From Non-Alcoholic Fatty Liver Disease to Liver Cancer: Microbiota and Inflammation as Key Players
Abstract
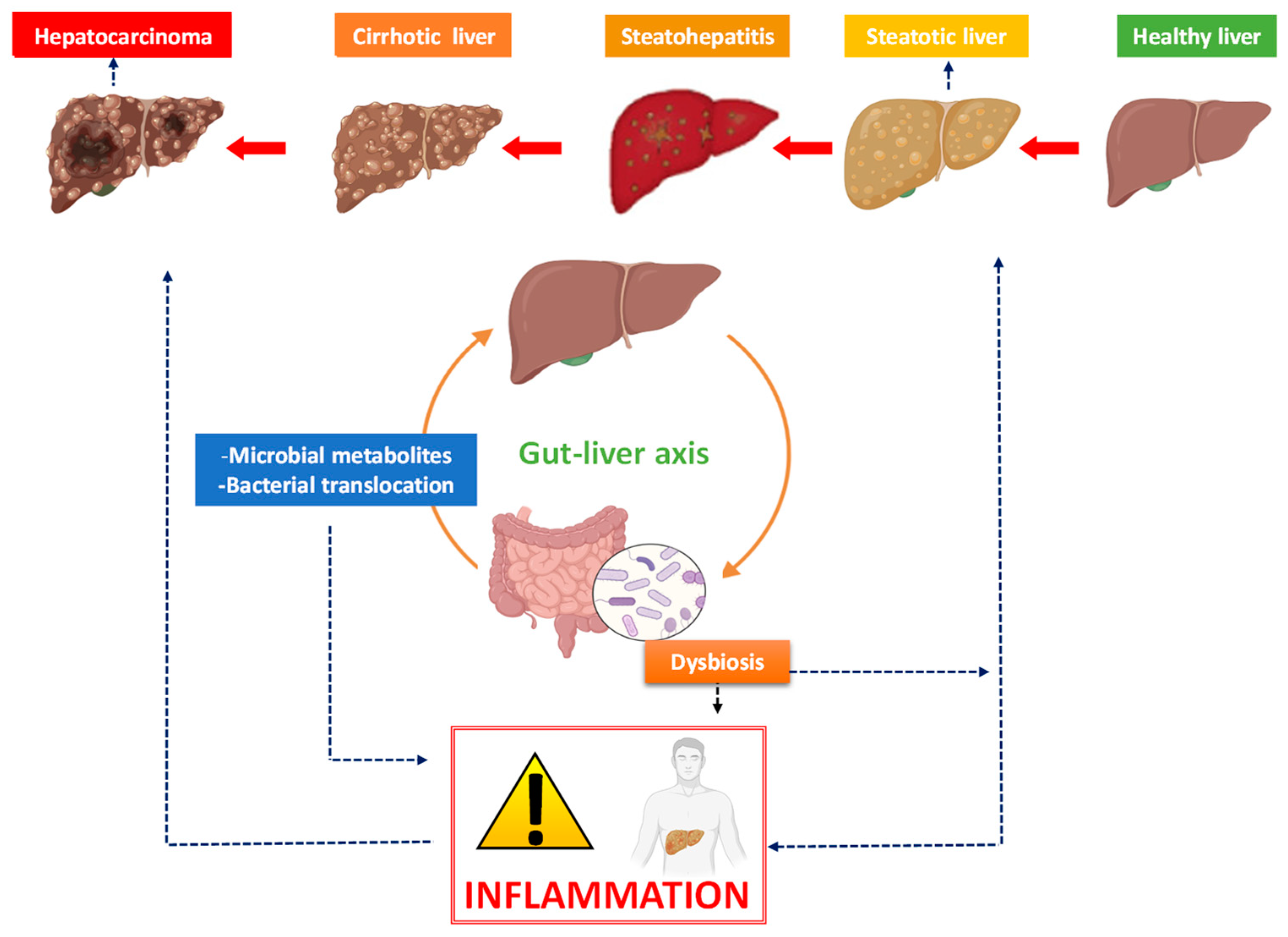
1. From Non-Alcoholic Fatty Liver Disease to Liver Cancer
2. The Bidirectional Liver–Gut Communication and Its Impact on Liver Disease
3. The Link between Inflammation, Microbiota and Hepatocarcinoma
3.1. Dysbiosis and Liver Cancer
3.2. Microbiota Manipulation in Clinical Trials for Patients with Liver Cancer
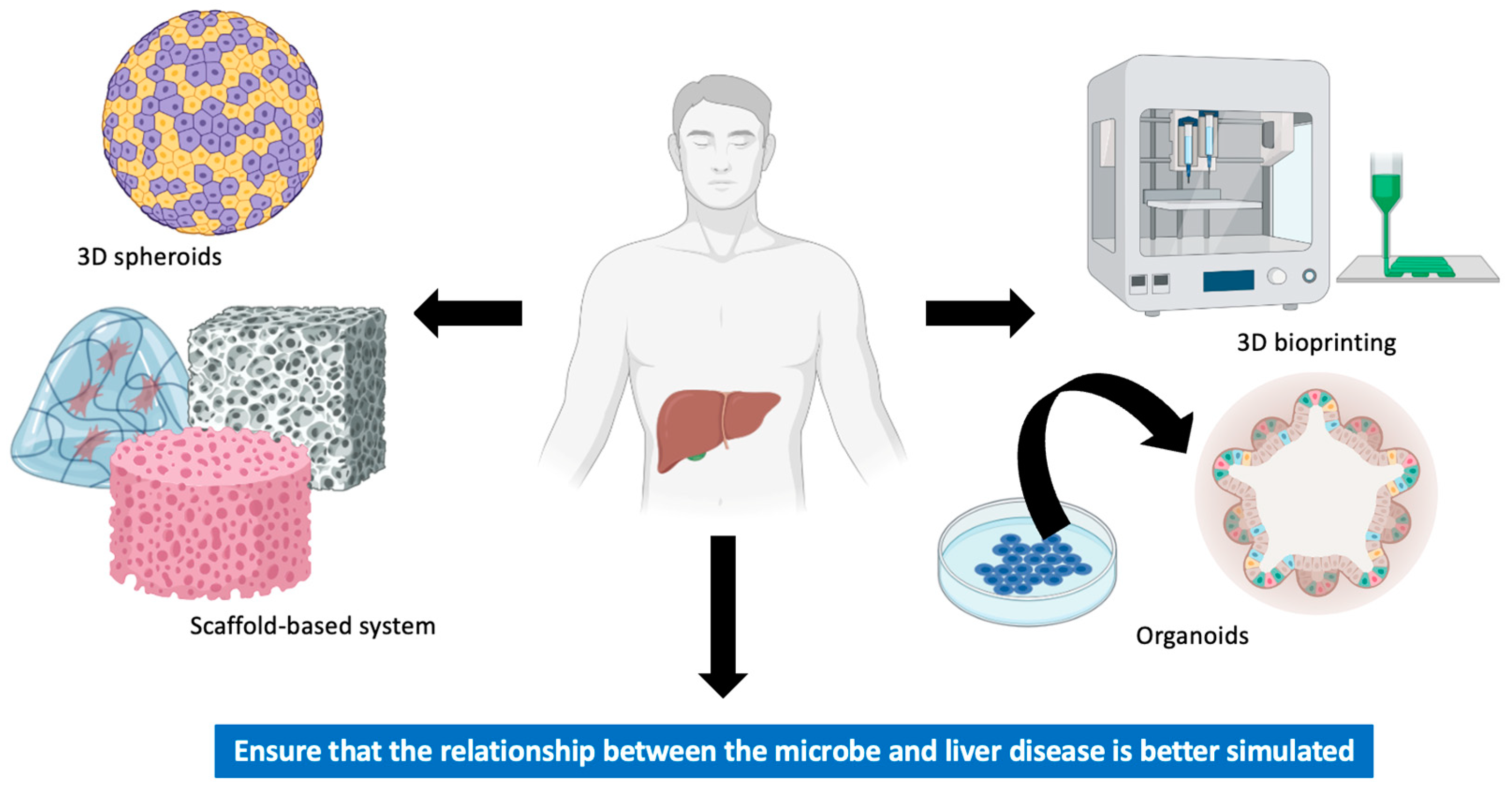
4. In Vitro Models of Liver Cancer
4.1. Spheroids
4.2. Scaffold-Based System
4.3. Bioprinting
4.4. Organoids
4.5. Microbiota-Based Models of Liver Cancer In Vitro
5. Further Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Huang, D.Q.; El-Serag, H.B.; Loomba, R. Global epidemiology of NAFLD-related HCC: Trends, predictions, risk factors and prevention. Nat. Rev. Gastroenterol. Hepatol. 2021, 18, 223–238. [Google Scholar] [CrossRef] [PubMed]
- Nassir, F.; Rector, R.S.; Hammoud, G.M.; Ibdah, J.A. Pathogenesis and Prevention of Hepatic Steatosis. Gastroenterol. Hepatol. 2015, 11, 167–175. [Google Scholar]
- Lee, J.; Hong, S.-W.; Rhee, E.-J.; Lee, W.-Y. GLP-1 Receptor Agonist and Non-Alcoholic Fatty Liver Disease. Diabetes Metab. J. 2012, 36, 262–267. [Google Scholar] [CrossRef] [PubMed]
- Di Ciaula, A.; Baj, J.; Garruti, G.; Celano, G.; De Angelis, M.; Wang, H.H.; Di Palo, D.M.; Bonfrate, L.; Wang, D.Q.-H.; Portincasa, P. Liver Steatosis, Gut-Liver Axis, Microbiome and Environmental Factors. A Never-Ending Bidirectional Cross-Talk. J. Clin. Med. 2020, 9, 2648. [Google Scholar] [CrossRef]
- Dietrich, P.; Hellerbrand, C. Non-alcoholic fatty liver disease, obesity and the metabolic syndrome. Best Prsct. Res. Clin. Gastroenterol. 2014, 28, 637–653. [Google Scholar] [CrossRef]
- LaBrecque, D.R.; Abbas, Z.; Anania, F.; Ferenci, P.; Khan, A.G.; Goh, K.-L.; Hamid, S.S.; Isakov, V.; Lizarzabal, M.; Peñaranda, M.M.; et al. World Gastroenterology Organisation Global Guidelines: Nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. J. Clin. Gastroenterol. 2014, 48, 467–473. [Google Scholar] [CrossRef]
- Brunt, E.M.; Wong, V.W.-S.; Nobili, V.; Day, C.P.; Sookoian, S.; Maher, J.J.; Bugianesi, E.; Sirlin, C.B.; Neuschwander-Tetri, B.A.; Rinella, M.E. Nonalcoholic fatty liver disease. Nat. Rev. Dis. Prim. 2015, 1, 15080. [Google Scholar] [CrossRef]
- Mattos, A.Z.; Debes, J.D.; Dhanasekaran, R.; Benhammou, J.N.; Arrese, M.; Patricio, A.L.V.; Zilio, A.C.; Mattos, A.A. Hepatocellular carcinoma in nonalcoholic fatty liver disease: A growing challenge. World J. Hepatol. 2021, 13, 1107–1121. [Google Scholar] [CrossRef]
- Albillos, A.; de Gottardi, A.; Rescigno, M. The gut-liver axis in liver disease: Pathophysiological basis for therapy. J. Hepatol. 2020, 72, 558–577. [Google Scholar] [CrossRef]
- Chanez-Paredes, S.D.; Abtahi, S.; Kuo, W.-T.; Turner, J.R. Differentiating between Tight Junction-Dependent and Tight Junction-Independent Intestinal Barrier Loss In Vivo. Methods Mol. Biol. 2021, 2367, 249–271. [Google Scholar] [CrossRef]
- Giannelli, V.; Di Gregorio, V.; Iebba, V.; Giusto, M.; Schippa, S.; Merli, M.; Thalheimer, U. Microbiota and the gut-liver axis: Bacterial translocation, inflammation and infection in cirrhosis. World J. Gastroenterol. 2014, 20, 16795–16810. [Google Scholar] [CrossRef] [PubMed]
- Wishart, D.S. Metabolomics for Investigating Physiological and Pathophysiological Processes. Physiol. Rev. 2019, 99, 1819–1875. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.-Y.; Zhang, D.-M.; Yishake, D.; Luo, Y.; Fang, A.-P.; Zhu, H.-L. Dietary choline, rather than betaine intake, is associated with hepatocellular carcinoma mortality. Food Funct. 2020, 11, 7866–7877. [Google Scholar] [CrossRef] [PubMed]
- Matthews, D.R.; Li, H.; Zhou, J.; Li, Q.; Glaser, S.; Francis, H.; Alpini, G.; Wu, C. Methionine- and Choline-Deficient Diet–Induced Nonalcoholic Steatohepatitis Is Associated with Increased Intestinal Inflammation. Am. J. Pathol. 2021, 191, 1743–1753. [Google Scholar] [CrossRef] [PubMed]
- Pohl, K.; Moodley, P.; Dhanda, A. The effect of increasing intestinal short-chain fatty acid concentration on gut permeability and liver injury in the context of liver disease: A systematic review. J. Gastroenterol. Hepatol. 2022, 37, 1498–1506. [Google Scholar] [CrossRef]
- Sydor, S.; Best, J.; Messerschmidt, I.; Manka, P.; Vilchez-Vargas, R.; Brodesser, S.; Lucas, C.; Wegehaupt, A.; Wenning, C.; Aßmuth, S.; et al. Altered Microbiota Diversity and Bile Acid Signaling in Cirrhotic and Noncirrhotic NASH-HCC. Clin. Transl. Gastroenterol. 2020, 11, e00131. [Google Scholar] [CrossRef]
- Thomas, C.E.; Luu, H.N.; Wang, R.; Xie, G.; Adams-Haduch, J.; Jin, A.; Koh, W.-P.; Jia, W.; Behari, J.; Yuan, J.-M. Association between Pre-Diagnostic Serum Bile Acids and Hepatocellular Carcinoma: The Singapore Chinese Health Study. Cancers 2021, 13, 2648. [Google Scholar] [CrossRef]
- Won, S.-M.; Park, E.; Jeong, J.-J.; Ganesan, R.; Gupta, H.; Gebru, Y.A.; Sharma, S.; Kim, D.-J.; Suk, K.-T. The Gut Microbiota-Derived Immune Response in Chronic Liver Disease. Int. J. Mol. Sci. 2021, 22, 8309. [Google Scholar] [CrossRef]
- Stolfi, C.; Maresca, C.; Monteleone, G.; Laudisi, F. Implication of Intestinal Barrier Dysfunction in Gut Dysbiosis and Diseases. Biomedicines 2022, 10, 289. [Google Scholar] [CrossRef]
- Ilhan, N. Gut microbiota and metabolism. Int. J. Med. Biochem. 2018, 1, 115–128. [Google Scholar] [CrossRef]
- De Muynck, K.; Vanderborght, B.; Van Vlierberghe, H.; Devisscher, L. The Gut–Liver Axis in Chronic Liver Disease: A Macrophage Perspective. Cells 2021, 10, 2959. [Google Scholar] [CrossRef] [PubMed]
- Chen, W.-Y.; Wang, M.; Zhang, J.; Barve, S.S.; McClain, C.J.; Joshi-Barve, S. Acrolein disrupts tight junction proteins and causes endoplasmic reticulum stress-mediated epithelial cell death leading to intestinal barrier dysfunction and permeability. Am. J. Pathol. 2017, 187, 2686–2697. [Google Scholar] [CrossRef]
- Vancells Lujan, P.; Viñas Esmel, E.; Sacanella Meseguer, E. Overview of non-alcoholic fatty liver disease (NAFLD) and the role of sugary food consumption and other dietary components in its development. Nutrients 2021, 13, 1442. [Google Scholar] [CrossRef] [PubMed]
- Stromsnes, K.; Correas, A.G.; Lehmann, J.; Gambini, J.; Olaso-Gonzalez, G. Anti-Inflammatory Properties of Diet: Role in Healthy Aging. Biomedicines 2021, 9, 922. [Google Scholar] [CrossRef] [PubMed]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Morrison, E.D.; Kowdley, K.V. Genetic liver disease in adults: Early recognition of the three most common causes. Postgrad. Med. 2000, 107, 147–159. [Google Scholar] [CrossRef]
- Hanahan, D.; Weinberg, R.A. Hallmarks of cancer: The next generation. Cell 2011, 144, 646–674. [Google Scholar] [CrossRef]
- Anand, P.; Kunnumakara, A.B.; Sundaram, C.; Harikumar, K.B.; Tharakan, S.T.; Lai, O.S.; Sung, B.; Aggarwal, B.B. Cancer is a Preventable Disease that Requires Major Lifestyle Changes. Pharm. Res. 2008, 25, 2097–2116. [Google Scholar] [CrossRef]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Coussens, L.M.; Werb, Z. Inflammation and cancer. Nature 2002, 420, 860–867. [Google Scholar] [CrossRef]
- Grivennikov, S.I.; Greten, F.R.; Karin, M. Immunity, inflammation, and cancer. Cell 2010, 140, 883–899. [Google Scholar] [CrossRef] [PubMed]
- Qian, S.; Golubnitschaja, O.; Zhan, X. Chronic inflammation: Key player and biomarker-set to predict and prevent cancer development and progression based on individualized patient profiles. EPMA J. 2019, 10, 365–381. [Google Scholar] [CrossRef] [PubMed]
- Shacter, E.; Weitzman, S.A. Chronic inflammation and cancer. Oncology 2002, 16, 217–226, discussion 230-2. [Google Scholar] [PubMed]
- Balkwill, F.; Mantovani, A. Inflammation and cancer: Back to Virchow? Lancet 2001, 357, 539–545. [Google Scholar] [CrossRef]
- Coussens, L.M.; Zitvogel, L.; Palucka, A.K. Neutralizing tumor-promoting chronic inflammation: A magic bullet? Science 2013, 339, 286–291. [Google Scholar] [CrossRef]
- Liu, M.; Tseng, T.-C.; Jun, D.W.; Yeh, M.-L.; Trinh, H.; Wong, G.L.H.; Chen, C.-H.; Peng, C.-Y.; Kim, S.E.; Oh, H.; et al. Transition rates to cirrhosis and liver cancer by age, gender, disease and treatment status in Asian chronic hepatitis B patients. Hepatol. Int. 2021, 15, 71–81. [Google Scholar] [CrossRef] [PubMed]
- Refolo, M.G.; Messa, C.; Guerra, V.; Carr, B.I.; D’alessandro, R. Inflammatory Mechanisms of HCC Development. Cancers 2020, 12, 641. [Google Scholar] [CrossRef]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef]
- Hilscher, M.B.; Shah, V.H. Neutrophil Extracellular Traps and Liver Disease. Semin. Liver Dis. 2020, 40, 171–179. [Google Scholar] [CrossRef]
- Arelaki, S.; Koletsa, T.; Sinakos, E.; Papadopoulos, V.; Arvanitakis, K.; Skendros, P.; Akriviadis, E.; Ritis, K.; Germanidis, G.; Hytiroglou, P. Neutrophil extracellular traps enriched with IL-1β and IL-17A participate in the hepatic inflammatory process of patients with non-alcoholic steatohepatitis. Virchows Arch. 2022, 481, 455–465. [Google Scholar] [CrossRef]
- Llovet, J.M.; Kelley, R.K.; Villanueva, A.; Singal, A.G.; Pikarsky, E.; Roayaie, S.; Lencioni, R.; Koike, K.; Zucman-Rossi, J.; Finn, R.S. Hepatocellular carcinoma. Nat. Rev. Dis. Primers 2021, 7, 6. [Google Scholar] [CrossRef] [PubMed]
- Kotsiliti, E.; Leone, V.; Schuehle, S.; Govaere, O.; Li, H.; Wolf, M.J.; Horvatic, H.; Bierwirth, S.; Hundertmark, J.; Inverso, D.; et al. Intestinal B cells license metabolic T-cell activation in NASH microbiota/antigen-independently and contribute to fibrosis by IgA-FcR signalling. J. Hepatol. 2023; online ahead of print. [Google Scholar] [CrossRef]
- Simon, T.G.; Roelstraete, B.; Sharma, R.; Khalili, H.; Hagström, H.; Ludvigsson, J.F. Cancer Risk in Patients with Biopsy-Confirmed Nonalcoholic Fatty Liver Disease: A Population-Based Cohort Study. Hepatology 2021, 74, 2410–2423. [Google Scholar] [CrossRef] [PubMed]
- Asfari, M.M.; Sarmini, M.T.; Alomari, M.; Lopez, R.; Dasarathy, S.; McCullough, A.J. The association of nonalcoholic steatohepatitis and hepatocellular carcinoma. Eur. J. Gastroenterol. Hepatol. 2020, 32, 1566–1570. [Google Scholar] [CrossRef]
- Kanwal, F.; Kramer, J.R.; Li, L.; Dai, J.; Natarajan, Y.; Yu, X.; Asch, S.M.; El-Serag, H.B. Effect of Metabolic Traits on the Risk of Cirrhosis and Hepatocellular Cancer in Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 808–819. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.D.; Ahmed, F.; Mara, K.C.; Addissie, B.D.; Allen, A.M.; Gores, G.J.; Roberts, L.R. Diabetes Is Associated with Increased Risk of Hepatocellular Carcinoma in Patients with Cirrhosis from Nonalcoholic Fatty Liver Disease. Hepatology 2020, 71, 907–916. [Google Scholar] [CrossRef]
- Sun, B.; Karin, M. Obesity, inflammation, and liver cancer. J. Hepatol. 2012, 56, 704–713. [Google Scholar] [CrossRef] [PubMed]
- Tangvarasittichai, S. Oxidative stress, insulin resistance, dyslipidemia and type 2 diabetes mellitus. World J. Diabetes 2015, 6, 456–480. [Google Scholar] [CrossRef]
- Cantero, I.; Abete, I.; Babio, N.; Arós, F.; Corella, D.; Estruch, R.; Fitó, M.; Hebert, J.R.; Martínez-González, M.; Pintó, X.; et al. Dietary Inflammatory Index and liver status in subjects with different adiposity levels within the PREDIMED trial. Clin. Nutr. 2018, 37, 1736–1743. [Google Scholar] [CrossRef]
- Luo, Y.; Lin, H. Inflammation initiates a vicious cycle between obesity and nonalcoholic fatty liver disease. Immun. Inflamm. Dis. 2021, 9, 59–73. [Google Scholar] [CrossRef]
- Leung, K.-C.; Doyle, N.; Ballesteros, M.; Waters, M.J.; Ho, K.K.Y. Insulin Regulation of Human Hepatic Growth Hormone Receptors: Divergent Effects on Biosynthesis and Surface Translocation1. J. Clin. Endocrinol. Metab. 2000, 85, 4712–4720. [Google Scholar] [CrossRef]
- Pollak, M.N.; Schernhammer, E.S.; Hankinson, S.E. Insulin-like growth factors and neoplasia. Nat. Rev. Cancer 2004, 4, 505–518. [Google Scholar] [CrossRef] [PubMed]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef] [PubMed]
- Shirazi, M.S.R.; Al-Alo, K.Z.K.; Al-Yasiri, M.H.; Lateef, Z.M.; Ghasemian, A. Microbiome Dysbiosis and Predominant Bacterial Species as Human Cancer Biomarkers. J. Gastrointest. Cancer 2020, 51, 725–728. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.; Jin, Y.; Chen, G.; Ma, X.; Zhang, L. Gut Microbiota Dysbiosis Drives the Development of Colorectal Cancer. Digestion 2021, 102, 508–515. [Google Scholar] [CrossRef]
- Xu, N.; Wang, L.; Li, C.; Ding, C.; Li, C.; Fan, W.; Cheng, C.; Gu, B. Microbiota dysbiosis in lung cancer: Evidence of association and potential mechanisms. Transl. Lung Cancer Res. 2020, 9, 1554–1568. [Google Scholar] [CrossRef]
- Parida, S.; Sharma, D. The power of small changes: Comprehensive analyses of microbial dysbiosis in breast cancer. Biochim. Biophys. Acta Rev. Cancer 2019, 1871, 392–405. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, Y.-N.; Chen, T.; Ren, C.-H.; Li, X.; Liu, G.-X. Relationship between intestinal microbial dysbiosis and primary liver cancer. Hepatobiliary Pancreat. Dis. Int. 2019, 18, 149–157. [Google Scholar] [CrossRef]
- Li, D.; Xi, W.; Zhang, Z.; Ren, L.; Deng, C.; Chen, J.; Sun, C.; Zhang, N.; Xu, J. Oral microbial community analysis of the patients in the progression of liver cancer. Microb. Pathog. 2020, 149, 104479. [Google Scholar] [CrossRef]
- Ren, Z.; Li, A.; Jiang, J.; Zhou, L.; Yu, Z.; Lu, H.; Xie, H.; Chen, X.; Shao, L.; Zhang, R.; et al. Gut microbiome analysis as a tool towards targeted non-invasive biomarkers for early hepatocellular carcinoma. Gut 2019, 68, 1014–1023. [Google Scholar] [CrossRef]
- Ponziani, F.R.; Bhoori, S.; Castelli, C.; Putignani, L.; Rivoltini, L.; Del Chierico, F.; Sanguinetti, M.; Morelli, D.; Sterbini, F.P.; Petito, V.; et al. Hepatocellular Carcinoma Is Associated with Gut Microbiota Profile and Inflammation in Nonalcoholic Fatty Liver Disease. Hepatology 2019, 69, 107–120. [Google Scholar] [CrossRef]
- Li, T.; Lin, X.; Shen, B.; Zhang, W.; Liu, Y.; Liu, H.; Wang, Y.; Zheng, L.; Zhi, F. Akkermansia muciniphila suppressing nonalcoholic steatohepatitis associated tumorigenesis through CXCR6+ natural killer T cells. Front. Immunol. 2022, 13, 1047570. [Google Scholar] [CrossRef] [PubMed]
- Montasser, K.; Osman, H.A.; Abozaid, H.; Hassan, M.H.; Abeer, M. Gut microbiota characterization in Egyptian patients with hepatocellular carcinoma post-chronic hepatitis C virus genotype 4 infection. J. Appl. Pharm. Sci. 2021, 11, 116–125. [Google Scholar]
- Zheng, R.; Wang, G.; Pang, Z.; Ran, N.; Gu, Y.; Guan, X.; Yuan, Y.; Zuo, X.; Pan, H.; Zheng, J.; et al. Liver cirrhosis contributes to the disorder of gut microbiota in patients with hepatocellular carcinoma. Cancer Med. 2020, 9, 4232–4250. [Google Scholar] [CrossRef] [PubMed]
- Effenberger, M.; Waschina, S.; Bronowski, C.; Sturm, G.; Tassiello, O.; Sommer, F.; Zollner, A.; Watschinger, C.; Grabherr, F.; Gstir, R.; et al. A gut bacterial signature in blood and liver tissue characterizes cirrhosis and hepatocellular carcinoma. Hepatol. Commun. 2023, 7, e00182. [Google Scholar] [CrossRef] [PubMed]
- Garrett, W.S. Cancer and the microbiota. Science 2015, 348, 80–86. [Google Scholar] [CrossRef] [PubMed]
- Behary, J.; Amorim, N.; Jiang, X.-T.; Raposo, A.; Gong, L.; McGovern, E.; Ibrahim, R.; Chu, F.; Stephens, C.; Jebeili, H.; et al. Gut microbiota impact on the peripheral immune response in non-alcoholic fatty liver disease related hepatocellular carcinoma. Nat. Commun. 2021, 12, 187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Gou, Y.; Liang, S.; Chen, N.; Liu, Y.; He, Q.; Zhang, J. Dysbiosis of Gut Microbiota Promotes Hepatocellular Carcinoma Progression by Regulating the Immune Response. J. Immunol. Res. 2021, 2021, 4973589. [Google Scholar] [CrossRef] [PubMed]
- Lee, P.-C.; Wu, C.-J.; Hung, Y.-W.; Lee, C.-J.; Chao, Y.; Hou, M.-C.; Kuo, Y.-L.; Chou, S.-H.; Huang, Y.-H. Association of gut microbiota and metabolites with tumor response to immune checkpoint inhibitors in patients with unresectable hepatocellular carcinoma. J. Clin. Oncol. 2021, 39, e16165. [Google Scholar] [CrossRef]
- Su, Y.-Y.; Lee, W.-H.; Wang, J.-H.; Wang, H.-W.; Chen, T.-W.; Chen, B.-B.; Ho, H.J.; Liu, T.-H.; Chou, S.-C.; Chen, B.-R.; et al. MO5-4 Potential roles of gut microbiome in patients with hepatocellular carcinoma treated with immune checkpoint inhibitors. Ann. Oncol. 2022, 33, S484. [Google Scholar] [CrossRef]
- Ma, J.; Li, J.; Jin, C.; Yang, J.; Zheng, C.; Chen, K.; Xie, Y.; Yang, Y.; Bo, Z.; Wang, J.; et al. Association of gut microbiome and primary liver cancer: A two-sample Mendelian randomization and case–control study. Liver Int. 2023, 43, 221–233. [Google Scholar] [CrossRef] [PubMed]
- Kootte, R.S.; Levin, E.; Salojärvi, J.; Smits, L.P.; Hartstra, A.V.; Udayappan, S.D.; Hermes, G.; Bouter, K.E.; Koopen, A.M.; Holst, J.J. Improvement of insulin sensitivity after lean donor feces in metabolic syndrome is driven by baseline intestinal microbiota composition. Cell Metab. 2017, 26, 611–619.e6. [Google Scholar] [CrossRef] [PubMed]
- Vrieze, A.; Van Nood, E.; Holleman, F.; Salojärvi, J.; Kootte, R.S.; Bartelsman, J.F.; Dallinga-Thie, G.M.; Ackermans, M.T.; Serlie, M.J.; Oozeer, R.; et al. Transfer of Intestinal Microbiota From Lean Donors Increases Insulin Sensitivity in Individuals with Metabolic Syndrome. Gastroenterology 2012, 143, 913–916.e7. [Google Scholar] [CrossRef] [PubMed]
- Kolodziejczyk, A.A.; Zheng, D.; Shibolet, O.; Elinav, E. The role of the microbiome in NAFLD and NASH. EMBO Mol. Med. 2019, 11, e9302. [Google Scholar] [CrossRef] [PubMed]
- Brooks, A.; Liang, X.; Zhang, Y.; Zhao, C.-X.; Roberts, M.S.; Wang, H.; Zhang, L.; Crawford, D.H. Liver organoid as a 3D in vitro model for drug validation and toxicity assessment. Pharmacol. Res. 2021, 169, 105608. [Google Scholar] [CrossRef] [PubMed]
- Blidisel, A.; Marcovici, I.; Coricovac, D.; Hut, F.; Dehelean, C.A.; Cretu, O.M. Experimental models of hepatocellular carci-noma—A preclinical perspective. Cancers 2021, 13, 3651. [Google Scholar] [CrossRef] [PubMed]
- Vicent, S.; Lieshout, R.; Saborowski, A.; Verstegen, M.M.; Raggi, C.; Recalcati, S.; Invernizzi, P.; van der Laan, L.J.; Alvaro, D.; Calvisi, D.F. Experimental models to unravel the molecular pathogenesis, cell of origin and stem cell properties of cholan-giocarcinoma. Liver Int. 2019, 39, 79–97. [Google Scholar] [CrossRef] [PubMed]
- Nuciforo, S.; Fofana, I.; Matter, M.S.; Blumer, T.; Calabrese, D.; Boldanova, T.; Piscuoglio, S.; Wieland, S.; Ringnalda, F.; Schwank, G.; et al. Organoid Models of Human Liver Cancers Derived from Tumor Needle Biopsies. Cell Rep. 2018, 24, 1363–1376. [Google Scholar] [CrossRef] [PubMed]
- Maier, C.F.; Zhu, L.; Nanduri, L.K.; Kühn, D.; Kochall, S.; Thepkaysone, M.-L.; William, D.; Grützmann, K.; Klink, B.; Betge, J. Patient-derived organoids of cholangiocarcinoma. Int. J. Mol. Sci. 2021, 22, 8675. [Google Scholar] [CrossRef] [PubMed]
- Bouwmeester, M.C.; Bernal, P.N.; Oosterhoff, L.A.; van Wolferen, M.E.; Lehmann, V.; Vermaas, M.; Buchholz, M.; Peiffer, Q.C.; Malda, J.; van der Laan, L.J.W.; et al. Bioprinting of Human Liver-Derived Epithelial Organoids for Toxicity Studies. Macromol. Biosci. 2021, 21, 2100327. [Google Scholar] [CrossRef] [PubMed]
- Gunti, S.; Hoke, A.T.; Vu, K.P.; London, N.R., Jr. Organoid and spheroid tumor models: Techniques and applications. Cancers 2021, 13, 874. [Google Scholar] [CrossRef] [PubMed]
- Massa, A.; Varamo, C.; Vita, F.; Tavolari, S.; Peraldo-Neia, C.; Brandi, G.; Rizzo, A.; Cavalloni, G.; Aglietta, M. Evolution of the Experimental Models of Cholangiocarcinoma. Cancers 2020, 12, 2308. [Google Scholar] [CrossRef] [PubMed]
- Ingelman-Sundberg, M.; Lauschke, V.M. 3D human liver spheroids for translational pharmacology and toxicology. Basic Clin. Pharmacol. Toxicol. 2022, 130, 5–15. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, E.; Veghini, L.; Corbo, V. Modeling cell communication in cancer with organoids: Making the complex simple. Front. Cell Dev. Biol. 2020, 8, 166. [Google Scholar] [CrossRef] [PubMed]
- De Siervi, S.; Turato, C. Liver Organoids as an In Vitro Model to Study Primary Liver Cancer. Int. J. Mol. Sci. 2023, 24, 4529. [Google Scholar] [CrossRef] [PubMed]
- Kozlowski, M.T.; Crook, C.J.; Ku, H.T. Towards organoid culture without Matrigel. Commun. Biol. 2021, 4, 1387. [Google Scholar] [CrossRef] [PubMed]
- Langhans, S.A. Three-Dimensional in Vitro Cell Culture Models in Drug Discovery and Drug Repositioning. Front. Pharmacol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Jensen, C.; Teng, Y. Is it time to start transitioning from 2D to 3D cell culture? Front. Mol. Biosci. 2020, 7, 33. [Google Scholar] [CrossRef] [PubMed]
- Benton, G.; Arnaoutova, I.; George, J.; Kleinman, H.K.; Koblinski, J. Matrigel: From discovery and ECM mimicry to assays and models for cancer research. Adv. Drug Deliv. Rev. 2014, 79, 3–18. [Google Scholar] [CrossRef]
- Datta, P.; Dey, M.; Ataie, Z.; Unutmaz, D.; Ozbolat, I.T. 3D bioprinting for reconstituting the cancer microenvironment. npj Precis. Oncol. 2020, 4, 18. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Kalva, S.N.; Ahmad, R.; Zahid, A.A.; Hasan, S.; Nayeem, A.; McClements, L.; Hasan, A. 3D Bioprinted cancer models: Revolutionizing personalized cancer therapy. Transl. Oncol. 2021, 14, 101015. [Google Scholar] [CrossRef]
- Bae, J.; Han, S.; Park, S. Recent Advances in 3D Bioprinted Tumor Microenvironment. BioChip J. 2020, 14, 137–147. [Google Scholar] [CrossRef]
- Huch, M.; Dorrell, C.; Boj, S.F.; Van Es, J.H.; Li, V.S.W.; Van De Wetering, M.; Sato, T.; Hamer, K.; Sasaki, N.; Finegold, M.J.; et al. In vitro expansion of single Lgr5+ liver stem cells induced by Wnt-driven regeneration. Nature 2013, 494, 247–250. [Google Scholar] [CrossRef] [PubMed]
- Dong, R.; Zhang, B.; Zhang, X. Liver organoids: An in vitro 3D model for liver cancer study. Cell Biosci. 2022, 12, 152. [Google Scholar] [CrossRef] [PubMed]
- Zhu, X.; Zhang, B.; He, Y.; Bao, J. Liver Organoids: Formation Strategies and Biomedical Applications. Tissue Eng. Regen. Med. 2021, 18, 573–585. [Google Scholar] [CrossRef] [PubMed]
- Huch, M.; Gehart, H.; van Boxtel, R.; Hamer, K.; Blokzijl, F.; Verstegen, M.M.; Ellis, E.; van Wenum, M.; Fuchs, S.A.; de Ligt, J.; et al. Long-Term Culture of Genome-Stable Bipotent Stem Cells from Adult Human Liver. Cell 2015, 160, 299–312. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, R.; Bae, S.D.W.; Zhou, G.; Read, S.A.; Ahlenstiel, G.; George, J.; Qiao, L. Application of organoids in translational research of human diseases with a particular focus on gastrointestinal cancers. Biochim. Biophys. Acta (BBA)-Rev. Cancer 2020, 1873, 188350. [Google Scholar] [CrossRef] [PubMed]
- Prior, N.; Inacio, P.; Huch, M. Liver organoids: From basic research to therapeutic applications. Gut 2019, 68, 2228–2237. [Google Scholar] [CrossRef] [PubMed]
- Khinsar, K.H.; Abdul, S.; Hussain, A.; Din, R.U.; Lei, L.; Cao, J.; Abbasi, M.; Rehman, A.U.; Farooqui, N.; Yi, X.; et al. Anti-tumor effect of polysaccharide from Pleurotus ostreatus on H22 mouse Hepatoma ascites in-vivo and hepatocellular carcinoma in-vitro model. AMB Express 2021, 11, 160. [Google Scholar] [CrossRef] [PubMed]
- Cianflone, A.; Zamparelli, M.S.; Guarracino, M.; Nardone, O.; Sgamato, C.; Varriale, V.; Nardone, G. Bifidobacterium longum up-regulates PPAR-gamma and PPAR-alpha levels in HepG2 hepatic cancer cell line. Dig. Liver Dis. 2013, 45, S63–S64. [Google Scholar] [CrossRef]
- Anstee, Q.M.; Day, C.P. The genetics of NAFLD. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 645–655. [Google Scholar] [CrossRef] [PubMed]
- Fotbolcu, H.; Zorlu, E. Nonalcoholic fatty liver disease as a multi-systemic disease. World J. Gastroenterol. 2016, 22, 4079–4090. [Google Scholar] [CrossRef] [PubMed]
- Mendes, F.D.; Lindor, K.D. Recent advances in the treatment of non-alcoholic fatty liver disease. Expert Opin. Investig. Drugs 2005, 14, 29–35. [Google Scholar] [CrossRef] [PubMed]
| Study Status | Study Title | Study Type | Locations |
|---|---|---|---|
| Recruiting | Anesthesia on gut microbiota and metabolomics, NCT04767503 | Interventional | Taiwan |
| Withdrawn | Gut microbiota in people with HCC, NCT02599909 | Observational | |
| Unknown | Microbiota study in liver transplanted patients, NCT03507140 | Observational | France |
| Enrolling by invitation | Relationship between microbiota and prognosis of HCC after systemic treatments, NCT05443217 | Observational | China |
| Not recruiting | FMT in liver cancer to overcome resistance to atezolizumab and bevacizumab (flora), NCT05690048 | Interventional | Germany |
| Completed | The effect of gut microbiota on postoperative liver function recovery in patients with HCC, NCT04303286 | Observational | China |
| Recruiting | Prebiotic effect of eicosapentaenoic acid treatment for colorectal cancer liver metastases, NCT04682665 | Observational | United Kingdom |
| Completed | Clinical study on Bifico accelerating postoperative liver function recovery in patients with HCC, NCT05178524 | Interventional | China |
| Recruiting | Early detection of HCC in a high-risk prospective cohort, NCT04965259 | Observational | Singapore |
| Recruiting | A multicentre study on features of the gut microbiota of patients with critical chronic diseases, NCT05638269 | Observational | China |
| Unknown | Probiotics in the prevention of HCC in cirrhosis, NCT03853928 | Interventional | |
| Enrolling by invitation | Volatiles in breath and headspace analysis, diagnostic markers, NCT03228095 | Observational | Latvia |
| Not recruiting | FMT in refractory HCC, NCT05750030 | Interventional | Austria |
| Completed | Tumor microenvironment surveillance on simultaneous liver metastases extensive stage small cell lung cancer, NCT05055999 | Observational | China |
| Recruiting | A prospective cohort study of changes in circulatory microRNA of resected HCC, NCT05148572 | Observational | Singapore |
| Unknown | HCC in patients with cirrhosis due to alcohol or a NAFLD, NCT03307408 | Observational | France |
| Active not recruiting | Guangzhou nutrition and health study (GNHS), NCT03179657 | Observational | China |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Lara, A.; Rueda-Robles, A.; Sáez-Lara, M.J.; Plaza-Diaz, J.; Álvarez-Mercado, A.I. From Non-Alcoholic Fatty Liver Disease to Liver Cancer: Microbiota and Inflammation as Key Players. Pathogens 2023, 12, 940. https://doi.org/10.3390/pathogens12070940
Rodríguez-Lara A, Rueda-Robles A, Sáez-Lara MJ, Plaza-Diaz J, Álvarez-Mercado AI. From Non-Alcoholic Fatty Liver Disease to Liver Cancer: Microbiota and Inflammation as Key Players. Pathogens. 2023; 12(7):940. https://doi.org/10.3390/pathogens12070940
Chicago/Turabian StyleRodríguez-Lara, Avilene, Ascensión Rueda-Robles, María José Sáez-Lara, Julio Plaza-Diaz, and Ana I. Álvarez-Mercado. 2023. "From Non-Alcoholic Fatty Liver Disease to Liver Cancer: Microbiota and Inflammation as Key Players" Pathogens 12, no. 7: 940. https://doi.org/10.3390/pathogens12070940
APA StyleRodríguez-Lara, A., Rueda-Robles, A., Sáez-Lara, M. J., Plaza-Diaz, J., & Álvarez-Mercado, A. I. (2023). From Non-Alcoholic Fatty Liver Disease to Liver Cancer: Microbiota and Inflammation as Key Players. Pathogens, 12(7), 940. https://doi.org/10.3390/pathogens12070940







