the Microbial Contamination of Fresh Vegetables in the Bekaa Valley, Lebanon. Am. J. Agric. For. 2018, 6, 191–197. [Google Scholar]
- Daou, C.; El Hoz, M.; Kassouf, A.; Legube, B. Multivariate Monitoring of Surface Water Quality: Physico-Chemical, Microbiological and 3D Fluorescence Characterization. Water 2020, 12, 1673. [Google Scholar] [CrossRef]
- Daou, C.; Salloum, M.; Legube, B.; Kassouf, A.; Ouaini, N. Characterization of spatial and temporal patterns in surface water quality: A case study of four major Lebanese rivers. Environ. Monit. Assess. 2018, 190, 485. [Google Scholar] [CrossRef] [PubMed]
- Massoud, M.A.; El-Fadel, M.; Scrimshaw, M.D.; Lester, J.N. Land Use Impact on the Spatial and Seasonal Variation of the Contaminant Loads to Abou Ali River and Its Coastal Zone in North Lebanon. Agric. Eng. Int. CIGR J. 2004, 6, 1–18. [Google Scholar]
- Jaafar, H.; Ahmad, F.; Holtmeier, L.; King-Okumu, C. Refugees, water balance, and water stress: Lessons learned from Lebanon. Ambio 2020, 49, 1179–1193. [Google Scholar] [CrossRef] [PubMed]
- Faour-Klingbeil, D.; Todd, E.C.D. Prevention and Control of Foodborne Diseases in Middle-East North African Countries: Review of National Control Systems. Int. J. Environ. Res. Public Health 2019, 17, 70. [Google Scholar] [CrossRef]
- Al Nahhas, S.; Aboualchamat, G. Investigation of parasitic contamination of salad vegetables sold by street vendors in city markets in Damascus, Syria. Food Waterborne Parasitol. 2020, 21, e00090. [Google Scholar] [CrossRef]
- Etewa, S.E.; Abdel-Rahman, S.A.; Fathy, G.M.; Abo El-Maaty, D.A.; Sarhan, M.H. Parasitic Contamination of Commonly Consumed Fresh Vegetables and Fruits in Some Rural Areas of Sharkyia Governorate, Egypt. Afro-Egypt. J. Infect. Endem. Dis. 2017, 7, 192–202. [Google Scholar] [CrossRef]
- Esboei, B.R.; Sharif, M.; Daryani, A.; Hosseini, F.; Pagheh, A.; Rahimi, M.; Nasrolahi, M. Parasitic Contamination in Commonly- Consumed Vegetables in Mazandaran Province, Northern Iran. J. Hum. Environ. Health Promot. 2017, 2, 89–95. [Google Scholar] [CrossRef][Green Version]
- Aydenizöz, M.; Gökpinar, S.; Gazyagci, A. Investigation of parasitological contamination in leafy vegetables in Kırıkkale of Turkey. Anim. Health Prod. Hyg. 2017, 1, 463–467. [Google Scholar]
- Gabre, R.M.; Shakir, A. Prevalence of Some Human Enteroparasites in Commonly Consumed Raw Vegetables in Tabuk, Saudi Arabia. J. Food Prot. 2016, 79, 655–658. [Google Scholar] [CrossRef]
- El Bakri, A.; Hussein, N.M.; Ibrahim, Z.A.; Hasan, H.; AbuOdeh, R. Intestinal Parasite Detection in Assorted Vegetables in the United Arab Emirates. Oman Med. J. 2020, 35, e128. [Google Scholar] [CrossRef]
- Attah, A.O.; Sanggari, A.; Li, L.I.; Nik Him, N.A.I.I.; Ismail, A.H.; Meor Termizi, F.H. Blastocystis occurrence in water sources worldwide from 2005 to 2022: A review. Parasitol. Res. 2023, 122, 1–10. [Google Scholar] [CrossRef]
- Greige, S.; El Safadi, D.; Khaled, S.; Gantois, N.; Baydoun, M.; Chemaly, M.; Benamrouz-Vanneste, S.; Chabé, M.; Osman, M.; Certad, G.; et al. First report on the prevalence and subtype distribution of Blastocystis sp. in dairy cattle in Lebanon and assessment of zoonotic transmission. Acta Trop. 2019, 194, 23–29. [Google Scholar] [CrossRef]
- Greige, S.; El Safadi, D.; Becu, N.; Gantois, N.; Pereira, B.; Chabe, M.; Benamrouz-Vanneste, S.; Certad, G.; El Hage, R.; Chemaly, M.; et al. Prevalence and subtype distribution of Blastocystis sp. isolates from poultry in Lebanon and evidence of zoonotic potential. Parasit. Vectors 2018, 11, 389. [Google Scholar] [CrossRef]
- Shirley, D.T.; Watanabe, K.; Moonah, S. Significance of amebiasis: 10 reasons why neglecting amebiasis might come back to bite us in the gut. PLoS Negl. Trop. Dis. 2019, 13, e0007744. [Google Scholar] [CrossRef] [PubMed]
- Else, K.J.; Keiser, J.; Holland, C.V.; Grencis, R.K.; Sattelle, D.B.; Fujiwara, R.T.; Bueno, L.L.; Asaolu, S.O.; Sowemimo, O.A.; Cooper, P.J. Whipworm and roundworm infections. Nat. Rev. Dis. Primers 2020, 6, 44. [Google Scholar] [CrossRef]
- WHO. L’utilisation des Eaux Usées en Agriculture et en Aquaculture: Recommandations à Visées Sanitaires; WHO: Geneva, Switzerland, 1989. [Google Scholar]
- Utaaker, K.S.; Skjerve, E.; Robertson, L.J. Keeping it cool: Survival of Giardia cysts and Cryptosporidium oocysts on lettuce leaves. Int. J. Food Microbiol. 2017, 255, 51–57. [Google Scholar] [CrossRef]
- Kanafani, Z.A.; Skoury, A.; Araj, G.F.; El-Khoury, M.; Sawaya, R.A.; Atweh, S.F.; Kanj, S.S. Seroprevalence of toxocariasis in Lebanon: A pilot study. Parasitology 2006, 132 Pt 5, 635–639. [Google Scholar] [CrossRef]
- Araj, G.F.; Musharrafieh, U.M.; Haydar, A.; Ghawi, A.; Itani, R.; Saliba, R. Trends and prevalence of intestinal parasites at a tertiary care center in Lebanon over a decade. J. Med. Liban. 2011, 59, 143–148. [Google Scholar] [PubMed]
- Ismail, Y. Prevalence of Parasitic Contamination in Salad Vegetables Collected from Supermarkets and Street Vendors in Amman and Baqa’a—Jordan. Pol. J. Microbiol. 2016, 65, 201–207. [Google Scholar] [CrossRef] [PubMed]
- UNHCR. Cholera Outbreak Situation Report No 8. 2023. Available online: https://reliefweb.int/report/lebanon/lebanon-cholera-response-interim-report-january-2023 (accessed on 10 April 2023).
- Alemu, G.; Mama, M.; Misker, D.; Haftu, D. Parasitic contamination of vegetables marketed in Arba Minch town, southern Ethiopia. BMC Infect. Dis. 2019, 19, 410. [Google Scholar] [CrossRef]
- Bekele, F.; Tefera, T.; Biresaw, G.; Yohannes, T. Parasitic contamination of raw vegetables and fruits collected from selected local markets in Arba Minch town, Southern Ethiopia. Infect. Dis. Poverty 2017, 6, 19. [Google Scholar] [CrossRef]
- Alemu, G.; Nega, M.; Alemu, M. Parasitic Contamination of Fruits and Vegetables Collected from Local Markets of Bahir Dar City, Northwest Ethiopia. Res. Rep. Trop. Med. 2020, 11, 17–25. [Google Scholar] [CrossRef]
- Avcioğlu, H.; Soykan, E.; Tarakci, U. Control of helminth contamination of raw vegetables by washing. Vector Borne Zoonotic Dis. 2011, 11, 189–191. [Google Scholar] [CrossRef]
- Shahnazi, M.; Jafari-Sabet, M. Prevalence of parasitic contamination of raw vegetables in villages of Qazvin Province, Iran. Foodborne Pathog. Dis. 2010, 7, 1025–1030. [Google Scholar] [CrossRef] [PubMed]
- do Nascimento Ramos, I.C.; Ramos, R.A.N.; Giannelli, A.; Lima, V.F.S.; Cringoli, G.; Rinaldi, L.; de Carvalho, G.A.; Alves, L.C. An Additional Asset for the FLOTAC Technique: Detection of Gastrointestinal Parasites in Vegetables. Acta Parasitol. 2019, 64, 423–425. [Google Scholar] [CrossRef] [PubMed]
- Barlaam, A.; Sannella, A.R.; Ferrari, N.; Temesgen, T.T.; Rinaldi, L.; Normanno, G.; Cacciò, S.M.; Robertson, L.J.; Giangaspero, A. Ready-to-eat salads and berry fruits purchased in Italy contaminated by Cryptosporidium spp., Giardia duodenalis, and Entamoeba histolytica. Int. J. Food Microbiol. 2022, 370, 109634. [Google Scholar] [CrossRef] [PubMed]
| Number of Vegetable Samples | Presence of Parasite (%) | Univariate Analysis X2 (p-Value) | Multivariable Analysis OR (95% CI) | |
|---|---|---|---|---|
| Date | ||||
| 2020 1 | 150 | 29 (19.3%) | 1.18 (0.28) | |
| 2021 | 150 | 21 (14.0%) | 0.77 (0.25–2.28) | |
| Sample type | ||||
| Arugula 1 | 60 | 7 (11.7%) | 3.36 (0.50) | |
| Lettuce | 60 | 14 (23.3%) | 2.36 (0.90–6.74) | |
| Mint | 60 | 11 (18.3%) | 1.72 (0.62–5.02) | |
| Parsley | 60 | 9 (15.0%) | 1.35 (0.47–4.05) | |
| Purslane | 60 | 9 (15.0%) | 1.37 (0.47–4.14) | |
| Market storing status | ||||
| Closed 1 | 112 | 16 (14.3%) | 0.48 (0.49) | |
| Open | 188 | 34 (18.1%) | 0.97 (0.37–2.44) | |
| Purchase status | ||||
| Dry 1 | 166 | 24 (14.5%) | 0.97 (0.32) | |
| Wet | 134 | 26 (19.4%) | 1.23 (0.51–3.04) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Safadi, D.; Osman, M.; Hanna, A.; Hajar, I.; Kassem, I.I.; Khalife, S.; Dabboussi, F.; Hamze, M. Parasitic Contamination of Fresh Leafy Green Vegetables Sold in Northern Lebanon. Pathogens 2023, 12, 1014. https://doi.org/10.3390/pathogens12081014
El Safadi D, Osman M, Hanna A, Hajar I, Kassem II, Khalife S, Dabboussi F, Hamze M. Parasitic Contamination of Fresh Leafy Green Vegetables Sold in Northern Lebanon. Pathogens. 2023; 12(8):1014. https://doi.org/10.3390/pathogens12081014
Chicago/Turabian StyleEl Safadi, Dima, Marwan Osman, Angel Hanna, Iman Hajar, Issmat I. Kassem, Sara Khalife, Fouad Dabboussi, and Monzer Hamze. 2023. "Parasitic Contamination of Fresh Leafy Green Vegetables Sold in Northern Lebanon" Pathogens 12, no. 8: 1014. https://doi.org/10.3390/pathogens12081014
APA StyleEl Safadi, D., Osman, M., Hanna, A., Hajar, I., Kassem, I. I., Khalife, S., Dabboussi, F., & Hamze, M. (2023). Parasitic Contamination of Fresh Leafy Green Vegetables Sold in Northern Lebanon. Pathogens, 12(8), 1014. https://doi.org/10.3390/pathogens12081014










