Epidemiological Assessment of African Swine Fever Spread in the Dominican Republic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Dataset
2.2. Descriptive Analysis
2.3. Descriptive Spatiotemporal Analysis
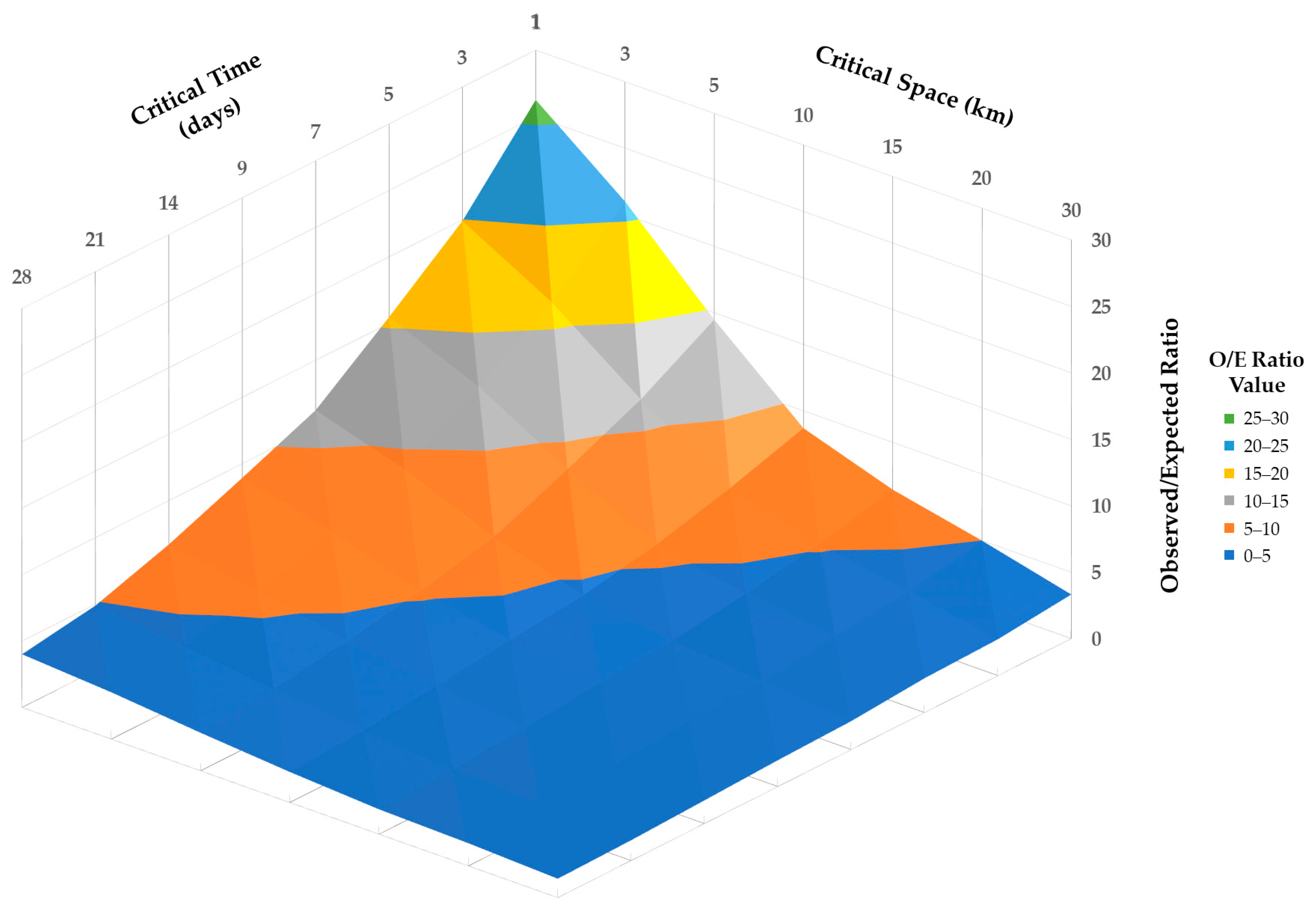
2.4. Cluster Detection
2.5. Space–Time Permutation Model
2.6. Estimation of Farm-Level Reproduction Ratio (R0)
3. Results
3.1. Descriptive Factors
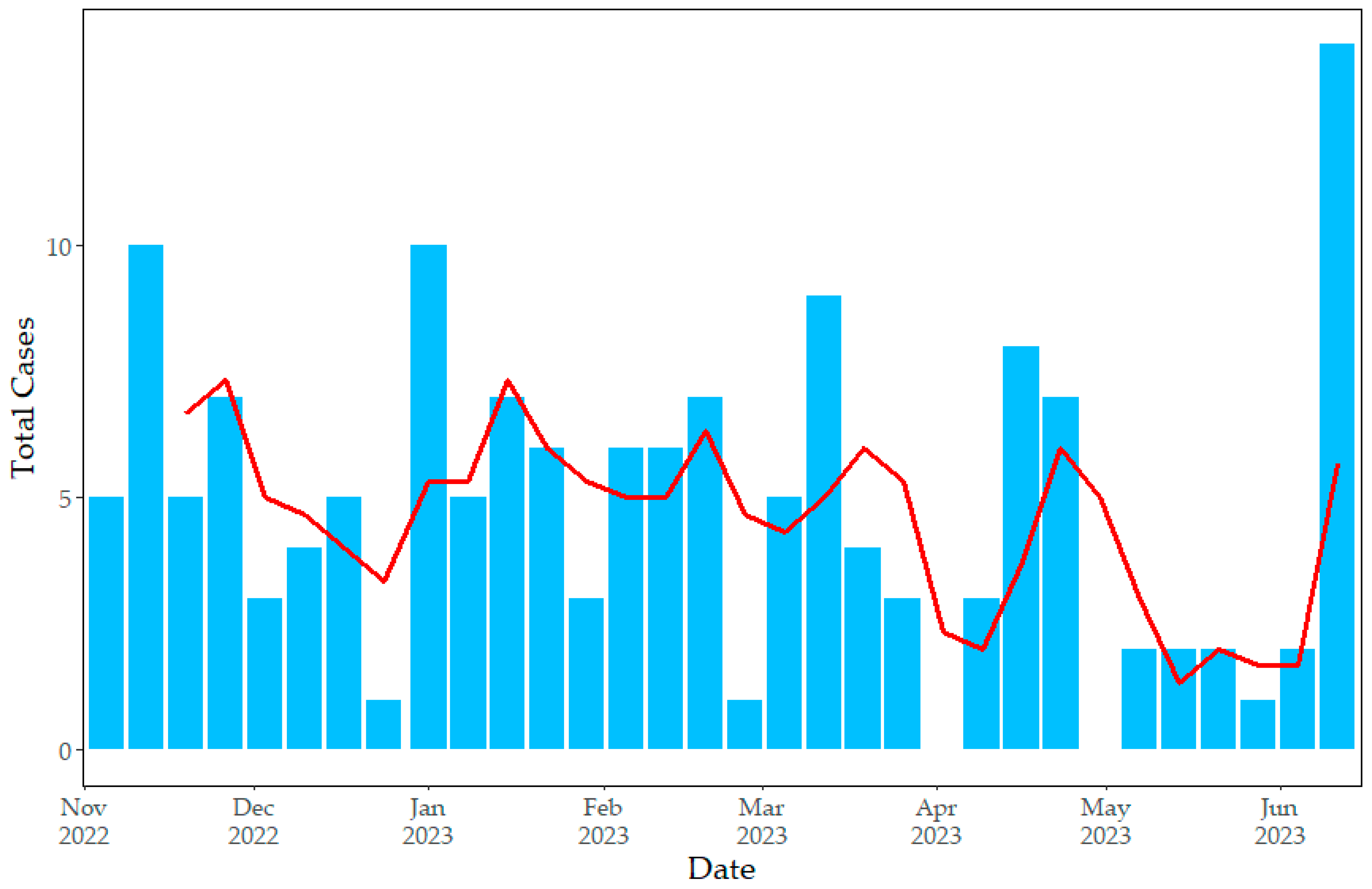
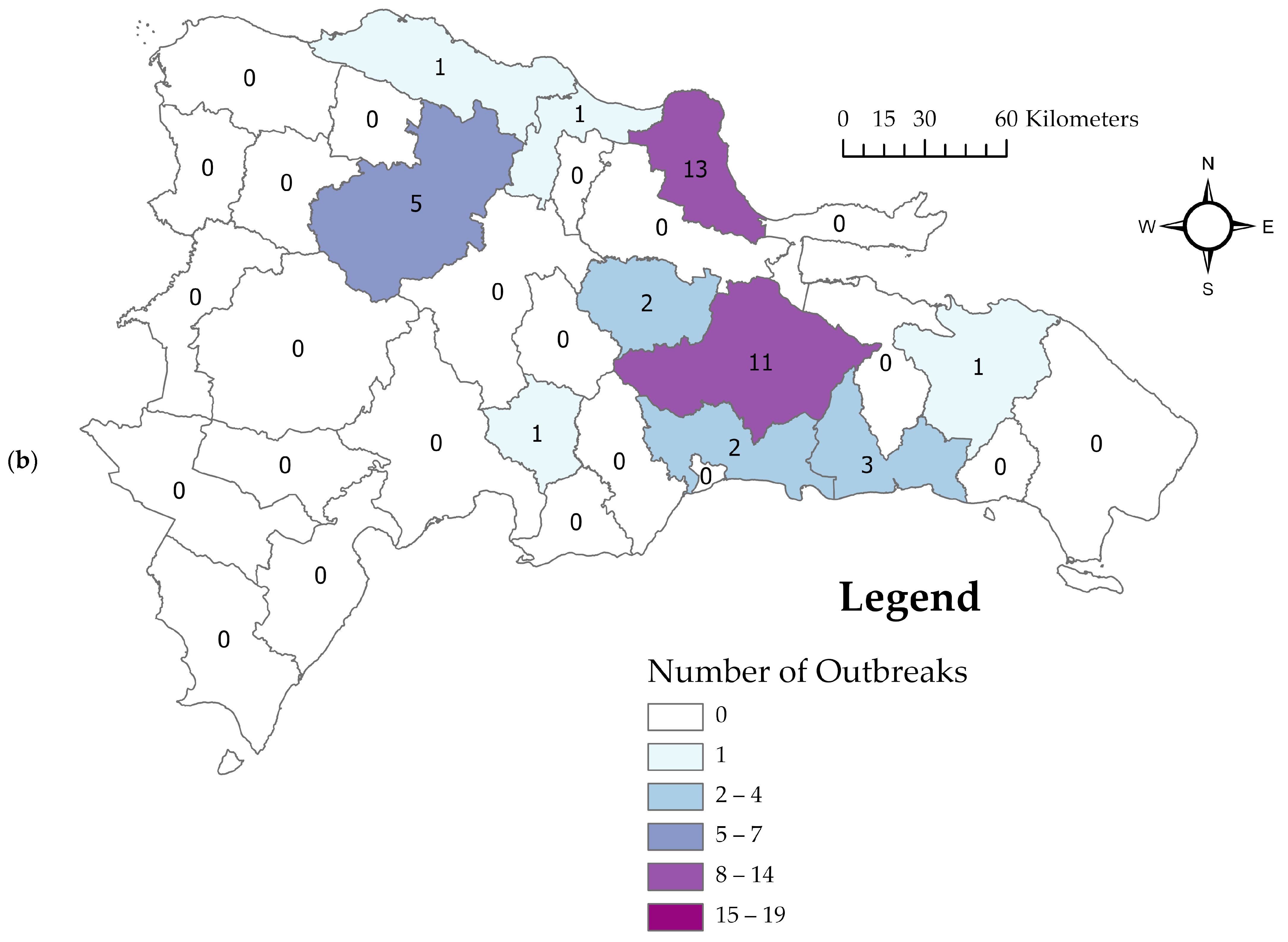
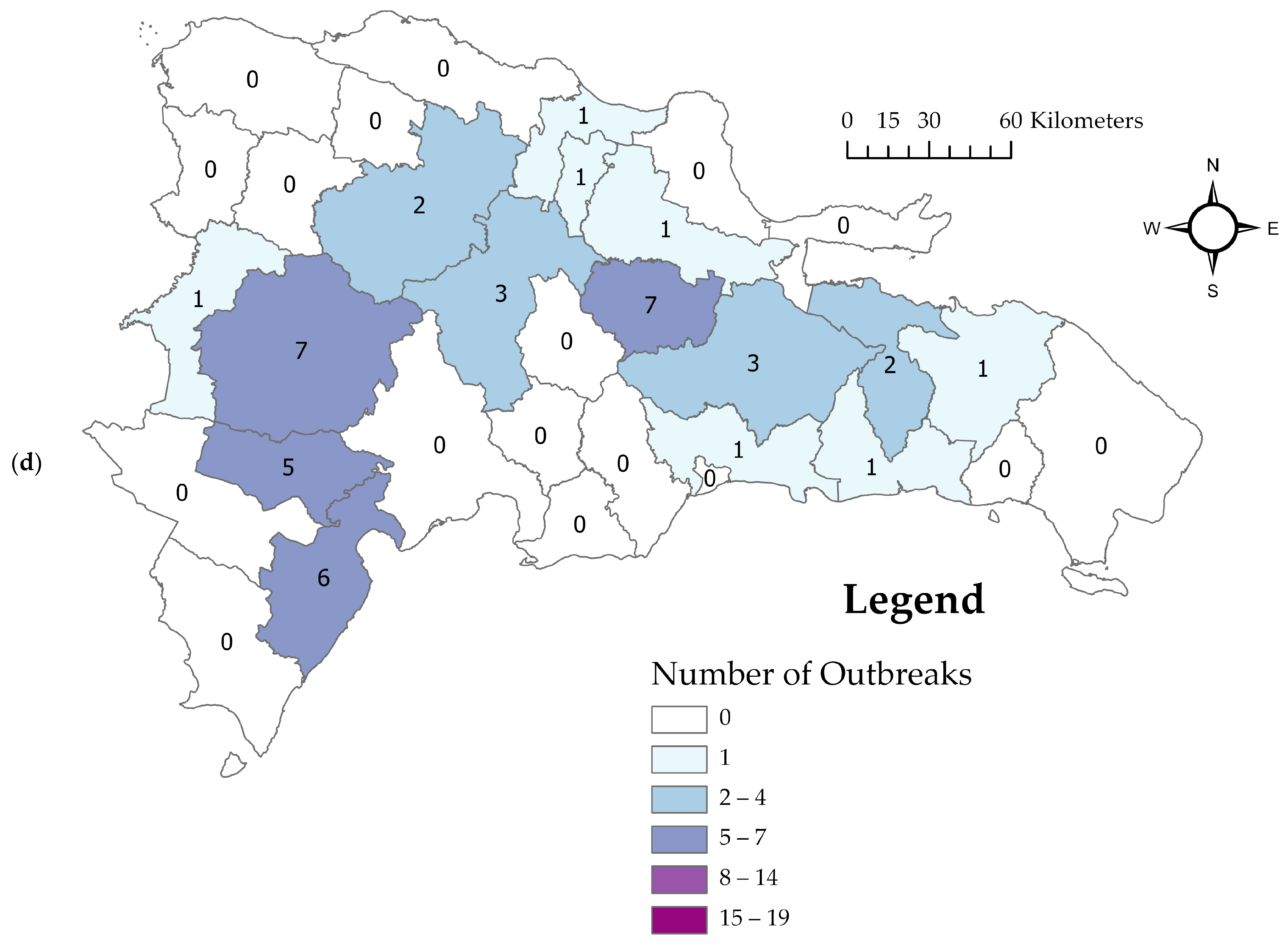
3.2. Descriptive Spatiotemporal Analysis
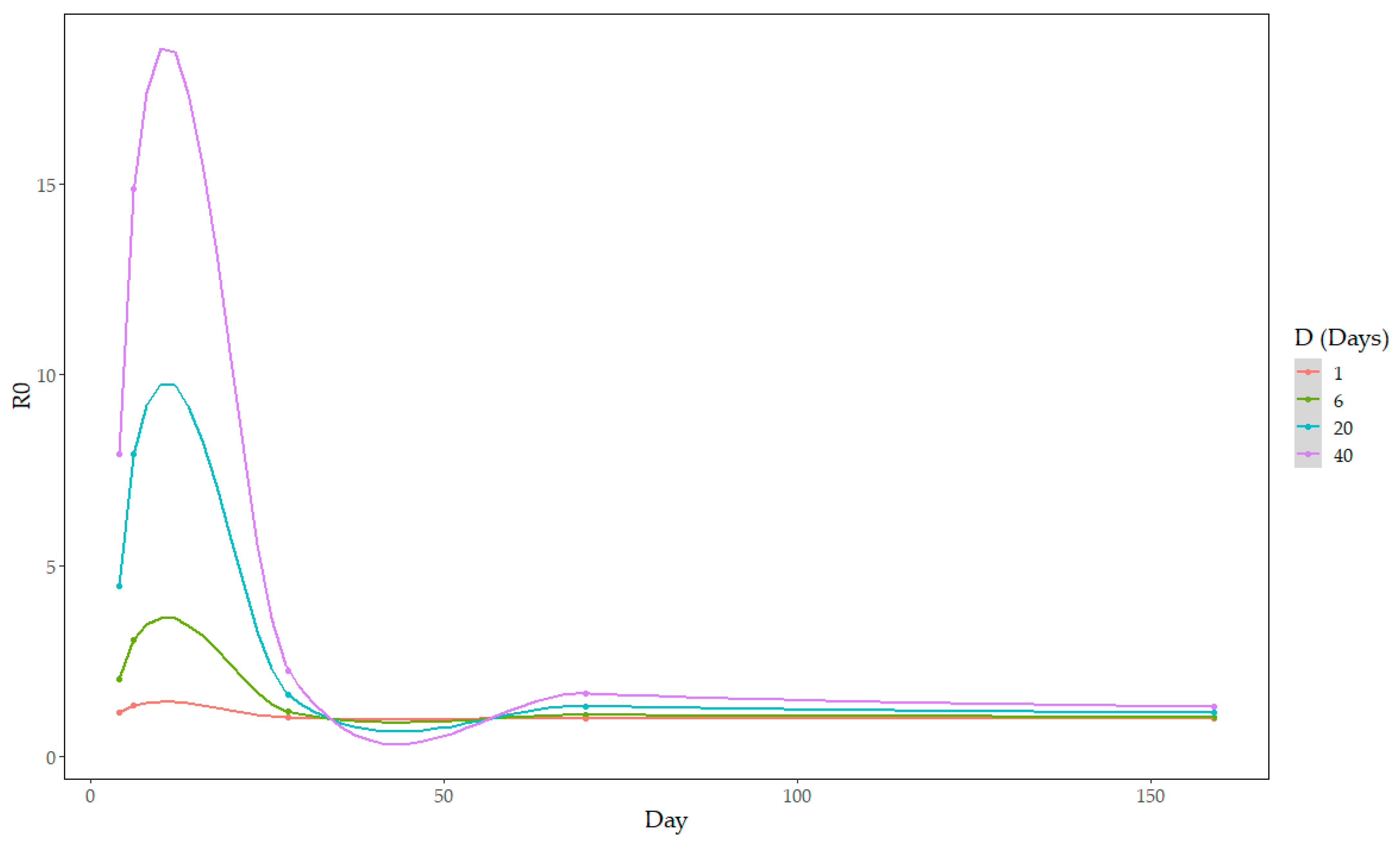
3.3. Between-Farm Reproduction Ratio
3.4. Cluster Analysis
3.5. Space–Time Permutation Model
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blome, S.; Franzke, K.; Beer, M. African swine fever—A review of current knowledge. Virus Res. 2020, 287, 198099. [Google Scholar] [CrossRef] [PubMed]
- Alonso, C.; Borca, M.; Dixon, L.; Revilla, Y.; Rodriguez, F.; Escribano, J.M.; ConsortiumYR, I.R. ICTV Virus Taxonomy Profile: Asfarviridae. J. Gen. Virol. 2018, 99, 613–614. [Google Scholar] [CrossRef] [PubMed]
- World Organisation for Animal Health. African Swine Fever; OIE Technical Disease Card; World Organisation for Animal Health: Paris, France, 2019; p. 5. [Google Scholar]
- Franzoni, G.; Dei Giudici, S.; Loi, F.; Sanna, D.; Floris, M.; Fiori, M.; Sanna, M.L.; Madrau, P.; Scarpa, F.; Zinellu, S.; et al. African Swine Fever Circulation among Free-Ranging Pigs in Sardinia: Data from the Eradication Program. Vaccines 2020, 8, 549. [Google Scholar] [CrossRef] [PubMed]
- Danzetta, M.L.; Marenzoni, M.L.; Iannetti, S.; Tizzani, P.; Calistri, P.; Feliziani, F. African Swine Fever: Lessons to Learn From Past Eradication Experiences. A Systematic Review. Front. Vet. Sci. 2020, 7, 296. [Google Scholar] [CrossRef] [PubMed]
- Dixon, L.K.; Stahl, K.; Jori, F.; Vial, L.; Pfeiffer, D.U. African Swine Fever Epidemiology and Control. Annu. Rev. Anim. Biosci. 2020, 8, 221–246. [Google Scholar] [CrossRef]
- USDA-APHIS. USDA Statement on Confirmation of African Swine Fever in the Dominican Republic; USDA: Washington, DC, USA, 2021.
- USDA-APHIS. USDA Statement on Confirmation of African Swine Fever in Haiti; USDA: Washington, DC, USA, 2021.
- World Organisation for Animal Health. African Swine Fever: Situation Report 39; World Organisation for Animal Health: Paris, France, 2023. [Google Scholar]
- EFSA Panel on Animal Health and Welfare (AHAW); More, S.; Miranda, M.A.; Bicout, D.; Bøtner, A.; Butterworth, A.; Calistri, P.; Edwards, S.; Garin-Bastuji, B.; Good, M.; et al. Scientific Opinion on the African swine fever in wild boar. EFSA J. 2018, 16, e05344. [Google Scholar] [CrossRef] [PubMed]
- USDA. History Highlight: APHIS Supports the Fight Against African Swine Fever; USDA: Washington, DC, USA, 2022.
- Jéssica Kayamori, L.; Trincado, C.; Souza, J.; Rojas, H.; Beltran-Alcrudo, D. Informe Misión Apoyo Técnico Emergencia Peste Porcina Africana; Food and Agriculture Organization of the United Nations: Rome, Italy, 2021. [Google Scholar]
- Mayol, V. Dominican Republic Swine Voluntary Report-ASF Outbreak Creates Increased Opportunities for US Swine Meat Exports to the DR; United States Department of Agriculture Foreign Agricultural Service: Santo Domingo, Dominican Republic, 2022; p. 8.
- Schambow, R.; Reyes, R.; Morales, J.; Diaz, A.; Perez, A.M. A qualitative assessment of alternative eradication strategies for African swine fever in the Dominican Republic. Front. Vet. Sci. 2022, 9, 1054271. [Google Scholar] [CrossRef]
- Sandoval, R. Inter-American Institute for Cooperation on Agriculture. Personal Communication, 2023. [Google Scholar]
- Arias, J. Inventario Nacional Porcino; Convenio Ministerio de Agricultura—OIRSA: Santo Domingo, Dominican Republic, 2022; p. 47. [Google Scholar]
- Kanankege, K.S.T.; Alvarez, J.; Zhang, L.; Perez, A.M. An Introductory Framework for Choosing Spatiotemporal Analytical Tools in Population-Level Eco-Epidemiological Research. Front. Vet. Sci. 2020, 7, 339. [Google Scholar] [CrossRef]
- Ward, M.P.; Carpenter, T.E. Analysis of time–space clustering in veterinary epidemiology. Prev. Vet. Med. 2000, 43, 225–237. [Google Scholar] [CrossRef]
- World Organisation for Animal Health. Terrestrial Animal Health Code: Glossary. Available online: https://www.woah.org/en/what-we-do/standards/codes-and-manuals/terrestrial-code-online-access/?id=169&L=1&htmfile=glossaire.htm (accessed on 10 February 2023).
- Cresswell, J. Research Design: Qualitative, Quantitative, and Mixed Methods Approaches, 3rd ed.; SAGE Publications: Thousand Oaks, CA, USA, 2009. [Google Scholar]
- Ward, M.P.; Carpenter, T.E. Techniques for analysis of disease clustering in space and in time in veterinary epidemiology. Prev. Vet. Med. 2000, 45, 257–284. [Google Scholar] [CrossRef]
- Moran, P.A.P. Notes on Continuous Stochastic Phenomena. Biometrika 1950, 37, 17–23. [Google Scholar] [CrossRef] [PubMed]
- George, K. Detection of Low Intensity Epidemicity. Br. J. Prev. Soc. Med. 1963, 17, 121. [Google Scholar] [CrossRef]
- Kulldorff, M.; Heffernan, R.; Hartman, J.; Assunção, R.; Mostashari, F. A Space–Time Permutation Scan Statistic for Disease Outbreak Detection. PLoS Med. 2005, 2, e59. [Google Scholar] [CrossRef] [PubMed]
- Diekmann, O.; Heesterbeek, J.A.P.; Metz, J.A.J. On the definition and the computation of the basic reproduction ratio R0 in models for infectious diseases in heterogeneous populations. J. Math. Biol. 1990, 28, 365–382. [Google Scholar] [CrossRef] [PubMed]
- Dohoo, I.; Martin, W.; Stryhn, H. Veterinary Epidemiologic Research, 2nd ed.; VER Inc.: Glendale, CA, USA, 2010. [Google Scholar]
- Iglesias, I.; Perez, A.M.; SÁNchez-VizcaÍNo, J.M.; MuÑOz, M.J.; MartÍNez, M.; De La Torre, A. Reproductive ratio for the local spread of highly pathogenic avian influenza in wild bird populations of Europe, 2005–2008. Epidemiol. Infect. 2011, 139, 99–104. [Google Scholar] [CrossRef] [PubMed]
- Iglesias, I.; Muñoz, M.J.; Montes, F.; Perez, A.; Gogin, A.; Kolbasov, D.; de la Torre, A. Reproductive Ratio for the Local Spread of African Swine Fever in Wild Boars in the Russian Federation. Transbound. Emerg. Dis. 2016, 63, e237–e245. [Google Scholar] [CrossRef]
- de Carvalho Ferreira, H.C.; Backer, J.A.; Weesendorp, E.; Klinkenberg, D.; Stegeman, J.A.; Loeffen, W.L.A. Transmission rate of African swine fever virus under experimental conditions. Vet. Microbiol. 2013, 165, 296–304. [Google Scholar] [CrossRef]
- Guinat, C.; Gogin, A.; Blome, S.; Keil, G.; Pollin, R.; Pfeiffer, D.U.; Dixon, L. Transmission routes of African swine fever virus to domestic pigs: Current knowledge and future research directions. Vet. Rec. 2016, 178, 262–267. [Google Scholar] [CrossRef]
- Guinat, C.; Porphyre, T.; Gogin, A.; Dixon, L.; Pfeiffer, D.U.; Gubbins, S. Inferring within-herd transmission parameters for African swine fever virus using mortality data from outbreaks in the Russian Federation. Transbound. Emerg. Dis. 2018, 65, e264–e271. [Google Scholar] [CrossRef]
- Gonzales, W.; Moreno, C.; Duran, U.; Henao, N.; Bencosme, M.; Lora, P.; Reyes, R.; Núñez, R.; De Gracia, A.; Perez, A.M. African swine fever in the Dominican Republic. Transbound. Emerg. Dis. 2021, 68, 3018–3019. [Google Scholar] [CrossRef]
- Barongo, M.B.; Ståhl, K.; Bett, B.; Bishop, R.P.; Fèvre, E.M.; Aliro, T.; Okoth, E.; Masembe, C.; Knobel, D.; Ssematimba, A. Estimating the Basic Reproductive Number (R0) for African Swine Fever Virus (ASFV) Transmission between Pig Herds in Uganda. PLoS ONE 2015, 10, e0125842. [Google Scholar] [CrossRef] [PubMed]
- Korennoy, F.I.; Gulenkin, V.M.; Gogin, A.E.; Vergne, T.; Karaulov, A.K. Estimating the Basic Reproductive Number for African Swine Fever Using the Ukrainian Historical Epidemic of 1977. Transbound. Emerg. Dis. 2017, 64, 1858–1866. [Google Scholar] [CrossRef] [PubMed]
- Gulenkin, V.M.; Korennoy, F.I.; Karaulov, A.K.; Dudnikov, S.A. Cartographical analysis of African swine fever outbreaks in the territory of the Russian Federation and computer modeling of the basic reproduction ratio. Prev. Vet. Med. 2011, 102, 167–174. [Google Scholar] [CrossRef] [PubMed]
- Thomas, J.C.; Weber, D.J. Epidemiologic Methods for the Study of Infectious Diseases; Oxford University Press: New York, NY, USA, 2001. [Google Scholar]
- Ramirez-Medina, E.; O’Donnell, V.; Silva, E.; Espinoza, N.; Velazquez-Salinas, L.; Moran, K.; Daite, D.A.; Barrette, R.; Faburay, B.; Holland, R.; et al. Experimental Infection of Domestic Pigs with an African Swine Fever Virus Field Strain Isolated in 2021 from the Dominican Republic. Viruses 2022, 14, 1090. [Google Scholar] [CrossRef]
- Nguyen-Thi, T.; Pham-Thi-Ngoc, L.; Nguyen-Ngoc, Q.; Dang-Xuan, S.; Lee, H.S.; Nguyen-Viet, H.; Padungtod, P.; Nguyen-Thu, T.; Nguyen-Thi, T.; Tran-Cong, T.; et al. An Assessment of the Economic Impacts of the 2019 African Swine Fever Outbreaks in Vietnam. Front. Vet. Sci. 2021, 8, 686038. [Google Scholar] [CrossRef]
- Gates, M.C.; Earl, L.; Enticott, G. Factors influencing the performance of voluntary farmer disease reporting in passive surveillance systems: A scoping review. Prev. Vet. Med. 2021, 196, 105487. [Google Scholar] [CrossRef]


| Inventory Type | Farm Type | Total Number (% of All Pigs) | Mean | SD | Median | Minimum | Maximum |
|---|---|---|---|---|---|---|---|
| All Pigs | Backyard | 857 (4) | 7 | 6.2 | 5 | 1 | 24 |
| CNT | 8819 (47) | 294 | 564 | 72 | 28 | 2855 | |
| ST | 9234 (49) | 2309 | 1439 | 1974 | 1015 | 4271 | |
| Live Pigs | Backyard | 631 (3) | 5 | 5.1 | 3 | 0 | 24 |
| CNT | 8503 (45) | 283 | 552 | 72 | 12 | 2811 | |
| ST | 9234 (49) | 2309 | 1439 | 1974 | 1015 | 4271 | |
| Dead Pigs | Backyard | 226 (1) | 2 | 3.7 | 0 | 0 | 18 |
| CNT | 316 (1) | 11 | 22 | 0 | 0 | 96 | |
| ST | 0 (0) | 0 | 0 | 0 | 0 | 0 |
| Clinical Sign or Necropsy Finding | Production Type (Number of Farms) * Number Reporting Sign (% By Production Stage) ** | |||||
|---|---|---|---|---|---|---|
| Piglet (63) | Nursery (35) | Boars (39) | Sows (60) | Fattening (40) | Total | |
| Dead | 26 (41) | 22 (63) | 19 (49) | 26 (43) | 11 (28) | 104 (44) |
| Anorexia | 25 (40) | 11 (31) | 10 (26) | 24 (40) | 20 (50) | 90 (38) |
| Fever | 12 (19) | 14 (40) | 11 (28) | 20 (33) | 16 (40) | 70 (30) |
| Diarrhea | 12 (19) | 6 (17) | 7 (18) | 11 (18) | 7 (18) | 43 (18) |
| Lethargy | 9 (14) | 7 (20) | 4 (10) | 13 (22) | 10 (25) | 39 (17) |
| Red skin | 5 (8) | 5 (14) | 5 (13) | 4 (7) | 4 (10) | 30 (13) |
| Epistaxis | 4 (6) | 3 (9) | 5 (13) | 8 (13) | 3 (8) | 23 (10) |
| No symptoms | 3 (5) | 0 (0) | 1 (3) | 4 (7) | 0 (0) | 8 (3) |
| Sudden death (specifically) | 4 (6) | 0 (0) | 2 (5) | 1 (2) | 0 (0) | 7 (3) |
| Vomiting | 1 (2) | 0 (0) | 2 (5) | 3 (5) | 1 (3) | 7 (3) |
| Hemorrhage (unspecified) | 1 (2) | 1 (3) | 0 (0) | 1 (2) | 0 (0) | 5 (2) |
| Nervous signs | 1 (2) | 0 (0) | 0 (0) | 1 (2) | 2 (5) | 4 (2) |
| Splenomegaly | 0 (0) | 0 (0) | 0 (0) | 2 (3) | 1 (3) | 3 (1) |
| Respiratory | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (3) | 1 (0.4) |
| Category | Biosecurity Factor | Number (%) | ||
|---|---|---|---|---|
| Backyard | CNT | ST | ||
| Farm Access | Clean/dirty line with change of clothes and boot covers | 0 (0) | 0 (0) | 2 (50) |
| Farm Access | Has a shower | 0 (0) | 5 (17) | 3 (75) |
| Farm Access | Has closed entrance doors | 2 (2) | 10 (33) | 3 (75) |
| Farm Access | Has visitor log | 0 (0) | 0 (0) | 1 (25) |
| Farm Access | Has vehicle disinfection arch | 0 (0) | 0 (0) | 1 (25) |
| Farm Access | Has foot baths and change of disinfectant | 0 (0) | 1 (3) | 1 (25) |
| Farm Access | Disinfects goods and equipment brought on farm | 0 (0) | 0 (0) | 3 (75) |
| Farm Access | Has unloading ramp | 1 (0.8) | 10 (33) | 4 (100) |
| Farm Access | Perimeter fence is in good condition | 1 (0.8) | 4 (13) | 3 (75) |
| Barn Access | Has cleaning and disinfection of the entrance | 0 (0) | 4 (13) | 3 (75) |
| Barn Access | Each barn has dedicated staff | 0 (0) * | 6 (20) | 3 (75) |
| Barn Access | Each barn has dedicated carts and work items | 1 (0.83) | 4 (13) | 3 (75) |
| Barn Access | Has rodent control | 0 (0) | 2 (7) | 3 (75) |
| Barn Access | Has bird curtain | 0 (0) | 5 (17) | 1 (25) |
| Barn Access | Has clean staff clothing | 0 (0) | 1 (3) | 2 (50) |
| Barn Access | Has footbath with frequent change of disinfectant | 0 (0) | 1 (3) | 1 (25) |
| Barn Access | Has cement floor with periodic disinfection | 6 (5.0) | 12 (40) | 4 (100) |
| Barn Access | Has feeders in good condition and periodic disinfection | 0 (0) | 6 (20) | 4 (100) |
| Staff | Staff receive biosecurity training | 0 (0) * | 0 (0) | 2 (50) |
| Staff | Staff have their own pigs | 0 (0) * | 0 (0) | 1 (25) |
| Staff | Staff have dedicated and clean clothes | 0 (0) * | 2 (7) | 3 (75) |
| Staff | Staff required to shower upon entering the barn or pig house | 0 (0) ** | 3 (10) | 3 (75) |
| Staff | Staff bring food into the barns | 32 (27) * | 26 (87) | 3 (75) |
| Breeding | Use natural mount | 67 (55) | 26 (87) | 4 (100) |
| Breeding | Use artificial insemination | 0 (0) | 1 (3) | 3 (75) |
| Breeding | Produces their own semen | 25 (21) | 21 (70) | 4 (100) |
| Breeding | Has boars for exclusive use | 16 (13) | 22 (73) | 4 (100) |
| Breeding | Produces their own replacement pigs | 28 (23) | 21 (70) | 3 (75) |
| General | Buries mortality on farm | 34 (28) | 21 (70) | 4 (100) |
| General | Feed enters with its own truck | 9 (7) | 16 (53) | 3 (75) |
| General | Trucks are disinfected at the entrance and exit | 0 (0) | 4 (13) | 3 (75) |
| General | Other species are present on premise | 27 (23) * | 18 (60) | 1 (25) |
| General | Uses one needle per pig for injections of drugs or vaccines | 7 (6) | 5 (17) | 4 (100) |
| General | Has weeds on the perimeter or close to the food | 62 (51) | 18 (60) | 0 (0) |
| General | Pens drain adequately without causing flooding | 1 (0.8) | 5 (17) | 4 (100) |
| General | Has clean animal loading and unloading area | 0 (0) | 6 (20) | 4 (100) |
| Water source for pigs *** | Drinking water | 67 (55) | 10 (33) | *** |
| Non-drinking water | 4 (3) | 0 (0) | *** | |
| Reason for ASF Introduction Suspected by Producer | Number of Farms (%) * | |||
|---|---|---|---|---|
| Backyard (n = 103) | CNT (n = 17) | ST (n = 2) | Total (n = 122) | |
| Proximity to other outbreaks and farms | 43 (42) | 0 (0) | 0 (0) | 43 (35) |
| Movements of pigs between farms or free-range domestic pigs | 26 (25) | 8 (47) | 0 (0) | 34 (28) |
| Swill or garbage feeding | 32 (31) | 0 (0) | 0 (0) | 32 (26) |
| Movements of and contact with people | 10 (10) | 4 (24) | 0 (0) | 14 (12) |
| Feed (unspecified source) | 11 (11) | 2 (12) | 0 (0) | 13 (11) |
| Sharing breeding boar | 8 (8) | 4 (24) | 0 (0) | 12 (10) |
| Non-pig animals | 5 (5) | 3 (18) | 2 (100) | 10 (8) |
| Water | 2 (2) | 1 (6) | 0 (0) | 3 (3) |
| Vehicles/Trucks | 0 (0) | 2 (12) | 0 (0) | 2 (2) |
| Carcass disposal method | 1 (1) | 0 (0) | 0 (0) | 1 (0.8) |
| Near landfill | 1 (1) | 0 (0) | 0 (0) | 1 (0.8) |
| Cluster | Radius (km) | Timeframe | Number of Cases | O/E Ratio | p-Value |
|---|---|---|---|---|---|
| 1 | 0.76 | 1 January 2023 to 7 January 2023 | 6 | 15.3 | <0.001 |
| 2 | 2.23 | 22 January 2023 to 28 January 2023 | 6 | 21.3 | <0.001 |
| 3 | 0.00042 | 11 June 2023 to 17 June 2023 | 7 | 10.9 | <0.001 |
| 4 | 0.21 | 5 March 2023 to 11 March 2023 | 4 | 30.6 | <0.001 |
| 5 | 0 | 5 February 2023 to 11 February 2023 | 4 | 25.5 | 0.001 |
| 6 | 0 | 12 February 2023 to 18 February 2023 | 4 | 25.5 | 0.001 |
| 7 | 0.63 | 23 April 2023 to 29 April 2023 | 4 | 21.9 | 0.003 |
| 8 | 0 | 12 March 2023 to 18 March 2023 | 4 | 17 | 0.011 |
| 9 | 0 | 11 June 2023 to 17 June 2023 | 5 | 10.9 | 0.014 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Schambow, R.A.; Hussain, S.; Antognoli, M.C.; Kreindel, S.; Reyes, R.; Perez, A.M. Epidemiological Assessment of African Swine Fever Spread in the Dominican Republic. Pathogens 2023, 12, 1414. https://doi.org/10.3390/pathogens12121414
Schambow RA, Hussain S, Antognoli MC, Kreindel S, Reyes R, Perez AM. Epidemiological Assessment of African Swine Fever Spread in the Dominican Republic. Pathogens. 2023; 12(12):1414. https://doi.org/10.3390/pathogens12121414
Chicago/Turabian StyleSchambow, Rachel A., Syed Hussain, Maria C. Antognoli, Silvia Kreindel, Raysa Reyes, and Andres M. Perez. 2023. "Epidemiological Assessment of African Swine Fever Spread in the Dominican Republic" Pathogens 12, no. 12: 1414. https://doi.org/10.3390/pathogens12121414
APA StyleSchambow, R. A., Hussain, S., Antognoli, M. C., Kreindel, S., Reyes, R., & Perez, A. M. (2023). Epidemiological Assessment of African Swine Fever Spread in the Dominican Republic. Pathogens, 12(12), 1414. https://doi.org/10.3390/pathogens12121414







