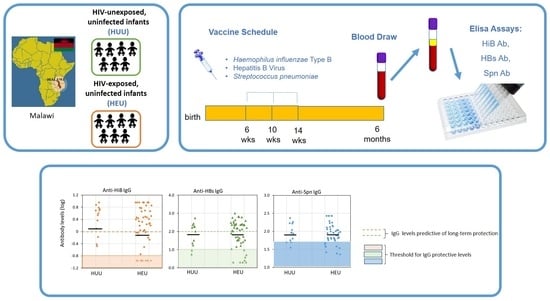
Rates of Seroprotection against Vaccine-Preventable Infectious Diseases in HIV-Exposed and -Unexposed Malawian Infants
Abstract
1. Introduction
2. Materials and Methods
2.1. Population Characteristics
2.2. Laboratory Evaluations
2.3. Statistical Analysis
3. Results
3.1. Population Characteristics
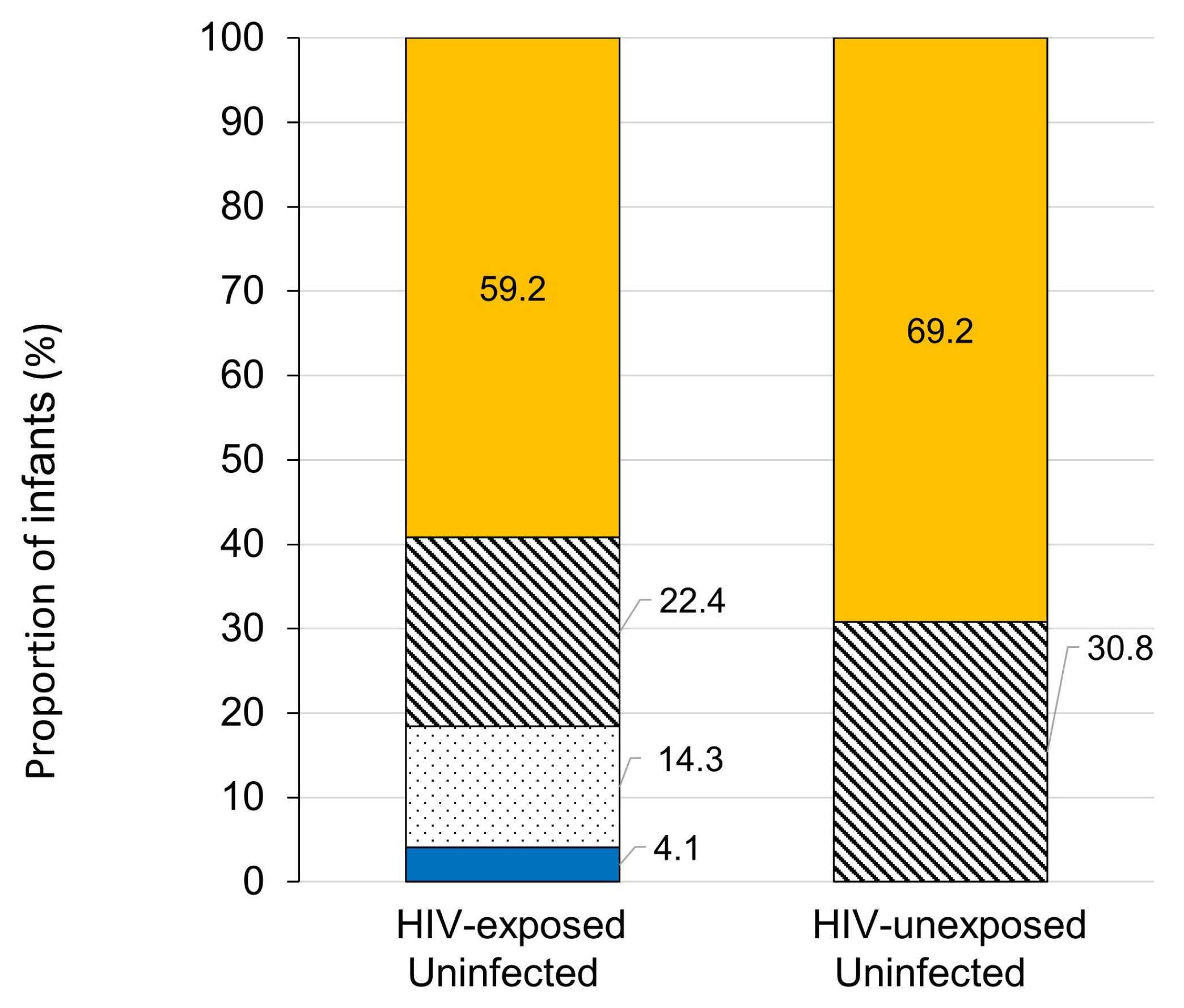
3.2. Seroprotection Rates
3.3. Vaccination Documentation
3.4. Analyses of Seroprotection Levels
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- The World Data Bank, 2022 Prevalence of HIV, Total (% of Population Ages 15–49)—Malawi. Available online: https://data.worldbank.org/indicator/SH.DYN.AIDS.ZS?locations=MW (accessed on 3 May 2023).
- UNICEF Data: Monitoring the Situation of Children and Women 2021. Available online: https://data.unicef.org/topic/child-survival/under-five-mortality/ (accessed on 3 May 2023).
- UNAIDS—Malawi Country Factsheet. 2020. Available online: https://www.unaids.org/en/regionscountries/countries/malawi (accessed on 6 February 2023).
- World Health Organization Regional Office for Africa 2014. T Implementation of Option B+ for Prevention of Mother-to-Child Transmission of HIV: Malawi Experience. Available online: https://www.afro.who.int/sites/default/files/2017-07/implementation-of-option-b%2B-for-prevention-of-mother-to-child-transmission.pdf (accessed on 15 March 2023).
- Redinger, S.; Udedi, E.; Richter, L.M.; Dovel, K.L.; Bruns, L.; Coates, T.J.; Rochat, T.J. Double benefit? Integrating an early childhood development programme into HIV PMTCT Option B+ services in Malawi. AIDS Care 2021, 33, 1595–1602. [Google Scholar] [CrossRef]
- Brennan, A.T.; Bonawitz, R.; Gill, C.J.; Thea, D.M.; Kleinman, M.; Long, L.; McCallum, C.; Fox, M.P. A Meta-Analysis Assessing Diarrhea and Pneumonia in HIV-Exposed Uninfected Compared with HIV-Unexposed Uninfected Infants and Children. J. Acquir. Immune Defic. Syndr. 2019, 82, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Anderson, K.; Kalk, E.; Madlala, H.P.; Nyemba, D.C.; Kassanjee, R.; Jacob, N.; Slogrove, A.; Smith, M.; Eley, B.S.; Cotton, M.F.; et al. Increased infectious-cause hospitalization among infants who are HIV-exposed uninfected compared to HIV-unexposed. AIDS 2021, 35, 2327–2339. [Google Scholar] [CrossRef] [PubMed]
- Labuda, S.M.; Huo, Y.; Kacanek, D.; Patel, K.; Huybrechts, K.; Jao, J.; Smith, C.; Hernandez-Diaz, S.; Scott, G.; Burchett, S.; et al. Rates of hospitalization and infection-related hospitalization among HIV-exposed uninfected children compared to HIV-unexposed uninfected children in the United States, 2007–2016. Clin. Infect. Dis. 2020, 71, 332–339. [Google Scholar] [CrossRef]
- Iroh Tam, P.Y.; Chirombo, J.; Henrion, M.; Newberry, L.; Mambule, I.; Everett, D.; Mwansambo, C.; Cunliffe, N.; French, N.; Heyderman, R.S.; et al. Clinical pneumonia in the hospitalised child in Malawi in the post-pneumococcal conjugate vaccine era: A prospective hospital-based observational study. BMJ Open 2022, 12, e050188. [Google Scholar] [CrossRef] [PubMed]
- Taylor, S.A.; Sharma, S.; Remmel, C.A.L.; Holder, B.; Jones, C.E.; Marchant, A.; Ackerman, M.E. HIV-associated alterations of the biophysical features of maternal antibodies correlate with their reduced transfer across the placenta. J. Infect. Dis. 2022, 226, 1441–1450. [Google Scholar] [CrossRef] [PubMed]
- Kidzeru, E.B.; Hesseling, A.C.; Passmore, J.A.; Myer, L.; Gamieldien, H.; Tchakoute, C.T.; Gray, C.M.; Sodora, D.L.; Jaspan, H.B. In-utero exposure to maternal HIV infection alters T-cell immune responses to vaccination in HIV-uninfected infants. AIDS 2014, 28, 1421–1430. [Google Scholar] [CrossRef]
- Afran, L.; Jambo, K.C.; Nedi, W.; Miles, D.J.C.; Kiran, A.; Banda, D.H.; Kamg’ona, R.; Tembo, D.; Pachnio, A.; Nastouli, E.; et al. Defective monocyte enzymatic function and an inhibitory immune phenotype in HIV-exposed uninfected African infants in the era of antiretroviral therapy. J. Infect. Dis. 2022, 226, 1243–1255. [Google Scholar] [CrossRef] [PubMed]
- Zimmermann, P.; Jones, C.E. Factors That Influence Infant Immunity and Vaccine Responses. Pediatr. Infect. Dis. J. 2021, 40, S40–S46. [Google Scholar] [CrossRef]
- World Health Organization Immunization Dashboard Malawi. 2021. Available online: https://immunizationdata.who.int/pages/profiles/mwi.html (accessed on 25 May 2023).
- Mmanga, K.; Mwenyenkulu, T.E.; Nkoka, O.; Ntenda, P.A.M. Tracking immunization coverage, dropout and equity gaps among children ages 12–23 months in Malawi—Bottleneck analysis of the Malawi Demographic and Health Survey. Int. Health 2022, 14, 250–259. [Google Scholar] [CrossRef]
- Open Edu. Immunization Module: Monitoring Your Immunization Programme. Available online: https://www.open.edu/openlearncreate/mod/oucontent/view.php?id=53371&printable=1 (accessed on 3 March 2023).
- Giuliano, M.; Orlando, S.; Andreotti, M.; Mthiko, B.; Mphwere, R.; Kavalo, T.; Ciccacci, F.; Marazzi, M.C.; Floridia, M. Maternal retention and early infant HIV diagnosis in a prospective cohort study of HIV-positive women and their children in Malawi. Int. J. STD AIDS 2023, 34, 54–61. [Google Scholar] [CrossRef]
- Floridia, M.; Orlando, S.; Andreotti, M.; Mphwere, R.; Kavalo, T.; Ciccacci, F.; Scarcella, P.; Marazzi, M.C.; Giuliano, M. A twelve-month prospective study of HIV-positive and HIV-negative women and their infants in Malawi: Comparative analysis of clinical events and infant growth. Am. J. Trop. Med. Hyg. 2022, 108, 394–402. [Google Scholar] [CrossRef] [PubMed]
- Triulzi, I.; Keiser, O.; Somerville, C.; Salimu, S.; Ciccacci, F.; Palla, I.; Sagno, J.B.; Gondwe, J.; Marazzi, C.; Orlando, S.; et al. Social determinants of male partner attendance in women’s prevention-of mother-to-child transmission program in Malawi. BMC Public Health 2020, 20, 1821. [Google Scholar] [CrossRef]
- World Health Organization. Weekly Epidemiology Record; World Health Organization: Geneva, Switzerland, 2013; Volume 88, pp. 413–428. Available online: https://apps.who.int/iris/handle/10665/242127 (accessed on 22 March 2023).
- Chua, I.; Lagos, M.; Charalambous, B.M.; Workman, S.; Chee, R.; Grimbacher, B. Pathogen-specific IgG antibody levels in immunodeficient patients receiving immunoglobulin replacement do not provide additional benefit to therapeutic management over total serum IgG. J. Allergy Clin. Immunol. 2011, 127, 1410–1411. [Google Scholar] [CrossRef]
- Giuliano, M.; Andreotti, M.; Liotta, G.; Jere, H.; Sagno, J.B.; Maulidi, M.; Mancinelli, S.; Buonomo, E.; Scarcella, P.; Pirillo, M.F.; et al. Maternal antiretroviral therapy for the prevention of mother-to-child transmission of HIV in Malawi: Maternal and infant outcomes two years after delivery. PLoS ONE 2013, 8, e68950. [Google Scholar] [CrossRef] [PubMed]
- Van Damme, P.; Leroux-Roels, G.; Suryakiran, P.; Folschweiller, N.; Van Der Meeren, O. Persistence of antibodies 20 y after vaccination with a combined hepatitis A and B vaccine. Hum. Vaccines Immunother. 2017, 13, 972–980. [Google Scholar] [CrossRef]
- Reikie, B.A.; Naidoo, S.; Ruck, C.E.; Slogrove, A.L.; de Beer, C.; la Grange, H.; Adams, R.C.; Ho, K.; Smolen, K.; Speert, D.P.; et al. Antibody responses to vaccination among South African HIV-exposed and unexposed uninfected infants during the first 2 years of life. Clin. Vaccine Immunol. 2013, 20, 33–38. [Google Scholar] [CrossRef]
- Simani, O.E.; Izu, A.; Violari, A.; Cotton, M.F.; van Niekerk, N.; Adrian, P.V.; Madhi, S.A. Effect of HIV-1 exposure and antiretroviral treatment strategies in HIV-infected children on immunogenicity of vaccines during infancy. AIDS 2014, 28, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Rutstein, R.M.; Rudy, B.; Codispoti, C.; Watson, B. Response to hepatitis B immunization by infants exposed to HIV. AIDS 1994, 8, 1281–1284. [Google Scholar] [CrossRef]
- Madhi, S.A.; Adrian, P.; Cotton, M.F.; McIntyre, J.A.; Jean-Philippe, P.; Meadows, S.; Nachman, S.; Käyhty, H.; Klugman, K.P.; Violari, A.; et al. Effect of HIV infection status and antiretroviral treatment on quantitative and qualitative antibody responses to pneumococcal conjugate vaccine in infants. J. Infect. Dis. 2010, 202, 355–361. [Google Scholar] [CrossRef]
- Bobo, F.T.; Asante, A.; Woldie, M.; Dawson, A.; Hayen, A. Child vaccination in sub-Saharan Africa: Increasing coverage addresses inequalities. Vaccine 2022, 40, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Ntenda, P.A.M. Factors associated with non- and under-vaccination among children aged 12–23 months in Malawi. A multinomial analysis of the population-based sample. Pediatr. Neonatol. 2019, 60, 623–633. [Google Scholar] [CrossRef] [PubMed]
- Zida-Compaore, W.I.C.; Ekouevi, D.K.; Gbeasor-Komlanvi, F.A.; Sewu, E.K.; Blatome, T.; Gbadoe, A.D.; Agbèrè, D.A.; Atakouma, Y. Immunization coverage and factors associated with incomplete vaccination in children aged 12 to 59 months in health structures in Lomé. BMC Res. Notes 2019, 12, 84. [Google Scholar] [CrossRef] [PubMed]
- Gadama, D.; Mvalo, T.; Ginsburg, M.S. Haemophilus influenzae type b and pneumococcal conjugate vaccination coverage in children aged 2–59 months in Malawi. Hum. Vaccine Immunother. 2021, 17, 397–399. [Google Scholar] [CrossRef]
- Zewdie, A.; Letebo, M.; Mekonnen, T. Reasons for defaulting from childhood immunization program: A qualitative study from Hadiya zone, Southern Ethiopia. BMC Public Health 2016, 16, 1240. [Google Scholar] [CrossRef]
- Mzumara, G.W.; Chawani, M.; Sakala, M.; Mwandira, L.; Phiri, E.; Milanzi, E.; Phiri, M.D.; Kazanga, I.; O’Byrne, T.; Zulu, E.M.; et al. The health policy response to COVID-19 in Malawi. BMJ Glob. Health 2021, 6, e006035. [Google Scholar] [CrossRef]
- Miles, M. Validity of vaccination cards and parental recall to estimate vaccination coverage: A systematic review of the literature. Vaccine 2013, 31, 1560–1568. [Google Scholar] [CrossRef]
- Ntenda, P.A.M.; Sixpence, A.; Mwenyenkulu, T.E.; Mmanga, K.; Chirambo, A.C.; Bauleni, A.; Nkoka, O. Determinants of pentavalent and measles vaccination dropouts among children aged 12–23 months in The Gambia. BMC Public Health 2022, 22, 520. [Google Scholar] [CrossRef]
- Myer, L.; Phillips, T.K.; Zerbe, A.; Brittain, K.; Lesosky, M.; Hsiao, N.Y.; Remien, R.H.; Mellins, C.A.; McIntyre, J.A.; Abrams, E.J. Integration of postpartum healthcare services for HIV-infected women and their infants in South Africa: A randomised controlled trial. PLoS Med. 2018, 15, e1002547. [Google Scholar] [CrossRef]
| HEU Infants | HUU Infants | p Value | |
|---|---|---|---|
| Number of infants | 49 | 13 | |
| Sex ratio (M/F) (%) | 30/19(61.2/38.8) | 4/9 (30.8/69.2) | 0.065 |
| Weight at birth (kg) | 3.50 (3.1–4.0) | 3.45 (3.0–3.8) | 0.651 |
| Weight gain over 1–6 months (kg) | 2.95 (2.48–3.43) | 2.80 (2.40–3.70) | 0.775 |
| Maternal age (years) | 30.0 (23.3–33.8) | 30.0 (25.5–32.5) | 0.994 |
| Maternal BMI | 25.0 (22.5–28.8) | 26.3 (22.5–28.8) | 0.970 |
| ART duration at enrolment among mothers living with HIV (years) | 0.7 (0.4–7.1) | - | - |
| Women with <1 year of ART (n, %) | 25 (51.0%) | - | |
| Women with >1 year of ART (n, %) | 24 (49.0%) | - | |
| Electricity at home, n, (%) | 18 (37.5%) | 4 (30.8%) | 0.654 |
| Water at home, n, (%) | 28 (58.3%) | 10 (76.9%) | 0.220 |
| Residency | 0.176 | ||
| Urban | 12 (24.5%) | 1 (7.7%) | |
| Semirural/rural | 36 (75.0.1%) | 12 (92.3%) | |
| Maternal Education | 0.835 | ||
| Primary or No Education | 28 (58.3%) | 8 (61.5%) | |
| Secondary or higher | 20 (40.8%) | 5 (38.5%) |
| All | HIV-Exposed Uninfected (HEU) | HIV-Unexposed Uninfected (HUU) | p Values | ||
|---|---|---|---|---|---|
| No. infants | 62 | 49 | 13 | ||
| Anti-HiB IgG (mg/L) | 1.58 (1.42–1.74) | 1.39 (0.75–1.21) | 2.54 (2.19–2.90) | 0.194 | |
| Infants with anti-HiB IgG (n, %) | >0.15 mg/mL | 53 (85.5%) | 40 (81.6%) | 13 (100%) | 0.095 |
| >1.00 mg/mL | 42 (67.7%) | 32 (65.3%) | 10 (76.9%) | 0.426 | |
| Anti-HBs IgG (mIU/L) | 64.9 (62.4–65.8) | 62.5 (57.02–58.4) | 74.7 (74.2–75.2) | 0.949 | |
| Infants with anti-HBs IgG (n, %) | >10 mIU/L | 51 (82.3%) | 40 (81.6%) | 11 (84.6%) | 0.802 |
| >100 mIU/L | 34 (54.8%) | 26 (53.1%) | 8 (61.5%) | 0.756 | |
| Anti-Spn IgG (mg/L) | 85.1 (84.7–86.9) | 83.7 (71.6–73.8) | 90.9 (89.5–92.4) | 0.809 | |
| Infants with anti-Spn IgG (n, %) | >50 mg/L | 49 (81.7%) | 38 (80.9%) | 11 (84.6%) | 0.576 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baroncelli, S.; Galluzzo, C.M.; Orlando, S.; Pirillo, M.F.; Luhanga, R.; Mphwere, R.; Kavalo, T.; Amici, R.; Floridia, M.; Andreotti, M.; et al. Rates of Seroprotection against Vaccine-Preventable Infectious Diseases in HIV-Exposed and -Unexposed Malawian Infants. Pathogens 2023, 12, 938. https://doi.org/10.3390/pathogens12070938
Baroncelli S, Galluzzo CM, Orlando S, Pirillo MF, Luhanga R, Mphwere R, Kavalo T, Amici R, Floridia M, Andreotti M, et al. Rates of Seroprotection against Vaccine-Preventable Infectious Diseases in HIV-Exposed and -Unexposed Malawian Infants. Pathogens. 2023; 12(7):938. https://doi.org/10.3390/pathogens12070938
Chicago/Turabian StyleBaroncelli, Silvia, Clementina Maria Galluzzo, Stefano Orlando, Maria Franca Pirillo, Richard Luhanga, Robert Mphwere, Thom Kavalo, Roberta Amici, Marco Floridia, Mauro Andreotti, and et al. 2023. "Rates of Seroprotection against Vaccine-Preventable Infectious Diseases in HIV-Exposed and -Unexposed Malawian Infants" Pathogens 12, no. 7: 938. https://doi.org/10.3390/pathogens12070938
APA StyleBaroncelli, S., Galluzzo, C. M., Orlando, S., Pirillo, M. F., Luhanga, R., Mphwere, R., Kavalo, T., Amici, R., Floridia, M., Andreotti, M., Ciccacci, F., Scarcella, P., Marazzi, M. C., & Giuliano, M. (2023). Rates of Seroprotection against Vaccine-Preventable Infectious Diseases in HIV-Exposed and -Unexposed Malawian Infants. Pathogens, 12(7), 938. https://doi.org/10.3390/pathogens12070938