Human Borrelia miyamotoi Infection in North America
Abstract
1. Epidemiology
1.1. Introduction
1.2. The Organism
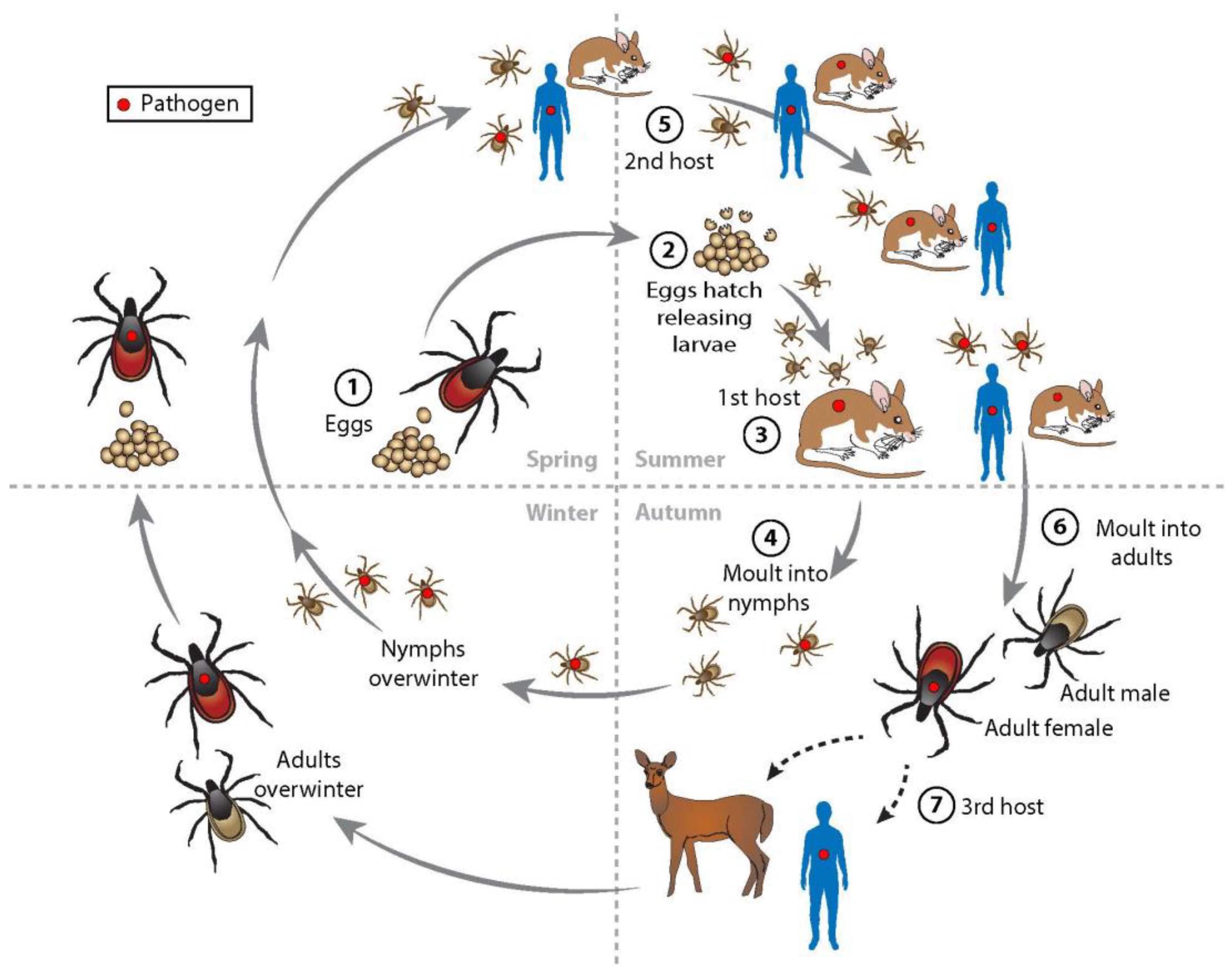
1.3. Ecology
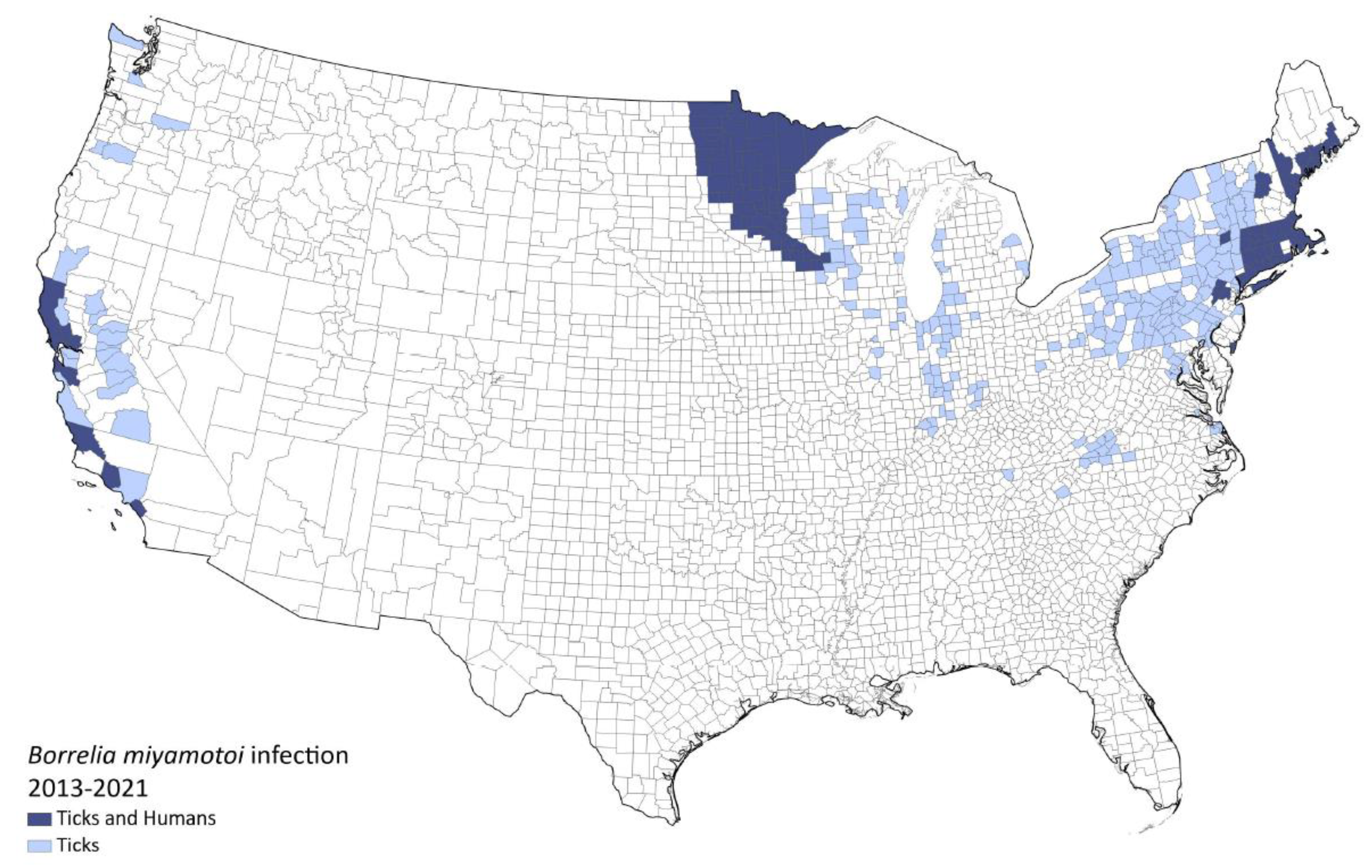
1.4. Location and Prevalence
1.4.1. USA
1.4.2. Canada
| Survey | Location | Dates | B. miyamotoi-Infected Average % (Range) | B. burgdorferi-Infected Average % (Range) |
|---|---|---|---|---|
| Human infection [16,59,70,71] (seroprevalence) | USA Northeast | 1991–2018 | 3.0 (0.6–5.2) | 10.8 (6.8–15.6) |
| I. scapularis infection [4,34,38,39,40,41,42,43,44,45,46,47] | USA Northeast | 1998–2019 | 1.5 (0–10.5) | 20.4 (2.6–49.7) |
| I. scapularis infection [34,40,44,45,46,63] | USA Midwest | 1998–2015 | 2.1 (0–12) | 17.7 (3.7–41) |
| I. pacificus infection [48,51,53,54,55,56,57,58,60] | USA Far West | 2000–2016 | 1.2 (0–3.7) | 3.9 (0.6–7.1) |
| I. scapularis infection [71,77] | Canada | 2011–2020 | 0.6 (0–0.7) | 17.4 (0–33.3) |
2. Clinical Manifestations
2.1. General Clinical Course
2.2. Coinfection
| Symptom | US Cases (85) No. (%) with Symptom | Worldwide Cases (504) No. (%) with Symptom |
|---|---|---|
| Fever | 80 (94%) | 479 (95%) |
| Chills/rigors | 67 (79%) | 343 (68%) |
| Headache | 61 (72%) | 432 (86%) |
| Myalgia | 60 (71%) | 328 (65%) |
| Fatigue | 59 (69%) | 197 (39%) |
| Arthralgia | 46 (54%) * | 225 (45%) |
| Abdominal complaints | 9 (11%) * | 214 (42%) |
| Relapsing fever | 3 (4%) | 47 (9%) |
| Erythema migrans rash | 0 | 21 (4%) |
2.3. Complications
3. Diagnosis, Treatment, and Prevention
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Disclaimer
References
- Fukunaga, M.; Takahashi, Y.; Tsuruta, Y.; Matsushita, O.; Ralph, D.; McClelland, M.; Nakao, M. Genetic and phenotypic analysis of Borrelia miyamotoi sp. nov., Isolated from the ixodid tick Ixodes persulcatus, the vector for Lyme disease in Japan. Int. J. Syst. Evol. Microbiol. 1995, 45, 804–810. [Google Scholar] [CrossRef] [PubMed]
- Dworkin, M.S.; Schwan, T.G.; Anderson, D.E., Jr.; Borchardt, S.M. Tick-borne relapsing fever. Infect. Dis. Clin. N. Am. 2008, 22, 449–468. [Google Scholar] [CrossRef]
- Larsson, C.; Andersson, M.; Bergström, S. Current issues in relapsing fever. Curr. Opin. Infect. Dis. 2009, 22, 443–449. [Google Scholar] [CrossRef] [PubMed]
- Scoles, G.A.; Papero, M.; Beati, L.; Fish, D. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 2001, 1, 21–34. [Google Scholar] [CrossRef] [PubMed]
- Platonov, A.E.; Karan, L.S.; Kolyasnikova, N.M.; Makhneva, N.A.; Toporkova, M.G.; Maleev, V.V.; Fish, D.; Krause, P.J. Humans infected with relapsing fever spirochete Borrelia miyamotoi, Russia. Emerg. Infect. Dis. 2011, 17, 1816–1823. [Google Scholar] [CrossRef]
- Hoornstra, D.; Azagi, T.; van Eck, J.A.; Wagemakers, A.; Koetsveld, J.; Spijker, R.; Platonov, A.E.; Sprong, H.; Hovius, J.W. Prevalence and clinical manifestation of Borrelia miyamotoi in Ixodes ticks and humans in the northern hemisphere: A systematic review and meta-analysis. Lancet Microbe 2022, 3, e772–e786. [Google Scholar] [CrossRef]
- Krause, P.; Fish, D.; Narasimhan, S.; Barbour, A. Borrelia miyamotoi infection in nature and in humans. Clin. Microbiol. Infect. 2015, 21, 631–639. [Google Scholar] [CrossRef]
- Wagemakers, A.; Staarink, P.J.; Sprong, H.; Hovius, J.W. Borrelia miyamotoi: A widespread tick-borne relapsing fever spirochete. Trends Parasitol. 2015, 31, 260–269. [Google Scholar] [CrossRef]
- Jiang, B.G.; Jia, N.; Jiang, J.F.; Zheng, Y.C.; Chu, Y.L.; Jiang, R.R.; Wang, Y.W.; Liu, H.B.; Wei, R.; Zhang, W.H.; et al. Borrelia miyamotoi infections in humans and ticks, Northeastern China. Emerg. Infect. Dis. 2018, 24, 236–241. [Google Scholar] [CrossRef]
- Talagrand-Reboul, E.; Boyer, P.H.; Bergström, S.; Vial, L.; Boulanger, N. Relapsing fevers: Neglected tick-borne diseases. Front. Cell Infect. Microbiol. 2018, 8, 98. [Google Scholar] [CrossRef]
- Kubiak, K.; Szczotko, M.; Dmitryjuk, M. Borrelia miyamotoi—An emerging human tick-borne pathogen in Europe. Microorganisms 2021, 9, 154. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Phylogeny of a relapsing fever Borrelia species transmitted by the hard tick Ixodes scapularis. Infect. Genet. Evol. 2014, 27, 551–558. [Google Scholar] [CrossRef]
- Telford, S.R., 3rd; Goethert, H.K.; Molloy, P.J.; Berardi, V.P.; Chowdri, H.R.; Gugliotta, J.L.; Lepore, T.J. Borrelia miyamotoi disease: Neither Lyme disease nor relapsing fever. Clin. Lab. Med. 2015, 35, 867–882. [Google Scholar] [CrossRef]
- Schwan, T.G.; Schrumpf, M.E.; Hinnebusch, B.J.; Anderson, D.E.; Konkel, M.E. GlpQ: An antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 1996, 34, 2483–2492. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Rollend, L.; Fikrig, E.; Lepore, T.; Barbour, A.; Fish, D. Human Borrelia miyamotoi infection in the United States. N. Engl. J. Med. 2013, 368, 291–293. [Google Scholar] [CrossRef]
- Krause, P.J.; Narasimhan, S.; Wormser, G.P.; Barbour, A.G.; Platonov, A.E.; Brancato, J.; Lepore, T.; Dardick, K.; Mamula, M.; Rollend, L.; et al. Borrelia miyamotoi sensu lato seroreactivity and seroprevalence in the northeastern United States. Emerg. Infect. Dis. 2014, 20, 1183–1190. [Google Scholar] [CrossRef] [PubMed]
- Rollend, L.; Fish, D.; Childs, J.E. Transovarial transmission of Borrelia spirochetes by Ixodes scapularis: A summary of the literature and recent observations. Ticks Tick-Borne Dis. 2013, 4, 46–51. [Google Scholar] [CrossRef]
- Lynn, G.E.; Breuner, N.E.; Hojgaard, A.; Oliver, J.; Eisen, L.; Eisen, R.J. A comparison of horizontal and transovarial transmission efficiency of Borrelia miyamotoi by Ixodes scapularis. Ticks Tick-Borne Dis. 2022, 13, 102003. [Google Scholar] [CrossRef] [PubMed]
- Nakao, R.; Kasama, K.; Boldbaatar, B.; Ogura, Y.; Kawabata, H.; Toyoda, A.; Hayashi, T.; Takano, A.; Maeda, K. The evolution of hard tick-borne relapsing fever borreliae is correlated with vector species rather than geographical distance. BMC Ecol. Evol. 2021, 21, 105. [Google Scholar] [CrossRef]
- Hue, F.; Langeroudi, A.G.; Barbour, A.G. Chromosome sequence of Borrelia miyamotoi, an uncultivable tick-borne agent of human infection. Genome Announc. 2013, 1, e00713-13. [Google Scholar] [CrossRef]
- Kuleshov, K.V.; Koetsveld, J.; Goptar, I.A.; Markelov, M.L.; Kolyasnikova, N.M.; Sarksyan, D.S.; Toporkova, M.G.; Kirdyashkina, N.P.; Shipulin, G.A.; Hovius, J.W.; et al. Whole-genome sequencing of six Borrelia miyamotoi clinical strains isolated in Russia. Genome Announc. 2018, 6, e01424-17. [Google Scholar] [CrossRef]
- Kingry, L.C.; Replogle, A.; Dolan, M.; Sexton, C.; Padgett, K.A.; Schriefer, M.E. Chromosome and large linear plasmid sequences of a Borrelia miyamotoi strain isolated from Ixodes pacificus ticks from California. Genome Announc. 2017, 5, e00960-17. [Google Scholar] [CrossRef] [PubMed]
- Kingry, L.C.; Replogle, A.; Batra, D.; Rowe, L.A.; Sexton, C.; Dolan, M.; Connally, N.; Petersen, J.M.; Schriefer, M.E. Toward a Complete North American Borrelia miyamotoi genome. Genome Announc. 2017, 5, e01557-16. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G. Multiple and diverse vsp and vlp sequences in Borrelia miyamotoi, a hard tick-borne zoonotic pathogen. PLoS ONE 2016, 11, e0146283. [Google Scholar] [CrossRef] [PubMed]
- Bergström, S.; Normark, J. Microbiological features distinguishing Lyme disease and relapsing fever spirochetes. Wien. Klin. Wochenschr. 2018, 130, 484–490. [Google Scholar] [CrossRef]
- Kuleshov, K.V.; Hoornstra, D.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Draft Whole-Genome Sequences of Two Western European Borrelia miyamotoi Isolates. Genome Announc. 2019, 8, e01314-19. [Google Scholar] [CrossRef] [PubMed]
- Kuleshov, K.V.; Margos, G.; Fingerle, V.; Koetsveld, J.; Goptar, I.A.; Markelov, M.L.; Kolyasnikova, N.M.; Sarksyan, D.S.; Kirdyashkina, N.P.; Shipulin, G.A.; et al. Whole genome sequencing of Borrelia miyamotoi isolate Izh-4: Reference for a complex bacterial genome. BMC Genom. 2020, 21, 16. [Google Scholar] [CrossRef]
- Hojgaard, A.; Osikowicz, L.M.; Maes, S.; Eisen, L.; Eisen, R.J. Detection of genetic variability in Borrelia miyamotoi (Spirochaetales: Spi-rochaetaceae) between and within the eastern and western United States. J. Med. Entomol. 2021, 58, 2154–2160. [Google Scholar] [CrossRef]
- Krause, P.J.; Carroll, M.; Fedorova, N.; Brancato, J.; Dumouchel, C.; Akosa, F.; Narasimhan, S.; Fikrig, E.; Lane, R.S. Human Borrelia miyamotoi infection in California: Serodiagnosis is complicated by multiple endemic Borrelia species. PLoS ONE 2018, 13, e0191725. [Google Scholar] [CrossRef]
- Fiorito, T.M.; Reece, R.; Flanigan, T.P.; Silverblatt, F.J. Borrelia miyamotoi polymerase chain reaction positivity on a tick-borne disease panel in an endemic region of Rhode Island: A case series. Infect. Dis. Clin. Pract. 2017, 25, 250–254. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Cucura, D.M.; Rochlin, I.; Sameroff, S.; Lipkin, W.I. Detection of Anaplasma phagocytophilum, Babesia microti, Borrelia burgdorferi, Borrelia miyamotoi, and Powassan virus in ticks by a multiplex real-time reverse transcription-PCR assay. Msphere 2017, 2, e00151-17. [Google Scholar] [CrossRef] [PubMed]
- Vannier, E.; Krause, P.J. Human babesiosis. N. Engl. J. Med. 2012, 366, 2397–2407. [Google Scholar] [CrossRef] [PubMed]
- Spielman, A.; Wilson, M.L.; Levine, J.F.; Piesman, J. Ecology of Ixodes Dammini-borne human babesiosis and Lyme Disease. Annu. Rev. Èntomol. 1985, 30, 439–460. [Google Scholar] [CrossRef] [PubMed]
- Barbour, A.G.; Fish, D.; Hoen, A.G.; Tsao, J.I.; Diuk-Wasser, M.A.; Bunikis, J.; Travinsky, B. Niche Partitioning of Borrelia burgdorferi and Borrelia miyamotoi in the same tick vector and mammalian reservoir species. Am. J. Trop. Med. Hyg. 2009, 81, 1120–1131. [Google Scholar] [CrossRef] [PubMed]
- Lommano, E.; Dvořák, C.; Vallotton, L.; Jenni, L.; Gern, L. Tick-borne pathogens in ticks collected from breeding and migratory birds in Switzerland. Ticks Tick-Borne Dis. 2014, 5, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Taylor, K.R.; Takano, A.; Konnai, S.; Shimozuru, M.; Kawabata, H.; Tsubota, T. Borrelia miyamotoi infections among wild rodents show age and month independence and correlation with Ixodes persulcatus larval attachment in Hokkaido, Japan. Vector-Borne Zoonotic Dis. 2013, 13, 92–97. [Google Scholar] [CrossRef]
- Cosson, J.-F.; Michelet, L.; Chotte, J.; Le Naour, E.; Cote, M.; Devillers, E.; Poulle, M.-L.; Huet, D.; Galan, M.; Geller, J.; et al. Genetic characterization of the human relapsing fever spirochete Borrelia miyamotoi in vectors and animal reservoirs of Lyme disease spirochetes in France. Parasites Vectors 2014, 7, 233. [Google Scholar] [CrossRef]
- Lehane, A.; Maes, S.E.; Graham, C.B.; Jones, E.; Delorey, M.; Eisen, R.J. Prevalence of single and coinfections of human pathogens in Ixodes ticks from five geographical regions in the United States, 2013–2019. Ticks Tick Borne Dis. 2021, 12, 101637. [Google Scholar] [CrossRef]
- Han, S.; Hickling, G.J.; Ogden, N.H.; Ginsberg, H.S.; Kobbekaduwa, V.; Rulison, E.L.; Beati, L.; Tsao, J.I. Seasonality of acarological risk of exposure to Borrelia miyamotoi from questing life stages of Ixodes scapularis collected from Wisconsin and Massachusetts, USA. Ticks Tick-Borne Dis. 2020, 12, 101556. [Google Scholar] [CrossRef]
- Tokarz, R.; Jain, K.; Bennett, A.; Briese, T.; Lipkin, W.I. Assessment of polymicrobial infections in ticks in New York state. Vector-Borne Zoonotic Dis. 2010, 10, 217–221. [Google Scholar] [CrossRef]
- Edwards, M.J.; Barbalato, L.A.; Makkapati, A.; Pham, K.D.; Bugbee, L.M. Relatively low prevalence of Babesia microti and Anaplasma phagocytophilum in Ixodes scapularis ticks collected in the Lehigh Valley region of eastern Pennsylvania. Ticks Tick-Borne Dis. 2015, 6, 812–819. [Google Scholar] [CrossRef]
- Johnson, T.L.; Graham, C.B.; Boegler, K.A.; Cherry, C.C.; Maes, S.E.; Pilgard, M.A.; Hojgaard, A.; Buttke, D.E.; Eisen, R.J. Prevalence and diversity of tick-borne pathogens in nymphal Ixodes scapularis(Acari: Ixodidae) in eastern national parks. J. Med. Èntomol. 2016, 54, 742–751. [Google Scholar]
- Graham, C.B.; Pilgard, M.A.; Maes, S.E.; Hojgaard, A.; Eisen, R.J. Paired real-time PCR assays for detection of Borrelia miyamotoi in North American Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae). Ticks Tick-Borne Dis. 2016, 7, 1230–1235. [Google Scholar] [CrossRef] [PubMed]
- Piedmonte, N.P.; Shaw, S.B.; Prusinski, M.; Fierke, M.K. Landscape features associated with blacklegged tick (Acari: Ixodidae) density and tick-borne pathogen prevalence at multiple spatial scales in central New York state. J. Med. Èntomol. 2018, 55, 1496–1508. [Google Scholar] [CrossRef]
- Edwards, M.J.; Russell, J.C.; Davidson, E.N.; Yanushefski, T.J.; Fleischman, B.L.; Heist, R.O.; Leep-Lazar, J.G.; Stuppi, S.L.; Esposito, R.A.; Suppan, L.M. A 4-yr survey of the range of ticks and tick-borne pathogens in the Lehigh Valley Region of Eastern Pennsylvania. J. Med. Èntomol. 2019, 56, 1122–1134. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Vicente, S.; Tagliafierro, T.; Coleman, J.L.; Benach, J.L.; Tokarz, R. Polymicrobial nature of tick-borne diseases. Mbio 2019, 10, e02055-19. [Google Scholar] [CrossRef] [PubMed]
- Adalsteinsson, S.A.; Shriver, W.G.; Hojgaard, A.; Bowman, J.L.; Brisson, D.; D’Amico, V.; Buler, J.J. Multiflora rose invasion amplifies prevalence of Lyme disease pathogen, but not necessarily Lyme disease risk. Parasites Vectors 2018, 11, 54. [Google Scholar] [CrossRef]
- Crowder, C.D.; Carolan, H.E.; Rounds, M.A.; Honig, V.; Mothes, B.; Haag, H.; Nolte, O.; Luft, B.J.; Grubhoffer, L.; Ecker, D.J.; et al. Prevalence of Borrelia miyamotoi in Ixodes ticks in Europe and the United States. Emerg. Infect. Dis. 2014, 20, 1678–1682. [Google Scholar] [CrossRef]
- Johnson, T.L.; Graham, C.B.; Maes, S.E.; Hojgaard, A.; Fleshman, A.; Boegler, K.A.; Delory, M.J.; Slater, K.S.; Karpathy, S.E.; Bjork, J.K.; et al. Prevalence and distribution of seven human pathogens in host-seeking Ixodes scapularis (Acari: Ixodidae) nymphs in Minnesota, USA. Ticks Tick-Borne Dis. 2018, 9, 1499–1507. [Google Scholar] [CrossRef]
- Hahn, M.B.; Bjork, J.K.; Neitzel, D.F.; Dorr, F.M.; Whitemarsh, T.; Boegler, K.A.; Graham, C.B.; Johnson, T.L.; Maes, S.E.; Eisen, R.J. Evaluating acarological risk for exposure to Ixodes scapularis and Ixodes scapularis-borne pathogens in recreational and residential settings in Washington County, Minnesota. Ticks Tick-Borne Dis. 2018, 9, 340–348. [Google Scholar] [CrossRef]
- Lynn, G.; Graham, C.B.; Horiuchi, K.; Eisen, L.; Johnson, T.L.; Lane, R.S.; Eisen, R.J. Prevalence and geographic distribution of Borrelia miyamotoi in host-seeking Ixodes pacificus (Acari: Ixodidae) nymphs in Mendocino County, California. J. Med. Èntomol. 2018, 55, 711–716. [Google Scholar] [CrossRef] [PubMed]
- Eshoo, M.W.; Carolan, H.E.; Massire, C.; Chou, D.M.; Crowder, C.D.; Rounds, M.A.; Phillipson, C.A.; Schutzer, S.E.; Ecker, D.J. Survey of Ixodes pacificus ticks in California reveals a diversity of microorganisms and a novel and widespread Anaplasmataceae species. PLoS ONE 2015, 10, e0135828. [Google Scholar] [CrossRef] [PubMed]
- Padgett, K.; Bonilla, D.; Kjemtrup, A.; Vilcins, I.-M.; Yoshimizu, M.H.; Hui, L.; Sola, M.; Quintana, M.; Kramer, V. Large scale spatial risk and comparative prevalence of Borrelia miyamotoi and Borrelia burgdorferi sensu lato in Ixodes pacificus. PLoS ONE 2014, 9, e110853. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Cinkovich, S.; Nieto, N.C. Tick-borne pathogens in northwestern California, USA. Emerg. Infect. Dis. 2014, 20, 493–494. [Google Scholar] [CrossRef]
- Salkeld, D.J.; Nieto, N.C.; Carbajales-Dale, P.; Carbajales-Dale, M.; Cinkovich, S.S.; Lambin, E.F. Disease risk & landscape attributes of tick-borne Borrelia pathogens in the San Francisco Bay area, California. PLoS ONE 2015, 10, e0134812. [Google Scholar]
- Fedorova, N.; Kleinjan, J.E.; James, D.; Hui, L.T.; Peeters, H.; Lane, R.S. Remarkable diversity of tick or mammalian-associated Borreliae in the metropolitan San Francisco Bay area, California. Ticks Tick-Borne Dis. 2014, 5, 951–961. [Google Scholar] [CrossRef]
- Dykstra, E.A.; Oltean, H.N.; Kangiser, D.; Marsden-Haug, N.; Rich, S.M.; Xu, G.; Lee, M.-K.; Morshed, M.G.; Graham, C.B.; Eisen, R.J. Ecology and epidemiology of tickborne pathogens, Washington, USA, 2011–2016. Emerg. Infect. Dis. 2020, 26, 648–657. [Google Scholar] [CrossRef]
- Mun, J.; Eisen, R.J.; Eisen, L.; Lane, R.S. Detection of a Borrelia miyamotoi sensu lato relapsing-fever group spirochete from Ixodes pacificus in California. J. Med. Èntomol. 2006, 43, 120–123. [Google Scholar] [CrossRef] [PubMed]
- Johnston, D.; Kelly, J.R.; Ledizet, M.; Lavoie, N.; Smith, R.P., Jr.; Parsonnet, J.; Schwab, J.; Stratidis, J.; Lee, G.; Espich, S.; et al. Geographic dispersion of Borrelia miyamotoi, Borrelia burgdorferi and Babesia microti in New England. Clin. Inf. Dis. 2022, 23, ciac107. [Google Scholar] [CrossRef]
- Lane, R.S.; Fedorova, N.; Kleinjan, J.E.; Maxwell, M. Eco-epidemiological factors contributing to the low risk of human exposure to ixodid tick-borne borreliae in southern California, USA. Ticks Tick-Borne Dis. 2013, 4, 377–385. [Google Scholar] [CrossRef]
- Fleshman, A.C.; Foster, E.; Maes, S.E.; Eisen, R.J. Reported county-level distribution of seven human pathogens detected in host-seeking Ixodes scapularis and Ixodes pacificus (Acari: Ixodidae) in the contiguous United States. J. Med. Èntomol. 2022, 59, 1328–1335. [Google Scholar] [CrossRef] [PubMed]
- Hamer, S.A.; Hickling, G.J.; Walker, E.D.; Tsao, J.I. Increased diversity of zoonotic pathogens and Borrelia burgdorferi strains in established versus incipient Ixodes scapularis populations across the midwestern United States. Infect. Genet Evol. 2014, 27, 531–542. [Google Scholar] [CrossRef] [PubMed]
- Han, S.; Hickling, G.J.; Tsao, J.I. High prevalence of Borrelia miyamotoi among adult blacklegged ticks from white-tailed deer. Emerg. Infect. Dis. 2016, 22, 316–318. [Google Scholar] [CrossRef]
- Keesing, F.; McHenry, D.J.; Hersh, M.H.; Ostfeld, R.S. Spatial and temporal patterns of the emerging tick-borne pathogen Borrelia miyamotoi in blacklegged ticks (Ixodes scapularis) in New York. Parasites Vectors 2021, 14, 51. [Google Scholar] [CrossRef]
- Livengood, J.; Hutchinson, M.L.; Thirumalapura, N.; Tewari, D. Detection of Babesia, Borrelia, Anaplasma, and Rickettsia spp. in adult black-legged ticks (Ixodes scapularis) from Pennsylvania, United States, with a Luminex Multiplex Bead Assay. Vector Borne Zoonotic Dis. 2020, 20, 406–411. [Google Scholar] [CrossRef]
- Tokarz, R.; Tagliafierro, T.; Sameroff, S.; Cucura, D.M.; Oleynik, A.; Che, X.; Jain, K.; Lipkin, W.I. Microbiome analysis of Ixodes scapularis ticks from New York and Connecticut. Ticks Tick Borne Dis. 2019, 10, 894–900. [Google Scholar] [CrossRef] [PubMed]
- Milholland, M.T.; Eisen, L.; Nadolny, R.M.; Hojgaard, A.; Machtinger, E.T.; Mullinax, J.M.; Li, A.Y. Surveillance of ticks and tick-borne pathogens in suburban natural habitats of central Maryland. J. Med. Èntomol. 2021, 58, 1352–1362. [Google Scholar] [CrossRef]
- Xu, G.; Luo, C.Y.; Ribbe, F.; Pearson, P.; Ledizet, M.; Rich, S.M. Borrelia miyamotoi in human-biting ticks, United States, 2013–2019. Emerg Infect Dis. 2021, 27, 3193–3195. [Google Scholar] [CrossRef]
- Padgett, K.A.; Bonilla, D.L. Novel exposure sites for nymphal Ixodes pacificus within picnic areas. Ticks Tick-Borne Dis. 2011, 2, 191–195. [Google Scholar] [CrossRef]
- Wroblewski, D.; Gebhardt, L.; Prusinski, M.A.; Meehan, L.J.; Halse, T.A.; Musser, K.A. Detection of Borrelia miyamotoi and other tick-borne pathogens in human clinical specimens and Ixodes scapularis ticks in New York State, 2012–2015. Ticks Tick-Borne Dis. 2017, 8, 407–411. [Google Scholar] [CrossRef]
- Smith, R.P., Jr.; Elias, S.P.; Cavanaugh, C.E.; Lubelczyk, C.B.; Lacombe, E.H.; Brancato, J.; Doyle, H.; Rand, P.W.; Ebel, G.D.; Krause, P.J. Seroprevalence of Borrelia burgdorferi, B. miyamotoi, and Powassan virus in residents bitten by Ixodes ticks, Maine, USA. Emerg Infect Dis. 2019, 25, 804–807. [Google Scholar] [CrossRef]
- Dibernardo, A.; Cote, T.; Ogden, N.H.; Lindsay, L.R. The prevalence of Borrelia miyamotoi infection, and co-infections with other Borrelia spp. in Ixodes scapularis ticks collected in Canada. Parasites Vectors 2014, 7, 183. [Google Scholar] [CrossRef] [PubMed]
- Clow, K.M.; Ogden, N.H.; Lindsay, L.R.; Michel, P.; Pearl, D.L.; Jardine, C.M. Distribution of ticks and the risk of Lyme disease and other tick-borne pathogens of public health significance in Ontario, Canada. Vector-Borne Zoonotic Dis. 2016, 16, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Milnes, E.L.; Thornton, G.; Léveillé, A.N.; Delnatte, P.; Barta, J.R.; Smith, D.A.; Nemeth, N. Babesia odocoilei and zoonotic pathogens identified from Ixodes scapularis ticks in southern Ontario, Canada. Ticks Tick-Borne Dis. 2019, 10, 670–676. [Google Scholar] [CrossRef] [PubMed]
- Kulkarni, M.; Kryuchkov, R.; Statculescu, A.; Thickstun, C.; Dibernardo, A.; Lindsay, L.; Talbot, B. Ixodes scapularis tick distribution and infection rates in Ottawa, Ontario, 2017. Can. Commun. Dis. Rep. 2018, 44, 237–242. [Google Scholar] [CrossRef] [PubMed]
- Nelder, M.P.; Russell, C.B.; Dibernardo, A.; Clow, K.M.; Johnson, S.; Cronin, K.; Patel, S.N.; Lindsay, L.R. Monitoring the patterns of submission and presence of tick-borne pathogens in Ixodes scapularis collected from humans and companion animals in Ontario, Canada (2011–2017). Parasites Vectors 2021, 14, 260. [Google Scholar] [CrossRef] [PubMed]
- Jacob, A.E.; Weese, J.S.; Rosseau, J.; Clow, K.M. Spatial patterns of Borrelia burgdorferi, Borrelia miyamotoi and Anaplasma phagocytophilum detected in Ixodes spp. ticks from Canadian companion animals, 2019–2020. Zoonoses Public Health 2022, 69, 944–955. [Google Scholar] [CrossRef] [PubMed]
- Guillot, C.; Badcock, J.; Clow, K.; Cram, J.; Dergousoff, S.; Dibernardo, A.; Evason, M.; Fraser, E.; Galanis, E.; Gasmi, S.; et al. Sentinel surveillance of Lyme disease risk in Canada, 2019: Results from the first year of the Canadian Lyme Sentinel Network (CaLSeN). Can. Commun. Dis. Rep. 2020, 46, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kadkhoda, K.; Dumouchel, C.; Brancato, J.; Ainsley, G.; Krause, P.J. Human seroprevalence of Borrelia miyamotoi in Manitoba, Canada: 2011–2014. A cross sectional study. CMAJ Open 2017, 5, E690–E693. [Google Scholar] [CrossRef] [PubMed]
- Ogden, N.H.; Ben Beard, C.; Ginsberg, H.S.; Tsao, J.I. Possible effects of climate change on Ixodid ticks and the pathogens they transmit: Predictions and observations. J. Med. Èntomol. 2020, 58, 1536–1545. [Google Scholar] [CrossRef]
- Gilbert, L. The impacts of climate change on ticks and tick-borne disease risk. Annu. Rev. Èntomol. 2021, 66, 373–388. [Google Scholar] [CrossRef] [PubMed]
- Eisen, R.J.; Eisen, L.; Ogden, N.H.; Beard, C.B. Linkages of weather and climate with Ixodes scapularis and Ixodes pacificus(Acari: Ixodidae), enzootic transmission of Borrelia burgdorferi, and Lyme disease in North America. J. Med. Èntomol. 2015, 53, 250–261. [Google Scholar] [CrossRef] [PubMed]
- Brownstein, J.S.; Holford, T.R.; Fish, D. Effect of climate change on Lyme disease risk in North America. EcoHealth 2005, 2, 38–46. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; McKay, K.; Gadbaw, J.; Christianson, D.; Closter, L.; Lepore, T.; Telford, S.; Sikand, V.; Ryan, R.; Persing, D.; et al. Increasing health burden of human babesiosis in endemic sites. Am. J. Trop. Med. Hyg. 2003, 68, 431–436. [Google Scholar] [CrossRef] [PubMed]
- Mead, P. Epidemiology of Lyme disease. Infect. Dis. Clin. N. Am. 2022, 36, 495–521. [Google Scholar] [CrossRef] [PubMed]
- Molloy, P.J.; Telford, S.R., 3rd; Chowdri, H.R.; Lepore, T.J.; Gugliotta, J.L.; Goethert, H.; Molloy, P.J.; Weeks, K.E.; Hewins, M.E.; Goethert, H.K.; et al. Borrelia miyamotoi disease in the northeastern United States: A case series. Ann. Intern. Med. 2015, 163, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Hoornstra, D.; Koetsveld, J.; Sprong, H.; Platonov, A.E.; Hovius, J.W. Borrelia miyamotoi disease in an immunocompetent patient, western Europe. Emerg Infect Dis. 2018, 24, 1770–1772. [Google Scholar] [CrossRef]
- Platonov, A.E.; Toporkova, M.G.; Kolyasnikova, N.M.; Stukolova, O.A.; Dolgova, A.S.; Brodovikova, A.V.; Makhneva, N.A.; Karan, L.S.; Koetsveld, J.; Shipulin, G.A.; et al. Clinical presentation of Ixodes tick-borne borreliosis caused by Borrelia miyamotoi in the context of an immune response to the pathogen. Ter. Arkhiv 2017, 89, 35–43. [Google Scholar] [CrossRef]
- Karan, L.; Makenov, M.; Kolyasnikova, N.; Stukolova, O.; Toporkova, M.; Olenkova, O. Dynamics of spirochetemia and early PCR detection of Borrelia miyamotoi. Emerg. Infect. Dis. 2018, 24, 860–867. [Google Scholar] [CrossRef]
- Sarksyan, D.; Platonov, A.; Karan, L.S.; Malinin, I.E.; Khalitova, L.I.; Shakhov, V.I.; Dudarev, M.V.; Malinin, O.; Maleev, V. Clinical presentation of “new” tick-borne borreliosis caused by Borrelia miyamotoi. Ter. Arkhiv 2012, 84, 34–41. [Google Scholar]
- Sarksyan, D.S.; Maleev, V.V.; Platonov, A.E.; Platonova, O.V.; Karan, L.S. Relapsing (recurrent) disease caused by Borrelia miyamotoi. Ter. Arkh. 2015, 87, 18–25. (In Russian) [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Telford, S.R.; Spielman, A.; Sikand, V.; Ryan, R.; Christianson, D.; Burke, G.; Brassard, P.; Pollack, R.; Peck, J.; et al. Concurrent Lyme disease and babesiosis. Evidence for increased severity and duration of illness. JAMA 1996, 275, 1657–1660. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; McKay, K.; Thompson, C.A.; Sikand, V.K.; Lentz, R.; Lepore, T.; Closter, L.; Christianson, D.; Telford, S.R.; Persing, D.; et al. Disease-specific diagnosis of coinfecting tickborne zoonoses: Babesiosis, human granulocytic ehrlichiosis, and Lyme disease. Clin. Infect. Dis. 2002, 4, 1184–1191. [Google Scholar] [CrossRef] [PubMed]
- Horowitz, H.W.; Aguero-Rosenfeld, M.E.; Holmgren, D.; McKenna, D.; Schwartz, I.; Cox, M.E.; Wormser, G.P. Lyme disease and human granulocytic anaplasmosis coinfection: Impact of case definition on coinfection rates and illness severity. Clin. Infect. Dis. 2012, 56, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Steere, A.C.; McHugh, G.; Suarez, C.; Hoitt, J.; Damle, N.; Sikand, V.K. Prospective study of coinfection in patients with erythema migrans. Clin Infect Dis. 2003, 36, 1078–1081. [Google Scholar] [CrossRef]
- Sato, K.; Takano, A.; Konnai, S.; Nakao, M.; Ito, T.; Koyama, K.; Kaneko, M.; Ohnishi, M.; Kawabata, H. Human infections with Borrelia miyamotoi, Japan. Emerg. Infect. Dis. 2014, 20, 1391–1393. [Google Scholar] [CrossRef]
- Jobe, D.A.; Lovrich, S.D.; Oldenburg, D.G.; Kowalski, T.J.; Callister, S.M. Borrelia miyamotoi infection in patients from upper midwestern United States, 2014–2015. Emerg. Infect. Dis. 2016, 22, 1471–1473. [Google Scholar] [CrossRef]
- Marcos, L.A.; Smith, K.; Reardon, K.; Weinbaum, F.; Spitzer, E. Presence of Borrelia miyamotoi infection in a highly endemic area of Lyme disease. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 22. [Google Scholar] [CrossRef]
- Mukerji, S.S.; Ard, K.L.; Schaefer, P.W.; Branda, J.A. Case 32-2020: A 63-Year-Old Man with confusion, fatigue, and garbled speech. N. Engl. J. Med. 2020, 383, 1578–1586. [Google Scholar] [CrossRef]
- Gandhi, S.; Narasimhan, S.; Workineh, A.; Mamula, M.; Jennifer Yoon, J.; Krause, P.J.; Farhadian, S.F. Borrelia miyamotoi meningoencephalitis in an immunocompetent patient. Open Forum Infect. Dis. 2022, 9, ofac295. [Google Scholar] [CrossRef]
- Hovius, J.W.; de Wever, B.; Sohne, M.; Brouwer, M.C.; Coumou, J.; Wagemakers, A.; Oei, A.; Knol, H.; Narasimhan, S.; Hodiamont, C.J.; et al. A case of meningoencephalitis by the relapsing fever spirochaete Borrelia miyamotoi in Europe. Lancet 2013, 382, 658. [Google Scholar] [CrossRef] [PubMed]
- Boden, K.; Lobenstein, S.; Hermann, B.; Margos, G.; Fingerle, V. Borrelia miyamotoi-associated neuroborreliosis in immunocompromised person. Emerg. Infect. Dis. 2016, 22, 1617–1620. [Google Scholar] [CrossRef] [PubMed]
- Henningsson, A.J.; Asgeirsson, H.; Hammas, B.; Karlsson, E.; Parke, Å.; Hoornstra, D.; Wilhelmsson, P.; Hovius, J.W. Two cases of Borrelia miyamotoi meningitis, Sweden, 2018. Emerg. Infect Dis. 2019, 25, 1965–1968. [Google Scholar] [CrossRef] [PubMed]
- Tobudic, S.; Burgmann, H.; Stanek, G.; Winkler, S.; Schötta, A.M.; Obermüller, M.; Markowicz, M.; Lagler, H. Human Borrelia miyamotoi infection, Austria. Emerg. Infect. Dis. 2020, 26, 2201–2204. [Google Scholar] [CrossRef]
- Gugliotta, J.L.; Goethert, H.K.; Berardi, V.P.; Telford, S.R., 3rd. Meningoencephalitis from Borrelia miyamotoi in an immunocompromised patient. N. Engl. J. Med. 2013, 368, 240–245. [Google Scholar] [CrossRef]
- Nadelman, R.B.; Wormser, G.P.; Sherer, C. Blood transfusion-associated relapsing fever. Transfusion 1990, 30, 380–381. [Google Scholar] [CrossRef] [PubMed]
- Krause, P.J.; Hendrickson, J.E.; Steeves, T.K.; Fish, D. Blood transfusion transmission of the tick borne relapsing fever spirochete Borrelia miyamotoi in mice. Transfusion 2015, 55, 593–597. [Google Scholar] [CrossRef]
- Thorp, A.M.; Tonetti, L. Distribution and survival of Borrelia miyamotoi in human blood components. Transfusion 2016, 56, 705–711. [Google Scholar] [CrossRef]
- Brummitt, S.I.; Kjemtrup, A.M.; Harvey, D.J.; Petersen, J.M.; Sexton, C.; Replogle, A.; Packham, A.E.; Bloch, E.M.; Barbour, A.G.; Krause, P.J.; et al. Borrelia burgdorferi and Borrelia miyamotoi seroprevalence in California blood donors. PLoS ONE 2020, 15, e0243950. [Google Scholar] [CrossRef]
- Jahfari, S.; Herremans, T.; Platonov, A.E.; Kuiper, H.; Karan, L.S.; Vasilieva, O.; Koopmans, M.P.; Hovius, J.W.; Sprong, H. High seroprevalence of Borrelia miyamotoi antibodies in forestry workers and individuals suspected of human granulocytic anaplasmosis in the Netherlands. New Microbes New Infect. 2014, 2, 144–149. [Google Scholar] [CrossRef]
- Reiter, M.; Stelzer, T.; Schotta, A.M.; Markowicz, M.; Leschnik, M.; Harsch, A.; Reiß, E.; Kneusel, R.E.; Stockinger, H.; Stanek, G. Glycerophosphodiester phosphodiesterase identified as non-reliable serological marker for Borrelia miyamotoi disease. Microorganisms 2020, 8, 1846. [Google Scholar] [CrossRef]
- Hussein, H.; Showler, A.; Tan, D.H. Tick-borne relapsing fever in pregnancy. Can. Med. Assoc. J. 2013, 186, 131–134. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.T.; Tsibris, A.M.; Branda, J.A. Case 24-2015—A 28-year-old pregnant woman with fever, chills, headache, and fatigue. N. Engl. J. Med. 2015, 373, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Wagemakers, A.; Koetsveld, J.; Narasimhan, S.; Wickel, M.; Deponte, K.; Bleijlevens, B.; Jahfari, S.; Sprong, H.; Karan, L.S.; Sarksyan, D.S.; et al. Variable major proteins as targets for specific antibodies against Borrelia miyamotoi. J. Immunol. 2016, 196, 4185–4195. [Google Scholar] [CrossRef] [PubMed]
- Koetsveld, J.; Kolyasnikova, N.M.; Wagemakers, A.; Stukolova, O.A.; Hoornstra, D.; Sarksyan, D.S.; Toporkova, M.G.; Henningsson, A.J.; Hvidsten, D.; Ang, W.; et al. Serodiagnosis of Borrelia miyamotoi disease by measuring antibodies against GlpQ and variable major proteins. Clin. Microbiol. Infect. 2018, 24, 1338.e1–1338.e7. [Google Scholar] [CrossRef] [PubMed]
- Hoornstra, D.; Stukolova, O.A.; Karan, L.S.; Sarksyan, D.S.; Kolyasnikova, N.M.; Markelov, M.L.; Cherkashina, A.S.; Dolgova, A.S.; Sudina, A.E.; Sokolova, M.I.; et al. Development and validation of a protein array for detection of antibodies against the tick-borne pathogen Borrelia miyamotoi. Microbiol. Spectr. 2022, 10, e02036-22. [Google Scholar] [CrossRef] [PubMed]
- Sudhindra, P.; Wang, G.; Schriefer, M.E.; McKenna, D.; Zhuge, J.; Krause, P.J.; Marques, A.R.; Wormser, G.P. Insights into Borrelia miyamotoi infection from an untreated case demonstrating relapsing fever, monocytosis and a positive C6 Lyme serology. Diagn. Microbiol. Infect. Dis. 2016, 86, 93–96. [Google Scholar] [CrossRef] [PubMed]
- Hayes, E.B.; Piesman, J. How can we prevent Lyme disease? N. Engl. J. Med. 2003, 348, 2424–2430. [Google Scholar] [CrossRef] [PubMed]
- Eisen, L.; Dolan, M.C. Evidence for personal protective measures to reduce human contact with blacklegged ticks and for environmentally based control methods to suppress host-seeking blacklegged ticks and reduce infection with Lyme disease spirochetes in tick vectors and rodent reservoirs. J. Med. Entomol. 2016, 53, 1063–1092. [Google Scholar]
- Kilpatrick, A.M.; Dobson, A.D.M.; Levi, T.; Salkeld, D.J.; Swei, A.; Ginsberg, H.S.; Kjemtrup, A.; Padgett, K.A.; Jensen, P.; Fish, D.; et al. Lyme disease ecology in a changing world: Consensus, uncertainty and critical gaps for improving control. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160117. [Google Scholar] [CrossRef]
- Eisen, L.; Stafford, K.C. Barriers to effective tick management and tick-bite prevention in the United States (Acari: Ixodidae). J. Med. Èntomol. 2020, 58, 1588–1600. [Google Scholar] [CrossRef] [PubMed]
- des Vignes, F.; Piesman, J.; Heffernan, R.; Schulze, T.L.; Stafford, K.C., 3rd; Fish, D. Effect of tick removal on transmission of Borrelia burgdorferi and Ehrlichia phagocytophila by Ixodes scapularis nymphs. J. Infect. Dis. 2001, 183, 773–778. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Burde, J.; Bloch, E.M.; Kelly, J.R.; Krause, P.J. Human Borrelia miyamotoi Infection in North America. Pathogens 2023, 12, 553. https://doi.org/10.3390/pathogens12040553
Burde J, Bloch EM, Kelly JR, Krause PJ. Human Borrelia miyamotoi Infection in North America. Pathogens. 2023; 12(4):553. https://doi.org/10.3390/pathogens12040553
Chicago/Turabian StyleBurde, Jed, Evan M. Bloch, Jill R. Kelly, and Peter J. Krause. 2023. "Human Borrelia miyamotoi Infection in North America" Pathogens 12, no. 4: 553. https://doi.org/10.3390/pathogens12040553
APA StyleBurde, J., Bloch, E. M., Kelly, J. R., & Krause, P. J. (2023). Human Borrelia miyamotoi Infection in North America. Pathogens, 12(4), 553. https://doi.org/10.3390/pathogens12040553





