Abstract
Swinepox virus (SWPV) is responsible for sporadic acute poxvirus infections in swine worldwide, causing a pathognomonic eruptive proliferative dermatitis. Beside direct and congenital transmission, the pig louse Haematopinus suis acts as a mechanical vector and favors virus infection through skin lesions. Infections are generally described in domestic pigs, while only a few cases have been reported in wild boars, in Austria and Germany. In September 2022, SWPV infection was suspected at post-mortem examination of a wild boar piglet with characteristic lesions in Liguria, Northwest Italy. The piglet was heavily parasitized by swine lice (H. suis). SWPV was then confirmed by histological and molecular analyses. Possible viral co-infections were also investigated (African swine fever virus, classical swine fever virus, parvovirus, circovirus, Aujeszky’s disease virus and hepatitis E virus). This article describes gross and histopathologic features of SWPV infection, differential diagnosis, and potential vector-borne transmission to domestic pigs, presenting a brief review of the literature on the topic. SWPV infection is reported in wild boars in Italy for the first time. The finding of SWPV in a wild boar in an area with a very limited pig population may suggest the existence of a “wildlife cycle” in the area. Further investigations are needed to understand the real risk of transmission of SWPV to domestic pigs as well as the role of other arthropod vectors.
Keywords:
infectious diseases; histopathology; vector-borne; pigs; wildlife; SWPV; molecular analysis 1. Introduction
Swinepox virus (SWPV) is a globally distributed swine pathogen causing sporadic acute poxvirus infections in pigs, characterized by a pathognomonic eruptive proliferative dermatitis and secondary ulcerations [1]. Its main mode of transmission is through the biting of the pig louse Haematopinus suis, which acts as a mechanical vector; however, direct animal contact and congenital transmission have also been reported [2,3,4]. Swinepox was first described as a disease of domestic pigs in Europe in 1842 [5] and in the USA in 1929 [6], but outbreaks have subsequently been found in pigs also in Africa, Asia, Australia and South America [7,8,9,10,11,12,13]. It is commonly associated with poor sanitation [4], since the virus shows a high environmental stability and is very resistant to drying, as all poxviruses are [14].
Piglets up to 3 months of age are the most susceptible to the clinical disease, while adults usually develop a mild form of the infection [15]. The disease occurring after congenital infection seems to be more severe, with higher mortality rates [1,4]. Despite its wide distribution, studies on SWPV infection are scarce, maybe due to its self-limiting and sporadic nature [2,4]. However, knowledge about the prevalence, strain diversity, evolutionary origin and wildlife reservoirs of SWPV is limited [1].
SWPV consists of a 146 kbp linear double-stranded DNA genome and is the only member of the Suipoxvirus genus, which belongs the Chordopoxvirinae subfamily, within the Poxviridae family [2]. Due to its large genome, the SWPV has been used as a delivery vector for several bacterial and virus vaccines [2]. Clinical studies and experimental and in vitro infections have shown that SWPV displays a high degree of host specificity [1]. Among swine, infections are mainly described in domestic pigs, while only a few cases have been reported in wild boars, in Austria [16] and Germany [1].
In Italy, SWPV has been very sporadically reported in domestic pigs. Its presence has been demonstrated in Northern Italy since 2002 [17] and in central Italy since 2006 [18]. In November 2013, an outbreak affecting a group of 3-months old piglets was registered in an extensive organic farm holding around 110 animals of a local breed in Tuscany (Central Italy) [18]. The aim of this study is to report a SWPV infection in a wild boar piglet with characteristic lesions in Liguria region (Northwest Italy), describing gross and histopathologic findings, differential diagnosis, and potential vector-borne transmission to domestic pigs. The presented case represents the first description of SWPV in wild boars in Italy, suggesting that the virus is circulating in this host in the wild.
2. Materials and Methods
2.1. Post-Mortem Examination
In September 2022, a male striated wild boar piglet accidentally found dead (lat. 44.445309, long. 9.191845) was submitted by the local veterinary authority to the Istituto Zooprofilattico Sperimentale of Piedmont, Liguria and Aosta Valley (IZSPLV), section of Genoa, for necrospy and molecular testing for African Swine Fever (ASF), as part of the ongoing national passive surveillance activity. A complete post-mortem examination was conducted, and samples were taken for subsequent histopathologic analysis.
2.2. Histopathology
Cutaneous lesions (from neck and ear skin) and a submandibular lymph node were fixed in 10% neutral-buffered formalin, paraffine embedded, sectioned by a microtome at 4 µm and subsequently stained with haematoxylin and eosin (HE). The slides were evaluated under a light microscope (Zeiss Axio Scope.A1, Jena, Germany) at increasing magnification (10×, 20×, 40×).
2.3. Molecular Analysis
Total DNA and RNA were extracted using a commercial virus extraction kit with Maxwell automatic extractor (Promega) from different anatomic samples: cutaneous lesions, submandibular and mesenteric lymph nodes, spleen, lungs, and liver. Molecular analyses for the detection of SWPV were conducted on cutaneous lesions, submandibular lymph node and spleen samples (Table 1). The SWPV amplicon was directly sequenced using PCR primers on a 3130XL Genetic Analyzer (Thermo Fisher Scientific Inc., Waltham, MA, USA). Sequences were aligned using the SeqMan software (Lasergene package. DNASTAR Inc., Madison, WI, USA) to obtain a consensus sequence and compared with available sequences retrieved from the National Center for Biotechnology Information (NCBI) database through the BLAST tool (http://blast.ncbi.nlm.nih.gov/Blast.cgi, accessed on 14 February 2023). Details on the other investigated viral infections and related tested samples are reported in Table 1. Negative and positive controls were used in every PCR reaction. Negative controls include an extraction control and an amplification control (water for molecular biology), positive controls consisted in reference material provided with the diagnostic kit (African Swine Fever) or by the National Reference Centre (Classical Swine Fever) or in DNA of field samples previously tested positive, with amplicons subjected to Sanger sequencing and confirmed by BLAST analysis.

Table 1.
Molecular analyses conducted to search for swinepox virus (SWPV) and co-infections and their results. NA: Not Available, as covered by industrial copyright.
3. Results
3.1. Post-Mortem Examination
The striated wild boar piglet (weight ~4 Kg) showed on external examination a heavy infection by the swine louse H. suis, a BCS of 2 and blood soiling on the snout. Macroscopically, multifocal to coalescing papular lesions (0.5–0.8 cm in diameter) were present on the skin of the snout, neck, torax, abdomen and paws, not involving the interdigital space (Figure 1). At necropsy, no apparent lesions affecting the abdominal viscera were observed, while, in the thorax, the lungs presented atelectasis affecting the apical pulmonary lobes. Traumatic lesions compatible with a car accident were found on examination of the head. The stomach was full of semi-digested material.

Figure 1.
Gross aspect of the affected wild boar: (a) left flak; (b) ventral view; (c) detail of the head and periocular region; (d) detail of the pinna; (e,f) front and back legs; (g) axillary region.
3.2. Histopathology
The histological examination of the skin lesions from the neck and ear showed areas of epidermal proliferation with foci of keratinocyte degeneration in the stratum spinosum, with rare intracytoplasmic eosinophilic inclusions. A severe multifocal infiltration of neutrophils was also observed, forming micro-abscesses at the intra-epidermal level with the formation of large surface crusted areas with the presence of bacterial aggregates. In some locations, the inflammatory process also involved adnexal structures. A severe and diffuse inflammatory infiltrate involving neutrophils, macrophages and lymphocytes in the dermis was also observed. The histopathologic picture is shown in Figure 2.
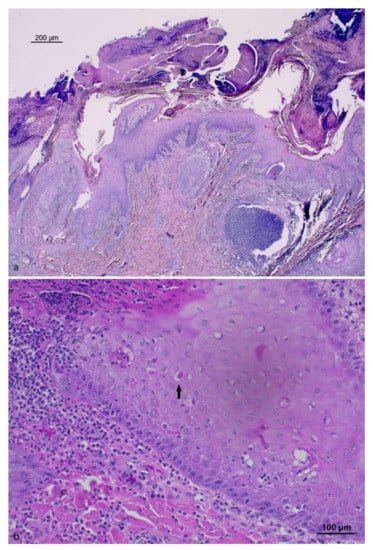
Figure 2.
Skin, wild boar pinna: (a) severe pustular epidermitis with crusted areas (HE), Bar = 200 μm; (b) hyperplastic epidermis with eosinophilic inclusion body (black arrow) in the cytoplasm of keratinocytes (HE), Bar = 100 μm.
The submandibular lymph node presented severe multifocal to coalescing inflammation characterized by intact and necrotic neutrophils with a large necrotic central area and the presence of bacterial aggregates.
3.3. Molecular Analysis
3.3.1. SWPV
DNA extracted from the cutaneous lesions and the submandibular lymph node tested positive for SWPV, while the spleen turned out negative (Figure 3). The positivity was confirmed by sequencing a 175 bp fragment of the putative metallo-protease gene. The SWPV sequence was submitted to NCBI GenBank under accession number OQ446858. Blast analysis revealed 100% identity with four sequences of the European–North American lineage (accession nrs. NC_003389, MZ682626, MZ773481, MZ773480) and 99.3% similarity with one sequence of the Indian lineage (accession nr. MW036632), according to the tentative classification recently proposed by Kumar et al. [25]. Since these five sequences are the only ones currently available for the target gene, with limited variability, a phylogenetic analysis was not carried out.
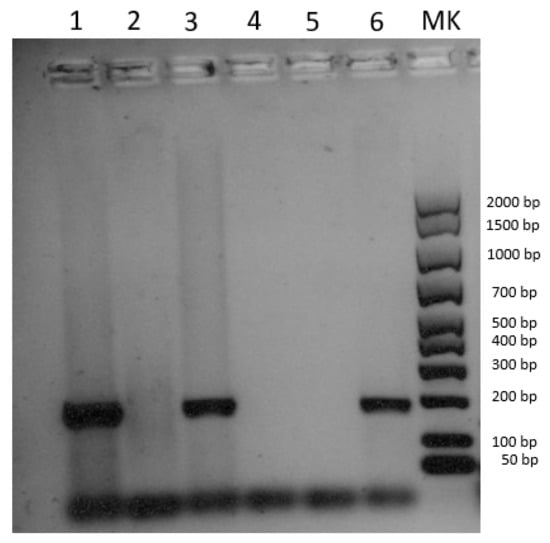
Figure 3.
Electrophoresis gel image of the pan poxvirus PCR according to Li et al. [19]. Line 1: cutaneous lesions; line 2: spleen; line 3: mesenteric lymph nodes; line 4: negative extraction control; line 5: negative amplification control; line 6: positive control, DNA of Lumpy skin disease virus (Capripoxvirus).
3.3.2. Other Viral Infections
Molecular analysis carried out to test for co-infections were all negative.
4. Discussion
4.1. Gross and Histopathologic Features of Swinepox Virus Infection
The macroscopic aspect of SWPV infection has been described as a multifocal, eruptive dermatitis, commonly affecting the abdomen, inner surface of the legs, pinnae and occasionally the snout, vulva and back [9,10,13]. The development of a generalized disease, with lesions affecting the whole body, has been reported for suckling piglets [2], as observed in the described case. It has been hypothesized that the main lesions, observed on the abdomen and inner surface of the legs, including the udder and vulva, correspond to the predilection sites of the pig louse [1]. Secondary bacterial infections, facilitated by SWPV infection and disruption of skin epithelium, lead to more severe lesions and formation of local abscesses [2,10]. According to the literature, clinical lesions are generally restricted to the skin, with occasional mild changes in the superficial lymph nodes [1]. In a study describing congenital infection in 14 piglets, pustular and ulcerative lesions were also found on the tongue and hard palate of four piglets, while no significant lesions were found on the internal organs [4]. No internal lesions were also found in an outbreak in Papua New Guinea [8]. These observations agree with our results, as we only observed typical lesions at the skin level, not involving internal organs.
The skin lesions typically start with petechiae, which may appear 2 days post-infection, and then evolve to papules, and to pustules that eventually originate crusts (scabs) after a week. A true vesicle stage is absent or transient [26]. The crusts ultimately shed, leaving skin discoloration [2]. The infection is usually self-limiting, with resolution in 3–4 weeks [27], although secondary bacterial infection prolongs the duration of symptoms [4]. A transient rise in temperature and appetite loss may occur. The infected pigs may exhibit conjunctivitis, unilateral or bilateral keratitis and/or pan-ophthalmia. Kerato-conjunctivitis without cutaneous eruption lesion has also been observed [15]. It is not fully understood how spreading from the primary site of replication to other parts of the organism occurs, as evidenced by the fact that viremia is lacking and SWPV could not be isolated from the blood of infected animals [28,29]. This could explain the negative PCR result obtained for DNA extracted from the spleen in our study.
The most readily visible histological changes of tissues from swinepox-infected pigs include hydropic degeneration of the epidermal stratum spinosum and keratinocytes, where viral replication occurs [1]. Eosinophilic, rounded, intracytoplasmic inclusion bodies 3–8μm in diameter can be observed in keratinocytes [9,12]. Indeed, the histological analysis of the lesions from the neck and ear skin of the described case showed keratinocyte degeneration at this level, with intracytoplasmic eosinophilic inclusions (Figure 2). Traditionally, the diagnosis of swinepox is based on the observation of the typical clinical signs and lesions, and on the observation of microscopic characteristics [30]: infected cell cytoplasm is enlarged and contains inclusion bodies, whereas the nucleus exhibits margination of chromatin and a large, central “vacuole” [2]. The secondary bacterial infection can complicate the histologic picture [8]. Histopathologic changes caused by SWPV are very similar to those of other poxviruses, which however are host specific.
The differential diagnosis of swinepox includes vaccinia virus (VACV) infection, vesicular diseases (including foot and mouth disease), parvovirus, pityriasis rosea, parakeratosis, parasitic skin disorders (including sarcoptic mange and Acarus (Tyroglyphus) spp. mite irritation), allergic skin reactions, insects’ bites, early stages of ringworm, thrombocytopenic purpura, localized staphylococcal or streptococcal epidermitis and cutaneous erysipelas [2,4,9,29]. The multispecies-infecting VACV is the etiological agent of the most similar disease, although lesions are smaller and the incubation period shorter. VACV still circulates in some countries, such as Brazil, but not in Western Europe and can be easily differentiated from SWPV by molecular tools [1].
4.2. Potential Vector-Borne Transmission
SWPV enters the host through a break in the skin, replicates in the cytoplasm of keratinocytes of the stratum spinosum [29], and it is shed from nasal and oral secretions and skin lesions. It is present in infected epithelium and in dry scabs produced in the later stages of the infection. Poxviruses in general show a high environmental stability and remain contagious over a period of several months. They are particularly resistant to drying, a feature which is further enhanced by the organic materials in which they are released into the environment, such as crusts [14]. Abraded skin can serve as the route of entry for the virus [15]. As mentioned, in addition to direct contact between infected and susceptible animals and to congenital transmission, insect vectors such as H. suis have been shown to facilitate viral spread between populations [31]. Early experiments investigated the role of H. suis in swinepox infections and demonstrated its function as a mechanical vector, but not as an intermediate host [3]. Flies and mosquitoes also seem to be implicated as mechanical vectors [2,15,29]. However, studies on vector-borne transmission are scanty and outdated.
In the most recently described outbreak in Italy, the source of SWPV infection could not be demonstrated, but a role in the transmission of the disease within the farm was attributed to H. suis, which was parasitizing the animals [18]. In two outbreaks in the northeast of India, the entire body of the affected animals was found to be heavily infected with swine lice [7]. Similarly, in five swinepox outbreaks in northeastern Brazil, affected backyard pigs from herds with poor hygienic conditions presented with severe fly and lice infestations [12]. In contrast, in an outbreak described in Papua New Guinea, lice were not observed, but a large number of stable flies, Stomoxys calcitrans, was present. Their role as a mechanical vector had already been hypothesized for fowl pox. Organophosphate insecticides were sprayed at the beginning of the outbreak to control the insects [8]. Moreover, in an outbreak described in Australia, sucking lice were not found, but mosquitoes, biting flies and midges were abundant in the affected environment. In such a case, pox-carrying insects could have been carried by the winds [9] and the spreading of SWPV could occur over longer distances.
In this study, SWPV infection in wild boars in Italy was reported for the first time, and only a few other reports in this wild host are known from the literature [1,16]. In a recent study conducted in Germany, SWPV strains isolated from a wild boar piglet and a congenitally infected domestic piglet were sequenced, finding only 0.076% divergence [1]. Interestingly, domestic pig breeding is traditionally very limited in Liguria, and the swine population has been further reduced due to the restrictions following the ASF outbreak, which started in January 2022. In contrast, the wild boar population is abundant and rising (authors’ note). Thus, it can be hypothesized that SWPV may be present in wild boars in the area, circulating in a wildlife (sylvatic) cycle. Although strict sanitary measures and high hygienic standards are usually implemented in industrialized countries to protect the livestock population against contact with pathogens present in the environment and to prevent potential direct or indirect contacts with wild boar, vector-borne transmission may be more challenging to control, especially in extensive farming. Further investigations are needed to understand if SWPV is present in wild boars in other geographical areas, as well as the real risk of transmission to domestic pigs, and the role of H. suis and other arthropod vectors.
Author Contributions
Conceptualization, L.G. and K.V.; methodology, L.G., V.L., K.V., E.B., L.M. and S.P.; software, S.P. and V.M.; formal analysis, S.P. and E.M.; investigation, L.G., V.L., L.W. and R.Z.; resources, E.R., P.A, E.B. and L.M.; writing—original draft preparation, L.G., K.V., L.W. and E.M.; writing—review and editing, E.R., V.L. and R.Z.; visualization, V.M.; supervision, P.A. and L.M.; project administration, E.R.; funding acquisition, E.R., P.A. and L.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Liguria Region, grant number 22ALA.
Institutional Review Board Statement
Ethical review and approval were waived for this study since the involved subject was accidentally found dead by local veterinary authorities.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data deriving from the study are given in the article text.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kaiser, F.K.; Wiedemann, A.; Kühl, B.; Menke, L.; Beineke, A.; Baumgärtner, W.; Wohlsein, P.; Rigbers, K.; Becher, P.; Peters, M.; et al. Swinepox Virus Strains Isolated from Domestic Pigs and Wild Boar in Germany Display Altered Coding Capacity in the Terminal Genome Region Encoding for Species-Specific Genes. Viruses 2021, 13, 2038. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishnan, M.A.; Ashokkumar, D. Swinepox virus. In Recent Advances in Animal Virology; Springer Nature: Singapore, 2019; pp. 161–169. [Google Scholar] [CrossRef]
- Shope, R.E. Swine Pox. Arch. Gesamte Virusforsch. 1940, 1, 457–467. [Google Scholar] [CrossRef]
- Thibault, S.; Drolet, R.; Alain, R.; Dea, S. Congenital swine pox: A sporadic skin disorder in nursing piglets. Swine Health Prod. 1998, 6, 276–278. [Google Scholar]
- Spinola, W.T.J. Die Krankheiten der Schweine (1842); Hieschwald, A., Ed.; Kessinger Publishing: Berlin, Germany, 2010; p. 204.2. [Google Scholar]
- McNutt, S.; Murray, C.; Purwin, P. Swine Pox. J. Am. Vet. Med. Assoc. 1929, 74, 752–761. [Google Scholar]
- Bora, M.; Borah, B.; Kakati, P.; Nehar, S.; Dutta, L.J.; Mech, P.; Barman, N.N.; Venkatesan, G.; Reddy, G.M.; Das, S.K. Detection and characterization of Swinepox virus from pig population of Assam, a North-Eastern state of India. Indian J. Anim. Res. 2017, B-3352, 1–6. [Google Scholar] [CrossRef]
- Copland, J.W. Swine pox in Papua New Guinea. Trop. Anim. Health Prod. 1974, 6, 153–157. [Google Scholar] [CrossRef]
- Jubb, T.; Ellis, T.; Peet, R.; Parkinson, J. Swinepox in pigs in northern Western Australia. Aust. Vet. J. 1992, 69, 99. [Google Scholar] [CrossRef]
- Mech, P.; Bora, D.P.; Neher, S.; Barman, N.N.; Borah, P.; Tamuly, S.; Dutta, L.J.; Das, S.K. Identification of swinepox virus from natural outbreaks in pig population of Assam. Virus Dis. 2018, 29, 395–399. [Google Scholar] [CrossRef]
- Medaglia, M.L.G.; Pereira, A.D.C.; Freitas, T.R.; Damaso, C.R. Swinepox Virus Outbreak, Brazil, 2011. Emerg. Infect. Dis. 2011, 17, 1976–1978. [Google Scholar] [CrossRef]
- Olinda, R.G.; Maia, L.A.; Cargnelutti, J.F.; Gois, R.C.; Batista, J.S.; Flores, E.F.; Riet-Correa, F.; Dantas, A.F. Swinepox dermatitis in backyard pigs in Northeastern Brazil. Pesqui. Vet. Bras. 2016, 36, 468–472. [Google Scholar] [CrossRef]
- Olufemi, B.E.; Ayoade, G.O.; Ikede, B.O.; Akpavie, S.O.; Nwufoh, K.J. Swine pox in Nigeria. Vet. Rec. 1981, 109, 278–280. [Google Scholar] [CrossRef]
- Rheinbaben, F.v.; Gebel, J.; Exner, M.; Schmidt, A. Environmental resistance, disinfection, and sterilization of poxviruses. In Poxviruses; Birkhäuser: Basel, Switzerland, 2007; pp. 397–405. [Google Scholar] [CrossRef]
- Delhon, G.; Tulman, E.R.; Afonso, C.L.; Rock, D.L. Diseases of Swine, 10th ed.; Wiley-Blackwell: Chichester/Ames, UK, 2012. [Google Scholar]
- Steineck, T.; Kolodziejek, J.; Nowotny, N.; Schilcher, F. A case of Swine Pox in a Wild Boar (Sus Scrofa) in Austria. In Proceedings of the 6th Conference of the European Wildlife Disease Association, Uppsala, Sweden, 8–12 September 2004. [Google Scholar]
- Tittarelli, C.; Sozzi, E.; Spaggiari, B.; Alborali, G.L.; Cordioli, P.; Lavazza, A. Diagnosi al microscopio elettronico di swinepoxvirus, agente di malattia cutanea sporadica, durante il periodo 2002–2008 nel Nord Italia. In Proceedings of the Atti della SIPAS, Società Italiana di Patologia ed Allevamento dei suini XXXV Meeting Annuale, Modena, Singapore, 12–13 March 2009; pp. 507–512. [Google Scholar]
- Mariano, V.; Nardi, A.; Vergari, E.; Carletti, F.; Barbieri, L.; Cardeti, G. Poxvirus in a swine farm in Italy: A sporadic outbreak. Large Anim. Rev. 2015, 21, 219–220. [Google Scholar]
- Li, Y.; Meyer, H.; Zhao, H.; Damon, I.K. GC Content-Based Pan-Pox Universal PCR Assays for Poxvirus Detection. J. Clin. Microbiol. 2010, 48, 268–276. [Google Scholar] [CrossRef]
- Hoffmann, B.; Beer, M.; Schelp, C.; Schirrmeier, H.; Depner, K. Validation of a real-time RT-PCR assay for sensitive and specific detection of classical swine fever. J. Virol. Methods 2005, 130, 36–44. [Google Scholar] [CrossRef]
- Ogawa, H.; Taira, O.; Hirai, T.; Takeuchi, H.; Nagao, A.; Ishikawa, Y.; Tuchiya, K.; Nunoya, T.; Ueda, S. Multiplex PCR and multiplex RT-PCR for inclusive detection of major swine DNA and RNA viruses in pigs with multiple infections. J. Virol. Methods 2009, 160, 210–214. [Google Scholar] [CrossRef]
- Larochelle, R.; Antaya, M.; Morin, M.; Magar, R. Typing of porcine circovirus in clinical specimens by multiplex PCR. J. Virol. Methods 1999, 80, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Ma, W.; Lager, K.M.; Richt, J.A.; Stoffregen, W.C.; Zhou, F.; Yoon, K.-J. Development of Real-Time Polymerase Chain Reaction Assays for Rapid Detection and Differentiation of Wild-Type Pseudorabies and Gene-Deleted Vaccine Viruses. J. Vet. Diagn. Investig. 2008, 20, 440–447. [Google Scholar] [CrossRef]
- Jothikumar, N.; Cromeans, T.L.; Robertson, B.H.; Meng, X.J.; Hill, V.R. A broadly reactive one-step real-time RT-PCR assay for rapid and sensitive detection of hepatitis E virus. J. Virol. Methods 2006, 131, 65–71. [Google Scholar] [CrossRef] [PubMed]
- Kumar, A.; Gupta, N.; Fayaz, A.; Mageswary, R.; Bano, R.; ChandraSekar, S.; Muthuchelvan, D.; Dhama, K.; Pandey, A.B.; Andavar, R.M. Molecular epidemiology of swinepox viruses circulating in India. Vet. Q. 2023, 43, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Munz, E.; Dumbell, K. Swinepox. In Infectious Diseases of Livestock; Coetzer, J.A.W., Thomson, G.R., Tustin, R.C., Eds.; Oxford University Press: New York, NY, USA, 1994; pp. 627–629. [Google Scholar]
- Bratke, K.A.; McLysaght, A.; Rothenburg, S. A survey of host range genes in poxvirus genomes. Infect. Genet. Evol. 2013, 14, 406–425. [Google Scholar] [CrossRef]
- Paton, D.J.; Brown, I.H.; Fitton, J.; Wrathall, A.E. Congenital pig pox: A case report. Vet. Rec. 1990, 127, 204. [Google Scholar] [PubMed]
- Freitas, T.R.P. Swinepox Virus. In Diseases of Swine; John Wiley & Sons: Hoboken, NJ, USA, 2019; pp. 709–714. [Google Scholar]
- Riyesh, T.; Barua, S.; Kumar, N.; Jindal, N.; Bera, B.C.; Narang, G.; Mahajan, N.K.; Arora, D.; Anand, T.; Vaid, R.K.; et al. Isolation and genetic characterization of swinepox virus from pigs in India. Comp. Immunol. Microbiol. Infect. Dis. 2016, 46, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Miller, R.B.; Olson, L.D. Experimental induction of cutaneous streptococcal abscesses in swine as a sequela to swinepox. Am. J. Vet. Res. 1980, 41, 341–347. [Google Scholar] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).