Oral Supplementation with AHCC®, a Standardized Extract of Cultured Lentinula edodes Mycelia, Enhances Host Resistance against SARS-CoV-2 Infection
Abstract
1. Introduction
2. Materials and Methods
2.1. Viruses
2.2. Mice
2.3. SARS-CoV-2 Infection in Mice
2.4. Oral Feeding with AHCC
2.5. Quantitative PCR (Q-PCR)
2.6. Plaque Assay
2.7. Histology
2.8. IgM and IgG ELISA
2.9. Flow Cytometry
2.10. Intracellular Cytokine Staining (ICS)
2.11. IFN-γ ELISPOT
2.12. Statistical Analysis
3. Results
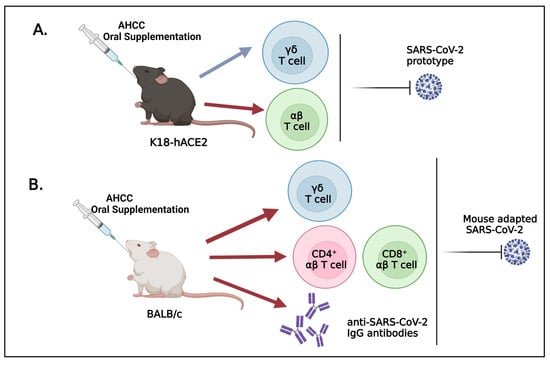
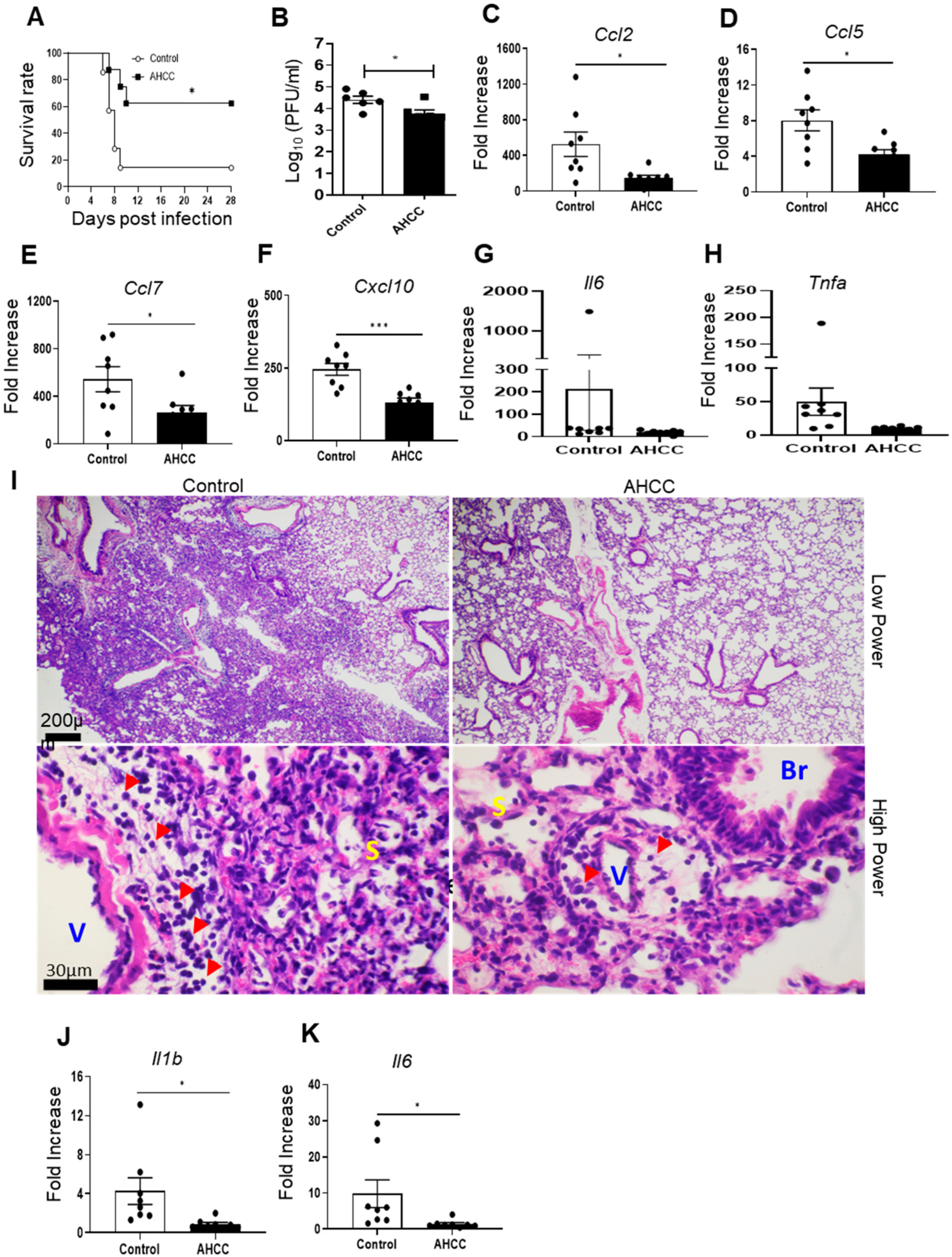
3.1. Oral Uptake of AHCC Enhances Host Resistance to SARS-CoV-2 Infection in K18-hACE2 and BALB/c Mice
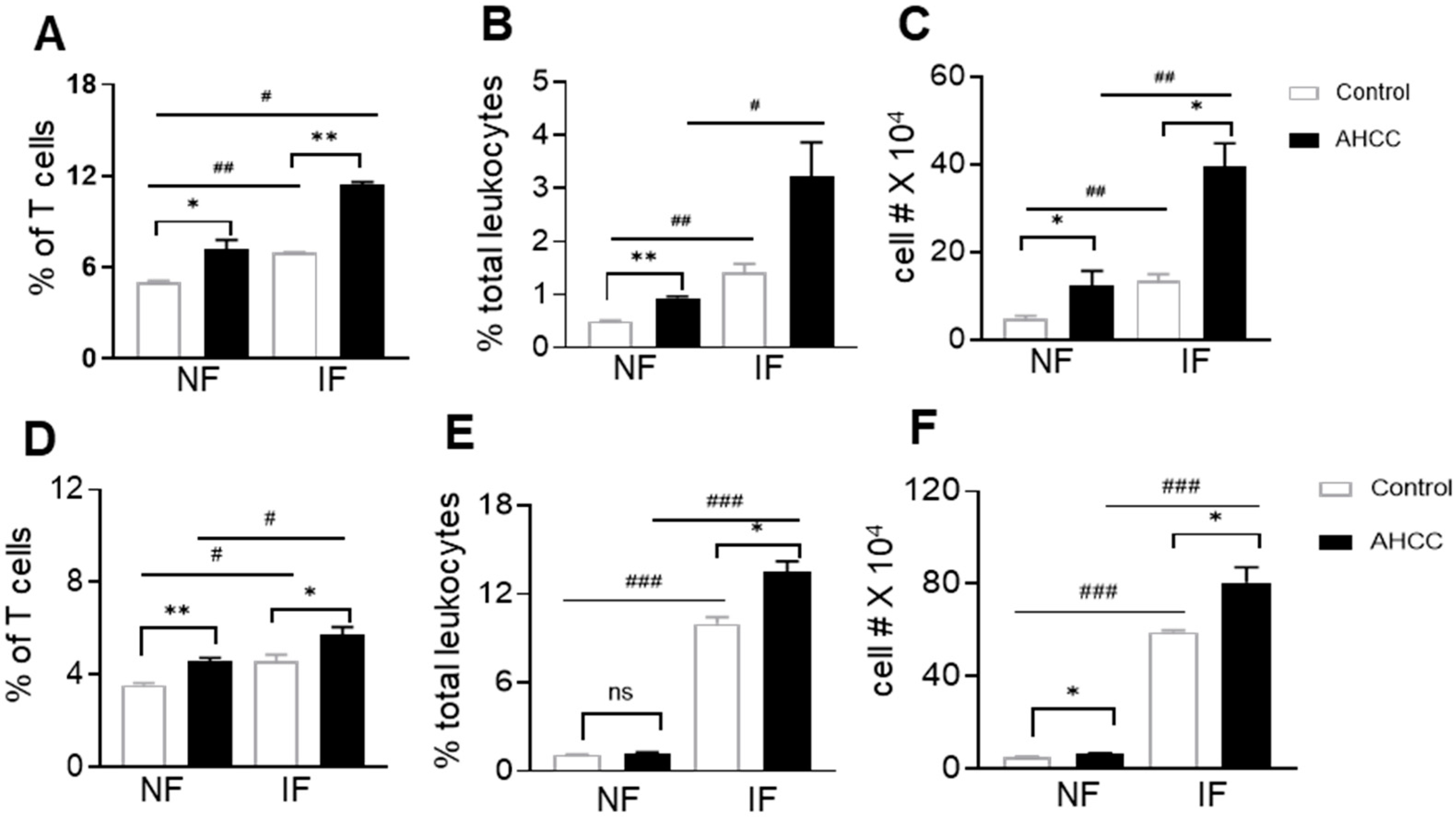
3.2. AHCC Supplementation Promotes Antiviral Innate and Adaptive T Cell Responses in Both Mouse Models following SARS-CoV-2 Infection and Increases IgG Titers in BALB/c Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gattinoni, L.; Chiumello, D.; Rossi, S. COVID-19 Pneumonia: Ards or Not? Crit. Care 2020, 24, 154. [Google Scholar] [CrossRef]
- Li, X.; Xu, S.; Yu, M.; Wang, K.; Tao, Y.; Zhou, Y.; Shi, J.; Zhou, M.; Wu, B.; Yang, Z.; et al. Risk Factors for Severity and Mortality in Adult COVID-19 Inpatients in Wuhan. J. Allergy Clin. Immunol. 2020, 146, 110–118. [Google Scholar] [CrossRef]
- Mao, L.; Jin, H.; Wang, M.; Hu, Y.; Chen, S.; He, Q.; Chang, J.; Hong, C.; Zhou, Y.; Wang, D.; et al. Neurologic Manifestations of Hospitalized Patients with Coronavirus Disease 2019 in Wuhan, China. JAMA Neurol. 2020, 77, 683–690. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.C.; Bai, W.Z.; Hashikawa, T. The Neuroinvasive Potential of SARS-COV2 May Play a Role in the Respiratory Failure of COVID-19 Patients. J. Med. Virol. 2020, 92, 552–555. [Google Scholar] [CrossRef] [PubMed]
- Koritala, T.; Hussain, A.; Pleshkova, Y.; Dondapati, L.; Tirupathi, R.; Rabaan, A.A.; Al Mutair, A.; Alhumaid, S.; Al-Tawfiq, J.A.; Kashyap, R. A Narrative Review of Emergency Use Authorization Versus Full Fda Approval and Its Effect on COVID-19 Vaccination Hesitancy. Infez. Med. 2021, 29, 339–344. [Google Scholar] [PubMed]
- Quinn, S.C.; Jamison, A.M.; Freimuth, V. Communicating Effectively about Emergency Use Authorization and Vaccines in the COVID-19 Pandemic. Am. J. Public Health 2021, 111, 355–358. [Google Scholar] [CrossRef]
- Lu, M.; Dravid, P.; Zhang, Y.; Trivedi, S.; Li, A.; Harder, O.; Kc, M.; Chaiwatpongsakorn, S.; Zani, A.; Kenney, A. A Safe and Highly Efficacious Measles Virus-Based Vaccine Expressing SARS-COV-2 Stabilized Prefusion Spike. Proc. Natl. Acad. Sci. USA 2021, 118, e2026153118. [Google Scholar] [CrossRef]
- Mullick, J.B.; Simmons, C.S.; Gaire, J. Animal Models to Study Emerging Technologies against SARS-COV-2. Cell. Mol. Bioeng. 2020, 13, 293–303. [Google Scholar] [CrossRef]
- Zhou, F.; Yu, T.; Du, R.; Fan, G.; Liu, Y.; Liu, Z.; Xiang, J.; Wang, Y.; Song, B.; Gu, X.; et al. Clinical Course and Risk Factors for Mortality of Adult Inpatients with COVID-19 in Wuhan, China: A Retrospective Cohort Study. Lancet 2020, 395, 1054–1062. [Google Scholar] [CrossRef]
- Kumari, P.; Rothan, H.A.; Natekar, J.P.; Stone, S.; Pathak, H.; Strate, P.G.; Arora, K.; Brinton, M.A.; Kumar, M. Neuroinvasion and Encephalitis following Intranasal Inoculation of SARS-COV-2 in K18-Hace2 Mice. Viruses 2021, 13, 132. [Google Scholar] [CrossRef]
- Oladunni, F.S.; Park, J.G.; Pino, P.A.; Gonzalez, O.; Akhter, A.; Allué-Guardia, A.; Olmo-Fontánez, A.; Gautam, S.; Garcia-Vilanova, A.; Ye, C.; et al. Lethality of SARS-COV-2 Infection in K18 Human Angiotensin-Converting Enzyme 2 Transgenic Mice. Nat. Commun. 2020, 11, 6122. [Google Scholar] [CrossRef] [PubMed]
- Israelow, B.; Song, E.; Mao, T.; Lu, P.; Meir, A.; Liu, F.; Alfajaro, M.M.; Wei, J.; Dong, H.; Homer, R.J.; et al. Mouse Model of SARS-COV-2 Reveals Inflammatory Role of Type I Interferon Signaling. J. Exp. Med. 2020, 217, e20201241. [Google Scholar] [CrossRef] [PubMed]
- Dinnon, K.H., 3rd; Leist, S.R.; Schafer, A.; Edwards, C.E.; Martinez, D.R.; Montgomery, S.A.; West, A.; Yount, B.L., Jr.; Hou, Y.J.; Adams, L.E.; et al. A Mouse-Adapted Model of SARS-COV-2 to Test COVID-19 Countermeasures. Nature 2020, 586, 560–566. [Google Scholar] [CrossRef] [PubMed]
- Muruato, A.; Vu, M.N.; Johnson, B.A.; Davis-Gardner, M.E.; Vanderheiden, A.; Lokugamage, K.; Schindewolf, C.; Crocquet-Valdes, P.A.; Langsjoen, R.M.; Plante, J.A.; et al. Mouse-Adapted SARS-COV-2 Protects Animals from Lethal SARS-COV Challenge. PLoS Biol. 2021, 19, e3001284. [Google Scholar] [CrossRef]
- Ritz, B.W.; Nogusa, S.; Ackerman, E.A.; Gardner, E.M. Supplementation with Active Hexose Correlated Compound Increases the Innate Immune Response of Young Mice to Primary Influenza Infection. J. Nutr. 2006, 136, 2868–2873. [Google Scholar] [CrossRef]
- Cao, Z.; Chen, X.; Lan, L.; Zhang, Z.; Du, J.; Liao, L. Active Hexose Correlated Compound Potentiates the Antitumor Effects of Low-Dose 5-Fluorouracil through Modulation of Immune Function in Hepatoma 22 Tumor-Bearing Mice. Nutr. Res. Pract. 2015, 9, 129–136. [Google Scholar] [CrossRef]
- Gao, Y.; Zhang, D.; Sun, B.; Fujii, H.; Kosuna, K.; Yin, Z. Active Hexose Correlated Compound Enhances Tumor Surveillance through Regulating Both Innate and Adaptive Immune Responses. Cancer Immunol. Immunother. 2006, 55, 1258–1266. [Google Scholar] [CrossRef]
- Ritz, B.W. Supplementation with Active Hexose Correlated Compound Increases Survival following Infectious Challenge in Mice. Nutr. Rev. 2008, 66, 526–531. [Google Scholar] [CrossRef]
- Wang, S.; Welte, T.; Fang, H.; Chang, G.J.; Born, W.K.; O’Brien, R.L.; Sun, B.; Fujii, H.; Kosuna, K.; Wang, T. Oral Administration of Active Hexose Correlated Compound Enhances Host Resistance to West Nile Encephalitis in Mice. J. Nutr. 2009, 139, 598–602. [Google Scholar] [CrossRef]
- Belay, T.; Fu, C.L.; Woart, A. Active Hexose Correlated Compound Activates Immune Function to Decrease Chlamydia Trachomatis Shedding in a Murine Stress Model. J. Nutr. Med. Diet Care 2015, 1, JNMDC-1-006. [Google Scholar] [CrossRef]
- Fujii, H.; Nishioka, N.; Simon, R.R.; Kaur, R.; Lynch, B.; Roberts, A. Genotoxicity and Subchronic Toxicity Evaluation of Active Hexose Correlated Compound (Ahcc). Regul. Toxicol. Pharmacol. 2011, 59, 237–250. [Google Scholar] [CrossRef]
- Spierings, E.L.; Fujii, H.; Sun, B.; Walshe, T. A Phase I Study of the Safety of the Nutritional Supplement, Active Hexose Correlated Compound, Ahcc, in Healthy Volunteers. J. Nutr. Sci. Vitaminol. 2007, 53, 536–539. [Google Scholar] [CrossRef]
- Chan, J.F.; Yuan, S.; Kok, K.H.; To, K.K.; Chu, H.; Yang, J.; Xing, F.; Liu, J.; Yip, C.C.; Poon, R.W.; et al. A Familial Cluster of Pneumonia Associated with the 2019 Novel Coronavirus Indicating Person-to-Person Transmission: A Study of a Family Cluster. Lancet 2020, 395, 514–523. [Google Scholar] [CrossRef] [PubMed]
- Klein, R.S.; Lin, E.; Zhang, B.; Luster, A.D.; Tollett, J.; Samuel, M.A.; Engle, M.; Diamond, M.S. Neuronal Cxcl10 Directs Cd8+ T-Cell Recruitment and Control of West Nile Virus Encephalitis. J. Virol. 2005, 79, 11457–11466. [Google Scholar] [CrossRef]
- Wang, T.; Town, T.; Alexopoulou, L.; Anderson, J.F.; Fikrig, E.; Flavell, R.A. Toll-Like Receptor 3 Mediates West Nile Virus Entry into the Brain Causing Lethal Encephalitis. Nat. Med. 2004, 10, 1366–1373. [Google Scholar] [CrossRef]
- Xie, G.; Luo, H.; Pang, L.; Peng, B.H.; Winkelmann, E.; McGruder, B.; Hesse, J.; Whiteman, M.; Campbell, G.; Milligan, G.N.; et al. Dysregulation of Toll-Like Receptor 7 Compromises Innate and Adaptive T Cell Responses and Host Resistance to an Attenuated West Nile Virus Infection in Old Mice. J. Virol. 2016, 90, 1333–1344. [Google Scholar] [CrossRef] [PubMed]
- Welte, T.; Aronson, J.; Gong, B.; Rachamallu, A.; Mendell, N.; Tesh, R.; Paessler, S.; Born, W.K.; O’Brien, R.L.; Wang, T. Vgamma4+ T Cells Regulate Host Immune Response to West Nile Virus Infection. FEMS Immunol. Med. Microbiol. 2011, 63, 183–192. [Google Scholar] [CrossRef] [PubMed]
- Adam, A.; Shi, Q.; Wang, B.; Zou, J.; Mai, J.; Osman, S.R.; Wu, W.; Xie, X.; Aguilar, P.V.; Bao, X.; et al. A Modified Porous Silicon Microparticle Potentiates Protective Systemic and Mucosal Immunity for SARS-COV-2 Subunit Vaccine. Transl. Res. 2022, 249, 13–27. [Google Scholar] [CrossRef]
- Del Valle, D.M.; Kim-Schulze, S.; Huang, H.-H.; Beckmann, N.D.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.H.; Madduri, D.; Stock, A.; et al. An Inflammatory Cytokine Signature Predicts COVID-19 Severity and Survival. Nat. Med. 2020, 26, 1636–1643. [Google Scholar] [CrossRef]
- Merad, M.; Martin, J.C. Pathological Inflammation in Patients with COVID-19: A Key Role for Monocytes and Macrophages. Nat. Rev. Immunol. 2020, 20, 355–362. [Google Scholar] [CrossRef]
- Yang, Y.; Shen, C.; Li, J.; Yuan, J.; Wei, J.; Huang, F.; Wang, F.; Li, G.; Li, Y.; Xing, L.; et al. Plasma Ip-10 and Mcp-3 Levels Are Highly Associated with Disease Severity and Predict the Progression of COVID-19. J. Allergy Clin. Immunol. 2020, 146, 119–127.e4. [Google Scholar] [CrossRef] [PubMed]
- Tu, W.; Zheng, J.; Liu, Y.; Sia, S.F.; Liu, M.; Qin, G.; Ng, I.H.; Xiang, Z.; Lam, K.T.; Peiris, J.S.; et al. The Aminobisphosphonate Pamidronate Controls Influenza Pathogenesis by Expanding a Gammadelta T Cell Population in Humanized Mice. J. Exp. Med. 2011, 208, 1511–1522. [Google Scholar] [CrossRef]
- von Massow, G.; Oh, S.; Lam, A.; Gustafsson, K. Gamma Delta T Cells and Their Involvement in COVID-19 Virus Infections. Front. Immunol. 2021, 12, 741218. [Google Scholar] [CrossRef]
- Israelow, B.; Mao, T.; Klein, J.; Song, E.; Menasche, B.; Omer, S.B.; Iwasaki, A. Adaptive Immune Determinants of Viral Clearance and Protection in Mouse Models of SARS-COV-2. Sci. Immunol. 2021, 6, eabl4509. [Google Scholar] [CrossRef]
- Peng, Y.; Mentzer, A.J.; Liu, G.; Yao, X.; Yin, Z.; Dong, D.; Dejnirattisai, W.; Rostron, T.; Supasa, P.; Liu, C.; et al. Investigators. Broad and Strong Memory Cd4+ and Cd8+ T Cells Induced by SARS-COV-2 in Uk Convalescent Individuals following COVID-19. Nat. Immunol. 2020, 21, 1336–1345. [Google Scholar] [CrossRef]
- Hayday, A.C. [Gamma][Delta] Cells: A Right Time and a Right Place for a Conserved Third Way of Protection. Annu. Rev. Immunol. 2000, 18, 975–1026. [Google Scholar] [CrossRef] [PubMed]
- Bieback, K.; Breer, C.; Nanan, R.; Meulen, V.T.; Schneider-Schaulies, S. Expansion of Human Gamma/Delta T Cells in Vitro Is Differentially Regulated by the Measles Virus Glycoproteins. J. Gen. Virol. 2003, 84 Pt 5, 1179–1188. [Google Scholar] [CrossRef] [PubMed]
- Lo Presti, E.; Dieli, F.; Meraviglia, S. Lymphopenia in COVID-19: Gammadelta T Cells-Based Therapeutic Opportunities. Vaccines 2021, 9, 562. [Google Scholar] [CrossRef]
- Le Bert, N.; Tan, A.T.; Kunasegaran, K.; Tham, C.Y.L.; Hafezi, M.; Chia, A.; Chng, M.H.Y.; Lin, M.; Tan, N.; Linster, M.; et al. SARS-COV-2-Specific T Cell Immunity in Cases of COVID-19 and Sars, and Uninfected Controls. Nature 2020, 584, 457–462. [Google Scholar] [CrossRef]
- Long, Q.X.; Liu, B.Z.; Deng, H.J.; Wu, G.C.; Deng, K.; Chen, Y.K.; Liao, P.; Qiu, J.F.; Lin, Y.; Cai, X.F.; et al. Antibody Responses to SARS-COV-2 in Patients with COVID-19. Nat. Med. 2020, 26, 845–848. [Google Scholar] [CrossRef]
- Luo, H.; Jia, T.; Chen, J.; Zeng, S.; Qiu, Z.; Wu, S.; Li, X.; Lei, Y.; Wang, X.; Wu, W.; et al. The Characterization of Disease Severity Associated Igg Subclasses Response in COVID-19 Patients. Front. Immunol. 2021, 12, 632814. [Google Scholar] [CrossRef] [PubMed]
- Atyeo, C.; Fischinger, S.; Zohar, T.; Slein, M.D.; Burke, J.; Loos, C.; McCulloch, D.J.; Newman, K.L.; Wolf, C.; Yu, J.; et al. Distinct Early Serological Signatures Track with SARS-COV-2 Survival. Immunity 2020, 53, 524–532e4. [Google Scholar] [CrossRef] [PubMed]
- Roltgen, K.; Powell, A.E.; Wirz, O.F.; Stevens, B.A.; Hogan, C.A.; Najeeb, J.; Hunter, M.; Wang, H.; Sahoo, M.K.; Huang, C.; et al. Defining the Features and Duration of Antibody Responses to SARS-COV-2 Infection Associated with Disease Severity and Outcome. Sci. Immunol. 2020, 5, eabe0240. [Google Scholar] [CrossRef] [PubMed]
- Masuda, Y.; Nakayama, Y.; Tanaka, A.; Naito, K.; Konishi, M. Antitumor Activity of Orally Administered Maitake Alpha-Glucan by Stimulating Antitumor Immune Response in Murine Tumor. PLoS ONE 2017, 12, e0173621. [Google Scholar] [CrossRef]
- Yin, Z.; Fujii, H.; Walshe, T. Effects of Active Hexose Correlated Compound on Frequency of Cd4+ and Cd8+ T Cells Producing Interferon-Gamma and/or Tumor Necrosis Factor-Alpha in Healthy Adults. Hum. Immunol. 2010, 71, 1187–1190. [Google Scholar] [CrossRef] [PubMed]
- Shin, M.S.; Park, H.-J.; Maeda, T.; Nishioka, H.; Fujii, H.; Kang, I. The Effects of Ahcc®, a Standardized Extract of Cultured Lentinura Edodes Mycelia, on Natural Killer and T Cells in Health and Disease: Reviews on Human and Animal Studies. J. Immunol. Res. 2019, 2019, 3758576. [Google Scholar] [CrossRef] [PubMed]
- Bittencourt, V.C.; Figueiredo, R.T.; da Silva, R.B.; Mourao-Sa, D.S.; Fernandez, P.L.; Sassaki, G.L.; Mulloy, B.; Bozza, M.T.; Barreto-Bergter, E. An Alpha-Glucan of Pseudallescheria Boydii Is Involved in Fungal Phagocytosis and Toll-Like Receptor Activation. J. Biol. Chem. 2006, 281, 22614–22623. [Google Scholar] [CrossRef]
- Nair, P.K.; Melnick, S.J.; Ramachandran, R.; Escalon, E.; Ramachandran, C. Mechanism of Macrophage Activation by (1,4)-Alpha-D-Glucan Isolated from Tinospora Cordifolia. Int. Immunopharmacol. 2006, 6, 1815–1824. [Google Scholar] [CrossRef]
- Daddaoua, A.; Martinez-Plata, E.; Ortega-Gonzalez, M.; Ocon, B.; Aranda, C.J.; Zarzuelo, A.; Suarez, M.D.; de Medina, F.S.; Martinez-Augustin, O. The Nutritional Supplement Active Hexose Correlated Compound (Ahcc) Has Direct Immunomodulatory Actions on Intestinal Epithelial Cells and Macrophages Involving Tlr/Myd88 and Nf-Kappab/Mapk Activation. Food Chem. 2013, 136, 1288–1295. [Google Scholar] [CrossRef]
- Mallet, J.F.; Graham, E.; Ritz, B.W.; Homma, K.; Matar, C. Active Hexose Correlated Compound (Ahcc) Promotes an Intestinal Immune Response in Balb/C Mice and in Primary Intestinal Epithelial Cell Culture Involving Toll-Like Receptors Tlr-2 and Tlr-4. Eur. J. Nutr. 2016, 55, 139–146. [Google Scholar] [CrossRef]
- Wesch, D.; Peters, C.; Oberg, H.H.; Pietschmann, K.; Kabelitz, D. Modulation of Gammadelta T Cell Responses by Tlr Ligands. Cell Mol Life Sci 2011, 68, 2357–2370. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, J.; Pang, L.; Xie, G.; Welte, T.; Saxena, V.; Wicker, J.; Mann, B.; Soong, L.; Barrett, A.; et al. The Co-Stimulatory Effects of Myd88-Dependent Toll-Like Receptor Signaling on Activation of Murine Gammadelta T Cells. PLoS ONE 2014, 9, e108156. [Google Scholar]
- Hoshi, H.; Yagi, Y.; Iijima, H.; Matsunaga, K.; Ishihara, Y.; Yasuhara, T. Isolation and Characterization of a Novel Immunomodulatory Alpha-Glucan-Protein Complex from the Mycelium of Tricholoma Matsutake in Basidiomycetes. J. Agric. Food Chem. 2005, 53, 8948–8956. [Google Scholar] [CrossRef] [PubMed]
- Allegra, A.; Tonacci, A.; Pioggia, G.; Musolino, C.; Gangemi, S. Vitamin Deficiency as Risk Factor for SARS-COV-2 Infection: Correlation with Susceptibility and Prognosis. Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9721–9738. [Google Scholar]
- Bogan-Brown, K.; Nkrumah-Elie, Y.; Ishtiaq, Y.; Redpath, P.; Shao, A. Potential Efficacy of Nutrient Supplements for Treatment or Prevention of COVID-19. J. Diet. Suppl. 2022, 19, 336–365. [Google Scholar] [CrossRef] [PubMed]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Singh, A.; Adam, A.; Rodriguez, L.; Peng, B.-H.; Wang, B.; Xie, X.; Shi, P.-Y.; Homma, K.; Wang, T. Oral Supplementation with AHCC®, a Standardized Extract of Cultured Lentinula edodes Mycelia, Enhances Host Resistance against SARS-CoV-2 Infection. Pathogens 2023, 12, 554. https://doi.org/10.3390/pathogens12040554
Singh A, Adam A, Rodriguez L, Peng B-H, Wang B, Xie X, Shi P-Y, Homma K, Wang T. Oral Supplementation with AHCC®, a Standardized Extract of Cultured Lentinula edodes Mycelia, Enhances Host Resistance against SARS-CoV-2 Infection. Pathogens. 2023; 12(4):554. https://doi.org/10.3390/pathogens12040554
Chicago/Turabian StyleSingh, Ankita, Awadalkareem Adam, Leslie Rodriguez, Bi-Hung Peng, Binbin Wang, Xuping Xie, Pei-Yong Shi, Kohei Homma, and Tian Wang. 2023. "Oral Supplementation with AHCC®, a Standardized Extract of Cultured Lentinula edodes Mycelia, Enhances Host Resistance against SARS-CoV-2 Infection" Pathogens 12, no. 4: 554. https://doi.org/10.3390/pathogens12040554
APA StyleSingh, A., Adam, A., Rodriguez, L., Peng, B.-H., Wang, B., Xie, X., Shi, P.-Y., Homma, K., & Wang, T. (2023). Oral Supplementation with AHCC®, a Standardized Extract of Cultured Lentinula edodes Mycelia, Enhances Host Resistance against SARS-CoV-2 Infection. Pathogens, 12(4), 554. https://doi.org/10.3390/pathogens12040554