Lysine Methyltransferase EhPKMT2 Is Involved in the In Vitro Virulence of Entamoeba histolytica
Abstract
1. Introduction
2. Materials and Methods
2.1. Entamoeba histolytica Cultures
2.2. Structural 3D Modeling
2.3. Expression of EhPKMT2 in Trophozoites Exposed to Different Conditions
2.4. Immunofluorescence and Confocal Microscopy
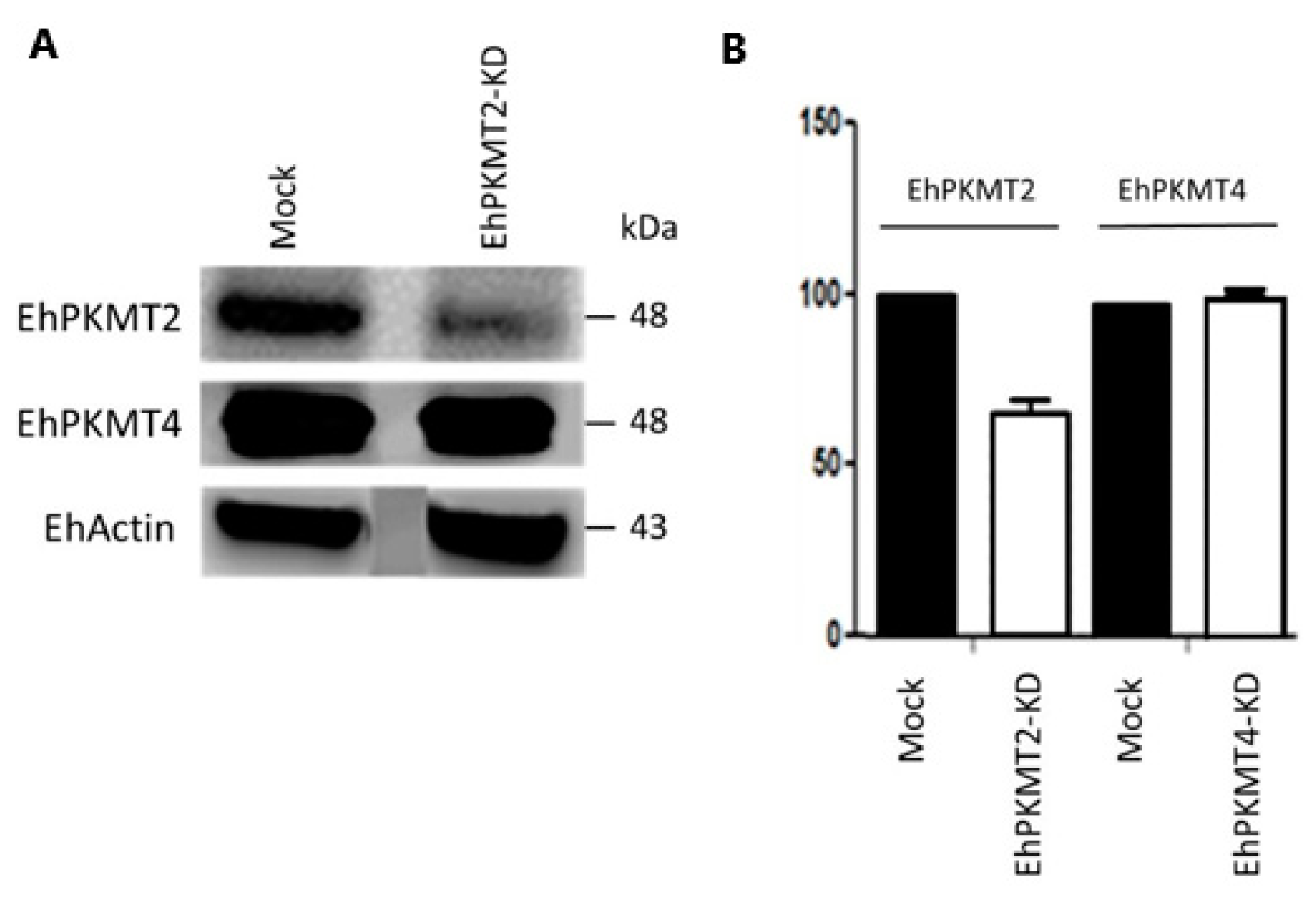
2.5. Knockdown of Ehpkmt2
2.6. Effect of Ehkmt2 Knockdown on Heat Shock Response
2.7. Effect of Ehpkmt2 Knockdown on In Vitro Virulence of E. histolytica
2.8. Statistical Analysis
3. Results
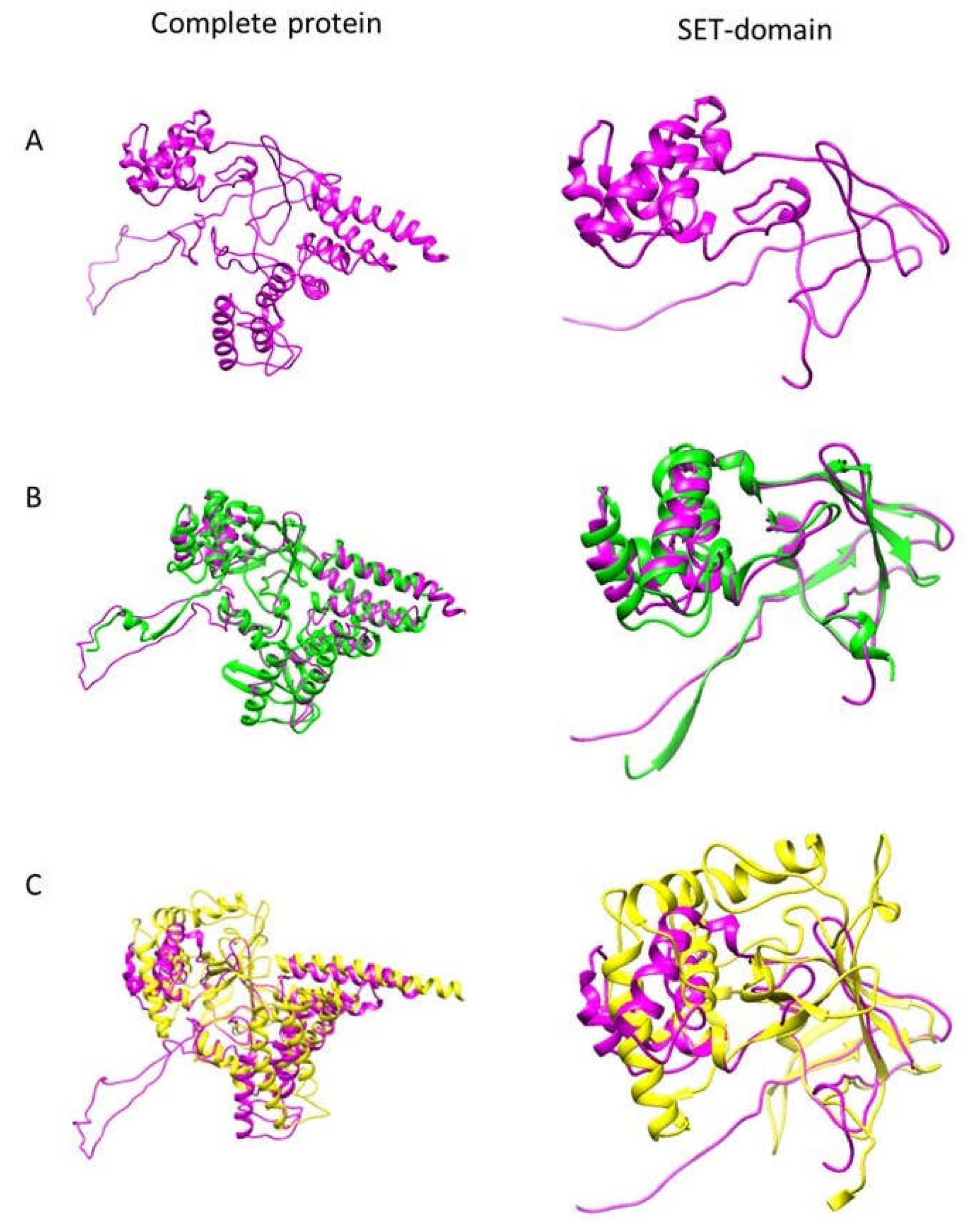
3.1. EhPKMT2 Is Structurally Related to AKMT of T. gondii and Human SMYD1
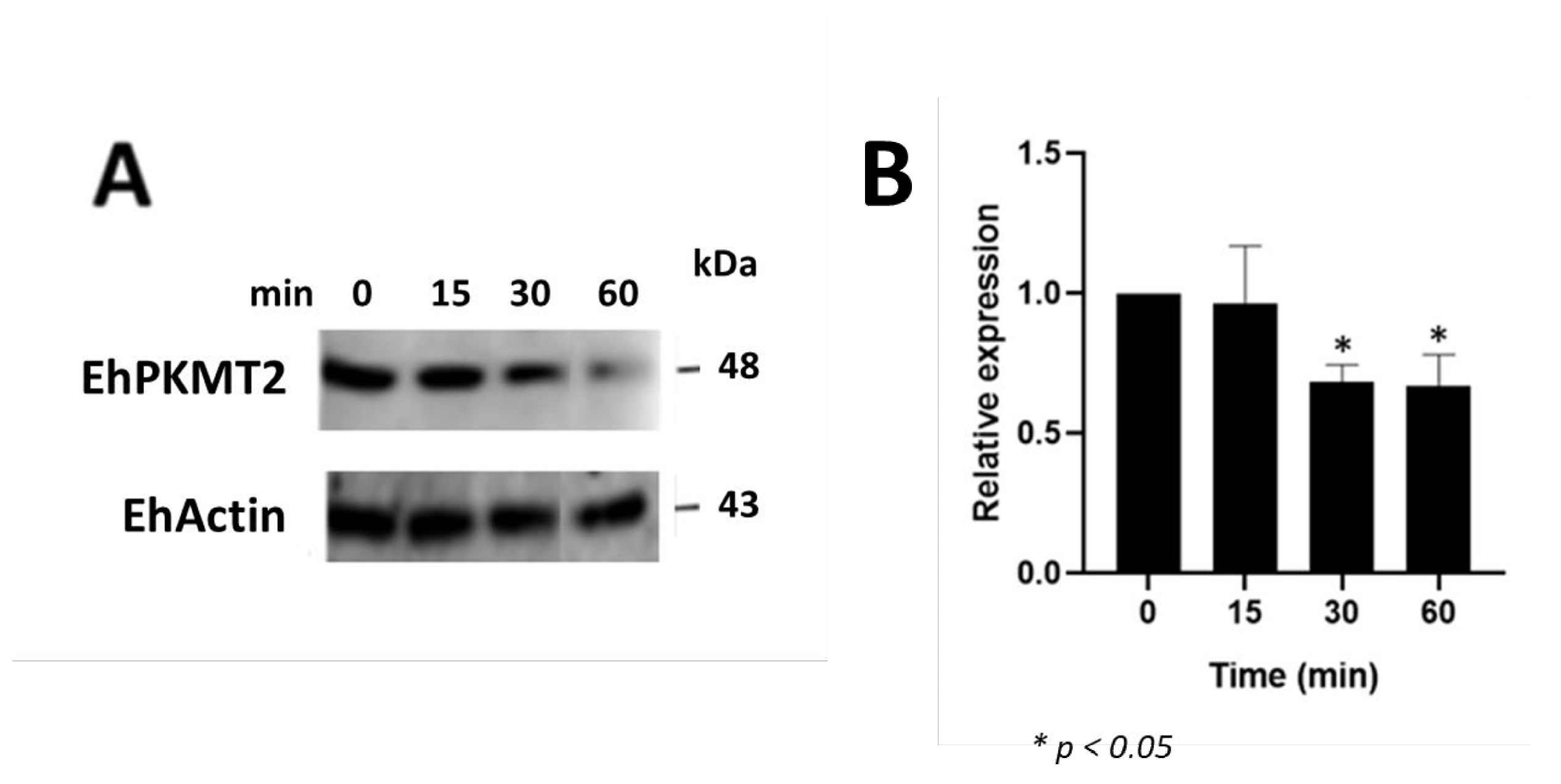
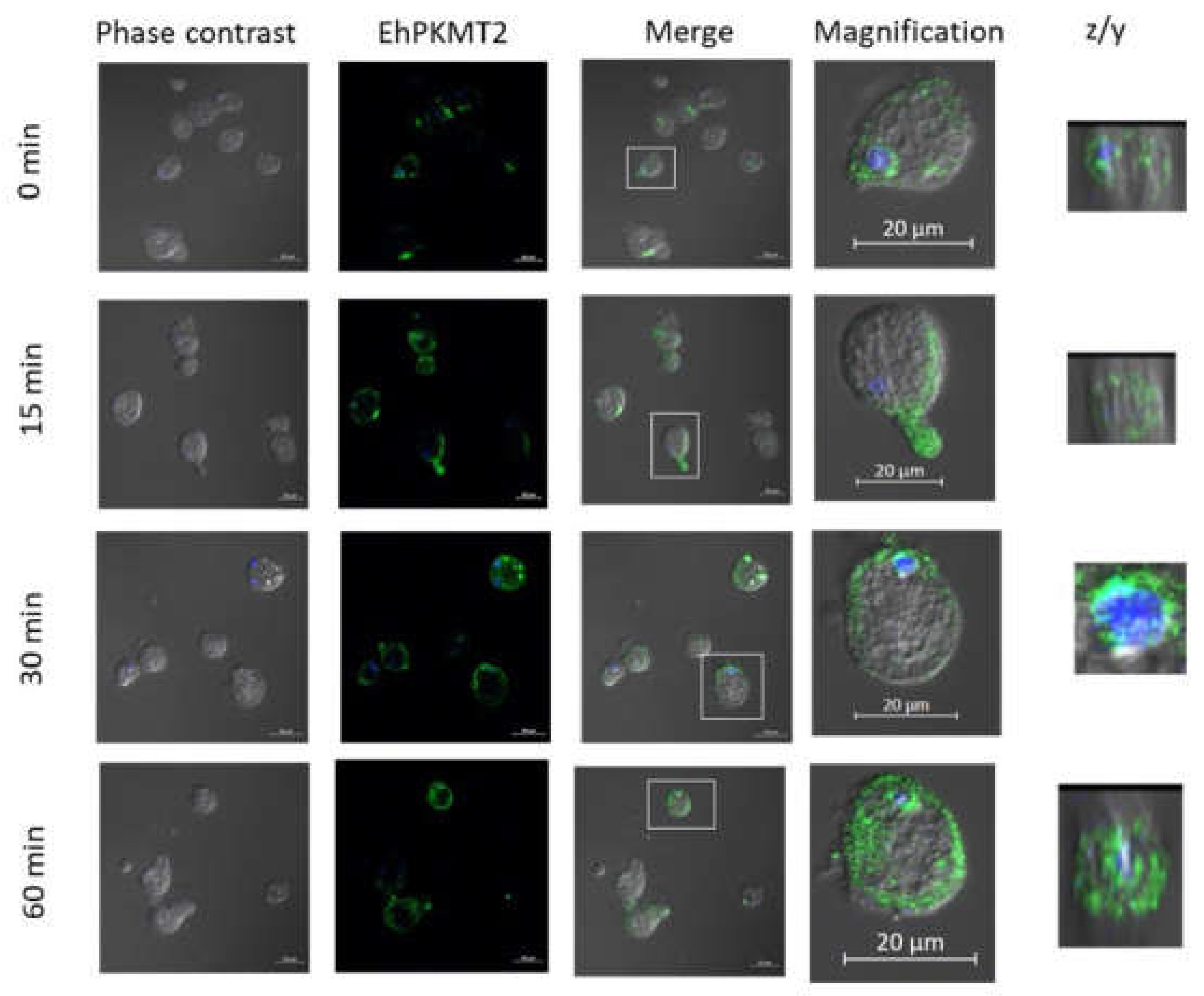
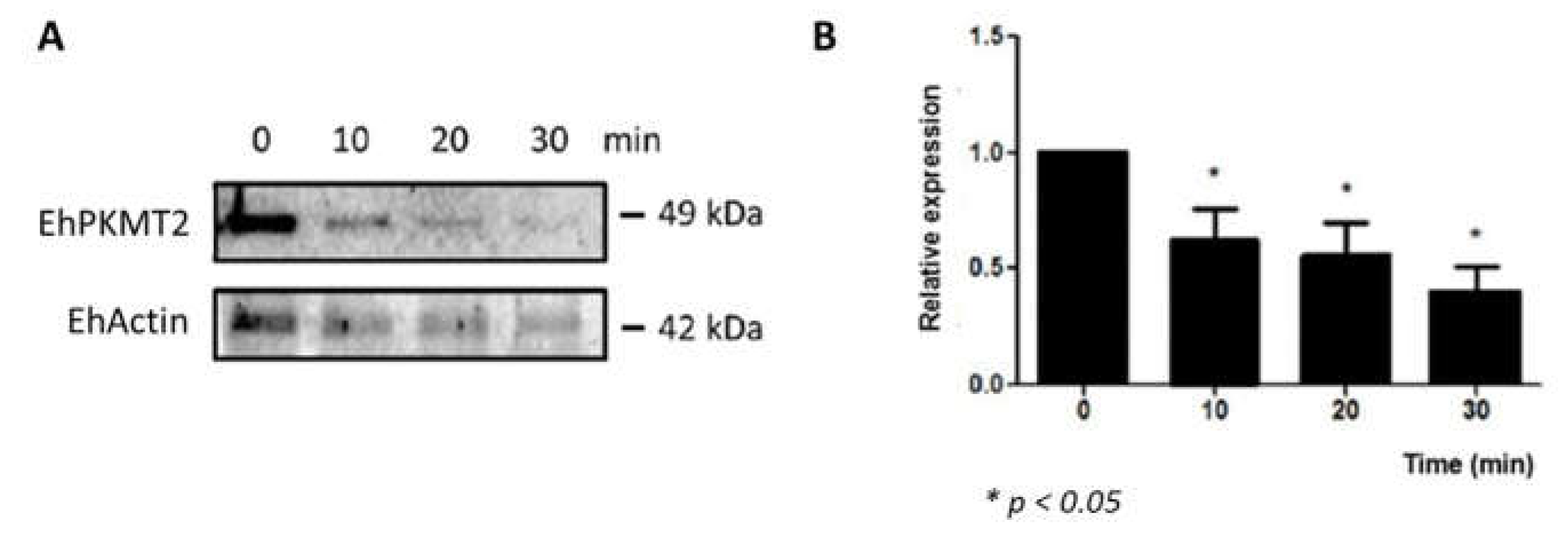
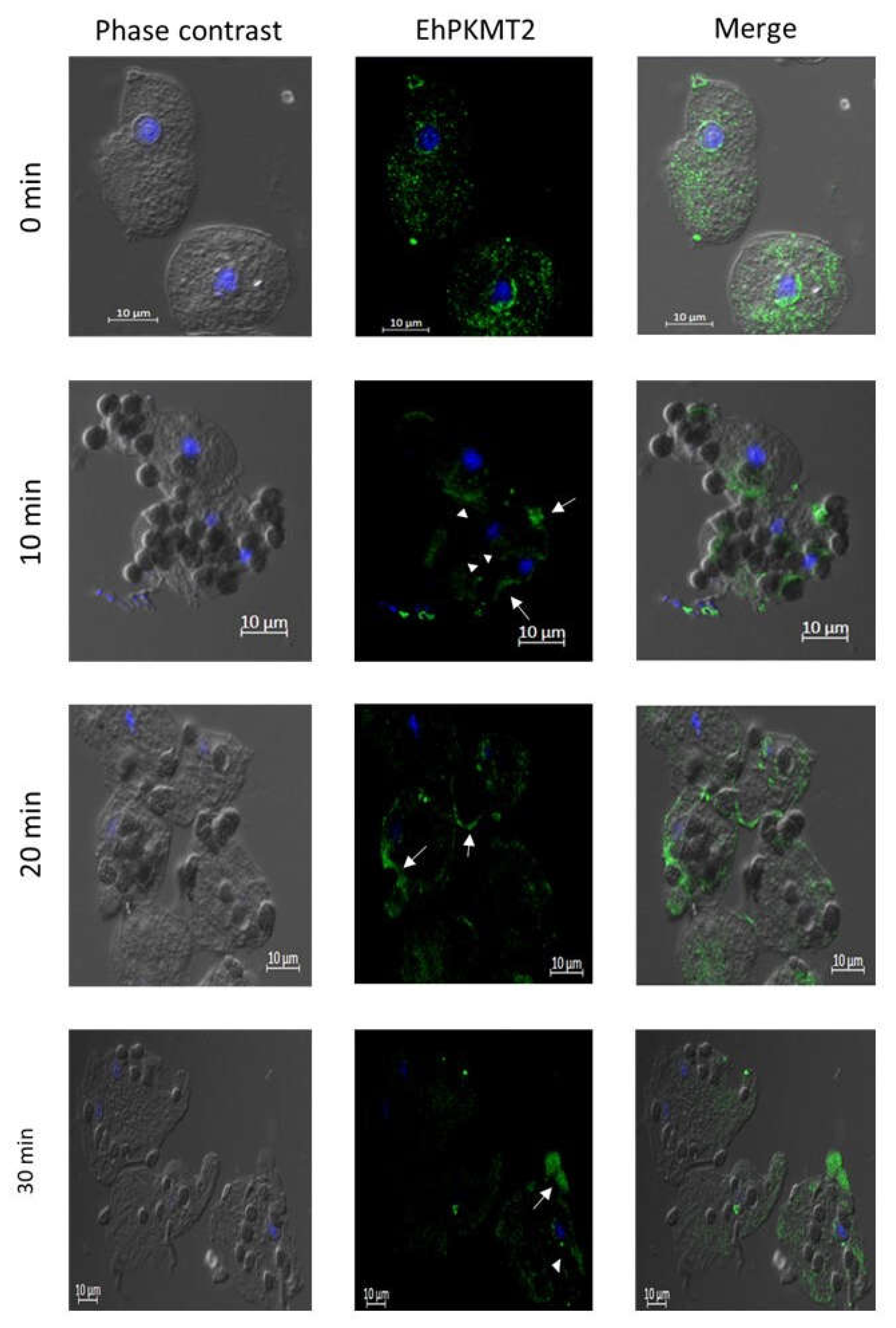
3.2. Changes in Expression and Localization of EhPKMT2 during Heat Shock and Erythrophagocytosis
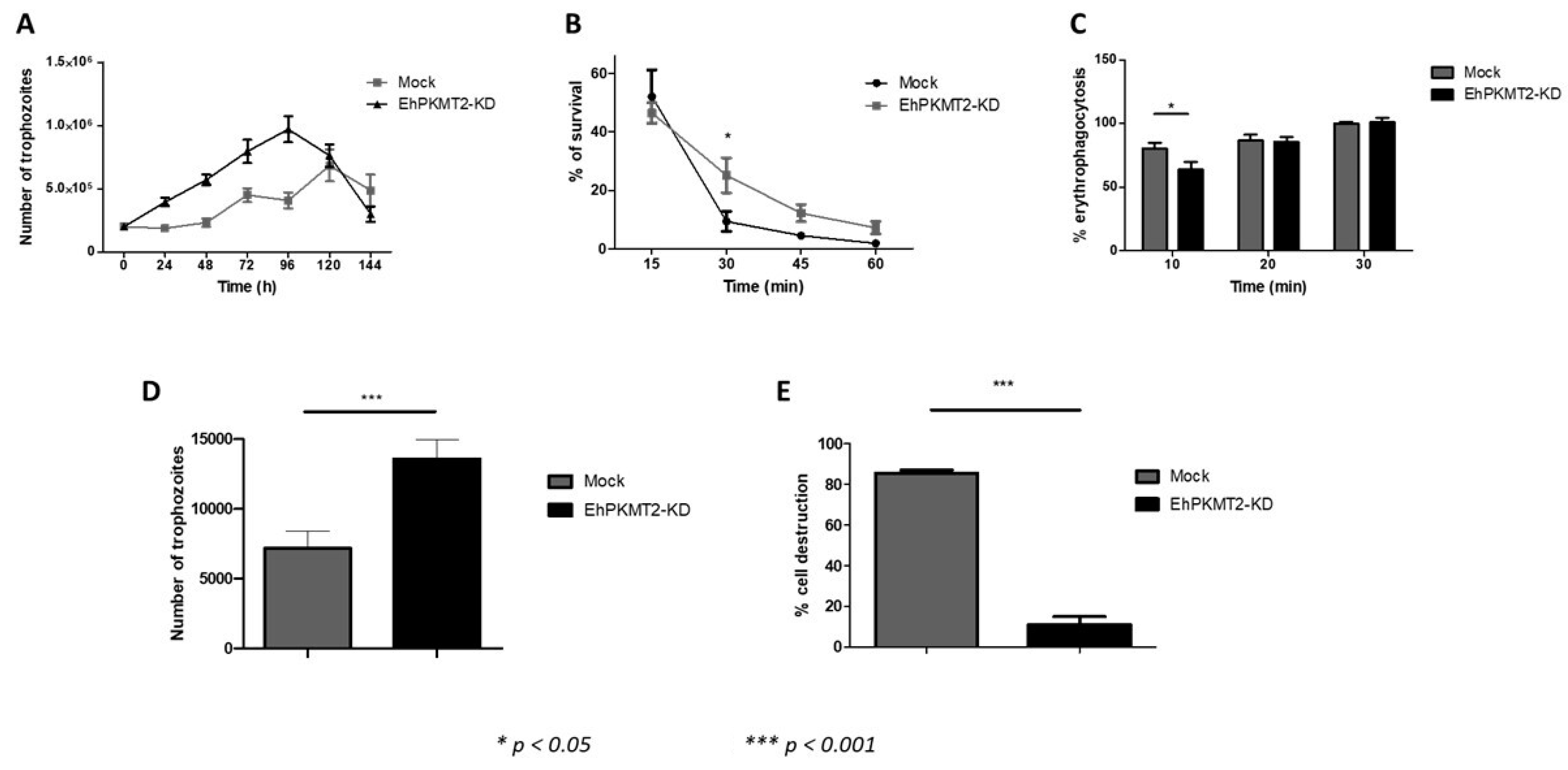
3.3. Ehpkmt2 Knockdown Modified Cellular Proliferation
3.4. Knockdown of EhPKMT2 Alter Different Virulence Factors
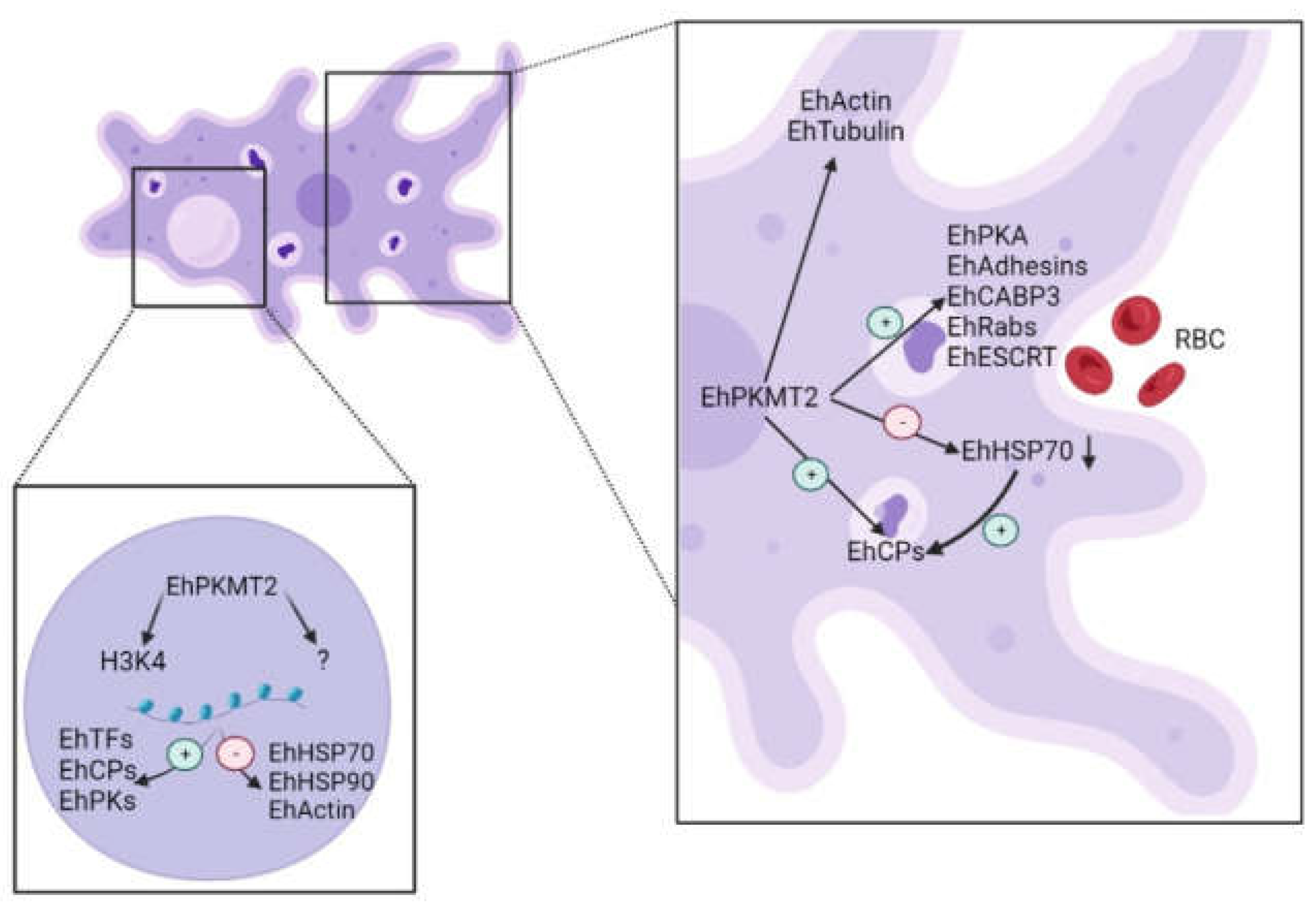
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Luo, M. Chemical and Biochemical Perspectives of Protein Lysine Methylation. Chem. Rev. 2018, 118, 6656–6705. [Google Scholar] [CrossRef] [PubMed]
- Nimura, K.; Ura, K.; Kaneda, Y. Histone methyltransferases: Regulation of transcription and contribution to human disease. J. Mol. Med. 2010, 88, 1213–1220. [Google Scholar] [CrossRef] [PubMed]
- Moore, K.E.; Gozani, O. An unexpected journey: Lysine methylation across the proteome. Biochim. Et Biophys. Acta Gene Regul. Mech. 2014, 1839, 1395–1403. [Google Scholar] [CrossRef]
- Schneider, R.; Bannister, A.J.; Kouzarides, T. Unsafe SETs: Histone lysine methyltransferases and cancer. Trends Biochem. Sci. 2002, 27, 396–402. [Google Scholar] [CrossRef]
- Richon, V.M.; Johnston, D.; Sneeringer, C.J.; Jin, L.; Majer, C.R.; Elliston, K.; Jerva, L.F.; Scott, M.P.; Copeland, R.A. Chemogenetic analysis of human protein methyltransferases. Chem. Biol. Drug. Des. 2011, 78, 199–210. [Google Scholar] [CrossRef]
- Cui, L.; Fan, Q.; Cui, L.; Miao, J. Histone lysine methyltransferases and demethylases in Plasmodium falciparum. Int. J. Parasitol. 2008, 38, 1083–1097. [Google Scholar] [CrossRef]
- Jiang, L.; Mu, J.; Zhang, Q.; Ni, T.; Srinivasan, P.; Rayavara, K.; Yang, W.; Turner, L.; Lavstsen, T.; Theander, T.G.; et al. PfSETvs methylation of histone H3K36 represses virulence genes in Plasmodium falciparum. Nature 2013, 499, 223–227. [Google Scholar] [CrossRef]
- Sivagurunathan, S.; Heaslip, A.; Liu, J.; Hu, K. Identification of functional modules of AKMT, a novel lysine methyltransferase regulating the motility of Toxoplasma gondii. Mol. Biochem. Parasitol. 2013, 189, 43–53. [Google Scholar] [CrossRef]
- Chen, P.B.; Ding, S.; Zanghi, G.; Soulard, V.; DiMaggio, P.A.; Fuchter, M.J.; Mecheri, S.; Mazier, D.; Scherf, A.; Malmquist, N.A. Plasmodium falciparum PfSET7: Enzymatic characterization and cellular localization of a novel protein methyltransferase in sporozoite, liver and erythrocytic stage parasites. Sci. Rep. 2016, 6, 21802. [Google Scholar] [CrossRef]
- Walsh, J.A. Problems in recognition and diagnosis of amebiasis: Estimation of the global magnitude of morbidity and mortality. Rev. Infect. Dis. 1986, 8, 228–238. [Google Scholar] [CrossRef]
- Santos, F.; Nequiz, M.; Hernandez-Cuevas, N.A.; Hernandez, K.; Pineda, E.; Encalada, R.; Guillen, N.; Luis-Garcia, E.; Saralegui, A.; Saavedra, E.; et al. Maintenance of intracellular hypoxia and adequate heat shock response are essential requirements for pathogenicity and virulence of Entamoeba histolytica. Cell. Microbiol. 2015, 17, 1037–1051. [Google Scholar] [CrossRef] [PubMed]
- Borbolla-Vazquez, J.; Orozco, E.; Medina-Gomez, C.; Martinez-Higuera, A.; Javier-Reyna, R.; Chavez, B.; Betanzos, A.; Rodriguez, M.A. Identification and functional characterization of lysine methyltransferases of Entamoeba histolytica. Mol. Microbiol. 2016, 101, 351–365. [Google Scholar] [CrossRef] [PubMed]
- Diamond, L.S.; Harlow, D.R.; Cunnick, C.C. A new medium for the axenic cultivation of Entamoeba histolytica and other Entamoeba. Trans. R. Soc. Trop. Med. Hyg. 1978, 72, 431–432. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y. I-TASSER server for protein 3D structure prediction. BMC Bioinform. 2008, 9, 40. [Google Scholar] [CrossRef]
- Yang, J.; Yan, R.; Roy, A.; Xu, D.; Poisson, J.; Zhang, Y. The I-TASSER Suite: Protein structure and function prediction. Nat. Methods 2015, 12, 7–8. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, Y. I-TASSER server: New development for protein structure and function predictions. Nucleic Acids Res. 2015, 43, W174–W181. [Google Scholar] [CrossRef]
- Ankri, S.; Stolarsky, T.; Mirelman, D. Antisense inhibition of expression of cysteine proteinases does not affect Entamoeba histolytica cytopathic or haemolytic activity but inhibits phagocytosis. Mol. Microbiol. 1998, 28, 777–785. [Google Scholar] [CrossRef]
- Valle-Solis, M.; Bolanos, J.; Orozco, E.; Huerta, M.; Garcia-Rivera, G.; Salas-Casas, A.; Chavez-Munguia, B.; Rodriguez, M.A. A Calcium/Cation Exchanger Participates in the Programmed Cell Death and in vitro Virulence of Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 342. [Google Scholar] [CrossRef]
- Medina-Gomez, C.; Bolanos, J.; Borbolla-Vazquez, J.; Munguia-Robledo, S.; Orozco, E.; Rodriguez, M.A. The atypical protein arginine methyltrasferase of Entamoeba histolytica (EhPRMTA) is involved in cell proliferation, heat shock response and in vitro virulence. Exp. Parasitol. 2021, 222, 108077. [Google Scholar] [CrossRef]
- Rodriguez, M.A.; Orozco, E. Isolation and characterization of phagocytosis- and virulence-deficient mutants of Entamoeba histolytica. J. Infect. Dis. 1986, 154, 27–32. [Google Scholar] [CrossRef]
- Gherardini, P.F.; Helmer-Citterich, M. Structure-based function prediction: Approaches and applications. Brief. Funct. Genom. Proteom. 2008, 7, 291–302. [Google Scholar] [CrossRef]
- Heaslip, A.T.; Nishi, M.; Stein, B.; Hu, K. The motility of a human parasite, Toxoplasma gondii, is regulated by a novel lysine methyltransferase. PLoS Pathog. 2011, 7, e1002201. [Google Scholar] [CrossRef] [PubMed]
- Rueda-Robles, A.; Audano, M.; Alvarez-Mercado, A.I.; Rubio-Tomas, T. Functions of SMYD proteins in biological processes: What do we know? An updated review. Arch. Biochem. Biophys. 2021, 712, 109040. [Google Scholar] [CrossRef]
- Krissinel, E. On the relationship between sequence and structure similarities in proteomics. Bioinformatics 2007, 23, 717–723. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.; Park, S.; Kwon, S.; Lee, E.J.; Lee, J.S. Set1-mediated H3K4 methylation is required for Candida albicans virulence by regulating intracellular level of reactive oxygen species. Virulence 2021, 12, 2648–2658. [Google Scholar] [CrossRef] [PubMed]
- Ren, K.; Mou, Y.N.; Tong, S.M.; Ying, S.H.; Feng, M.G. DIM5/KMT1 controls fungal insect pathogenicity and genome stability by methylation of histone H3K4, H3K9 and H3K36. Virulence 2021, 12, 1306–1322. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, N.; Yin, Y.N.; Chen, Y.; Jiang, J.H.; Ma, Z.H. Histone H3K4 methylation regulates hyphal growth, secondary metabolism and multiple stress responses in Fusarium graminearum. Environ. Microbiol. 2015, 17, 4615–4630. [Google Scholar] [CrossRef]
- Orozco, E.; Guarneros, G.; Martinez-Palomo, A.; Sanchez, T. Entamoeba histolytica. Phagocytosis as a virulence factor. J. Exp. Med. 1983, 158, 1511–1521. [Google Scholar] [CrossRef]
- Hornbeck, P.V.; Zhang, B.; Murray, B.; Kornhauser, J.M.; Latham, V.; Skrzypek, E. PhosphoSitePlus, 2014: Mutations, PTMs and recalibrations. Nucleic Acids Res. 2015, 43, D512–D520. [Google Scholar] [CrossRef]
- Cornett, E.M.; Ferry, L.; Defossez, P.A.; Rothbart, S.B. Lysine Methylation Regulators Moonlighting outside the Epigenome. Mol. Cell 2019, 75, 1092–1101. [Google Scholar] [CrossRef]
- Kaur, I.; Zeeshan, M.; Saini, E.; Kaushik, A.; Mohmmed, A.; Gupta, D.; Malhotra, P. Widespread occurrence of lysine methylation in Plasmodium falciparum proteins at asexual blood stages. Sci. Rep. 2016, 6, 35432. [Google Scholar] [CrossRef] [PubMed]
- Malmquist, N.A.; Moss, T.A.; Mecheri, S.; Scherf, A.; Fuchter, M.J. Small-molecule histone methyltransferase inhibitors display rapid antimalarial activity against all blood stage forms in Plasmodium falciparum. Proc. Natl. Acad. Sci. USA 2012, 109, 16708–16713. [Google Scholar] [CrossRef] [PubMed]
- Spellmon, N.; Holcomb, J.; Trescott, L.; Sirinupong, N.; Yang, Z. Structure and function of SET and MYND domain-containing proteins. Int. J. Mol. Sci. 2015, 16, 1406–1428. [Google Scholar] [CrossRef] [PubMed]
- Han, N.; Jeschke, U.; Kuhn, C.; Hester, A.; Czogalla, B.; Mahner, S.; Rottmann, M.; Mayr, D.; Schmoeckel, E.; Trillsch, F. H3K4me3 Is a Potential Mediator for Antiproliferative Effects of Calcitriol (1alpha,25(OH)2D3) in Ovarian Cancer Biology. Int. J. Mol. Sci. 2020, 21, 2151. [Google Scholar] [CrossRef]
- Yang, L.; Jin, M.L.; Jeong, K.W. Histone H3K4 Methyltransferases as Targets for Drug-Resistant Cancers. Biology 2021, 10, 581. [Google Scholar] [CrossRef]
- Daks, A.; Vasileva, E.; Fedorova, O.; Shuvalov, O.; Barlev, N.A. The Role of Lysine Methyltransferase SET7/9 in Proliferation and Cell Stress Response. Life 2022, 12, 362. [Google Scholar] [CrossRef]
- Daks, A.; Mamontova, V.; Fedorova, O.; Petukhov, A.; Shuvalov, O.; Parfenyev, S.; Netsvetay, S.; Venina, A.; Kizenko, A.; Imyanitov, E.; et al. Set7/9 controls proliferation and genotoxic drug resistance of NSCLC cells. Biochem. Biophys. Res. Commun. 2021, 572, 41–48. [Google Scholar] [CrossRef]
- Vermillion, K.L.; Lidberg, K.A.; Gammill, L.S. Cytoplasmic protein methylation is essential for neural crest migration. J. Cell Biol. 2014, 204, 95–109. [Google Scholar] [CrossRef]
- Yokoyama, Y.; Hieda, M.; Nishioka, Y.; Matsumoto, A.; Higashi, S.; Kimura, H.; Yamamoto, H.; Mori, M.; Matsuura, S.; Matsuura, N. Cancer-associated upregulation of histone H3 lysine 9 trimethylation promotes cell motility in vitro and drives tumor formation in vivo. Cancer Sci. 2013, 104, 889–895. [Google Scholar] [CrossRef]
- Park, I.Y.; Powell, R.T.; Tripathi, D.N.; Dere, R.; Ho, T.H.; Blasius, T.L.; Chiang, Y.C.; Davis, I.J.; Fahey, C.C.; Hacker, K.E.; et al. Dual Chromatin and Cytoskeletal Remodeling by SETD2. Cell 2016, 166, 950–962. [Google Scholar] [CrossRef]
- Seervai, R.N.H.; Jangid, R.K.; Karki, M.; Tripathi, D.N.; Jung, S.Y.; Kearns, S.E.; Verhey, K.J.; Cianfrocco, M.A.; Millis, B.A.; Tyska, M.J.; et al. The Huntingtin-interacting protein SETD2/HYPB is an actin lysine methyltransferase. Sci. Adv. 2020, 6, eabb7854. [Google Scholar] [CrossRef]
- Gunawan, M.; Venkatesan, N.; Loh, J.T.; Wong, J.F.; Berger, H.; Neo, W.H.; Li, L.Y.; La Win, M.K.; Yau, Y.H.; Guo, T.; et al. The methyltransferase Ezh2 controls cell adhesion and migration through direct methylation of the extranuclear regulatory protein talin. Nat. Immunol. 2015, 16, 505–516. [Google Scholar] [CrossRef]
- Kantor, M.; Abrantes, A.; Estevez, A.; Schiller, A.; Torrent, J.; Gascon, J.; Hernandez, R.; Ochner, C. Entamoeba histolytica: Updates in Clinical Manifestation, Pathogenesis, and Vaccine Development. Can. J. Gastroenterol. Hepatol. 2018, 2018, 4601420. [Google Scholar] [CrossRef]
- Baxt, L.A.; Singh, U. New insights into Entamoeba histolytica pathogenesis. Curr. Opin. Infect. Dis. 2008, 21, 489–494. [Google Scholar] [CrossRef] [PubMed]
- Nakada-Tsukui, K.; Nozaki, T. Immune Response of Amebiasis and Immune Evasion by Entamoeba histolytica. Front. Immunol. 2016, 7, 175. [Google Scholar] [CrossRef] [PubMed]
- Ding, W.; Higgins, D.P.; Yadav, D.K.; Godbole, A.A.; Pukkila-Worley, R.; Walker, A.K. Stress-responsive and metabolic gene regulation are altered in low S-adenosylmethionine. PLoS Genet. 2018, 14, e1007812. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Arrington, J.; Ratliff, A.C.; Chen, J.; Horton, H.E.; Nie, Y.; Yue, F.; Hrycyna, C.A.; Tao, W.A.; Kuang, S. Methyltransferase-like 21c methylates and stabilizes the heat shock protein Hspa8 in type I myofibers in mice. J. Biol. Chem. 2019, 294, 13718–13728. [Google Scholar] [CrossRef]
- MacFarlane, R.C.; Shah, P.H.; Singh, U. Transcriptional profiling of Entamoeba histolytica trophozoites. Int. J. Parasitol. 2005, 35, 533–542. [Google Scholar] [CrossRef]
- Wang, L.; Wang, Q.T.; Liu, Y.P.; Dong, Q.Q.; Hu, H.J.; Miao, Z.; Li, S.; Liu, Y.; Zhou, H.; Zhang, T.C.; et al. ATM Signaling Pathway Is Implicated in the SMYD3-mediated Proliferation and Migration of Gastric Cancer Cells. J. Gastric Cancer 2017, 17, 295–305. [Google Scholar] [CrossRef]
- Sierra-Lopez, F.; Baylon-Pacheco, L.; Espiritu-Gordillo, P.; Lagunes-Guillen, A.; Chavez-Munguia, B.; Rosales-Encina, J.L. Influence of Micropatterned Grill Lines on Entamoeba histolytica Trophozoites Morphology and Migration. Front. Cell. Infect. Microbiol. 2018, 8, 295. [Google Scholar] [CrossRef]
- Avalos-Padilla, Y.; Knorr, R.L.; Javier-Reyna, R.; Garcia-Rivera, G.; Lipowsky, R.; Dimova, R.; Orozco, E. The Conserved ESCRT-III Machinery Participates in the Phagocytosis of Entamoeba histolytica. Front. Cell. Infect. Microbiol. 2018, 8, 53. [Google Scholar] [CrossRef] [PubMed]
- Javier-Reyna, R.; Montano, S.; Garcia-Rivera, G.; Rodriguez, M.A.; Gonzalez-Robles, A.; Orozco, E. EhRabB mobilises the EhCPADH complex through the actin cytoskeleton during phagocytosis of Entamoeba histolytica. Cell. Microbiol. 2019, 21, e13071. [Google Scholar] [CrossRef] [PubMed]
- Batista Ede, J.; de Souza, W. Involvement of protein kinases on the process of erythrophagocytis by Entamoeba histolytica. Cell Biol. Int. 2004, 28, 243–248. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Munguía-Robledo, S.; Orozco, E.; García-Rivera, G.; Bolaños, J.; Valdés, J.; Azuara-Licéaga, E.; Rodríguez, M.A. Lysine Methyltransferase EhPKMT2 Is Involved in the In Vitro Virulence of Entamoeba histolytica. Pathogens 2023, 12, 474. https://doi.org/10.3390/pathogens12030474
Munguía-Robledo S, Orozco E, García-Rivera G, Bolaños J, Valdés J, Azuara-Licéaga E, Rodríguez MA. Lysine Methyltransferase EhPKMT2 Is Involved in the In Vitro Virulence of Entamoeba histolytica. Pathogens. 2023; 12(3):474. https://doi.org/10.3390/pathogens12030474
Chicago/Turabian StyleMunguía-Robledo, Susana, Esther Orozco, Guillermina García-Rivera, Jeni Bolaños, Jesús Valdés, Elisa Azuara-Licéaga, and Mario Alberto Rodríguez. 2023. "Lysine Methyltransferase EhPKMT2 Is Involved in the In Vitro Virulence of Entamoeba histolytica" Pathogens 12, no. 3: 474. https://doi.org/10.3390/pathogens12030474
APA StyleMunguía-Robledo, S., Orozco, E., García-Rivera, G., Bolaños, J., Valdés, J., Azuara-Licéaga, E., & Rodríguez, M. A. (2023). Lysine Methyltransferase EhPKMT2 Is Involved in the In Vitro Virulence of Entamoeba histolytica. Pathogens, 12(3), 474. https://doi.org/10.3390/pathogens12030474





