Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease
Abstract
:1. Introduction
2. AMPs and CPPs and Neglected Zoonotic Diseases: An Overview
3. How Do AMPs and CPPs Specifically Target Protozoan Parasites without Harming the Infected Mammalian Cell? Cell Entry Mechanisms
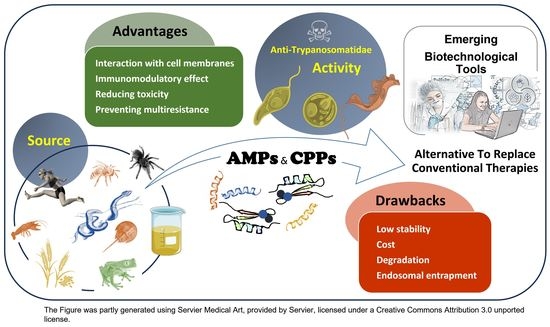
4. AMPs and CPPs as Alternative Therapies to Conventional Drugs against Leishmaniosis and Chagas Disease
5. Antiparasitic and Immunomodulatory Activity of AMPs and CPPs
5.1. Synthetic and Bioinformatic Tools
5.2. AMPs and CPPs for Combatting Different Forms of Leishmaniosis
| Peptide Molecule | Source | Antiprotozoal Activity | Reference |
|---|---|---|---|
| Andropin | Synthetic | L. panamensis L. major | [104] |
| Anti-lipopolysaccharide factor | Penaeus monodon (marine crustacean) | L. braziliensis | [105] |
| BatxC | Bothrops atrox (snake) | T. cruzi (Y strain) | [133] |
| Bombinins H2 and H4 | Bombina variegata (frog) | L. donovani | [124] |
| Cathelicidins (SMAP 29, PG-1) | Synthetic | L. major L. amazonensis | [134] |
| Cecropin A, D | Drosophila Hyalaphora cecropia | L. aethiopica L. panamensis | [69,95] |
| Cecropin A-melittin | Hybrid peptide | L. donovani L. pifanoi | [96,128] |
| Cecropin A, B, and P1 | Synthetic | L. panamensis | [104] |
| L. major | |||
| T. cruzi (Tulahuen strain) | [135] | ||
| Chyral cyclobutanes | Synthetic | L. donovani | [30] |
| Clavanin A | Styela clava (sea squirt) | L. braziliensis | [105] |
| CM11 (cecropin–melittin hybrid) | Synthetic | L. major | [49] |
| Cryptdin-1 and -4 | Macaca mulatta (rhesus macaque) | L. major L. amazonensis | [134] |
| Ctn | Crotalus durissus terrificus (rattlesnake) | T. cruzi (Y strain) | [136] |
| Defensin | Phlebotomus duboscqi (sandfly) | L. major L. amazonensis | [69,137] |
| Defensin α1 | Human | T. cruzi (Tulahuen strain) | [138,139] |
| Defensin (fragments D, P, B, Q, and E) | Mytilus galloprovincialis (mussel) | L. major | [140] |
| Dermaseptin | Phyllomedusa sauvagii (frog) | L. mexicana L. panamensis L. major | [104,122] |
| Dermaseptin 01 | Synthetic | L. infantum | [123] |
| Dermaseptin 01, 02, 03, 04, 06, and 07 | Phyllomedusa hypochondrialis (frog) | L. amazonensis | [121] |
| Dermaseptin S1 analogs | Synthetic | L. major | [120] |
| Dhvar4 (histatin 5 analog) | Synthetic | L. donovani | [141] |
| DS 01 | Phyllomedusa oreades (frog) | T. cruzi (Y strain) | [142] |
| Enterocin AS-48 | Enterococcus faecalis | L. pifanoi | [143] |
| Enterocin AS-48 homologs | Synthetic | L. donovani | [47] |
| Eumenitin | Eumenes rubronotatus (wasp venom) | L. major | [69] |
| Gomesin | Acanthoscurria gomesiana (tarantula) | L. amazonensis | [144] |
| Histatin 5 (L- and D-enantiomers) | Synthetic | L. donovani L. pifanoi | [141] |
| Hmc364-382 | Dpenaeus monodon (shrimp) | T. cruzi (Y strain) | [145] |
| Indolicidin | Synthetic | L. donovani | [115] |
| Lactoferricin (17–30) Lactoferrampin (265–284) LFchimera | Bovine milk lactoferrin (domain N1) | L. pifanoi L. donovani | [107] |
| LTP2 α-1 | Hordeum vulgare (barley) | L. donovani | [130] |
| M-PONTX-Dq3a[1-15]/[Lys]3-M-PONTX-Dq3a[1-15] | Dinoponera quadriceps (ant) synthetic modification | T. cruzi (Y strain) | [146,147] |
| Magainin Magainin analogs (MG-H1/H2) and F5W-magainin 2 | Xenopus laevis (frog) | L. braziliensis | [69,105] |
| L. major | |||
| L. donovani | |||
| Synthetic | L. amazonensis | [148] | |
| Melittin | Bee venom Apis mellifera | L. donovani L. infantum L. panamensis L. major T. cruzi (CL Brener strain) | [69,104,149] |
| Mylitin A | Mytilus edulis (mussel) | L. braziliensis | [105] |
| NK2 | Synthetic | T. cruzi (Tehuantepec strain) | [150] |
| Ovispirin | Synthetic | L. major L. amazonensis | [134] |
| p-Acl and analog p-AclR7 | Synthetic | L. amazonensis L. infantum | [102] |
| Penaeidian-3 | Whiteleg shrimp Litopenaeus vannamei | L. braziliensis | [105] |
| Rhesus | Synthetic | L. major L. amazonensis | [134] |
| Phylloseptin-1 | Synthetic | L. amazonensis | [118] |
| Polybia-CP | Polybia paulista (wasp) | T. cruzi (Y strain) | [151] |
| PTH-1 | Solanum tuberosum (potato) | L. donovani | [130] |
| Pr-1, 2, and 3 | Synthetic | L. panamensis L. major | [104] |
| Pylloseptin 7 | Phyllomedusa nordestina (frog) | T. cruzi (Y strain) | [152] |
| SALPs | Synthetic | L. major | [69] |
| Snakin-1 | Solanum tuberosum (potato) | L. donovani | [130] |
| Seminalplasmin (SPK and 27RP) | Synthetic | L. donovani | [115] |
| StigA25 | Tityus stigmurus (scorpion) Synthetic | T. cruzi (Y strain) | [153] |
| Tachyplesin | Tachypleus tridentatus (horseshoe crab) | L. panamensis L. major L. braziliensis L. donovani T. cruzi (Y strain) | [104,105,106,154] |
| TAT (48–57) peptide TAT (48–60) peptide TAT and polyarginine | TAT (transactivator of transcription) protein from HIV-1 | L. donovani L. infantum | [30,111,112] |
| Temporins A and B | Rana temporaria (frog) | L. donovani L. pifanoi | [69,126] |
| Temporin-1Sa, 1Sb, and 1Sc | Pelophylax saharica (frog) | L. infantum | [125] |
| Temporizin-1 | Synthetic | T. cruzi (Y strain) | [155] |
| Thionin α-1, α-2, and β type I | Triticum aestivum (wheat) Hordeum vulgare (barley) | L. donovani | [130] |
| [Arg]11-VmCT1 | Vaejovis mexicanus (scorpion) | T. cruzi (Y strain) | [156] |
5.3. AMPs and CPPs to Combat T. cruzi Infection
6. Biological Models to Evaluate the Activity of AMPs and CPPs
6.1. Cell Lines and Primary Cell Cultures for Cytotoxicity Assays
6.2. Antiparasitic Activity
6.3. In Vivo Models
7. Challenges to Overcome Regarding the Current Limitations of AMPs and CPPs
8. Emerging Biotechnological Tools: Future Directions
9. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Perez-Molina, J.A.; Molina, I. Chagas disease. Lancet 2018, 391, 82–94. [Google Scholar] [CrossRef] [PubMed]
- Burza, S.; Croft, S.L.; Boelaert, M. Leishmaniasis. Lancet 2018, 392, 951–970. [Google Scholar] [CrossRef] [PubMed]
- Donelson, J.E.; Hill, K.L.; El-Sayed, N.M. Multiple mechanisms of immune evasion by African trypanosomes. Mol. Biochem. Parasitol. 1998, 91, 51–66. [Google Scholar] [CrossRef] [PubMed]
- Pauli, I.; Rezende, C.O., Jr.; Slafer, B.W.; Dessoy, M.A.; de Souza, M.L.; Ferreira, L.L.G.; Adjanohun, A.L.M.; Ferreira, R.S.; Magalhaes, L.G.; Krogh, R.; et al. Multiparameter Optimization of Trypanocidal Cruzain Inhibitors with In Vivo Activity and Favorable Pharmacokinetics. Front. Pharmacol. 2021, 12, 774069. [Google Scholar] [CrossRef]
- Gupta, A.K.; Das, S.; Kamran, M.; Ejazi, S.A.; Ali, N. The pathogenicity and virulence of Leishmania—Interplay of virulence factors with host defenses. Virulence 2022, 13, 903–935. [Google Scholar] [CrossRef]
- Vazquez-Cabrera, N.; Espinosa-Marquez, A.; Cedillo-Ramirez, M.L. Historical evolution of World Health Organization guidelines on antimicrobial resistanceEvolucao historica da Organizacao Mundial da Saude e a resistencia aos antimicrobianos. Rev. Panam. Salud Publica 2023, 47, e51. [Google Scholar] [CrossRef]
- Murray, A.K.; Stanton, I.; Gaze, W.H.; Snape, J. Dawning of a new ERA: Environmental Risk Assessment of antibiotics and their potential to select for antimicrobial resistance. Water Res. 2021, 200, 117233. [Google Scholar] [CrossRef]
- Huang, X.; Li, G. Antimicrobial Peptides and Cell-Penetrating Peptides: Non-Antibiotic Membrane-Targeting Strategies Against Bacterial Infections. Infect. Drug Resist. 2023, 16, 1203–1219. [Google Scholar] [CrossRef]
- Parn, K.; Eriste, E.; Langel, U. The Antimicrobial and Antiviral Applications of Cell-Penetrating Peptides. Methods Mol. Biol. 2015, 1324, 223–245. [Google Scholar] [CrossRef]
- Cruz, G.S.; Santos, A.T.D.; Brito, E.H.S.; Radis-Baptista, G. Cell-Penetrating Antimicrobial Peptides with Anti-Infective Activity against Intracellular Pathogens. Antibiotics 2022, 11, 1772. [Google Scholar] [CrossRef]
- WHO. Neglected Tropical Diseases. Available online: https://www.who.int/health-topics/neglected-tropical-diseases#tab=tab_1 (accessed on 1 June 2023).
- The, L. Neglected tropical diseases: Ending the neglect of populations. Lancet 2022, 399, 411. [Google Scholar] [CrossRef]
- World Health Organization. Ending the Neglect to Attain the Sustainable Development Goals: A Road Map for Neglected Tropical Diseases 2021–2030; World Health Organization: Geneva, Switzerland, 2021. [Google Scholar]
- Roquero, I.; Cantizani, J.; Cotillo, I.; Manzano, M.P.; Kessler, A.; Martin, J.J.; McNamara, C.W. Novel chemical starting points for drug discovery in leishmaniasis and Chagas disease. Int. J. Parasitol. Drugs Drug Resist. 2019, 10, 58–68. [Google Scholar] [CrossRef]
- Rojas-Pirela, M.; Kemmerling, U.; Quinones, W.; Michels, P.A.M.; Rojas, V. Antimicrobial Peptides (AMPs): Potential Therapeutic Strategy against Trypanosomiases? Biomolecules 2023, 13, 599. [Google Scholar] [CrossRef]
- Robles-Loaiza, A.A.; Pinos-Tamayo, E.A.; Mendes, B.; Teixeira, C.; Alves, C.; Gomes, P.; Almeida, J.R. Peptides to Tackle Leishmaniasis: Current Status and Future Directions. Int. J. Mol. Sci. 2021, 22, 4400. [Google Scholar] [CrossRef]
- Rima, M.; Rima, M.; Fajloun, Z.; Sabatier, J.M.; Bechinger, B.; Naas, T. Antimicrobial Peptides: A Potent Alternative to Antibiotics. Antibiotics 2021, 10, 1095. [Google Scholar] [CrossRef]
- APD. The Antimicrobial Peptide Database. Available online: https://aps.unmc.edu/ (accessed on 8 March 2023).
- Mazurkiewicz-Pisarek, A.; Baran, J.; Ciach, T. Antimicrobial Peptides: Challenging Journey to the Pharmaceutical, Biomedical, and Cosmeceutical Use. Int. J. Mol. Sci. 2023, 24, 9031. [Google Scholar] [CrossRef]
- Neundorf, I. Antimicrobial and Cell-Penetrating Peptides: How to Understand Two Distinct Functions Despite Similar Physicochemical Properties. Adv. Exp. Med. Biol. 2019, 1117, 93–109. [Google Scholar] [CrossRef]
- CPPdatabase. The Cell Penetrating Peptides Database. Available online: https://webs.iiitd.edu.in/raghava/cppsite/ (accessed on 9 March 2023).
- Khairkhah, N.; Namvar, A.; Bolhassani, A. Application of Cell Penetrating Peptides as a Promising Drug Carrier to Combat Viral Infections. Mol. Biotechnol. 2023, 1–16. [Google Scholar] [CrossRef]
- Kardani, K.; Bolhassani, A. Cppsite 2.0: An Available Database of Experimentally Validated Cell-Penetrating Peptides Predicting their Secondary and Tertiary Structures. J. Mol. Biol. 2021, 433, 166703. [Google Scholar] [CrossRef]
- Batista, M.F.; Najera, C.A.; Meneghelli, I.; Bahia, D. The Parasitic Intracellular Lifestyle of Trypanosomatids: Parasitophorous Vacuole Development and Survival. Front. Cell Dev. Biol. 2020, 8, 396. [Google Scholar] [CrossRef]
- Rennick, J.J.; Johnston, A.P.R.; Parton, R.G. Key principles and methods for studying the endocytosis of biological and nanoparticle therapeutics. Nat. Nanotechnol. 2021, 16, 266–276. [Google Scholar] [CrossRef] [PubMed]
- Buccini, D.F.; Cardoso, M.H.; Franco, O.L. Antimicrobial Peptides and Cell-Penetrating Peptides for Treating Intracellular Bacterial Infections. Front. Cell Infect. Microbiol. 2020, 10, 612931. [Google Scholar] [CrossRef] [PubMed]
- Rivas, L.; Nácher-Vázquez, M.; Andreu, D. The Physical Matrix of the Plasma Membrane as a Target: The Charm of Drugs with Low Specificity. In Drug Discovery for Leishmaniasis; Rivas, L., Gil, C., Eds.; The Royal Society of Chemistry: London, UK, 2017; pp. 248–281. [Google Scholar]
- Field, M.C.; Carrington, M. The trypanosome flagellar pocket. Nat. Rev. Microbiol. 2009, 7, 775–786. [Google Scholar] [CrossRef] [PubMed]
- Halliday, C.; de Castro-Neto, A.; Alcantara, C.L.; Cunha, E.S.N.L.; Vaughan, S.; Sunter, J.D. Trypanosomatid Flagellar Pocket from Structure to Function. Trends Parasitol. 2021, 37, 317–329. [Google Scholar] [CrossRef] [PubMed]
- Illa, O.; Olivares, J.A.; Gaztelumendi, N.; Martinez-Castro, L.; Ospina, J.; Abengozar, M.A.; Sciortino, G.; Marechal, J.D.; Nogues, C.; Royo, M.; et al. Chiral Cyclobutane-Containing Cell-Penetrating Peptides as Selective Vectors for Anti-Leishmania Drug Delivery Systems. Int. J. Mol. Sci. 2020, 21, 7502. [Google Scholar] [CrossRef]
- Fernandes, A.C.S.; Soares, D.C.; Neves, R.F.C.; Koeller, C.M.; Heise, N.; Adade, C.M.; Frases, S.; Meyer-Fernandes, J.R.; Saraiva, E.M.; Souto-Padron, T. Endocytosis and Exocytosis in Leishmania amazonensis Are Modulated by Bromoenol Lactone. Front. Cell Infect. Microbiol. 2020, 10, 39. [Google Scholar] [CrossRef] [Green Version]
- Ansari, I.; Basak, R.; Mukhopadhyay, A. Hemoglobin Endocytosis and Intracellular Trafficking: A Novel Way of Heme Acquisition by Leishmania. Pathogens 2022, 11, 585. [Google Scholar] [CrossRef]
- Patel, N.; Singh, S.B.; Basu, S.K.; Mukhopadhyay, A. Leishmania requires Rab7-mediated degradation of endocytosed hemoglobin for their growth. Proc. Natl. Acad. Sci. USA 2008, 105, 3980–3985. [Google Scholar] [CrossRef]
- Rastogi, R.; Kapoor, A.; Verma, J.K.; Ansari, I.; Sood, C.; Kumar, K.; Mukhopadhyay, A. Rab5b function is essential to acquire heme from hemoglobin endocytosis for survival of Leishmania. Biochim. Biophys. Acta Mol. Cell Res. 2021, 1868, 118868. [Google Scholar] [CrossRef]
- Rastogi, R.; Verma, J.K.; Singh, V.; Krishnamurthy, G.; Sood, C.; Kapoor, A.; Kumar, K.; Ansari, I.; Mukhopadhyay, A. Identification and characterization of the hemoglobin-binding domain of hemoglobin receptor in Leishmania. FEBS Lett. 2021, 595, 548–558. [Google Scholar] [CrossRef]
- Pessoa, C.C.; Ferreira, E.R.; Bayer-Santos, E.; Rabinovitch, M.; Mortara, R.A.; Real, F. Trypanosoma cruzi Differentiates and Multiplies within Chimeric Parasitophorous Vacuoles in Macrophages Coinfected with Leishmania amazonensis. Infect. Immun. 2016, 84, 1603–1614. [Google Scholar] [CrossRef] [Green Version]
- Pessoa, C.C.; Reis, L.C.; Ramos-Sanchez, E.M.; Orikaza, C.M.; Cortez, C.; de Castro Levatti, E.V.; Badaro, A.C.B.; Yamamoto, J.; D’Almeida, V.; Goto, H.; et al. ATP6V0d2 controls Leishmania parasitophorous vacuole biogenesis via cholesterol homeostasis. PLoS Pathog. 2019, 15, e1007834. [Google Scholar] [CrossRef] [Green Version]
- Scariot, D.B.; Staneviciute, A.; Zhu, J.; Li, X.; Scott, E.A.; Engman, D.M. Leishmaniasis and Chagas disease: Is there hope in nanotechnology to fight neglected tropical diseases? Front. Cell Infect. Microbiol. 2022, 12, 1000972. [Google Scholar] [CrossRef]
- Sundar, S.; Rai, M. Treatment of visceral leishmaniasis. Expert. Opin. Pharmacother. 2005, 6, 2821–2829. [Google Scholar] [CrossRef]
- Chatelain, E.; Konar, N. Translational challenges of animal models in Chagas disease drug development: A review. Drug Des. Devel. Ther. 2015, 9, 4807–4823. [Google Scholar] [CrossRef] [Green Version]
- Mishra, J.; Saxena, A.; Singh, S. Chemotherapy of leishmaniasis: Past, present and future. Curr. Med. Chem. 2007, 14, 1153–1169. [Google Scholar] [CrossRef]
- Del Rio, G.; Trejo Perez, M.A.; Brizuela, C.A. Antimicrobial peptides with cell-penetrating activity as prophylactic and treatment drugs. Biosci. Rep. 2022, 42, BSR20221789. [Google Scholar] [CrossRef]
- Radis-Baptista, G. Cell-Penetrating Peptides Derived from Animal Venoms and Toxins. Toxins 2021, 13, 147. [Google Scholar] [CrossRef]
- Huan, Y.; Kong, Q.; Mou, H.; Yi, H. Antimicrobial Peptides: Classification, Design, Application and Research Progress in Multiple Fields. Front. Microbiol. 2020, 11, 582779. [Google Scholar] [CrossRef]
- Powers, J.P.; Hancock, R.E. The relationship between peptide structure and antibacterial activity. Peptides 2003, 24, 1681–1691. [Google Scholar] [CrossRef]
- Abengozar, M.A.; Fernandez-Reyes, M.; Salazar, V.A.; Torrent, M.; de la Torre, B.G.; Andreu, D.; Boix, E.; Rivas, L. Essential Role of Enzymatic Activity in the Leishmanicidal Mechanism of the Eosinophil Cationic Protein (RNase 3). ACS Infect. Dis. 2022, 8, 1207–1217. [Google Scholar] [CrossRef] [PubMed]
- Corman, H.N.; Ross, J.N.; Fields, F.R.; Shoue, D.A.; McDowell, M.A.; Lee, S.W. Rationally Designed Minimal Bioactive Domains of AS-48 Bacteriocin Homologs Possess Potent Antileishmanial Properties. Microbiol. Spectr. 2022, 10, e0265822. [Google Scholar] [CrossRef] [PubMed]
- Crauwels, P.; Bank, E.; Walber, B.; Wenzel, U.A.; Agerberth, B.; Chanyalew, M.; Abebe, M.; Konig, R.; Ritter, U.; Reiling, N.; et al. Cathelicidin Contributes to the Restriction of Leishmania in Human Host Macrophages. Front. Immunol. 2019, 10, 2697. [Google Scholar] [CrossRef] [PubMed]
- Khalili, S.; Ebrahimzade, E.; Mohebali, M.; Shayan, P.; Mohammadi-Yeganeh, S.; Moosazadeh Moghaddam, M.; Elikaee, S.; Akhoundi, B.; Sharifi-Yazdi, M.K. Investigation of the antimicrobial activity of a short cationic peptide against promastigote and amastigote forms of Leishmania major (MHRO/IR/75/ER): An in vitro study. Exp. Parasitol. 2019, 196, 48–54. [Google Scholar] [CrossRef]
- Naderer, T.; Vince, J.E.; McConville, M.J. Surface determinants of Leishmania parasites and their role in infectivity in the mammalian host. Curr. Mol. Med. 2004, 4, 649–665. [Google Scholar] [CrossRef]
- McConville, M.J.; Ferguson, M.A. The structure, biosynthesis and function of glycosylated phosphatidylinositols in the parasitic protozoa and higher eukaryotes. Biochem. J. 1993, 294 Pt 2, 305–324. [Google Scholar] [CrossRef]
- Torrent, M.; Pulido, D.; Rivas, L.; Andreu, D. Antimicrobial peptide action on parasites. Curr. Drug Targets 2012, 13, 1138–1147. [Google Scholar] [CrossRef]
- Yang, Z.; Zheng, J.; Chan, C.F.; Wong, I.L.K.; Heater, B.S.; Chow, L.M.C.; Lee, M.M.M.; Chan, M.K. Targeted delivery of antimicrobial peptide by Cry protein crystal to treat intramacrophage infection. Biomaterials 2019, 217, 119286. [Google Scholar] [CrossRef]
- Kauffman, W.B.; Fuselier, T.; He, J.; Wimley, W.C. Mechanism Matters: A Taxonomy of Cell Penetrating Peptides. Trends Biochem. Sci. 2015, 40, 749–764. [Google Scholar] [CrossRef] [Green Version]
- Cao, L.; Jiang, W.; Cao, S.; Zhao, P.; Liu, J.; Dong, H.; Guo, Y.; Liu, Q.; Gong, P. In vitro leishmanicidal activity of antimicrobial peptide KDEL against Leishmania tarentolae. Acta Biochim. Biophys. Sin. 2019, 51, 1286–1292. [Google Scholar] [CrossRef]
- Yu, Y.; Zhao, P.; Cao, L.; Gong, P.; Yuan, S.; Yao, X.; Guo, Y.; Dong, H.; Jiang, W. A Novel Anti-Microbial Peptide from Pseudomonas, REDLK Induced Growth Inhibition of Leishmania tarentolae Promastigote In Vitro. Korean J. Parasitol. 2020, 58, 173–179. [Google Scholar] [CrossRef]
- Pitale, D.M.; Kaur, G.; Baghel, M.; Kaur, K.J.; Shaha, C. Halictine-2 antimicrobial peptide shows promising anti-parasitic activity against Leishmania spp. Exp. Parasitol. 2020, 218, 107987. [Google Scholar] [CrossRef]
- Zahedifard, F.; Lee, H.; No, J.H.; Salimi, M.; Seyed, N.; Asoodeh, A.; Rafati, S. Comparative study of different forms of Jellein antimicrobial peptide on Leishmania parasite. Exp. Parasitol. 2020, 209, 107823. [Google Scholar] [CrossRef]
- Croft, S.L.; Olliaro, P. Leishmaniasis chemotherapy—Challenges and opportunities. Clin. Microbiol. Infect. 2011, 17, 1478–1483. [Google Scholar] [CrossRef] [Green Version]
- Pradhan, S.; Schwartz, R.A.; Patil, A.; Grabbe, S.; Goldust, M. Treatment options for leishmaniasis. Clin. Exp. Dermatol. 2022, 47, 516–521. [Google Scholar] [CrossRef]
- Kumar, P.; Kumar, P.; Singh, N.; Khajuria, S.; Patel, R.; Rajana, V.K.; Mandal, D.; Velayutham, R. Limitations of current chemotherapy and future of nanoformulation-based AmB delivery for visceral leishmaniasis-An updated review. Front. Bioeng. Biotechnol. 2022, 10, 1016925. [Google Scholar] [CrossRef]
- Zahedifard, F.; Lee, H.; No, J.H.; Salimi, M.; Seyed, N.; Asoodeh, A.; Rafati, S. Anti-leishmanial activity of Brevinin 2R and its Lauric acid conjugate type against L. major: In vitro mechanism of actions and in vivo treatment potentials. PLoS Negl. Trop. Dis. 2019, 13, e0007217. [Google Scholar] [CrossRef] [Green Version]
- Souza, G.S.; de Carvalho, L.P.; de Melo, E.J.T.; Gomes, V.M.; Carvalho, A.O. The toxic effect of Vu-Defr, a defensin from Vigna unguiculata seeds, on Leishmania amazonensis is associated with reactive oxygen species production, mitochondrial dysfunction, and plasma membrane perturbation. Can. J. Microbiol. 2018, 64, 455–464. [Google Scholar] [CrossRef] [Green Version]
- Jhingran, A.; Chawla, B.; Saxena, S.; Barrett, M.P.; Madhubala, R. Paromomycin: Uptake and resistance in Leishmania donovani. Mol. Biochem. Parasitol. 2009, 164, 111–117. [Google Scholar] [CrossRef] [Green Version]
- Pinto-Martinez, A.K.; Rodriguez-Duran, J.; Serrano-Martin, X.; Hernandez-Rodriguez, V.; Benaim, G. Mechanism of Action of Miltefosine on Leishmania donovani Involves the Impairment of Acidocalcisome Function and the Activation of the Sphingosine-Dependent Plasma Membrane Ca2+ Channel. Antimicrob. Agents Chemother. 2018, 62, e01614-17. [Google Scholar] [CrossRef] [Green Version]
- Paris, C.; Loiseau, P.M.; Bories, C.; Breard, J. Miltefosine induces apoptosis-like death in Leishmania donovani promastigotes. Antimicrob. Agents Chemother. 2004, 48, 852–859. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Souza, G.S.; de Carvalho, L.P.; de Melo, E.J.T.; da Silva, F.C.V.; Machado, O.L.T.; Gomes, V.M.; de Oliveira Carvalho, A. A synthetic peptide derived of the beta(2)-beta(3) loop of the plant defensin from Vigna unguiculata seeds induces Leishmania amazonensis apoptosis-like cell death. Amino Acids 2019, 51, 1633–1648. [Google Scholar] [CrossRef] [PubMed]
- Andre, S.; Raja, Z.; Humblot, V.; Piesse, C.; Foulon, T.; Sereno, D.; Oury, B.; Ladram, A. Functional Characterization of Temporin-SHe, a New Broad-Spectrum Antibacterial and Leishmanicidal Temporin-SH Paralog from the Sahara Frog (Pelophylax saharicus). Int. J. Mol. Sci. 2020, 21, 6713. [Google Scholar] [CrossRef] [PubMed]
- El-Dirany, R.; Fernandez-Rubio, C.; Pena-Guerrero, J.; Moreno, E.; Larrea, E.; Espuelas, S.; Abdel-Sater, F.; Brandenburg, K.; Martinez-de-Tejada, G.; Nguewa, P. Repurposing the Antibacterial Agents Peptide 19-4LF and Peptide 19-2.5 for Treatment of Cutaneous Leishmaniasis. Pharmaceutics 2022, 14, 2528. [Google Scholar] [CrossRef] [PubMed]
- Khanra, S.; Kumar, Y.P.; Dash, J.; Banerjee, R. In vitro screening of known drugs identified by scaffold hopping techniques shows promising leishmanicidal activity for suramin and netilmicin. BMC Res. Notes 2018, 11, 319. [Google Scholar] [CrossRef]
- Tiwari, B.; Pahuja, R.; Kumar, P.; Rath, S.K.; Gupta, K.C.; Goyal, N. Nanotized Curcumin and Miltefosine, a Potential Combination for Treatment of Experimental Visceral Leishmaniasis. Antimicrob. Agents Chemother. 2017, 61, e01169-16. [Google Scholar] [CrossRef] [Green Version]
- Aqeele, G.; Shayan, P.; Ebrahimzade Abkooh, E.; Mohebali, M. Evaluation of curcumin and CM11 peptide alone and in combination against amastigote form of Iranian strain of L. major (MRHO/IR75/ER) in vitro. Exp. Parasitol. 2021, 229, 108151. [Google Scholar] [CrossRef]
- Aqeele, G.; Shayan, P.; Ebrahimzadeh, E.; Mohebali, M.; Khalili, S. Determination of the Effective Dose of Curcumin alone and in Combination with Antimicrobial Peptide CM11 on Promastigote Forms of Iranian Strain of L. major (MRHO/IR/75/ER). Arch. Razi Inst. 2019, 74, 413–422. [Google Scholar] [CrossRef]
- Wijnant, G.-J.; Dumetz, F.; Dirkx, L.; Bulté, D.; Cuypers, B.; Van Bocxlaer, K.; Hendrickx, S. Tackling Drug Resistance and Other Causes of Treatment Failure in Leishmaniasis. Front. Trop. Dis. 2022, 3, 837460. [Google Scholar] [CrossRef]
- Sanchez-Valdez, F.J.; Padilla, A.; Wang, W.; Orr, D.; Tarleton, R.L. Spontaneous dormancy protects Trypanosoma cruzi during extended drug exposure. eLife 2018, 7, e34039. [Google Scholar] [CrossRef]
- Moretta, A.; Scieuzo, C.; Petrone, A.M.; Salvia, R.; Manniello, M.D.; Franco, A.; Lucchetti, D.; Vassallo, A.; Vogel, H.; Sgambato, A.; et al. Antimicrobial Peptides: A New Hope in Biomedical and Pharmaceutical Fields. Front. Cell Infect. Microbiol. 2021, 11, 668632. [Google Scholar] [CrossRef]
- Alberola, J.; Rodriguez, A.; Francino, O.; Roura, X.; Rivas, L.; Andreu, D. Safety and efficacy of antimicrobial peptides against naturally acquired leishmaniasis. Antimicrob. Agents Chemother. 2004, 48, 641–643. [Google Scholar] [CrossRef] [Green Version]
- Catisti, R.; Uyemura, S.A.; Docampo, R.; Vercesi, A.E. Calcium mobilization by arachidonic acid in trypanosomatids. Mol. Biochem. Parasitol. 2000, 105, 261–271. [Google Scholar] [CrossRef]
- Zhang, Q.Y.; Yan, Z.B.; Meng, Y.M.; Hong, X.Y.; Shao, G.; Ma, J.J.; Cheng, X.R.; Liu, J.; Kang, J.; Fu, C.Y. Antimicrobial peptides: Mechanism of action, activity and clinical potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef]
- Marr, A.K.; McGwire, B.S.; McMaster, W.R. Modes of action of Leishmanicidal antimicrobial peptides. Future Microbiol. 2012, 7, 1047–1059. [Google Scholar] [CrossRef]
- McGwire, B.S.; Kulkarni, M.M. Interactions of antimicrobial peptides with Leishmania and trypanosomes and their functional role in host parasitism. Exp. Parasitol. 2010, 126, 397–405. [Google Scholar] [CrossRef]
- Santos, F.A.; Cruz, G.S.; Vieira, F.A.; Queiroz, B.R.S.; Freitas, C.D.T.; Mesquita, F.P.; Souza, P.F.N. Systematic review of antiprotozoal potential of antimicrobial peptides. Acta Trop. 2022, 236, 106675. [Google Scholar] [CrossRef]
- Mesa-Galloso, H.; Valiente, P.A.; Valdes-Tresanco, M.E.; Epand, R.F.; Lanio, M.E.; Epand, R.M.; Alvarez, C.; Tieleman, D.P.; Ros, U. Membrane Remodeling by the Lytic Fragment of SticholysinII: Implications for the Toroidal Pore Model. Biophys. J. 2019, 117, 1563–1576. [Google Scholar] [CrossRef]
- Riezk, A.; Raynes, J.G.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Chitosan and Its Derivatives against Leishmania major and Leishmania mexicana In Vitro. Antimicrob. Agents Chemother. 2020, 64, e01772-19. [Google Scholar] [CrossRef] [Green Version]
- Soussi, S.; Essid, R.; Karkouch, I.; Saad, H.; Bachkouel, S.; Aouani, E.; Limam, F.; Tabbene, O. Effect of Lipopeptide-Loaded Chitosan Nanoparticles on Candida albicans Adhesion and on the Growth of Leishmania major. Appl. Biochem. Biotechnol. 2021, 193, 3732–3752. [Google Scholar] [CrossRef]
- Riezk, A.; Van Bocxlaer, K.; Yardley, V.; Murdan, S.; Croft, S.L. Activity of Amphotericin B-Loaded Chitosan Nanoparticles against Experimental Cutaneous Leishmaniasis. Molecules 2020, 25, 4002. [Google Scholar] [CrossRef] [PubMed]
- Registre, C.; Soares, R.; Rubio, K.T.S.; Santos, O.D.H.; Carneiro, S.P. A Systematic Review of Drug-Carrying Nanosystems Used in the Treatment of Leishmaniasis. ACS Infect. Dis. 2023, 9, 423–449. [Google Scholar] [CrossRef] [PubMed]
- Bahar, A.A.; Ren, D. Antimicrobial peptides. Pharmaceuticals 2013, 6, 1543–1575. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellavita, R.; Braccia, S.; Galdiero, S.; Falanga, A. Glycosylation and Lipidation Strategies: Approaches for Improving Antimicrobial Peptide Efficacy. Pharmaceuticals 2023, 16, 439. [Google Scholar] [CrossRef]
- Torres, M.D.T.; Sothiselvam, S.; Lu, T.K.; de la Fuente-Nunez, C. Peptide Design Principles for Antimicrobial Applications. J. Mol. Biol. 2019, 431, 3547–3567. [Google Scholar] [CrossRef]
- Abdildinova, A.; Kurth, M.J.; Gong, Y.D. Heterocycles as a Peptidomimetic Scaffold: Solid-Phase Synthesis Strategies. Pharmaceuticals 2021, 14, 449. [Google Scholar] [CrossRef]
- Pena-Carrillo, M.S.; Pinos-Tamayo, E.A.; Mendes, B.; Dominguez-Borbor, C.; Proano-Bolanos, C.; Miguel, D.C.; Almeida, J.R. Dissection of phospholipases A(2) reveals multifaceted peptides targeting cancer cells, Leishmania and bacteria. Bioorg. Chem. 2021, 114, 105041. [Google Scholar] [CrossRef]
- Diaz-Garrido, P.; Cardenas-Guerra, R.E.; Martinez, I.; Poggio, S.; Rodriguez-Hernandez, K.; Rivera-Santiago, L.; Ortega-Lopez, J.; Sanchez-Esquivel, S.; Espinoza, B. Differential activity on trypanosomatid parasites of a novel recombinant defensin type 1 from the insect Triatoma (Meccus) pallidipennis. Insect. Biochem. Mol. Biol. 2021, 139, 103673. [Google Scholar] [CrossRef]
- Xu, W.; Zhu, X.; Tan, T.; Li, W.; Shan, A. Design of embedded-hybrid antimicrobial peptides with enhanced cell selectivity and anti-biofilm activity. PLoS ONE 2014, 9, e98935. [Google Scholar] [CrossRef]
- Akuffo, H.; Hultmark, D.; Engstom, A.; Frohlich, D.; Kimbrell, D. Drosophila antibacterial protein, cecropin A, differentially affects non-bacterial organisms such as Leishmania in a manner different from other amphipathic peptides. Int. J. Mol. Med. 1998, 1, 77–82. [Google Scholar] [CrossRef]
- Diaz-Achirica, P.; Ubach, J.; Guinea, A.; Andreu, D.; Rivas, L. The plasma membrane of Leishmania donovani promastigotes is the main target for CA(1-8)M(1-18), a synthetic cecropin A-melittin hybrid peptide. Biochem. J. 1998, 330 Pt 1, 453–460. [Google Scholar] [CrossRef]
- Le, C.F.; Fang, C.M.; Sekaran, S.D. Intracellular Targeting Mechanisms by Antimicrobial Peptides. Antimicrob. Agents Chemother. 2017, 61, e02340-16. [Google Scholar] [CrossRef] [Green Version]
- Sabia Junior, E.F.; Menezes, L.F.S.; de Araujo, I.F.S.; Schwartz, E.F. Natural Occurrence in Venomous Arthropods of Antimicrobial Peptides Active against Protozoan Parasites. Toxins 2019, 11, 563. [Google Scholar] [CrossRef] [Green Version]
- Brogden, K.A. Antimicrobial peptides: Pore formers or metabolic inhibitors in bacteria? Nat. Rev. Microbiol. 2005, 3, 238–250. [Google Scholar] [CrossRef]
- Lazzaro, B.P.; Zasloff, M.; Rolff, J. Antimicrobial peptides: Application informed by evolution. Science 2020, 368, eaau5480. [Google Scholar] [CrossRef]
- De Barros, N.B.; Aragao Macedo, S.R.; Ferreira, A.S.; Tagliari, M.P.; Kayano, A.M.; Nicolete, L.D.F.; Soares, A.M.; Nicolete, R. ASP49-phospholipase A(2)-loaded liposomes as experimental therapy in cutaneous leishmaniasis model. Int. Immunopharmacol. 2018, 55, 128–132. [Google Scholar] [CrossRef]
- Mendes, B.; Almeida, J.R.; Vale, N.; Gomes, P.; Gadelha, F.R.; Da Silva, S.L.; Miguel, D.C. Potential use of 13-mer peptides based on phospholipase and oligoarginine as leishmanicidal agents. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2019, 226, 108612. [Google Scholar] [CrossRef]
- Amand, H.L.; Rydberg, H.A.; Fornander, L.H.; Lincoln, P.; Norden, B.; Esbjorner, E.K. Cell surface binding and uptake of arginine- and lysine-rich penetratin peptides in absence and presence of proteoglycans. Biochim. Biophys. Acta 2012, 1818, 2669–2678. [Google Scholar] [CrossRef] [Green Version]
- Perez-Cordero, J.J.; Lozano, J.M.; Cortes, J.; Delgado, G. Leishmanicidal activity of synthetic antimicrobial peptides in an infection model with human dendritic cells. Peptides 2011, 32, 683–690. [Google Scholar] [CrossRef]
- Lofgren, S.E.; Miletti, L.C.; Steindel, M.; Bachere, E.; Barracco, M.A. Trypanocidal and leishmanicidal activities of different antimicrobial peptides (AMPs) isolated from aquatic animals. Exp. Parasitol. 2008, 118, 197–202. [Google Scholar] [CrossRef]
- Kumar, V.; Chugh, A. Peptide-mediated leishmaniasis management strategy: Tachyplesin emerges as an effective anti-leishmanial peptide against Leishmania donovani. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183629. [Google Scholar] [CrossRef] [PubMed]
- Silva, T.; Abengozar, M.A.; Fernandez-Reyes, M.; Andreu, D.; Nazmi, K.; Bolscher, J.G.; Bastos, M.; Rivas, L. Enhanced leishmanicidal activity of cryptopeptide chimeras from the active N1 domain of bovine lactoferrin. Amino Acids 2012, 43, 2265–2277. [Google Scholar] [CrossRef] [PubMed]
- Keller, A.A.; Breitling, R.; Hemmerich, P.; Kappe, K.; Braun, M.; Wittig, B.; Schaefer, B.; Lorkowski, S.; Reissmann, S. Transduction of proteins into leishmania tarentolae by formation of non-covalent complexes with cell-penetrating peptides. J. Cell Biochem. 2014, 115, 243–252. [Google Scholar] [CrossRef] [PubMed]
- Ajasin, D.; Eugenin, E.A. HIV-1 Tat: Role in Bystander Toxicity. Front. Cell Infect. Microbiol. 2020, 10, 61. [Google Scholar] [CrossRef] [Green Version]
- Sadiq, I.Z.; Muhammad, A.; Mada, S.B.; Ibrahim, B.; Umar, U.A. Biotherapeutic effect of cell-penetrating peptides against microbial agents: A review. Tissue Barriers 2022, 10, 1995285. [Google Scholar] [CrossRef] [PubMed]
- de la Torre, B.G.; Hornillos, V.; Luque-Ortega, J.R.; Abengozar, M.A.; Amat-Guerri, F.; Acuna, A.U.; Rivas, L.; Andreu, D. A BODIPY-embedding miltefosine analog linked to cell-penetrating Tat(48-60) peptide favors intracellular delivery and visualization of the antiparasitic drug. Amino Acids 2014, 46, 1047–1058. [Google Scholar] [CrossRef] [Green Version]
- Defaus, S.; Gallo, M.; Abengozar, M.A.; Rivas, L.; Andreu, D. A Synthetic Strategy for Conjugation of Paromomycin to Cell-Penetrating Tat(48-60) for Delivery and Visualization into Leishmania Parasites. Int. J. Pept. 2017, 2017, 4213037. [Google Scholar] [CrossRef] [Green Version]
- Luque-Ortega, J.R.; Rivas, L. Characterization of the leishmanicidal activity of antimicrobial peptides. Methods Mol. Biol. 2010, 618, 393–420. [Google Scholar] [CrossRef]
- Gulsen, S.H.; Tileklioglu, E.; Bode, E.; Cimen, H.; Ertabaklar, H.; Ulug, D.; Ertug, S.; Wenski, S.L.; Touray, M.; Hazir, C.; et al. Antiprotozoal activity of different Xenorhabdus and Photorhabdus bacterial secondary metabolites and identification of bioactive compounds using the easyPACId approach. Sci. Rep. 2022, 12, 10779. [Google Scholar] [CrossRef]
- Bera, A.; Singh, S.; Nagaraj, R.; Vaidya, T. Induction of autophagic cell death in Leishmania donovani by antimicrobial peptides. Mol. Biochem. Parasitol. 2003, 127, 23–35. [Google Scholar] [CrossRef]
- Dong, Z.; Hu, H.; Yu, X.; Tan, L.; Ma, C.; Xi, X.; Li, L.; Wang, L.; Zhou, M.; Chen, T.; et al. Novel Frog Skin-Derived Peptide Dermaseptin-PP for Lung Cancer Treatment: In vitro/vivo Evaluation and Anti-tumor Mechanisms Study. Front. Chem. 2020, 8, 476. [Google Scholar] [CrossRef]
- Brand, G.D.; Santos, R.C.; Arake, L.M.; Silva, V.G.; Veras, L.M.; Costa, V.; Costa, C.H.; Kuckelhaus, S.S.; Alexandre, J.G.; Feio, M.J.; et al. The skin secretion of the amphibian Phyllomedusa nordestina: A source of antimicrobial and antiprotozoal peptides. Molecules 2013, 18, 7058–7070. [Google Scholar] [CrossRef] [Green Version]
- Kuckelhaus, S.A.; Leite, J.R.; Muniz-Junqueira, M.I.; Sampaio, R.N.; Bloch, C., Jr.; Tosta, C.E. Antiplasmodial and antileishmanial activities of phylloseptin-1, an antimicrobial peptide from the skin secretion of Phyllomedusa azurea (Amphibia). Exp. Parasitol. 2009, 123, 11–16. [Google Scholar] [CrossRef]
- Kuckelhaus, S.A.S.; Aquino, D.S.; Borges, T.K.; Moreira, D.C.; Leite, L.M.; Muniz-Junqueira, M.I.; Kuckelhaus, C.S.; Romero, G.A.S.; Prates, M.V.; Bloch, C., Jr.; et al. Phylloseptin-1 is Leishmanicidal for Amastigotes of Leishmaniaamazonensis Inside Infected Macrophages. Int. J. Environ. Res. Public Health 2020, 17, 4856. [Google Scholar] [CrossRef]
- Savoia, D.; Guerrini, R.; Marzola, E.; Salvadori, S. Synthesis and antimicrobial activity of dermaseptin S1 analogues. Bioorg. Med. Chem. 2008, 16, 8205–8209. [Google Scholar] [CrossRef] [Green Version]
- Brand, G.D.; Leite, J.R.; de Sa Mandel, S.M.; Mesquita, D.A.; Silva, L.P.; Prates, M.V.; Barbosa, E.A.; Vinecky, F.; Martins, G.R.; Galasso, J.H.; et al. Novel dermaseptins from Phyllomedusa hypochondrialis (Amphibia). Biochem. Biophys. Res. Commun. 2006, 347, 739–746. [Google Scholar] [CrossRef]
- Hernandez, C.; Mor, A.; Dagger, F.; Nicolas, P.; Hernandez, A.; Benedetti, E.L.; Dunia, I. Functional and structural damage in Leishmania mexicana exposed to the cationic peptide dermaseptin. Eur. J. Cell Biol. 1992, 59, 414–424. [Google Scholar]
- Zampa, M.F.; Araujo, I.M.; Costa, V.; Nery Costa, C.H.; Santos, J.R., Jr.; Zucolotto, V.; Eiras, C.; Leite, J.R. Leishmanicidal activity and immobilization of dermaseptin 01 antimicrobial peptides in ultrathin films for nanomedicine applications. Nanomed. Nanotechnol. Biol. Med. 2009, 5, 352–358. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Papo, N.; Saugar, J.M.; Barra, D.; Shai, Y.; Simmaco, M.; Rivas, L. Effect of natural L- to D-amino acid conversion on the organization, membrane binding, and biological function of the antimicrobial peptides bombinins H. Biochemistry 2006, 45, 4266–4276. [Google Scholar] [CrossRef]
- Abbassi, F.; Oury, B.; Blasco, T.; Sereno, D.; Bolbach, G.; Nicolas, P.; Hani, K.; Amiche, M.; Ladram, A. Isolation, characterization and molecular cloning of new temporins from the skin of the North African ranid Pelophylax saharica. Peptides 2008, 29, 1526–1533. [Google Scholar] [CrossRef]
- Mangoni, M.L.; Saugar, J.M.; Dellisanti, M.; Barra, D.; Simmaco, M.; Rivas, L. Temporins, small antimicrobial peptides with leishmanicidal activity. J. Biol. Chem. 2005, 280, 984–990. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Luque-Ortega, J.R.; Saugar, J.M.; Chiva, C.; Andreu, D.; Rivas, L. Identification of new leishmanicidal peptide lead structures by automated real-time monitoring of changes in intracellular ATP. Biochem. J. 2003, 375, 221–230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chicharro, C.; Granata, C.; Lozano, R.; Andreu, D.; Rivas, L. N-terminal fatty acid substitution increases the leishmanicidal activity of CA(1-7)M(2-9), a cecropin-melittin hybrid peptide. Antimicrob. Agents Chemother. 2001, 45, 2441–2449. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fernandez-Reyes, M.; Diaz, D.; de la Torre, B.G.; Cabrales-Rico, A.; Valles-Miret, M.; Jimenez-Barbero, J.; Andreu, D.; Rivas, L. Lysine N(epsilon)-trimethylation, a tool for improving the selectivity of antimicrobial peptides. J. Med. Chem. 2010, 53, 5587–5596. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A.; Rodriguez-Palenzuela, P.; Garcia-Olmedo, F.; Rivas, L. Leishmania donovani: Thionins, plant antimicrobial peptides with leishmanicidal activity. Exp. Parasitol. 2009, 122, 247–249. [Google Scholar] [CrossRef] [Green Version]
- Vila-Perello, M.; Sanchez-Vallet, A.; Garcia-Olmedo, F.; Molina, A.; Andreu, D. Synthetic and structural studies on Pyrularia pubera thionin: A single-residue mutation enhances activity against Gram-negative bacteria. FEBS Lett. 2003, 536, 215–219. [Google Scholar] [CrossRef] [Green Version]
- Stec, B. Plant thionins--the structural perspective. Cell Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef]
- Mello, C.P.; Lima, D.B.; Menezes, R.R.; Bandeira, I.C.; Tessarolo, L.D.; Sampaio, T.L.; Falcao, C.B.; Radis-Baptista, G.; Martins, A.M. Evaluation of the antichagasic activity of batroxicidin, a cathelicidin-related antimicrobial peptide found in Bothrops atrox venom gland. Toxicon 2017, 130, 56–62. [Google Scholar] [CrossRef]
- Kulkarni, M.M.; McMaster, W.R.; Kamysz, E.; Kamysz, W.; Engman, D.M.; McGwire, B.S. The major surface-metalloprotease of the parasitic protozoan, Leishmania, protects against antimicrobial peptide-induced apoptotic killing. Mol. Microbiol. 2006, 62, 1484–1497. [Google Scholar] [CrossRef]
- Jaynes, J.M.; Burton, C.A.; Barr, S.B.; Jeffers, G.W.; Julian, G.R.; White, K.L.; Enright, F.M.; Klei, T.R.; Laine, R.A. In vitro cytocidal effect of novel lytic peptides on Plasmodium falciparum and Trypanosoma cruzi. FASEB J. 1988, 2, 2878–2883. [Google Scholar] [CrossRef] [Green Version]
- Bandeira, I.C.J.; Bandeira-Lima, D.; Mello, C.P.; Pereira, T.P.; De Menezes, R.; Sampaio, T.L.; Falcao, C.B.; Radis-Baptista, G.; Martins, A.M.C. Antichagasic effect of crotalicidin, a cathelicidin-like vipericidin, found in Crotalus durissus terrificus rattlesnake’s venom gland. Parasitology 2018, 145, 1059–1064. [Google Scholar] [CrossRef]
- Boulanger, N.; Lowenberger, C.; Volf, P.; Ursic, R.; Sigutova, L.; Sabatier, L.; Svobodova, M.; Beverley, S.M.; Spath, G.; Brun, R.; et al. Characterization of a defensin from the sand fly Phlebotomus duboscqi induced by challenge with bacteria or the protozoan parasite Leishmania major. Infect. Immun. 2004, 72, 7140–7146. [Google Scholar] [CrossRef] [Green Version]
- Madison, M.N.; Kleshchenko, Y.Y.; Nde, P.N.; Simmons, K.J.; Lima, M.F.; Villalta, F. Human defensin alpha-1 causes Trypanosoma cruzi membrane pore formation and induces DNA fragmentation, which leads to trypanosome destruction. Infect. Immun. 2007, 75, 4780–4791. [Google Scholar] [CrossRef] [Green Version]
- Kleschenko, Y.E.; Karpenko, L.P.; Villalta, F. Effects of human defensin-alpha(1)on Trypanosoma cruzi trypomastigotes in vitro. Bull. Exp. Biol. Med. 2010, 149, 731–733. [Google Scholar] [CrossRef]
- Roch, P.; Beschin, A.; Bernard, E. Antiprotozoan and Antiviral Activities of Non-cytotoxic Truncated and Variant Analogues of Mussel Defensin. Evid. Based Complement Alternat. Med. 2004, 1, 167–174. [Google Scholar] [CrossRef] [Green Version]
- Luque-Ortega, J.R.; van’t Hof, W.; Veerman, E.C.; Saugar, J.M.; Rivas, L. Human antimicrobial peptide histatin 5 is a cell-penetrating peptide targeting mitochondrial ATP synthesis in Leishmania. FASEB J. 2008, 22, 1817–1828. [Google Scholar] [CrossRef] [Green Version]
- Brand, G.D.; Leite, J.R.; Silva, L.P.; Albuquerque, S.; Prates, M.V.; Azevedo, R.B.; Carregaro, V.; Silva, J.S.; Sa, V.C.; Brandao, R.A.; et al. Dermaseptins from Phyllomedusa oreades and Phyllomedusa distincta. Anti-Trypanosoma cruzi activity without cytotoxicity to mammalian cells. J. Biol. Chem. 2002, 277, 49332–49340. [Google Scholar] [CrossRef] [Green Version]
- Abengozar, M.A.; Cebrian, R.; Saugar, J.M.; Garate, T.; Valdivia, E.; Martinez-Bueno, M.; Maqueda, M.; Rivas, L. Enterocin AS-48 as Evidence for the Use of Bacteriocins as New Leishmanicidal Agents. Antimicrob. Agents Chemother. 2017, 61, e02288-16. [Google Scholar] [CrossRef] [Green Version]
- Silva, P.I., Jr.; Daffre, S.; Bulet, P. Isolation and characterization of gomesin, an 18-residue cysteine-rich defense peptide from the spider Acanthoscurria gomesiana hemocytes with sequence similarities to horseshoe crab antimicrobial peptides of the tachyplesin family. J. Biol. Chem. 2000, 275, 33464–33470. [Google Scholar] [CrossRef] [Green Version]
- Monteiro, M.L.; Lima, D.B.; Menezes, R.; Sampaio, T.L.; Silva, B.P.; Serra Nunes, J.V.; Cavalcanti, M.M.; Morlighem, J.E.; Martins, A.M.C. Antichagasic effect of hemocyanin derived from antimicrobial peptides of penaeus monodon shrimp. Exp. Parasitol. 2020, 215, 107930. [Google Scholar] [CrossRef]
- Lima, D.B.; Mello, C.P.; Bandeira, I.C.J.; Pessoa Bezerra de Menezes, R.R.P.; Sampaio, T.L.; Falcao, C.B.; Morlighem, J.R.L.; Radis-Baptista, G.; Martins, A.M.C. The dinoponeratoxin peptides from the giant ant Dinoponera quadriceps display in vitro antitrypanosomal activity. Biol. Chem. 2018, 399, 187–196. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, M.L.; Lima, D.B.; Freire, K.A.; Nicolaski Pedron, C.; Magalhaes, E.P.; Silva, B.P.; Garcia-Jareno, A.B.; De Oliveira, C.S.; Nunes, J.V.S.; Marinho, M.M.; et al. Rational design of a trypanocidal peptide derived from Dinoponera quadriceps venom. Eur. J. Med. Chem. 2022, 241, 114624. [Google Scholar] [CrossRef] [PubMed]
- Guerrero, E.; Saugar, J.M.; Matsuzaki, K.; Rivas, L. Role of positional hydrophobicity in the leishmanicidal activity of magainin 2. Antimicrob. Agents Chemother. 2004, 48, 2980–2986. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adade, C.M.; Oliveira, I.R.; Pais, J.A.; Souto-Padron, T. Melittin peptide kills Trypanosoma cruzi parasites by inducing different cell death pathways. Toxicon 2013, 69, 227–239. [Google Scholar] [CrossRef]
- Jacobs, T.; Bruhn, H.; Gaworski, I.; Fleischer, B.; Leippe, M. NK-lysin and its shortened analog NK-2 exhibit potent activities against Trypanosoma cruzi. Antimicrob. Agents Chemother. 2003, 47, 607–613. [Google Scholar] [CrossRef] [Green Version]
- Freire, K.A.; Torres, M.T.; Lima, D.B.; Monteiro, M.L.; Bezerra de Menezes, R.; Martins, A.M.C.; Oliveira, V.X., Jr. Wasp venom peptide as a new antichagasic agent. Toxicon 2020, 181, 71–78. [Google Scholar] [CrossRef]
- Pinto, E.G.; Pimenta, D.C.; Antoniazzi, M.M.; Jared, C.; Tempone, A.G. Antimicrobial peptides isolated from Phyllomedusa nordestina (Amphibia) alter the permeability of plasma membrane of Leishmania and Trypanosoma cruzi. Exp. Parasitol. 2013, 135, 655–660. [Google Scholar] [CrossRef]
- Amorim-Carmo, B.; Daniele-Silva, A.; Parente, A.M.S.; Furtado, A.A.; Carvalho, E.; Oliveira, J.W.F.; Santos, E.C.G.; Silva, M.S.; Silva, S.R.B.; Silva-Junior, A.A.; et al. Potent and Broad-Spectrum Antimicrobial Activity of Analogs from the Scorpion Peptide Stigmurin. Int. J. Mol. Sci. 2019, 20, 623. [Google Scholar] [CrossRef] [Green Version]
- Souto-Padron, T. The surface charge of trypanosomatids. An. Acad. Bras. Cienc. 2002, 74, 649–675. [Google Scholar] [CrossRef] [Green Version]
- Souza, A.L.; Faria, R.X.; Calabrese, K.S.; Hardoim, D.J.; Taniwaki, N.; Alves, L.A.; De Simone, S.G. Temporizin and Temporizin-1 Peptides as Novel Candidates for Eliminating Trypanosoma cruzi. PLoS ONE 2016, 11, e0157673. [Google Scholar] [CrossRef] [Green Version]
- Pedron, C.N.; Freire, K.A.; Torres, M.T.; Lima, D.B.; Monteiro, M.L.; Menezes, R.; Martins, A.M.C.; Oliveira, V.X. Arg-substituted VmCT1 analogs reveals promising candidate for the development of new antichagasic agent. Parasitology 2020, 147, 1810–1818. [Google Scholar] [CrossRef]
- Clemente, C.M.; Pineda, T.; Yepes, L.M.; Upegui, Y.; Allemandi, D.A.; Robledo, S.M.; Ravetti, S. Eugenol carbonate activity against Plasmodium falciparum, Leishmania braziliensis, and Trypanosoma cruzi. Arch. Pharm. 2022, 355, e2100432. [Google Scholar] [CrossRef]
- Franco, C.H.; Alcantara, L.M.; Chatelain, E.; Freitas-Junior, L.; Moraes, C.B. Drug Discovery for Chagas Disease: Impact of Different Host Cell Lines on Assay Performance and Hit Compound Selection. Trop. Med. Infect. Dis. 2019, 4, 82. [Google Scholar] [CrossRef] [Green Version]
- Martinez-Peinado, N.; Martori, C.; Cortes-Serra, N.; Sherman, J.; Rodriguez, A.; Gascon, J.; Alberola, J.; Pinazo, M.J.; Rodriguez-Cortes, A.; Alonso-Padilla, J. Anti-Trypanosoma cruzi Activity of Metabolism Modifier Compounds. Int. J. Mol. Sci. 2021, 22, 688. [Google Scholar] [CrossRef]
- Milagre, M.M.; Branquinho, R.T.; Goncalves, M.F.; de Assis, G.; de Oliveira, M.T.; Reis, L.; Saude-Guimaraes, D.A.; de Lana, M. Activity of the sesquiterpene lactone goyazensolide against Trypanosoma cruzi in vitro and in vivo. Parasitology 2020, 147, 108–119. [Google Scholar] [CrossRef]
- Andriani, G.; Chessler, A.D.; Courtemanche, G.; Burleigh, B.A.; Rodriguez, A. Activity in vivo of anti-Trypanosoma cruzi compounds selected from a high throughput screening. PLoS Negl. Trop. Dis. 2011, 5, e1298. [Google Scholar] [CrossRef]
- Buckner, F.S.; Verlinde, C.L.; La Flamme, A.C.; Van Voorhis, W.C. Efficient technique for screening drugs for activity against Trypanosoma cruzi using parasites expressing beta-galactosidase. Antimicrob. Agents Chemother. 1996, 40, 2592–2597. [Google Scholar] [CrossRef] [Green Version]
- Lin, C.; Ferreira de Almeida Fiuza, L.; Cardoso Santos, C.; Ferreira Nunes, D.; Cruz Moreira, O.; Bouton, J.; Karalic, I.; Maes, L.; Caljon, G.; Hulpia, F.; et al. 6-Methyl-7-Aryl-7-Deazapurine Nucleosides as Anti-Trypanosoma cruzi Agents: Structure-Activity Relationship and in vivo Efficacy. ChemMedChem 2021, 16, 2231–2253. [Google Scholar] [CrossRef]
- Lin, C.; Jaen Batista, D.D.G.; Mazzeti, A.L.; Donola Girao, R.; de Oliveira, G.M.; Karalic, I.; Hulpia, F.; Soeiro, M.N.C.; Maes, L.; Caljon, G.; et al. N(6)-modification of 7-Deazapurine nucleoside analogues as Anti-Trypanosoma cruzi and anti-Leishmania agents: Structure-activity relationship exploration and In vivo evaluation. Eur. J. Med. Chem. 2022, 231, 114165. [Google Scholar] [CrossRef]
- Ortiz, J.E.; Pineiro, M.; Martinez-Peinado, N.; Barrera, P.; Sosa, M.; Bastida, J.; Alonso-Padilla, J.; Feresin, G.E. Candimine from Hippeastrum escoipense (Amaryllidaceae): Anti-Trypanosoma cruzi activity and synergistic effect with benznidazole. Phytomedicine 2023, 114, 154788. [Google Scholar] [CrossRef]
- Tayama, Y.; Mizukami, S.; Toume, K.; Komatsu, K.; Yanagi, T.; Nara, T.; Tieu, P.; Huy, N.T.; Hamano, S.; Hirayama, K. Anti-Trypanosoma cruzi activity of Coptis rhizome extract and its constituents. Trop. Med. Health 2023, 51, 12. [Google Scholar] [CrossRef] [PubMed]
- Bortoluzzi, A.A.M.; Staffen, I.V.; Banhuk, F.W.; Griebler, A.; Matos, P.K.; Ayala, T.S.; da Silva, E.A.A.; Sarragiotto, M.H.; Schuquel, I.T.A.; Jorge, T.C.M.; et al. Determination of chemical structure and anti-Trypanosoma cruzi activity of extracts from the roots of Lonchocarpus cultratus (Vell.) A.M.G. Azevedo & H.C. Lima. Saudi J. Biol. Sci. 2021, 28, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Meira, C.S.; Guimaraes, E.T.; Dos Santos, J.A.; Moreira, D.R.; Nogueira, R.C.; Tomassini, T.C.; Ribeiro, I.M.; de Souza, C.V.; Ribeiro Dos Santos, R.; Soares, M.B. In vitro and in vivo antiparasitic activity of Physalis angulata L. concentrated ethanolic extract against Trypanosoma cruzi. Phytomedicine 2015, 22, 969–974. [Google Scholar] [CrossRef] [PubMed]
- Beer, M.F.; Reta, G.F.; Puerta, A.; Bivona, A.E.; Alberti, A.S.; Cerny, N.; Malchiodi, E.L.; Tonn, C.E.; Padron, J.M.; Sulsen, V.P.; et al. Oxonitrogenated Derivatives of Eremophilans and Eudesmans: Antiproliferative and Anti-Trypanosoma cruzi Activity. Molecules 2022, 27, 3067. [Google Scholar] [CrossRef] [PubMed]
- Morilla, M.J.; Montanari, J.; Frank, F.; Malchiodi, E.; Corral, R.; Petray, P.; Romero, E.L. Etanidazole in pH-sensitive liposomes: Design, characterization and in vitro/in vivo anti-Trypanosoma cruzi activity. J. Control. Release Off. J. Control. Release Soc. 2005, 103, 599–607. [Google Scholar] [CrossRef]
- Musikant, D.; Leverrier, A.; Bernal, D.; Ferri, G.; Palermo, J.A.; Edreira, M.M. Hybrids of Cinchona Alkaloids and Bile Acids as Antiparasitic Agents Against Trypanosoma cruzi. Molecules 2019, 24, 3168. [Google Scholar] [CrossRef] [Green Version]
- Gulin, J.E.N.; Bisio, M.M.C.; Rocco, D.; Altcheh, J.; Solana, M.E.; Garcia-Bournissen, F. Miltefosine and Benznidazole Combination Improve Anti-Trypanosoma cruzi In Vitro and In Vivo Efficacy. Front. Cell Infect. Microbiol. 2022, 12, 855119. [Google Scholar] [CrossRef]
- Pereira, P.S.; Oliveira, C.V.B.; Maia, A.J.; Tintino, S.R.; Oliveira-Tintino, C.D.M.; Vega-Gomez, M.C.; Rolon, M.; Coronel, C.; Duarte, A.E.; Barros, L.M.; et al. Cytotoxicity of Essential Oil Cordia verbenaceae against Leishmania brasiliensis and Trypanosoma cruzi. Molecules 2021, 26, 4485. [Google Scholar] [CrossRef]
- Roldos, V.; Nakayama, H.; Rolon, M.; Montero-Torres, A.; Trucco, F.; Torres, S.; Vega, C.; Marrero-Ponce, Y.; Heguaburu, V.; Yaluff, G.; et al. Activity of a hydroxybibenzyl bryophyte constituent against Leishmania spp. and Trypanosoma cruzi: In silico, in vitro and in vivo activity studies. Eur. J. Med. Chem. 2008, 43, 1797–1807. [Google Scholar] [CrossRef]
- Swearengen, J.R. Choosing the right animal model for infectious disease research. Anim. Model Exp. Med. 2018, 1, 100–108. [Google Scholar] [CrossRef]
- Laing, G.; Vigilato, M.A.N.; Cleaveland, S.; Thumbi, S.M.; Blumberg, L.; Salahuddin, N.; Abdela-Ridder, B.; Harrison, W. One Health for neglected tropical diseases. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 182–184. [Google Scholar] [CrossRef]
- Kiani, A.K.; Pheby, D.; Henehan, G.; Brown, R.; Sieving, P.; Sykora, P.; Marks, R.; Falsini, B.; Capodicasa, N.; Miertus, S.; et al. Ethical considerations regarding animal experimentation. J. Prev. Med. Hyg. 2022, 63, E255–E266. [Google Scholar] [CrossRef]
- Fischer, M.R.; John, D.; Kautz-Neu, K.; Schermann, A.I.; Schwonberg, K.; von Stebut, E. Animal model for cutaneous leishmaniasis. Methods Mol. Biol. 2013, 961, 389–402. [Google Scholar] [CrossRef]
- Nieto, A.; Domínguez-Bernal, G.; Orden, J.A.; De La Fuente, R.; Madrid-Elena, N.; Carrión, J. Mechanisms of resistance and susceptibility to experimental visceral leishmaniosis: BALB/c mouse versus Syrian hamster model. Vet. Res. 2011, 42, 39. [Google Scholar] [CrossRef] [Green Version]
- Robledo, S.M.; Carrillo, L.M.; Daza, A.; Restrepo, A.M.; Munoz, D.L.; Tobon, J.; Murillo, J.D.; Lopez, A.; Rios, C.; Mesa, C.V.; et al. Cutaneous leishmaniasis in the dorsal skin of hamsters: A useful model for the screening of antileishmanial drugs. J. Vis. Exp. 2012, 3533. [Google Scholar] [CrossRef]
- Murillo, J.; Montoya, A.; Carrillo-Bonilla, L.; Rodriguez, B.; Velez, I.D.; Robledo, S.M. Verification and monitoring of visceral leishmaniasis in hamsters caused by Leishmania infantum, using non-invasive approaches involving ultrasound imaging and blood gases. Exp. Parasitol. 2019, 201, 78–89. [Google Scholar] [CrossRef]
- Chatelain, E.; Scandale, I. Animal models of Chagas disease and their translational value to drug development. Expert. Opin. Drug Discov. 2020, 15, 1381–1402. [Google Scholar] [CrossRef]
- Loria-Cervera, E.N.; Andrade-Narvaez, F.J. Animal models for the study of leishmaniasis immunology. Rev. Inst. Med. Trop. Sao Paulo 2014, 56, 1–11. [Google Scholar] [CrossRef]
- De Lana, M.; Giunchetti, R.C. Dogs as a Model for Chemotherapy of Chagas Disease and Leishmaniasis. Curr. Pharm. Des. 2021, 27, 1741–1756. [Google Scholar] [CrossRef]
- Guedes, P.M.; Veloso, V.M.; Afonso, L.C.; Caliari, M.V.; Carneiro, C.M.; Diniz, L.F.; Marques-da-Silva, E.A.; Caldas, I.S.; Do Valle Matta, M.A.; Souza, S.M.; et al. Development of chronic cardiomyopathy in canine Chagas disease correlates with high IFN-gamma, TNF-alpha, and low IL-10 production during the acute infection phase. Vet. Immunol. Immunopathol. 2009, 130, 43–52. [Google Scholar] [CrossRef]
- Ribeiro, F.F.F.; Moreira, H.T.; de Barros-Filho, A.C.L.; Tanaka, D.M.; Fabricio, C.G.; Oliveira, L.F.L.; Prado, C.M.; Simoes, M.V.; Schmidt, A.; Maciel, B.C.; et al. Prospective analysis of myocardial strain through the evolution of Chagas disease in the hamster animal model. Int. J. Cardiovasc. Imaging 2022, 38, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Greber, K.E.; Dawgul, M. Antimicrobial Peptides Under Clinical Trials. Curr. Top Med. Chem. 2017, 17, 620–628. [Google Scholar] [CrossRef] [PubMed]
- Bellotti, D.; Remelli, M. Lights and Shadows on the Therapeutic Use of Antimicrobial Peptides. Molecules 2022, 27, 4584. [Google Scholar] [CrossRef] [PubMed]
- Perez-Molina, J.A.; Crespillo-Andujar, C.; Bosch-Nicolau, P.; Molina, I. Trypanocidal treatment of Chagas disease. Enferm. Infecc. Microbiol. Clin. Engl. Ed. 2021, 39, 458–470. [Google Scholar] [CrossRef]
- Diamond, G.; Beckloff, N.; Weinberg, A.; Kisich, K.O. The roles of antimicrobial peptides in innate host defense. Curr. Pharm. Des. 2009, 15, 2377–2392. [Google Scholar] [CrossRef] [Green Version]
- Guryanova, S.V.; Ovchinnikova, T.V. Immunomodulatory and Allergenic Properties of Antimicrobial Peptides. Int. J. Mol. Sci. 2022, 23, 2499. [Google Scholar] [CrossRef]
- Martin-Serrano, A.; Gomez, R.; Ortega, P.; de la Mata, F.J. Nanosystems as Vehicles for the Delivery of Antimicrobial Peptides (AMPs). Pharmaceutics 2019, 11, 448. [Google Scholar] [CrossRef] [Green Version]
- Knauer, N.; Pashkina, E.; Apartsin, E. Topological Aspects of the Design of Nanocarriers for Therapeutic Peptides and Proteins. Pharmaceutics 2019, 11, 91. [Google Scholar] [CrossRef] [Green Version]
- Urban, P.; Valle-Delgado, J.J.; Moles, E.; Marques, J.; Diez, C.; Fernandez-Busquets, X. Nanotools for the delivery of antimicrobial peptides. Curr. Drug Targets 2012, 13, 1158–1172. [Google Scholar] [CrossRef]
- Ellington, A.D.; Szostak, J.W. In vitro selection of RNA molecules that bind specific ligands. Nature 1990, 346, 818–822. [Google Scholar] [CrossRef]
- Tuerk, C.; Gold, L. Systematic evolution of ligands by exponential enrichment: RNA ligands to bacteriophage T4 DNA polymerase. Science 1990, 249, 505–510. [Google Scholar] [CrossRef]
- Lee, B.; Park, J.; Ryu, M.; Kim, S.; Joo, M.; Yeom, J.H.; Kim, S.; Park, Y.; Lee, K.; Bae, J. Antimicrobial peptide-loaded gold nanoparticle-DNA aptamer conjugates as highly effective antibacterial therapeutics against Vibrio vulnificus. Sci. Rep. 2017, 7, 13572. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Shin, E.; Yeom, J.H.; Choi, Y.; Joo, M.; Lee, M.; Kim, J.H.; Bae, J.; Lee, K. Gold nanoparticle-DNA aptamer-assisted delivery of antimicrobial peptide effectively inhibits Acinetobacter baumannii infection in mice. J. Microbiol. 2022, 60, 128–136. [Google Scholar] [CrossRef]
- Liu, Y.; Wu, X.; Gao, Y.; Zhang, J.; Zhang, D.; Gu, S.; Zhu, G.; Liu, G.; Li, X. Aptamer-functionalized peptide H3CR5C as a novel nanovehicle for codelivery of fasudil and miRNA-195 targeting hepatocellular carcinoma. Int. J. Nanomed. 2016, 11, 3891–3905. [Google Scholar] [CrossRef] [Green Version]
- Frezza, V.; Pinto-Diez, C.; Fernandez, G.; Soto, M.; Martin, M.E.; Garcia-Sacristan, A.; Gonzalez, V.M. DNA aptamers targeting Leishmania infantum H3 protein as potential diagnostic tools. Anal. Chim. Acta 2020, 1107, 155–163. [Google Scholar] [CrossRef]
- Guerra-Perez, N.; Ramos, E.; Garcia-Hernandez, M.; Pinto, C.; Soto, M.; Martin, M.E.; Gonzalez, V.M. Molecular and Functional Characterization of ssDNA Aptamers that Specifically Bind Leishmania infantum PABP. PLoS ONE 2015, 10, e0140048. [Google Scholar] [CrossRef] [Green Version]
- Martin, M.E.; Garcia-Hernandez, M.; Garcia-Recio, E.M.; Gomez-Chacon, G.F.; Sanchez-Lopez, M.; Gonzalez, V.M. DNA aptamers selectively target Leishmania infantum H2A protein. PLoS ONE 2013, 8, e78886. [Google Scholar] [CrossRef]
- Moreno, M.; Rincon, E.; Pineiro, D.; Fernandez, G.; Domingo, A.; Jimenez-Ruiz, A.; Salinas, M.; Gonzalez, V.M. Selection of aptamers against KMP-11 using colloidal gold during the SELEX process. Biochem. Biophys. Res. Commun. 2003, 308, 214–218. [Google Scholar] [CrossRef]
- Goringer, H.U.; Homann, M.; Lorger, M. In vitro selection of high-affinity nucleic acid ligands to parasite target molecules. Int. J. Parasitol. 2003, 33, 1309–1317. [Google Scholar] [CrossRef]
- Nagarkatti, R.; Bist, V.; Sun, S.; Fortes de Araujo, F.; Nakhasi, H.L.; Debrabant, A. Development of an aptamer-based concentration method for the detection of Trypanosoma cruzi in blood. PLoS ONE 2012, 7, e43533. [Google Scholar] [CrossRef]
- Nagarkatti, R.; de Araujo, F.F.; Gupta, C.; Debrabant, A. Aptamer based, non-PCR, non-serological detection of Chagas disease biomarkers in Trypanosoma cruzi infected mice. PLoS Negl. Trop. Dis. 2014, 8, e2650. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, H.; Magdesian, M.H.; Alves, M.J.; Colli, W. In vitro selection of RNA aptamers that bind to cell adhesion receptors of Trypanosoma cruzi and inhibit cell invasion. J. Biol. Chem. 2002, 277, 20756–20762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zahedifard, F.; Rafati, S. Prospects for antimicrobial peptide-based immunotherapy approaches in Leishmania control. Expert. Rev. Anti Infect. Ther. 2018, 16, 461–469. [Google Scholar] [CrossRef] [PubMed]
| Cell Type/Tissue, Product Code | Organism |
|---|---|
| Normal epithelial kidney cells VERO (ATCC® CCL-81TM) | Cercopithecus aethiops (African green monkey) |
| Normal epithelial kidney LLC-MK2 (ATCC® CCL-7TM) | Maccaca mulata (rhesus monkey) |
| Embryo fibroblast NIH/3T3 (ATCC® CRL-1658TM) | Mus musculus (mouse) |
| Macrophages from reticulum cell sarcoma J774A.1 (ATTC TIB-67™) | Mus musculus (mouse) |
| Myoblast H9c2(2-1) (ATCC® CRL 1446TM) | Rattus norvegicus (rat) |
| Macrophage DH82 cell line | Canis familiaris (dog) |
| Normal tissue lung fibroblast MRC-5 (ATCC® CCL-171™) | Homo sapiens (rat) |
| Epithelial bone osteosarcoma U-2 OS (ATCC® HTB-96™) | Homo sapiens (human) |
| Tissue monocytes U-937 (ATCC® CRL-1593.2TM) | Homo sapiens (human) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robledo, S.M.; Pérez-Silanes, S.; Fernández-Rubio, C.; Poveda, A.; Monzote, L.; González, V.M.; Alonso-Collado, P.; Carrión, J. Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease. Pathogens 2023, 12, 939. https://doi.org/10.3390/pathogens12070939
Robledo SM, Pérez-Silanes S, Fernández-Rubio C, Poveda A, Monzote L, González VM, Alonso-Collado P, Carrión J. Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease. Pathogens. 2023; 12(7):939. https://doi.org/10.3390/pathogens12070939
Chicago/Turabian StyleRobledo, Sara M., Silvia Pérez-Silanes, Celia Fernández-Rubio, Ana Poveda, Lianet Monzote, Víctor M. González, Paloma Alonso-Collado, and Javier Carrión. 2023. "Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease" Pathogens 12, no. 7: 939. https://doi.org/10.3390/pathogens12070939
APA StyleRobledo, S. M., Pérez-Silanes, S., Fernández-Rubio, C., Poveda, A., Monzote, L., González, V. M., Alonso-Collado, P., & Carrión, J. (2023). Neglected Zoonotic Diseases: Advances in the Development of Cell-Penetrating and Antimicrobial Peptides against Leishmaniosis and Chagas Disease. Pathogens, 12(7), 939. https://doi.org/10.3390/pathogens12070939