Abstract
Canine Vector-Borne Diseases (CVBDs) are widespread in Europe and enzootic in many other countries. Though severe illnesses may occur, dogs living in enzootic areas often show vague or no clinical signs of CVBDs. Undiagnosed infections/co-infections in subclinically infected animals favor the spread of CVBDs and increase the risk of transmission to other animals and, in some cases, humans. This study has evaluated the exposure of dogs living in key enzootic countries, i.e., Italy and Greece, to major CVBDs via the use of in-clinic diagnostic kits. Overall, 300 privately owned dogs without/with single mild clinical signs living in different regions of Italy (n. 150) and Greece (n. 150) were included in the study. As part of a clinical examination, a blood sample was collected from each dog and subjected to two serological rapid tests, i.e., the SNAP® 4Dx®Plus (IDEXX Laboratories Inc.) for the detection of antibodies against Ehrlichia spp., Anaplasma spp., Borrelia burgdorferi s.l. and Dirofilaria immitis antigen and the SNAP® Leishmania (IDEXX Laboratories Inc.) for the detection of antibodies against Leishmania infantum. In all, 51 dogs (17%; 95% CI 12.9–21.7) were seropositive to at least 1 pathogen, i.e., 4 in Italy (2.7%; 95% CI 1.4–13.1) and 47 in Greece (31.3%; 95% CI 24–39.4). Dirofilaria immitis antigens were found in 39 dogs (13%; 95% CI 9.4–17.3), while antibodies against Ehrlichia, Anaplasma and Leishmania were detected in 25 (8.3%; 95% CI 5.5–12.1), 8 (2.7%; 95% CI 1.2–5.2) and 5 (1.7%; 95% CI 0.5–3.8) dogs, respectively. None of the dogs tested seropositive for B. burgdorferi s.l. Statistical analyses were performed to evaluate associations between exposure to CVBDs and possible risk factors. The present results indicate that dogs living in enzootic areas may be seropositive for one or more CVBDs in absence of clinical signs. Rapid kits are among first line tools for the detection of CVBDs in clinical settings, as they are cost-effective, straightforward and quick to use. Also, in-clinic tests used herein allowed detection of co-exposure to CVBDs investigated.
1. Introduction
In enzootic areas, dogs are exposed to the bite of arthropods able to transmit several pathogens, representing a primary threat to animal and human health [1,2,3,4,5,6]. In Europe, the most important agents of Canine Vector-Borne Diseases (CVBDs) include the tick-borne bacteria Ehrlichia canis, Anaplasma platys, Anaplasma phagocytophilum, Borrelia spp. and Rickettsia spp., the nematode Dirofilaria immitis and the protozoans Leishmania infantum and Babesia spp., transmitted by mosquitoes, sandflies and ticks, respectively [7,8,9,10,11,12,13]. European regions have suitable environments for the occurrence of diseases transmitted by invertebrates and, among countries, Italy and Greece are epizootiologically significant areas for many CVBDs [11,14,15,16,17,18,19,20,21].
Infected dogs may display signs of varying severity, from none or mild to severe and life-threatening clinical manifestations and abnormalities [9,11,12]. However, especially in enzootic areas, infected dogs do not often show significant clinical signs and may still act as a source of infection for the vectors. Though the role of subclinically infected dogs as carriers of certain CVBPs should be further investigated, undetected infections may lead to (i) underdiagnosis, which could prevent the detection of subclinical laboratory alterations, e.g., early kidney disease, anemia, thrombocytopenia, increased CRP (especially in co-infections), (ii) underestimation of disease prevalence and (iii) spread of CVBDs in both enzootic and free regions. On the other hand, dogs are epidemiological sentinels in enzootic areas and continuous serological monitoring is useful to assess their exposure to major CVBDs [4,11,22]. In fact, the detection of seropositive dogs may assist in investigating their potential role as sources of infection for vectors (seropositive dogs are not a certain source of infection, especially for tick-borne bacteria, as seropositivity may indicate a past exposure rather than a current infection), provide information on their clinical assessment and indicate the risk for animals and humans. The epidemiological history and the environment where dogs live should be taken into account and the persistence and the ability to cause subclinical infections are variable among pathogens.
Various diagnostic techniques are used to assess the serological status of canine populations living in regions enzootic for CVBDs. While assays that quantitatively evaluate the seropositivity and the antibody titers in exposed/infected animals (e.g., IFAT, ELISA) are typically applied on large-scale surveys, individual diagnosis in the clinic often requires the use of rapid, cost-effective and straightforward qualitative or semi-quantitative tests. Also, some specific-peptides-based rapid kits allow the detection of co-exposure to different pathogens, compared to other serological methods that in many cases are based on the use of whole cells. In addition to the usefulness for preliminary screening and the aid in diagnosis for individual animals, the same rapid tests may be used also in epizootiological studies.
In this survey, two in-clinic rapid kits were used to evaluate apparently healthy dogs living in enzootic regions of major CVBDs, to provide further surveillance on the exposure to CVBDs in key enzootic countries and data for continued refinement of control programs, aiming to protect animal and human health.
2. Materials and Methods
2.1. Study Design and Sampling
A total of 300 privately owned dogs living in different regions of Italy and Greece were included in the study. In Italy, 50 dogs were selected in Northern (Site A), Central (Site B) and Southern regions (Sites C and D) each. In Northeastern Greece, 25 dogs from each of the six selected sites (Sites E–J) were included in the study (Figure 1). The sampling was divided into two windows within 2022, with half of the dogs included between February and April (timeframe 1—TF1) and the other half between May and September (timeframe 2—TF2).
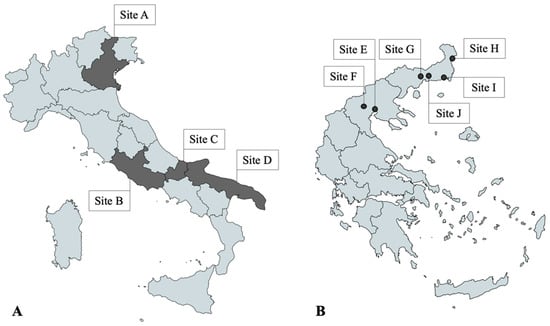
Figure 1.
(A) Study sites in Italy: regions of Veneto (Site A), Lazio (Site B), Molise (Site C), Puglia (Site D); (B) Study sites in Greece: cities of Thessaloniki (Site E), Pella (Site F), Xanthi (Site G), Didimoticho (Site H), Alexandroupoli (Site I), Komotini (Site J). Dogs in Site C were included only during timeframe 1, while dogs from D were included during timeframe 2.
The survey was conducted in the framework of routine medical checks coordinated by local veterinarians and dogs were selected based on the following criteria: (i) willingness of the owners to monitor the health status of their dogs; (ii) dogs living in areas enzootic for CVBDs; (iii) dog age that permitted at least one vector season experienced; (iv) absence of clinical diseases compatible with any of the major CVBDs under study.
Prior to enrollment, each dog was subjected to a physical examination to evaluate the presence of clinical signs possibly related to CVBDs. For every single clinical sign, a score ranging from 0 to 3 was assigned, as follows: 0 (normal status or absent), 1 (mild), 2 (moderate) and 3 (severe). A clinical score was calculated for each dog based on the sum of the scores of each clinical sign and each dog was categorized as follows: clinically healthy dog (score 0–5), mild disease (score 5–30), moderate disease (score 31–55), severe disease (score 55–75). Only clinically healthy dogs were included in the study.
Signalment and anamnesis, including data on age, sex and breed, were recorded for each dog. A consent form was signed by the owner before blood collection, which was performed via venipuncture of jugular, cephalic or saphenous veins. The blood was transferred into a tube without anticoagulant and centrifuged after clot formation to separate serum and then tested immediately.
2.2. Study Animals
Overall, 124 (41.3%) dogs included were male and 176 (58.7%) were female. A total of 36 dogs (24%) enrolled in Italy were ≤2 years old and 114 (76%) were >2 years old. In Greece, 42 (28%) and 108 (72%) were ≤2 years old and > 2 years old, respectively. Among the enrolled dogs, 174 (58%) were crossbred and 126 (42%) were purebred dogs.
2.3. Serological Tests and Statistical Analysis
Each serum sample was subjected to two different serological examinations performed by the veterinarians according to the manufacturer’s instructions:
- -
- SNAP® 4Dx® Plus Test (IDEXX Laboratories, Inc., Westbrook, ME, USA) for the detection of Dirofilaria immitis circulating antigen (Sensitivity 98.9%, Specificity 99.3%) and of antibodies against specific peptides of Anaplasma phagocytophilum (Sensitivity 93.2%, Specificity 99.2%), Anaplasma platys (Sensitivity 89.2%, Specificity 99.2%), Ehrlichia canis (Sensitivity 97.8%, Specificity 92.3%), Ehrlichia ewingii (Sensitivity 96.5%, Specificity 93.9%) and the C6-peptide of Borrelia burgdorferi s.l. (Sensitivity 96.7%, Specificity 98.8%) [23];
- -
- SNAP® Leishmania Test (IDEXX Laboratories, Inc., USA) (Sensitivity 91.1–93.4%, Specificity 99.2–100%) for the detection of antibodies against L. infantum [24].
The GraphPad Prism 9 Software was used for the statistical analysis, based on Fisher’s exact test to evaluate the presence of significant associations (p < 0.05) between possible risk factors and exposure to CVBDs. Possible risk factors (i.e., presence of clinical signs, age, sex, breed, timeframe) were evaluated with a binomial logistic regression with a strength measured using the Odds Ratio (OR) and a 95% Confidence Interval.
3. Results
Fifty-one out of three hundred (17%; 95% CI 12.9–21.7) dogs included in the study were seropositive to at least one CVBD pathogen (Table 1).

Table 1.
Overall number and percentage of dogs seropositive for different vector-borne pathogens in the present study. A total of 300 dogs were examined, i.e., 150 dogs for each timeframe (TF): 75 in sites of Italy and 75 in sites of Greece.
Three dogs (2%; 95% CI 0.4–5.7) sampled in Italy—one and two from central and Southern Italy (Sites B and D), respectively—in TF2 were seropositive for L. infantum. Also, one dog sampled in Southern Italy in TF2 was seropositive for the D. immitis antigen (Table 2). Overall, 47 dogs (31.3%; 95% CI 31.3; 24–39.4) sampled in Greece were seropositive for at least one pathogen, i.e., 4, 7, 5, 17, 6 and 8 in Sites E to J, respectively (Table 3). Of these dogs, 27 (18%; 95% CI 12.2–25.1) were seropositive to only one pathogen, whilst 20 (13.3%; 95% CI 8.3–19.8) were seropositive for more than one pathogen, i.e., 15, 4 and 1 dogs were seropositive for 2, 3 or 4 pathogens, respectively. The highest seroprevalence was found for D. immitis (38 dogs, 25.3%; 95% CI 18.6–33.1), followed by Ehrlichia spp. (25 dogs, 16.7%; 95% CI; 11.1–23.6). Accordingly, D. immitis and Ehrlichia spp. were the most-recorded pathogens in dogs with a single seropositive result (19 and 7 dogs, 12.7%; 95% CI 7.8–19.1 and 4.7%; 95% CI 1.9–9.4).

Table 2.
Number and percentage of dogs seropositive for different vector-borne pathogens enrolled in Italy. In each study site, 25 dogs have been examined, for a total of 75 dogs for each timeframe (TF). Study sites = regions of Veneto (Site A), Lazio (Site B), Molise (Site C), Puglia (Site D).

Table 3.
Number and percentage of dogs seropositive for different vector-borne pathogens enrolled in Greece. In each study site, 25 dogs have been examined, for a total of 75 dogs for each timeframe (TF). Study sites = cities of Thessaloniki (Site E), Pella (Site F), Xanthi (Site G), Didimoticho (Site H), Alexandruopoli (Site I), Komotini (Site J).
The most frequent combinations in dogs seropositive for 2 or more pathogens were Ehrlichia spp. + D. immitis (12 dogs, 4%) and Ehrlichia spp. + A. platys/phagocytophilum + D. immitis (4 dogs, 1.3%) (Table 4).

Table 4.
Number, percentage and different combinations of mixed seropositivity to vector-borne pathogens in the 300 dogs of the present study.
Among the dogs included in the study, 45 (15%; 95% CI 11.2–19.5) displayed at least one clinical sign (though with a total clinical score between 0 and 5) and 5 (11.1%; 95% CI 3.7–24) of them were seropositive for at least one CVBD.
Statistical Analysis
The Fisher’s exact test revealed three statistically significant factors associated with the seropositivity to at least one CVBD pathogen: age more than 2 years (p = 0.0062; OR = 0.3265), crossbred dogs (p < 0.0001; OR = 6.769; 95% CI = 2.84–15.36) and sampling during timeframe 2 (p = 0.005; OR = 0.3923; 95% CI = 0.2065–0.7454). Being a crossbred dog was a risk factor for seropositivity to CVBDs from the results of the binomial logistic regression (p < 0.0001; OR = 5.823; 95% CI = 2.39–14.19). No other statistically significant associations were detected.
4. Discussion
This study demonstrates that dogs without or with single, mild clinical signs living in enzootic areas may be seropositive for one or more major CVBDs. All study animals were residing in Mediterranean territories where several epizootiological, biological and ecological drivers may foster their exposure to bites of infected arthropods [11,25,26,27,28,29]. Relevant and interesting differences have been found among Italy and Greece and among sites of these countries in terms of seropositivity to CVBDs in animals with no evidence of clinical signs.
The low seropositivity rates for L. infantum and other CVBDs herein detected in Italy has been most likely influenced by the inclusion of urban dogs only, i.e., at lower risk of CVBD exposure, instead of a randomly selected population, as has been described in classical epizootiological or previous studies [18,19,27]. The same accounts for the seropositivity for Ehrlichia spp., A. platys/A. phagocytophilum and B. burgdorferi s.l. [19,30,31,32,33]. It should be considered that the seropositivity rates of CVBDs could vary depending on the diagnostic test used [9,34] and overestimation could occur when low specificity, whole-cell or crude antigen-based tests are used [35].
Dirofilaria immitis is traditionally considered enzootic in Northern Italy and has recently been spreading southward [29,36,37]. However, the present results support the decrease in the infection rates detected in other studies, which could be attributed to intensive prophylactic measures applied throughout Italy to privately owned dogs [19,36,37,38]. Nevertheless, to the authors’ best knowledge, chemoprevention against D. immitis is not yet routinely performed in Southern and Central Italy, where a further increase in seroprevalence could occur in the near future.
Regarding Greece, the results confirm that the studied CVBDs are enzootic in this country and may occur at a relatively high seroprevalence in apparently healthy dogs, particularly since these pathogens can produce subclinical infections that could be associated with diseases detectable only with laboratory analyses, e.g., early kidney disease [39]. The higher seroprevalence in this group was not surprising, as many of the dogs examined in Greece were previously strays, having little veterinary or preventive care prior to the time of the study.
Leishmania infantum is enzootic and widespread in all areas of Greece. The present results suggest that the occurrence of infection in owned dogs seems to be decreasing, probably due to the development and application of effective preventive measures (vaccination, insect repellents) [40,41]. With all likelihood, the seroreactivity to Ehrlichia spp. herein detected represented exposure to E. canis, as this is the only species occurring in dogs in Europe, and due to the vast distribution of its main vector, Rhipicephalus sanguineus. Northern Greece historically displays the highest seroprevalence of D. immitis compared to the rest of the country [42]. Despite preventive treatments being applied in most owned dogs in Northern Greece [43], a combination of factors, e.g., wetlands, mosquito populations, stray dogs and wildlife abundance (e.g., foxes, jackals, wolves), result in this epizootiological scenario [42,44].
Such a marked difference in the seropositivity rate of CVBD pathogens in dogs from Italy and Greece was not detected in recent studies; by contrast, a similar occurrence of CVBDs was reported [11,30]. The discrepancy is likely due to the geographic area herein studied, i.e., Northern and Northeastern Greece, where CVBDs are significantly more prevalent than in other areas of the country, probably due to environmental drivers [15,40,42], while the previous study was conducted in Aegean islands, where no differences with the seroprevalence rates in Italy were detected [11]. Furthermore, in the present study, in Italy, almost only urban dogs were enrolled, while most of the dogs enrolled in Greece were from towns located in rural areas that lived outdoors, i.e., gardens and yards, and in environments where the presence of vectors and the infection pressure by CVBD pathogens is particularly high. Dogs living in less urbanized areas may be subjected to higher parasitological pressure, due to the higher density of wild reservoir hosts of both arthropod vectors and transmitted pathogens [45].
It should be kept in mind that dogs that tested seropositive for vector-borne pathogens (e.g., L. infantum, E. canis) and/or that were repeatedly exposed to one or more CVBDs may be clinically healthy for a certain period of time and then develop overt disease, e.g., kidney disease, only in a later stage of the infection [46,47,48]. Clinically healthy dogs have an important role in the maintenance of the circulation of L. infantum within canine and human populations, as they are more rarely examined and screened compared to dogs displaying clinical signs compatible with leishmaniosis [49]. This category of dogs plays an important epizootiological/epidemiological role, as they favor the spread of the infection, especially in cases where routine prophylaxis for leishmaniosis is not performed. As an example, unnoticed infections in non-endemic areas may facilitate the circulation of L. infantum among vectors and animal hosts.
The results of the statistical analyses should be interpreted in consideration of some features of the study dogs. The correlation between dogs over 2 years of age and the seropositivity to at least one CVBD is likely due to more chances of contact with the vectors than younger dogs. The apparent higher risk of infection in crossbred dogs has been likely influenced by the inclusion of a high number of crossbreeds (n. 120 dogs) in sites of Greece, especially during TF2.
5. Conclusions
In conclusion, rapid kits are among the first-line tools for the diagnosis of CVBDs in clinical settings, due to their limited cost and speed of execution and due to the high specificity ensured by the specific peptide targets for antibody detection. In-clinic kits are useful for the diagnosis of D. immitis infection (in combination with appropriate microfilariae-based test), for starting an in-depth diagnostic approach in the case of L. infantum infection or for steering the diagnosis towards tick-borne diseases in presence of compatible clinical signs. Testing for CVBDs should become routine in clinical settings, as this would allow the detection of subclinical infections that may subsequently exacerbate (especially co-infections), and when dogs are adopted or do not receive regular veterinary preventive care. While rapid kits are useful and easy to use routinely in veterinary practices, other laboratory serological tests (IFAT, ELISA) are often necessary for the confirmation of infection and/or the quantitative assessment of seropositivity. It is important to avoid unnecessary treatments and to set up a proper diagnostic and therapeutic strategy after a careful clinical evaluation by the veterinarian and on a case-by-case basis. Although clinically healthy dogs generally do not need therapy (unless laboratory alterations are present and/or in presence of high antibody titer), knowledge of their parasitological/serological status is necessary to set a proper follow-up and to apply prophylactic measures to limit the spread of the pathogen.
No rapid kit is currently available for the serodiagnosis of other important CVBDs (e.g., Rickettsia spp., Hepatozoon spp.) and the presence of the latter pathogens can be investigated only using laboratory serological tests (IFAT, ELISA) and/or molecular analyses (PCR). New rapid diagnostic tools would be highly appreciated by veterinarians in clinical settings, as a laboratory diagnosis using IFAT/ELISA or PCR is usually more expensive, possibly reducing the compliance of the owners to perform the investigation, and is more prone to cross-reactions in some cases [50]. Therefore, future studies aimed at (i) comparing the diagnostic performance of rapid kits vs. IFAT/ELISA and (ii) the development of new sensitive and specific rapid tools for the in-clinic diagnosis of CVBDs are advocated [51].
Author Contributions
Conceptualization, M.B., R.C., D.T. and N.P.; methodology and investigation, S.M., A.D., A.F.d.R., M.C., G.S., A.D.C., A.P., C.P., Z.T. and A.B.; validation and data curation, S.M., M.B. and R.C., writing—original draft preparation, S.M., D.T. and N.P.; writing—review and editing, S.M., A.D., D.T., M.B. and R.C.; supervision, M.B. and D.T.; project administration, M.B., R.C., S.M. and A.D.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by IDEXX Laboratories Inc., Westbrook, ME, USA by a donation to the University of Teramo. No funding number is available.
Institutional Review Board Statement
Ethical review and approval were waived for this study, as all the study activities have been performed in the frame of routine medical checks already needed by each animal involved in the present study and coordinated by local veterinarians. No procedures have been performed on the animal solely for the purposes of the present study.
Informed Consent Statement
Informed consent was obtained from all owners of animals involved in the study.
Data Availability Statement
All the data generated are described in the present article.
Acknowledgments
The authors would like to thank Dimitris Dimzas for his help in collecting and examining part of the samples.
Conflicts of Interest
Melissa Beall, Ramaswamy Chandrashekar and Nikola Pantchev are employees at IDEXX. All other authors declare no conflict of interest.
References
- Beugnet, F.; Marié, J.L. Emerging arthropod-borne diseases of companion animals in Europe. Vet. Parasitol. 2009, 163, 298–305. [Google Scholar] [CrossRef] [PubMed]
- Simón, F.; Siles-Lucas, M.; Morchón, R.; González-Miguel, J.; Mellado, I.; Carretón, E.; Montoya-Alonso, J.A. Human and animal dirofilariasis: The emergence of a zoonotic mosaic. Clin. Microbiol. Rev. 2012, 25, 507–544. [Google Scholar] [CrossRef] [PubMed]
- Gharbi, M.; Mhadhbi, M.; Rejeb, A.; Jaouadi, K.; Rouatbi, M.; Darghouth, M.A. Leishmaniosis (Leishmania infantum infection) in dogs. Rev. Sci. Tech. Off. Int. Epizoot. 2015, 342, 613–626. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Sainz, Á.; Roura, X.; Estrada-Peña, A.; Miró, G. A review of canine babesiosis: The European perspective. Parasites Vectors 2016, 9, 336. [Google Scholar] [CrossRef] [PubMed]
- Barash, N.R.; Thomas, B.; Birkenheuer, A.J.; Breitschwerdt, E.B.; Lemler, E.; Qurollo, B.A. Prevalence of Babesia spp. and clinical characteristics of Babesia vulpes infections in North American dogs. J. Vet. Intern. Med. 2019, 33, 2075–2081. [Google Scholar] [CrossRef] [PubMed]
- Guo, W.P.; Xie, G.C.; Li, D.; Su, M.; Jian, R.; Du, L.Y. Molecular detection and genetic characteristics of Babesia gibsoni in dogs in Shaanxi Province, China. Parasites Vectors 2020, 13, 366. [Google Scholar] [CrossRef] [PubMed]
- McCall, J.W.; Genchi, C.; Kramer, L.H.; Guerrero, J.; Venco, L. Heartworm disease in animals and humans. Adv. Parasitol. 2008, 66, 193–285. [Google Scholar] [PubMed]
- Paltrinieri, S.; Solano-Gallego, L.; Fondati, A.; Lubas, G.; Gradoni, L.; Castagnaro, M.; Crotti, A.; Maroli, M.; Oliva, G.; Roura, X.; et al. Guidelines for diagnosis and clinical classification of leishmaniasis in dogs. J. Am. Vet. Med. Assoc. 2010, 236, 1184–1191. [Google Scholar] [CrossRef]
- Sainz, Á.; Roura, X.; Miró, G.; Estrada-Peña, A.; Kohn, B.; Harrus, S.; Solano-Gallego, L. Guideline for veterinary practitioners on canine ehrlichiosis and anaplasmosis in Europe. Parasites Vectors 2015, 8, 75. [Google Scholar] [CrossRef]
- Mrljak, V.; Kuleš, J.; Mihaljević, Ž.; Torti, M.; Gotić, J.; Crnogaj, M.; Živičnjak, T.; Mayer, I.; Šmit, I.; Bhide, M.; et al. Prevalence and Geographic Distribution of Vector-Borne Pathogens in Apparently Healthy Dogs in Croatia. Vector-Borne Zoonotic Dis. 2017, 17, 398–408. [Google Scholar] [CrossRef]
- Diakou, A.; Di Cesare, A.; Morelli, S.; Colombo, M.; Halos, L.; Simonato, G.; Tamvakis, A.; Beugnet, F.; Paoletti, B.; Traversa, D. Endoparasites and vector-borne pathogens in dogs from Greek islands: Pathogen distribution and zoonotic implications. PLoS Negl. Trop. Dis. 2019, 13, e0007003. [Google Scholar] [CrossRef] [PubMed]
- Springer, A.; Glass, A.; Topp, A.K.; Strube, C. Zoonotic Tick-Borne Pathogens in Temperate and Cold Regions of Europe–A Review on the Prevalence in Domestic Animals. Front. Vet. Sci. 2020, 7, 604910. [Google Scholar] [CrossRef] [PubMed]
- Strobl, A.; Künzel, F.; Tichy, A.; Leschnik, M. Complications and risk factors regarding the outcomes of canine babesiosis in Central Europe–A retrospective analysis of 240 cases. Acta Vet. Hung. 2020, 68, 160–168. [Google Scholar] [CrossRef] [PubMed]
- Magi, M.; Guardone, L.; Dell’omodarme, M.; Prati, M.; Mignone, W.; Torracca, B.; Monni, G.; Macchioni, F. Angiostrongylus vasorum in red foxes (Vulpes vulpes) and badgers (Meles meles) from Central and Northern Italy. Hystrix Ital. J. Mammal. 2009, 20, 121–126. [Google Scholar]
- Angelou, A.; Gelasakis, A.I.; Verde, N.; Pantchev, N.; Schaper, R.; Chandrashekar, R.; Papadopoulos, E. Prevalence and risk factors for selected canine vector-borne diseases in Greece. Parasites Vectors 2019, 12, 283. [Google Scholar] [CrossRef]
- Gizzarelli, M.; Foglia Manzillo, V.; Ciuca, L.; Moroglione, M.E.; El Houda Ben Fayala, N.; Cringoli, G.; Oliva, G.; Rinaldi, L.; Maurelli, M.P. Simultaneous detection of parasitic vector borne diseases: A robust cross-sectional survey in hunting, stray and sheep dogs in a Mediterranean area. Front. Vet. Sci. 2019, 6, 288. [Google Scholar] [CrossRef]
- Morelli, S.; Crisi, P.E.; Di Cesare, A.; De Santis, F.; Barlaam, A.; Santoprete, G.; Parrinello, C.; Palermo, S.; Mancini, P.; Traversa, D. Exposure of client-owned cats to zoonotic vector-borne pathogens: Clinic-pathological alterations and infection risk analysis. Comp. Immunol. Microbiol. Infect. Dis. 2019, 66, 101344. [Google Scholar] [CrossRef]
- Traversa, D.; Morelli, S.; Cassini, R.; Crisi, P.E.; Russi, I.; Grillotti, E.; Manzocchi, S.; Simonato, G.; Beraldo, P.; Viglietti, A.; et al. Occurrence of canine and feline extra-intestinal nematodes in key endemic regions of Italy. Acta Trop. 2019, 193, 227–235. [Google Scholar] [CrossRef]
- Colombo, M.; Morelli, S.; Simonato, G.; Di Cesare, A.; Veronesi, F.; Frangipane di Regalbono, A.; Grassi, L.; Russi, I.; Tiscar, P.G. Exposure to major vector-borne diseases in dogs subjected to different preventative regimens in endemic areas of Italy. Pathogens 2021, 10, 507. [Google Scholar] [CrossRef]
- De Zan, G.; Citterio, C.V.; Danesi, P.; Gaspardis, G.; Gabassi, E.; Panciera, L.; Zanardello, C.; Binato, G.; Cocchi, M. Angiostrongylosis in northeastern Italy: First report of two autochthonous fatal cases in dogs and first detection in a wild red fox. Vet. Parasitol. Reg. Stud. Rep. 2021, 23, 100505. [Google Scholar] [CrossRef]
- Miró, G.; Wright, I.; Michael, H.; Burton, W.; Hegarty, E.; Rodón, J.; Buch, J.; Pantchev, N.; von Samson-Himmelstjerna, G. Seropositivity of main vector-borne pathogens in dogs across Europe. Parasites Vectors 2022, 15, 189. [Google Scholar] [CrossRef]
- Tennant, K.V.; Barker, E.N.; Polizopoulou, Z.; Helps, C.R.; Tasker, S. Real-time quantitative polymerase chain reaction detection of haemoplasmas in healthy and unhealthy dogs from Central Macedonia, Greece. J. Small Anim. Pract. 2011, 52, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Stillman, B.A.; Monn, M.; Liu, J.; Thatcher, B.; Foster, P.; Andrews, B.; Little, S.; Eberts, M.; Breitschwerdt, E.B.; Beall, M.J.; et al. Performance of a commercially available in-clinic ELISA for detection of antibodies against Anaplasma phagocytophilum, Anaplasma platys, Borrelia burgdorferi, Ehrlichia canis, and Ehrlichia ewingii and Dirofilaria immitis antigen in dogs. J. Am. Vet. Med. Assoc. 2014, 245, 80–86. [Google Scholar] [CrossRef]
- Ferroglio, E.; Centaro, E.; Mignone, W.; Trisciuoglio, A. Evaluation of an ELISA rapid device for the serological diagnosis of Leishmania infantum infection in dog as compared with immunofluorescence assay and Western blot. Vet. Parasitol. 2007, 144, 162–166. [Google Scholar] [CrossRef] [PubMed]
- Di Cesare, A.; Morelli, S.; Colombo, M.; Simonato, G.; Veronesi, F.; Marcer, F.; Diakou, A.; D’Angelosante, R.; Pantchev, N.; Psaralexi, E.; et al. Is Angiostrongylosis a Realistic Threat for Domestic Cats? Front. Vet. Sci. 2020, 7, 195. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Alonso, J.A.; Morchón, R.; Costa-Rodríguez, N.; Matos, J.I.; Falcón-Cordón, Y.; Carretón, E. Current Distribution of Selected Vector-Borne Diseases in Dogs in Spain. Front. Vet. Sci. 2020, 7, 564429. [Google Scholar] [CrossRef]
- Morelli, S.; Colombo, M.; Dimzas, D.; Barlaam, A.; Traversa, D.; Di Cesare, A.; Russi, I.; Spoletini, R.; Paoletti, B.; Diakou, A. Leishmania infantum Seroprevalence in Cats From Touristic Areas of Italy and Greece. Front. Vet. Sci. 2020, 7, 616566. [Google Scholar] [CrossRef]
- Morelli, S.; Diakou, A.; Di Cesare, A.; Schnyder, M.; Colombo, M.; Strube, C.; Dimzas, D.; Latino, R.; Traversa, D. Feline lungworms in Greece: Copromicroscopic, molecular and serological study. Parasitol. Res. 2020, 119, 2877–2883. [Google Scholar] [CrossRef]
- Panarese, R.; Iatta, R.; Latrofa, M.S.; Zatelli, A.; Ćupina, A.I.; Montarsi, F.; Pombi, M.; Mendoza-Roldan, J.A.; Beugnet, F.; Otranto, D. Hyperendemic Dirofilaria immitis infection in a sheltered dog population: An expanding threat in the Mediterranean region. Int. J. Parasitol. 2020, 50, 555–559. [Google Scholar] [CrossRef]
- Traversa, D.; Di Cesare, A.; Simonato, G.; Cassini, R.; Merola, C.; Diakou, A.; Halos, L.; Beugnet, F.; Frangipane di Regalbono, A. Zoonotic intestinal parasites and vector-borne pathogens in Italian shelter and kennel dogs. Comp. Immunol. Microbiol. Infect. Dis. 2017, 51, 69–75. [Google Scholar] [CrossRef]
- Silveira, C.A. Ehrlichiose Canina. Estudo Clinico De Uma População Animal, Na Região Urbana E Rural De Setúbal,–Implicações Em Saúde Publica E Saude Publica Veterinaria. Master’s Thesis, Universidade Tecnica de Lisboa, Lisboa, Portugal, 1992. [Google Scholar]
- Cardoso, L.; Mendão, C.; Madeira de Carvalho, L. Prevalence of Dirofilaria immitis, Ehrlichia canis, Borrelia burgdorferi sensu lato, Anaplasma spp. and Leishmania infantum in apparently healthy and CVBD-suspect dogs in Portugal-a national serological study. Parasites Vectors 2012, 5, 62. [Google Scholar] [CrossRef] [PubMed]
- Dordio, A.M.; Beck, R.; Nunes, T.; Pereira da Fonseca, I.; Gomes, J. Molecular survey of vector-borne diseases in two groups of domestic dogs from Lisbon, Portugal. Parasites Vectors 2021, 14, 163. [Google Scholar] [CrossRef] [PubMed]
- Ebani, V.V. Serological Survey of Ehrlichia canis and Anaplasma phagocytophilum in Dogs from Central Italy: An Update (2013–2017). Pathogens 2019, 8, 3. [Google Scholar] [CrossRef] [PubMed]
- Pantchev, N.; Schnyder, M.; Vrhovec, M.G.; Schaper, R.; Tsachev, I. Current Surveys of the Seroprevalence of Borrelia burgdorferi, Ehrlichia canis, Anaplasma phagocytophilum, Leishmania infantum, Babesia canis, Angiostrongylus vasorum and Dirofilaria immitis in Dogs in Bulgaria. Parasitol. Res. 2015, 114 (Suppl. 1), S117–S130. [Google Scholar] [CrossRef]
- Santoro, M.; Miletti, G.; Vangone, L.; Spadari, L.; Reccia, S.; Fusco, G. Heartworm disease (Dirofilaria immitis) in two roaming dogs from the urban area of Castel Volturno, Southern Italy. Front. Vet. Sci. 2019, 6, 270. [Google Scholar] [CrossRef]
- Mendoza-Roldan, J.; Benelli, G.; Panarese, R.; Iatta, R.; Furlanello, T.; Beugnet, F.; Zatelli, A.; Otranto, D. Leishmania infantum and Dirofilaria immitis infections in Italy, 2009–2019: Changing distribution patterns. Parasites Vectors 2020, 13, 193. [Google Scholar] [CrossRef] [PubMed]
- Matos, M.; Alho, A.M.; Owen, S.P.; Nunes, T.; de Carvalho, L.M. Parasite control practices and public perception of parasitic diseases: A survey of dog and cat owners. Prev. Vet. Med. 2015, 122, 174–180. [Google Scholar] [CrossRef]
- Beasley, E.A.; Pessôa-Pereira, D.; Scorza, B.M.; Petersen, C.A. Epidemiologic, Clinical and Immunological Consequences of Co-Infections during Canine Leishmaniosis. Animals 2021, 11, 3206. [Google Scholar] [CrossRef]
- Symeonidou, I.; Angelou, A.; Theodoridis, A.; Sioutas, G.; Papadopoulos, E. Canine Leishmaniosis in Greece: An Updated Countrywide Serological Study and Associated Risk Factors. Pathogens 2021, 10, 1129. [Google Scholar] [CrossRef]
- Athanasiou, L.V.; Kontos, V.I.; Saridomichelakis, M.N.; Rallis, T.S.; Diakou, A. A cross-sectional seroepidemiological study of canine leishmaniasis in Greek mainland. Acta Trop. 2012, 122, 291–295. [Google Scholar] [CrossRef]
- Diakou, A.; Kapantaidakis, E.; Tamvakis, A.; Giannakis, V.; Strus, N. Dirofilaria infections in dogs in different areas of Greece. Parasites Vectors 2016, 9, 508. [Google Scholar] [CrossRef] [PubMed]
- Diakou, A.; Mylonakis, M.; Polizopoulou, Z.; Koutinas, C. Awareness and strategies about canine heartworm (Dirofilaria immitis) infection in private practices in Greece: Preliminary results of an ongoing questionnaire survey. In Proceedings of the Fifth European Dirofilaria and Angiostrongylus Days (FiEDAD) 2016, Vienna, Austria, 11–13 July 2016. [Google Scholar]
- Diakou, A.; Gewehr, S.; Kapantaidakis, E.; Mourelatos, S. Can mosquito population dynamics predict Diroflaria hyperendemic foci? In Proceedings of the E-SOVE 2014 the 19th Conference, Thessaloniki, Greece, 13–17 October 2014. [Google Scholar]
- de Macedo, L.O.; Bezerra-Santos, M.A.; Filho, C.R.C.U.; da Silva Sales, K.G.; de Sousa-Paula, L.C.; da Silva, L.G.; Dantas-Torres, F.; do Nascimento Ramos, R.A.; Otranto, D. Vector-borne pathogens of zoonotic concern in dogs from a Quilombola community in northeastern Brazil. Parasitol. Res. 2022, 121, 3305–3311. [Google Scholar] [CrossRef]
- Drake, C.; Coyne, M.; McCrann, D.J.; Buch, J.; Mack, R. Risk of development of chronic kidney disease after exposure to Borrelia burgdorferi and Anaplasma spp. Top. Companion Anim. Med. 2021, 42, 100491. [Google Scholar] [CrossRef] [PubMed]
- Burton, W.; Drake, C.; Ogeer, J.; Buch, J.; Mack, R.; McCrann, D.; Coyne, M.J. Association between exposure to Ehrlichia spp. and risk of developing chronic kidney disease in dogs. J. Am. Anim. Hosp. Assoc. 2020, 56, 159–164. [Google Scholar] [CrossRef] [PubMed]
- Solano-Gallego, L.; Miró, G.; Koutinas, A.; Cardoso, L.; Pennisi, M.G.; Ferrer, L.; Bourdeau, P.; Oliva, G.; Baneth, G.; The LeishVet Group. LeishVet guidelines for the practical management of canine leishmaniosis. Parasites Vectors 2011, 4, 86. [Google Scholar] [CrossRef]
- Moshfe, A.; Mohebali, M.; Edrissian, G.; Zarei, Z.; Akhoundi, B.; Kazemi, B.; Jamshidi, S.; Mahmoodi, M. Canine visceral leishmaniasis: Asymptomatic infected dogs as a source of L. infantum infection. Acta trop. 2009, 112, 101–105. [Google Scholar] [CrossRef]
- Qurollo, B.A.; Stillman, B.A.; Beall, M.J.; Foster, P.; Hegarty, B.C.; Breitschwerdt, E.B.; Chandrashekar, R. Comparison of Anaplasma and Ehrlichia species-specific peptide ELISAs with whole organism-based immunofluorescent assays for serologic diagnosis of anaplasmosis and ehrlichiosis in dogs. Am. J. Vet. Res. 2021, 82, 71–80. [Google Scholar] [CrossRef]
- Beall, M.J.; Mainville, C.A.; Arguello-Marin, A.; Clark, G.; Lemieux, C.; Saucier, J.; Thatcher, B.; Breitschwerdt, E.B.; Cohn, L.A.; Qurollo, B.A.; et al. An Improved Point-of-Care ELISA for the Diagnosis of Anaplasmosis and Ehrlichiosis During the Acute Phase of Tick-Borne Infections in Dogs. Top. Companion Anim. Med. 2022, 51, 100735. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).