Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita
Abstract
:1. Introduction
2. Results
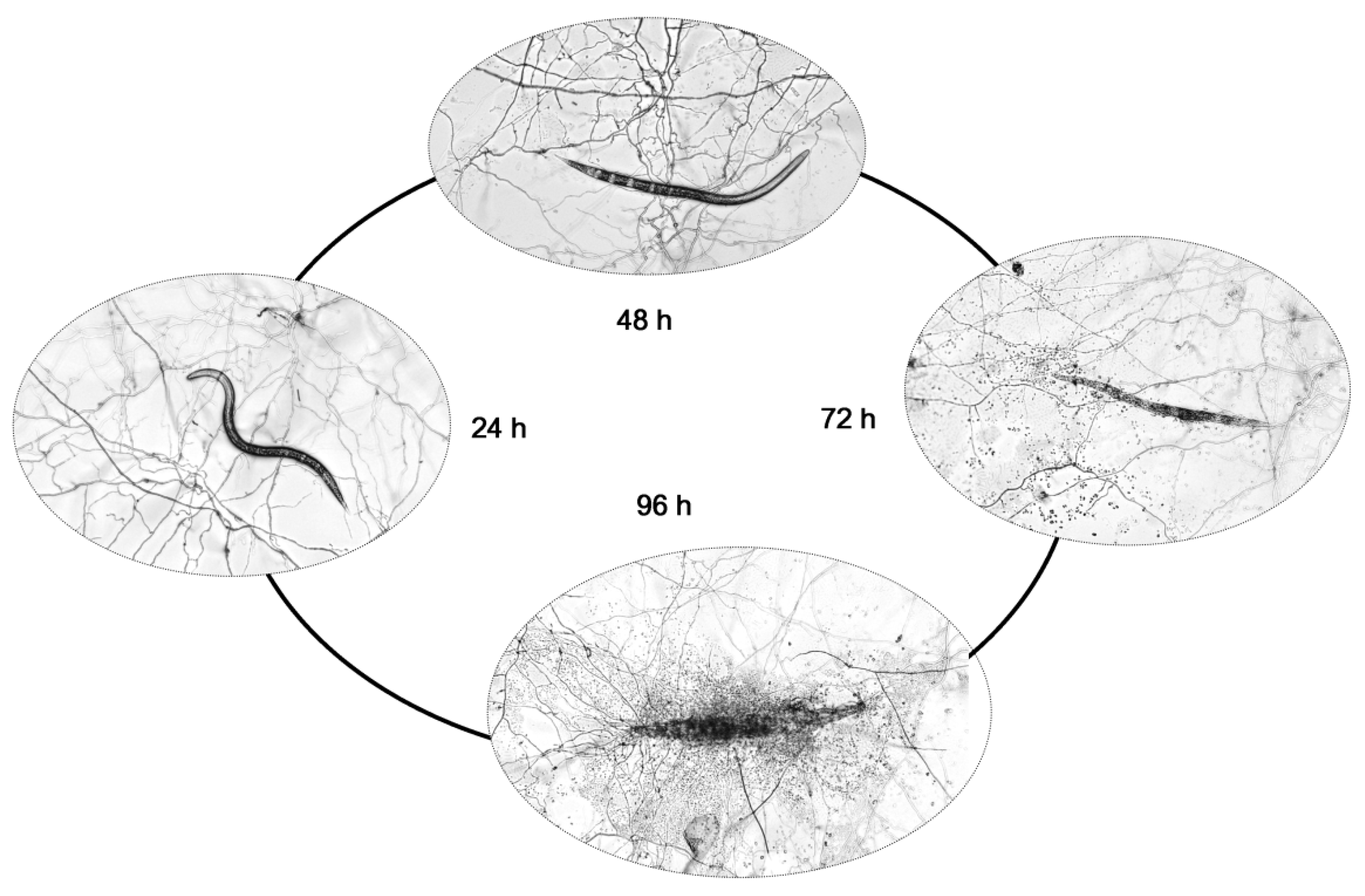
2.1. Pathogenicity of P. lavendulum YMF1.00683 against M. incognita
2.2. Isolation and Structural Identification of Compounds
2.3. Activity
2.3.1. Effects of Compounds on the Egg Hatching of M. incognita
2.3.2. Evaluation of the Nematocidal Activity of Compounds against M. incognita
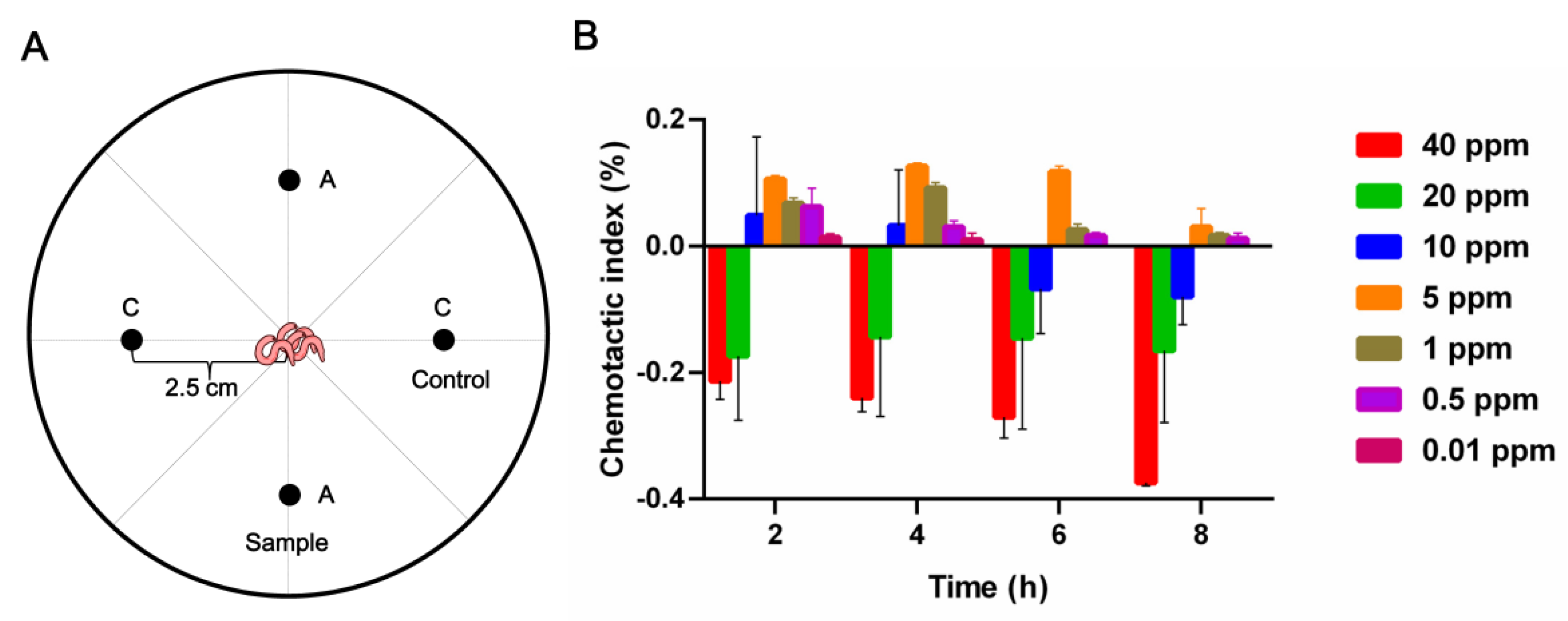
2.3.3. Chemotaxis Activities of Compound 7 against M. incognita
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Infection of M. incognita by P. lavendulum
4.3. Extraction and Separation of Metabolites
4.4. Spectroscopic Data
4.5. Assay Activity against M. incognita
4.5.1. Effects of Compounds on the Hatching of M. incognita Eggs
4.5.2. Assay of Nematocidal Activity against M. incognita
4.5.3. Assay of Chemotaxis Activity against M. incognita
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Abad, P.; Gouzy, J.; Aury, J.M.; Castagnone-Sereno, P.; Danchin, E.G.J.; Deleury, E.; Perfus-Barbeoch, L.; Anthouard, V.; Artiguenave, F.; Blok, V.C.; et al. Genome sequence of the metazoan plant-parasitic nematode Meloidogyne incognita. Nat. Biotechnol. 2008, 26, 909–915. [Google Scholar] [CrossRef] [Green Version]
- Yu, Z.Q.; Xiong, J.; Zhou, Q.N.; Luo, H.Y.; Hu, S.B.; Xia, L.Q.; Sun, M.; Li, L.; Yu, Z.N. The diverse nematicidal properties and biocontrol efficacy of Bacillus thuringiensis Cry6A against the root-knot nematode Meloidogyne hapla. J. Invertebr. Pathol. 2015, 125, 73–80. [Google Scholar] [CrossRef]
- Francisco, B.G.F.; Ponce, I.M.; Espinosa, M.A.P.; Moctezuma, A.M.; Lopez, V.E.L.Y. Advances in the biological control of phytoparasitic nematodes via the use of nematophagous fungi. World J. Microb. Biot. 2021, 37, 180. [Google Scholar] [CrossRef]
- Perdomo, H.; Cano, J.; Gene, J.; Garcia, D.; Hernandez, M.; Guarro, J. Polyphasic analysis of Purpureocillium lilacinum isolates from different origins and proposal of the new species Purpureocillium lavendulum. Mycologia 2013, 105, 151–161. [Google Scholar] [CrossRef]
- Siddiqui, Z.A.; Mahmood, I. Biological control of plant parasitic nematodes by fungi: A review. Bioresour.Technol. 1996, 58, 229–239. [Google Scholar] [CrossRef]
- Dube, B.; Smart, G.C. Biological control of Meloidogyne incognita by Paecilomyces lilacinus and Pasteuria penetrans. J. Nematol. 1987, 19, 222–227. [Google Scholar]
- Cabanillas, E.; Barker, K.R.; Nelson, L.A. Growth of isolates of Paecilomyces lilacinus and their efficacy in biocontrol of Meloidogyne incognita on tomato. J. Nematol. 1989, 21, 164–172. [Google Scholar]
- Fiedler, Z.; Sosnowska, D. Nematophagous fungus Paecilomyces lilacinus (Thom) Samson is also a biological agent for control of greenhouse insects and mite pests. BioControl 2007, 52, 547–558. [Google Scholar] [CrossRef]
- Goffre, D.; Folgarait, P.J. Purpureocillium lilacinum, potential agent for biological control of the leaf-cutting ant Acromyrmex lundii. J. Invertebr. Pathol. 2015, 130, 107–115. [Google Scholar] [CrossRef]
- Wan, J.; Dai, Z.B.; Zhang, K.Q.; Li, G.H.; Zhao, P.J. Pathogenicity and metabolites of endoparasitic nematophagous fungus Drechmeria coniospora YMF1.01759 against nematodes. Microorganisms 2021, 9, 1735. [Google Scholar] [CrossRef]
- Park, J.O.; Hargreaves, J.R.; McConville, E.J.; Stirling, G.R.; Ghisalberti, E.L.; Sivasithamparam, K. Production of leucinostatins and nematicidal activity of Australian isolates of Paecilomyces lilacinus (Thom) Samson. Lett. Appl. Microbiol. 2004, 38, 271–276. [Google Scholar] [CrossRef]
- Gapasin, R.M.; Vasquez, E.A.; Rendon, G.A. Bioassay-guided identification of the nematicidal secondary metabolites from Paecilomyces lilicanus for the control of root-knot nematode (Meloidogyne graminicola, Golden and Birchfield). Ann. Tropic. Res. 2011, 33, 22–43. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.P.; Xu, J.; Yue, J.M. Sterols from the fungus Catathelasma imperiale. Chin. J. Chem. 2003, 21, 1390–1394. [Google Scholar] [CrossRef]
- Cheng, S.M.; Zhai, Y.C.; Xue, F.; Wang, J.H. Chemical constituents of Bombyx batryticatus. Zhong Cao Yao 2015, 46, 3630–3636. [Google Scholar] [CrossRef]
- Zhao, J.L.; Mou, Y.; Shan, T.J.; Li, Y.; Zhou, L.G.; Wang, M.G.; Wang, J.G. Antimicrobial metabolites from the endophytic fungus Pichia guilliermondii Isolated from Paris polyphylla var. yunnanensis. Molecules 2010, 15, 7961–7970. [Google Scholar] [CrossRef] [Green Version]
- Cai, H.H.; Liu, X.M.; Chen, Z.Y.; Liao, S.T.; Zou, Y.X. Isolation, purification and identification of nine chemical compounds from Flammulina velutipes fruiting bodies. Food Chem. 2013, 141, 2873–2879. [Google Scholar] [CrossRef]
- Yue, J.M.; Chen, S.N.; Lin, Z.W.; Sun, H.D. Sterols from the fungus Lactarium volemus. Phytochemistry 2001, 56, 801–806. [Google Scholar] [CrossRef]
- Wang, F.; Fang, Y.; Zhang, M.; Lin, A.; Zhu, A.; Gu, Q.; Zhu, W. Six new ergosterols from the marine-derived fungus Rhizopus sp. Steroids 2008, 73, 19–26. [Google Scholar] [CrossRef]
- Andrioli, W.J.; Lopes, A.A.; Cavalcanti, B.C.; Pessoa, C.; Nanayakkara, N.P.D.; Bastos, J.K. Isolation and characterization of 2-pyridone alkaloids and alloxazines from Beauveria bassiana. Nat. Prod. Res. 2017, 31, 1920–1929. [Google Scholar] [CrossRef]
- Don, M.-J.; Shen, C.-C.; Lin, Y.-L.; Syu, W., Jr.; Ding, Y.-H.; Sun, C.-M. Nitrogen-containing compounds from Salvia miltiorrhiza. J. Nat. Prod. 2005, 68, 1066–1070. [Google Scholar] [CrossRef]
- Silva, S.D.; Carneiro, R.M.D.G.; Faria, M.; Souza, D.A.; Monnerat, R.G.; Lopes, R.B. Evaluation of Pochonia chlamydosporia and Purpureocillium lilacinum for suppression of Meloidogyne enterolobii on tomato and banana. J. Nematol. 2017, 49, 77–85. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.P.; Wang, J.X.; Liu, F.; Pan, C.S. Enhancing the virulence of Paecilomyces lilacinus against Meloidogyne incognita eggs by overexpression of a serine protease. Biotechnol. Lett. 2010, 32, 1159–1166. [Google Scholar] [CrossRef] [Green Version]
- Ishiyama, A.; Otoguro, K.; Iwatsuki, M.; Namatame, M.; Nishihara, A.; Nonaka, K.; Kinoshita, Y.; Takahashi, Y.; Masuma, R.; Shiomi, K.; et al. In Vitro and in Vivo antitrypanosomal activities of three peptide antibiotics: Leucinostatin A and B, alamethicin I and tsushimycin. J. Antibiot. 2009, 62, 343. [Google Scholar] [CrossRef] [Green Version]
- Mori, Y.; Tsuboi, M.; Suzuki, M.; Fukushima, K.; Arai, T. Isolation of leucinostatin A and one of its constituents, the new amino acid, 4-methyl-6-(2-oxobutyl)-2-piperidinecarboxylic acid, from Paecilomyces lilacinus A-267. J. Antibiot. 1982, 35, 543–544. [Google Scholar] [CrossRef] [Green Version]
- Ban, S.; Azuma, Y.; Sato, H.; Suzuki, K.-I.; Nakagiri, A. Isaria takamizusanensis is the anamorph of Cordyceps ryogamimontana, warranting a new combination, Purpureocillium takamizusanense comb. nov. Int. J. Syst. Evol. Microbiol. 2015, 65, 2459–2465. [Google Scholar] [CrossRef]
- Huang, R.; Kim, H.J.; Min, D.B. Photosensitizing effect of riboflavin, lumiflavin, and lumichrome on the generation of volatiles in soy milk. J. Agric. Food Chem. 2006, 54, 2359–2364. [Google Scholar] [CrossRef]
- Ahmad, I.; Fasiliullah, Q.; Vaid, F.H.M. A study of simultaneous photolysis and photoaddition reactions of riboflavin in aqueous solution. J. Photochem. Photobiol. B-Biol. 2004, 75, 13–20. [Google Scholar] [CrossRef]
- Schmidt, F.; Koch, B.P.; Elvert, M.; Schmidt, G.; Witt, M.; Hinrichs, K.U. Diagenetic transformation of dissolved organic nitrogen compounds under contrasting sedimentary redox conditions in the Black Sea. Environ. Sci. Technol. 2011, 45, 5223–5229. [Google Scholar] [CrossRef]
- Xu, H.; Chakrabarty, Y.; Philmus, B.; Mehta, A.P.; Bhandari, D.; Hohmann, H.P.; Begley, T.P. Identification of the first riboflavin catabolic gene cluster isolated from Microbacterium maritypicum G10. J. Biol. Chem. 2016, 291, 23506–23515. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, K.; Asano, Y. Efficient production of lumichrome by Microbacterium sp. Strain TPU3598. Appl. Environ. Microbiol. 2015, 81, 7360–7367. [Google Scholar] [CrossRef] [Green Version]
- Rajamani, S.; Bauer, W.D.; Robinson, J.B.; Farrow, J.M.; Pesci, E.C.; Teplitski, M.; Gao, M.S.; Sayre, R.T.; Phillips, D.A. The vitamin riboflavin and its derivative lumichrome activate the lasR Bacterial quorum-sensing receptor. Mol. Plant-Microbe Interact. 2008, 21, 1184–1192. [Google Scholar] [CrossRef] [Green Version]
- Youn, U.J.; Lee, J.Y.; Kil, Y.S.; Han, A.R.; Chae, C.H.; Ryu, S.Y.; Seo, E.K. Identification of new pyrrole alkaloids from the fruits of Lycium chinense. Arch. Pharm. Res. 2016, 39, 321–327. [Google Scholar] [CrossRef]
- Feng, K.J.; Cheng, M.J.; Yang, S.S.; Wu, M.D.; Hsieh, S.Y.; Chan, H.Y.; Su, Y.S.; Chou, Y.T.; Chang, H.S. Chemical constituents of the endophytic fungus Ophiocordyceps sobolifera. Chem. Nat. Compd. 2019, 55, 309–312. [Google Scholar] [CrossRef]
- Chen, M.; Yang, H.Y.; Cao, Y.R.; Hui, Q.Q.; Fan, H.F.; Zhang, C.C.; Han, J.J.; Guo, Z.Y.; Xu, J.P.; Zhang, K.Q.; et al. Functional characterization of core regulatory genes involved in sporulation of the nematophagous fungus Purpureocillium lavendulum. mSphere 2020, 5, e00932-20. [Google Scholar] [CrossRef]
- Giannakou, I.O.; Karpouzas, D.G.; Anastasiades, I.; Tsiropoulos, N.G.; Georgiadou, A. Factors affecting the efficacy of non-fumigant nematicides for controlling root-knot nematodes. Pest Manag. Sci. 2005, 61, 961–972. [Google Scholar] [CrossRef]
- Huang, D.; Yu, C.; Shao, Z.Z.; Cai, M.M.; Li, G.Y.; Zheng, L.Y.; Yu, Z.N.; Zhang, J.B. Identification and characterization of nematicidal volatile organic compounds from deep-sea Virgibacillus dokdonensis MCCC 1A00493. Molecules 2020, 25, 744. [Google Scholar] [CrossRef] [Green Version]
- Hsueh, Y.P.; Gronquist, M.R.; Schwarz, E.M.; Nath, R.D.; Lee, C.H.; Gharib, S.; Schroeder, F.C.; Sternberg, P.W. Nematophagous fungus Arthrobotrys oligospora mimics olfactory cues of sex and food to lure its nematode prey. eLife 2017, 6, e20023. [Google Scholar] [CrossRef]
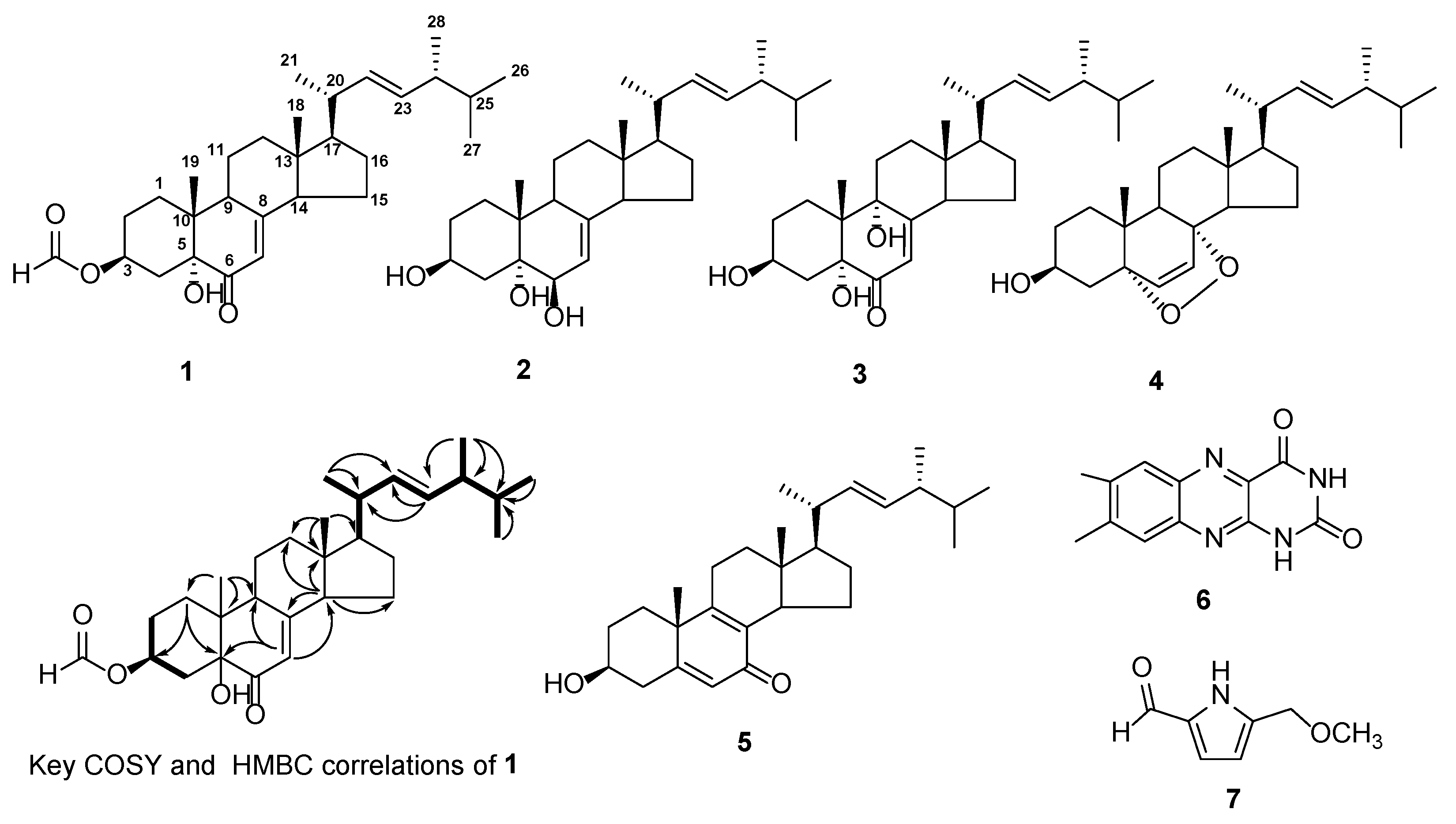
| Position | 1H | 13C | HMBC |
|---|---|---|---|
| 1 | 1.61 (1H, m) | 30.5, t | C-3, C-5 |
| 2.10 (1H, dt, J = 3.8, 9.7 Hz) | C-2, C-3, C-5, C-10 | ||
| 2 | 1.61 (1H, m) | 27.1, t | - |
| 2.00 (1H, m) | - | ||
| 3 | 5.76 (1H, m) | 71.4, d | - |
| 4 | 2.16 (1H, dd, J = 11.8, 13.1 Hz) | 33.5, t | C-3, C-5, C-10 |
| 2.74 (1H, dd, J = 5.0, 13.1 Hz) | C-3, C-5, C-10 | ||
| 5 | - | 77.0, s | - |
| 6 | - | 199.0, s | - |
| 7 | 5.90 (1H, s) | 120.2, d | C-5, C-9, C-14 |
| 8 | - | 164.2, s | - |
| 9 | 2.92 (1H, m) | 44.0, d | C-19, C-11, C-10, C-8, C-7 |
| 10 | - | 40.9, s | - |
| 11 | 1.46 (2H, m) | 21.9, t | - |
| 2.00 (1H, m) | - | ||
| 12 | 1.56 (1H, m) | 38.9, t | - |
| 2.00 (1H, m) | - | ||
| 13 | - | 44.5, s | - |
| 14 | 1.98 (1H, m) | 55.6, d | C-7, C-8, C-18, C-15, C-12, C-13 |
| 15 | 1.45 (2H, m) | 22.6, t | C-13, C-14 |
| 16 | 1.68 (1H, m) | 28.1, t | C-18, C-13, C-14, C-17 |
| 2.10 (1H, m) | C-18, C-13, C-14 | ||
| 17 | 1.21 (1H, m) | 55.9, d | C-16, C-13 |
| 18 | 0.57 (3H, s) | 12.6, q | C-12, C-13, C-14, C-17 |
| 19 | 1.02 (3H, s) | 16.0, q | C-1, C-10, C-9, C-5 |
| 20 | 2.00 (1H, m, overlap) | 40.5, d | - |
| 21 | 1.03 (3H, d, J = 6.6 Hz) | 21.2, q | C-17, C-20, C-22 |
| 22 | 5.14 (1H, dd, J = 8.4, 15.2 Hz) | 135.6, d | C-20, C-24, C-23 |
| 23 | 5.26 (1H, dd, J = 7.8, 15.2 Hz) | 132.3, d | C-20, C-24, C-22 |
| 24 | 1.87 (1H, m, overlap) | 43.0, d | - |
| 25 | 1.45 (1H, m, overlap) | 33.2, d | - |
| 26 | 0.95 (3H, d, J = 6.5 Hz) | 17.7, q | C-25, C-24, C-23 |
| 27 | 0.85 (3H, d, J = 6.6 Hz) | 19.7, q | C-24, C-25, C-28 |
| 28 | 0.86 (3H, d, J = 6.7 Hz) | 20.0, q | C-24, C-25, C-27 |
| 29 | 8.31 (1H, s) | 161.2, d | C-3 |
| Compounds | Hatching Rate % (Inhibition Rate %) | |||
|---|---|---|---|---|
| 24 h | 48 h | 72 h | 96 h | |
| Control | 13.77 b | 29.27 d | 37.81 d | 47.39 e |
| 1 | 12.79 b (9.8) | 27.09 d (7.4) | 37.28 d (1.40) | 47.30 e (0.19) |
| 2 | 11.98 b (13.0) | 28.35 d (3.1) | 36.95 d (2.27) | 46.58 e (1.71) |
| 3 | 11.92a b (13.44) | 24.28 c (17.05) | 37.21 d (1.59) | 47.24 e (0.32) |
| 4 | 12.36 b(10.2) | 29.05 d (0.75) | 37.19 d (1.64) | 47.21 e(0.38) |
| 5 | 10.38 ab (24.62) | 22.15 b (24.33) | 30.36 c (19.70) | 39.40 d (16.86) |
| 6 | 9.67 ab (29.77) | 13.07 ab (55.59) | 16.78 b (54.33) | 20.79 c (56.13) |
| 7 | 7.1 a (48.44) | 12.36 a (57.77) | 14.32 a (62.13) | 19.22 b (59.44) |
| Compounds | Adjusted Mortality (%) | ||||
|---|---|---|---|---|---|
| 12 h | 24 h | 48 h | 72 h | 96 h | |
| Control | (2.06 ± 0.22) bc | (2.07 ± 0.12) c | (2.67 ± 0.58) d | (4.40 ± 0.56) d | (5.73 ± 1.10) e |
| 1 | (0) c | (2.50 ± 1.30) b | (13.56 ± 1.90) b | (22.83 ± 1.82) b | (30.45 ± 2.05) c |
| 2 | (0) c | (2.38 ± 0.90) b | (15.47 ± 1.90) b | (21.76 ± 1.39) b | (29.06 ± 1.98) c |
| 3 | (1.87 ± 2.14) bc | (7.03 ± 0.49) b | (19.20 ± 2.21) b | (27.70 ± 0.82) b | (32.17 ± 2.32) c |
| 4 | (0) c | (2.63 ± 0.77) c | (8.73 ± 1.08) d | (17.60 ± 2.73) d | (22.06 ± 2.4) d |
| 5 | (0) c | (2.83 ± 0.76) c | (4.73 ± 1.00) d | (14.60 ± 4.33) c | (18.63 ± 2.01) d |
| 6 | (2.90 ± 0.79) bc | (3.67 ± 0.38) c | (12.40 ± 2.19) c | (16.47 ± 1.24) c | (31.20 ± 1.82) c |
| 7 | (23.20 ± 1.51) a | (29.30 ± 2.33) a | (41.60 ± 3.80) a | (68.87 ± 3.63) a | (98.23 ± 0.81) a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bao, Z.-X.; Liu, R.; Li, C.-Q.; Pan, X.-R.; Zhao, P.-J. Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita. Pathogens 2022, 11, 795. https://doi.org/10.3390/pathogens11070795
Bao Z-X, Liu R, Li C-Q, Pan X-R, Zhao P-J. Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita. Pathogens. 2022; 11(7):795. https://doi.org/10.3390/pathogens11070795
Chicago/Turabian StyleBao, Zheng-Xue, Rui Liu, Chun-Qiang Li, Xue-Rong Pan, and Pei-Ji Zhao. 2022. "Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita" Pathogens 11, no. 7: 795. https://doi.org/10.3390/pathogens11070795
APA StyleBao, Z.-X., Liu, R., Li, C.-Q., Pan, X.-R., & Zhao, P.-J. (2022). Pathogenicity and Metabolites of Purpureocillium lavendulum YMF1.00683 against Meloidogyne incognita. Pathogens, 11(7), 795. https://doi.org/10.3390/pathogens11070795






