Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection
Abstract
1. Introduction
2. Microbiota Associated with Asymptomatic Colonization and CDI
2.1. Composition of the Normal Human Gut Microbiota
2.2. Factors Influencing the Healthy Gut Microbiota
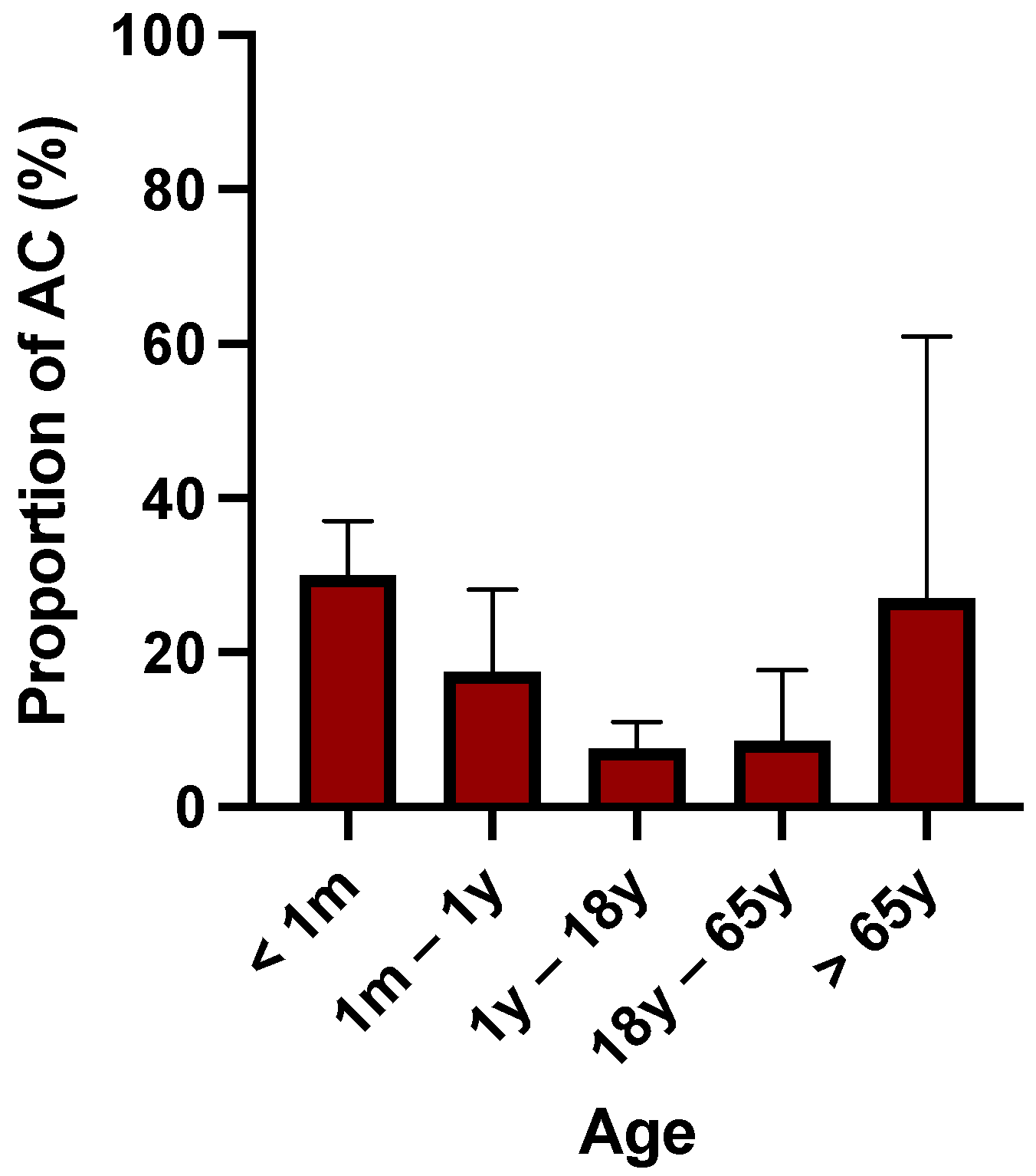
2.3. Composition of Microbiota among Patients with AC
2.4. Microbiota Composition of Adults Suffering from CDI
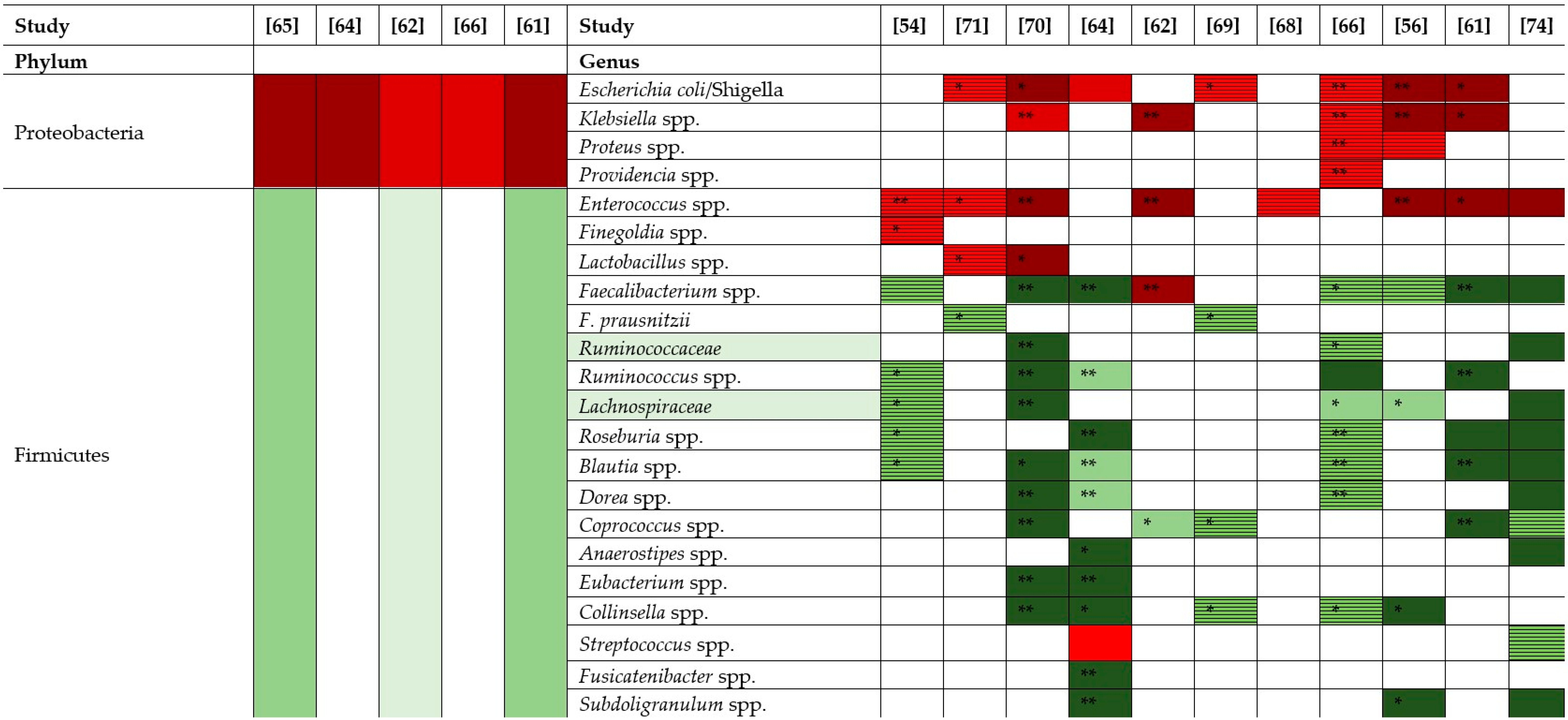
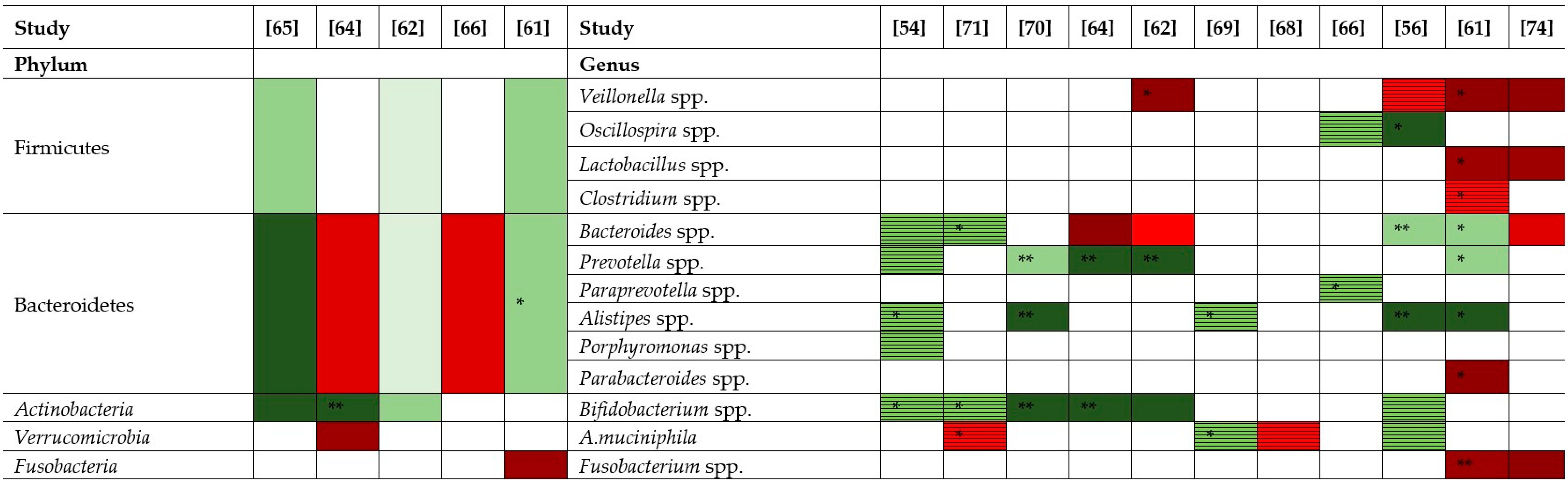
 ) represents an increase of +10 to 33 %, red (
) represents an increase of +10 to 33 %, red ( ) an increase of +34 to 66%, dark red (
) an increase of +34 to 66%, dark red ( ) an increase of +67 to 100%. Bright green (
) an increase of +67 to 100%. Bright green ( ) represents a decrease of −10 to −33%, green (
) represents a decrease of −10 to −33%, green ( ) a decrease of −34 to −66%, dark green (
) a decrease of −34 to −66%, dark green ( ) a decrease of −67 to −100%. Striped red (
) a decrease of −67 to −100%. Striped red ( ) represents an increase by unspecified value and striped green (
) represents an increase by unspecified value and striped green ( ) a decrease by unspecified value.
) a decrease by unspecified value.3. Fecal Microbiota Transplantation (FMT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Centers for Disease Control and Prevention (U.S.). Antibiotic Resistance Threats in the United States, 2019; Centers for Disease Control and Prevention: Atlanta, GA, USA, 2019.
- Colomb-Cotinat, M.; Assouvie, L.; Durand, J.; Daniau, C.; Leon, L.; Maugat, S.; Soing-Altrach, S.; Gateau, C.; Couturier, J.; Arnaud, I.; et al. Epidemiology of Clostridioides difficile infections, France, 2010 to 2017. Eurosurveillance 2019, 24, 1800638. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Healthcare-associated infections: Clostridium difficile infections. In Annual Epidemiological Report for 2016; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- Furuya-Kanamori, L.; Marquess, J.; Yakob, L.; Riley, T.V.; Paterson, D.L.; Foster, N.F.; Huber, C.A.; Clements, A.C.A. Asymptomatic Clostridium difficile colonization: Epidemiology and clinical implications. BMC Infect. Dis. 2015, 15, 516. [Google Scholar] [CrossRef] [PubMed]
- D’Silva, K.M.; Mehta, R.; Mitchell, M.; Lee, T.C.; Singhal, V.; Wilson, M.G.; McDonald, E.G. Proton pump inhibitor use and risk for recurrent Clostridioides difficile infection: A systematic review and meta-analysis. Clin. Microbiol. Infect. 2021, 27, 697–703. [Google Scholar] [CrossRef] [PubMed]
- Bavishi, C.; DuPont, H.L. Systematic Review: The use of proton pump inhibitors and increased susceptibility to enteric infection: Systematic review: Proton pump inhibitors and bacterial diarrhoea. Aliment. Pharmacol. Ther. 2011, 34, 1269–1281. [Google Scholar] [CrossRef] [PubMed]
- Jump, R.L.P.; Pultz, M.J.; Donskey, C.J. Vegetative Clostridium difficile survives in room air on moist surfaces and in gastric contents with reduced acidity: A potential mechanism to explain the association between proton pump inhibitors and C. difficile-associated diarrhea? Antimicrob. Agents Chemother. 2007, 51, 5. [Google Scholar] [CrossRef]
- Rao, A.; Jump, R.L.P.; Pultz, N.J.; Pultz, M.J.; Donskey, C.J. In vitro killing of nosocomial pathogens by acid and acidified nitrite. Antimicrob. Agents Chemother. 2006, 50, 3901–3904. [Google Scholar] [CrossRef]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-analysis of antibiotics and the risk of community-associated Clostridium difficile infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef]
- Nasiri, M.J.; Goudarzi, M.; Hajikhani, B.; Ghazi, M.; Goudarzi, H.; Pouriran, R. Clostridioides (Clostridium) difficile infection in hospitalized patients with antibiotic-associated diarrhea: A systematic review and meta-analysis. Anaerobe 2018, 50, 32–37. [Google Scholar] [CrossRef]
- Webb, B.J.; Subramanian, A.; Lopansri, B.; Goodman, B.; Jones, P.B.; Ferraro, J.; Stenehjem, E.; Brown, S.M. Antibiotic exposure and risk for hospital-associated Clostridioides difficile infection. Antimicrob. Agents Chemother. 2020, 64, e02169-19. [Google Scholar] [CrossRef]
- Zomer, T.P.; Van Duijkeren, E.; Wielders, C.C.H.; Veenman, C.; Hengeveld, P.; Van Der Hoek, W.; De Greeff, S.C.; Smit, L.A.M.; Heederik, D.J.; Yzermans, C.J.; et al. Prevalence and risk factors for colonization of Clostridium difficile among adults living near livestock farms in the netherlands. Epidemiol. Infect. 2017, 145, 2745–2749. [Google Scholar] [CrossRef]
- The ANTICIPATE Study Group; van Werkhoven, C.H.; Ducher, A.; Berkell, M.; Mysara, M.; Lammens, C.; Torre-Cisneros, J.; Rodríguez-Baño, J.; Herghea, D.; Cornely, O.A.; et al. Incidence and predictive biomarkers of Clostridioides difficile infection in hospitalized patients receiving broad-spectrum antibiotics. Nat. Commun. 2021, 12, 2240. [Google Scholar] [CrossRef] [PubMed]
- Anjewierden, S.; Han, Z.; Brown, A.M.; Donskey, C.J.; Deshpande, A. Risk factors for Clostridioides difficile colonization among hospitalized adults: A meta-analysis and systematic review. Infect. Contr. Hosp. Epidemiol. 2021, 42, 565–572. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Cai, H.; Sussman, D.A.; Donet, J.; Dholaria, K.; Yang, J.; Panara, A.; Croteau, R.; Barkin, J.S. Association between immunosuppressive therapy and outcome of Clostridioides difficile infection: Systematic review and meta-analysis. Dig. Dis. Sci. 2021. [Google Scholar] [CrossRef] [PubMed]
- Avni, T.; Babitch, T.; Ben-Zvi, H.; Hijazi, R.; Ayada, G.; Atamna, A.; Bishara, J. Clostridioides difficile infection in immunocompromised hospitalized patients is associated with a high recurrence rate. Int. J. Infect. Dis. 2020, 90, 237–242. [Google Scholar] [CrossRef]
- Rea, M.C.; O’Sullivan, O.; Shanahan, F.; O’Toole, P.W.; Stanton, C.; Ross, R.P.; Hill, C. Clostridium difficile carriage in elderly subjects and associated changes in the intestinal microbiota. J. Clin. Microbiol. 2012, 50, 867–875. [Google Scholar] [CrossRef]
- Dumic, I.; Nordin, T.; Jecmenica, M.; Stojkovic Lalosevic, M.; Milosavljevic, T.; Milovanovic, T. Gastrointestinal Tract Disorders in Older Age. Can. J. Gastroenterol. Hepatol. 2019, 2019, 6757524. [Google Scholar] [CrossRef]
- Lucado, J.; Gould, C.; Elixhauser, A. Clostridium difficile Infections (CDI) in Hospital Stays, 2009; HCUP Statistical Brief #124; Agency for Healthcare Research and Quality: Rockville, MD, USA, 2012. Available online: http://www.hcup-us.ahrq.gov/reports/statbriefs/sb124.pdf (accessed on 23 June 2022).
- Crobach, M.J.T.; Vernon, J.J.; Loo, V.G.; Kong, L.Y.; Péchiné, S.; Wilcox, M.H.; Kuijper, E.J. Understanding Clostridium difficile colonization. Clin. Microbiol. Rev. 2018, 31, e00021-17. [Google Scholar] [CrossRef]
- Nagy, E. What do we know about the diagnostics, treatment and epidemiology of Clostridioides (Clostridium) difficile infection in Europe? J. Infect. Chemother. 2018, 24, 164–170. [Google Scholar] [CrossRef]
- Galdys, A.L.; Nelson, J.S.; Shutt, K.A.; Schlackman, J.L.; Pakstis, D.L.; Pasculle, A.W.; Marsh, J.W.; Harrison, L.H.; Curry, S.R. Prevalence and duration of asymptomatic Clostridium difficile carriage among healthy subjects in Pittsburgh, pennsylvania. J Clin. Microbiol. 2014, 52, 2406–2409. [Google Scholar] [CrossRef]
- Rousseau, C.; Levenez, F.; Fouqueray, C.; Doré, J.; Collignon, A.; Lepage, P. Clostridium difficile colonization in early infancy is accompanied by changes in intestinal microbiota composition. J. Clin. Microbiol. 2011, 49, 858–865. [Google Scholar] [CrossRef]
- Lees, E.A.; Miyajima, F.; Pirmohamed, M.; Carrol, E.D. The role of Clostridium difficile in the paediatric and neonatal gut—A narrative review. Eur. J. Clin. Microbiol. Infect. Dis. 2016, 35, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Miyajima, F.; Roberts, P.; Swale, A.; Price, V.; Jones, M.; Horan, M.; Beeching, N.; Brazier, J.; Parry, C.; Pendleton, N.; et al. Characterisation and carriage ratio of Clostridium difficile strains isolated from a community-dwelling elderly population in the United Kingdom. PLoS ONE 2011, 6, e22804. [Google Scholar] [CrossRef] [PubMed]
- Jangi, S.; Lamont, J.T. Asymptomatic colonization by Clostridium difficile in infants: Implications for disease in later life. J. Pediatr. Gastroenterol. Nutr. 2010, 51, 2–7. [Google Scholar] [CrossRef] [PubMed]
- Penders, J.; Thijs, C.; Vink, C.; Stelma, F.F.; Snijders, B.; Kummeling, I.; van den Brandt, P.A.; Stobberingh, E.E. Factors influencing the composition of the intestinal microbiota in early infancy. Pediatrics 2006, 118, 511–521. [Google Scholar] [CrossRef]
- Leffler, D.A. Clostridium difficile infection. N. Engl. J. Med. 2015, 372, 1539–1548. [Google Scholar] [CrossRef]
- Pepin, J. Mortality attributable to nosocomial Clostridium difficile-associated disease during an epidemic caused by a hypervirulent strain in Quebec. Can. Med. Assoc. J. 2005, 173, 1037–1042. [Google Scholar] [CrossRef]
- Péchiné, S.; Collignon, A. Immune responses induced by Clostridium difficile. Anaerobe 2016, 41, 68–78. [Google Scholar] [CrossRef]
- Engevik, M.A.; Yacyshyn, M.B.; Engevik, K.A.; Wang, J.; Darien, B.; Hassett, D.J.; Yacyshyn, B.R.; Worrell, R.T. Human Clostridium Difficile infection: Altered mucus production and composition. Am. J. Physiol.-Gastrointest. Liver Physiol. 2015, 308, G510–G524. [Google Scholar] [CrossRef]
- Francis, M.B.; Allen, C.A.; Shrestha, R.; Sorg, J.A. Bile acid recognition by the Clostridium difficile germinant receptor, CspC, is important for establishing infection. PLoS Pathog. 2013, 9, e1003356. [Google Scholar] [CrossRef]
- Wilson, K.H.; Silva, J.; Fekety, F.R. Suppression of Clostridium difficile by Normal Hamster Cecal Flora and Prevention of Antibiotic-Associated Cecitis. Infect. Immun. 1981, 34, 626–628. [Google Scholar] [CrossRef]
- Babcock, G.J.; Broering, T.J.; Hernandez, H.J.; Mandell, R.B.; Donahue, K.; Boatright, N.; Stack, A.M.; Lowy, I.; Graziano, R.; Molrine, D.; et al. Human monoclonal antibodies directed against toxins a and b prevent Clostridium difficile-induced mortality in hamsters. Infect. Immun. 2006, 74, 6339–6347. [Google Scholar] [CrossRef] [PubMed]
- Young, V.B. Old and new models for studying host-microbe interactions in health and disease: C. difficile as an example. Am. J. Physiol.-Gastrointest. Liver Physiol. 2017, 312, G623–G627. [Google Scholar] [CrossRef] [PubMed]
- Steele, J.; Feng, H.; Parry, N.; Tzipori, S. Piglet models of acute or chronic Clostridium difficile Illness. J. Infect. Dis. 2010, 201, 428–434. [Google Scholar] [CrossRef] [PubMed]
- Pultz, N.J.; Donskey, C.J. Effect of antibiotic treatment on growth of and toxin production by Clostridium difficile in the cecal contents of mice. Antimicrob. Agents Chemother. 2005, 49, 3529–3532. [Google Scholar] [CrossRef]
- Yamamoto-Osaki, T.; Kamiya, S.; Sawamura, S.; Kai, M.; Ozawa, A. growth inhibition of Clostridium difficile by intestinal flora of infant faeces in continuous flow culture. J. Med. Microbiol. 1994, 40, 179–187. [Google Scholar] [CrossRef]
- Hopkins, M.J.; Macfarlane, G.T. Changes in predominant bacterial populations in human faeces with age and with Clostridium difficile infection. J. Med. Microbiol. 2002, 51, 448–454. [Google Scholar] [CrossRef] [PubMed]
- Macfarlane, G.T.; Macfarlane, S.; Gibson, G.R. Validation of a three-stage compound continuous culture system for investigating the effect of retention time on the ecology and metabolism of bacteria in the human colon. Microb. Ecol. 1998, 35, 180–187. [Google Scholar] [CrossRef]
- Best, E.L.; Freeman, J.; Wilcox, M.H. Models for the study of Clostridium difficile infection. Gut Microbes 2012, 3, 145–167. [Google Scholar] [CrossRef]
- Van Nuenen, M.H.M.C.; Diederick Meyer, P.; Venema, K. The effect of various inulins and Clostridium difficile on the metabolic activity of the human colonic microbiota in vitro. Microb. Ecol. Health Dis. 2003, 15, 137–144. [Google Scholar] [CrossRef]
- Cheng, M.; Ning, K. Stereotypes about enterotype: The old and new ideas. Genom. Proteom. Bioinform. 2019, 17, 4–12. [Google Scholar] [CrossRef]
- Rinninella, E.; Raoul, P.; Cintoni, M.; Franceschi, F.; Miggiano, G.; Gasbarrini, A.; Mele, M. What is the healthy gut microbiota composition? A changing ecosystem across age, environment, diet, and diseases. Microorganisms 2019, 7, 14. [Google Scholar] [CrossRef] [PubMed]
- MetaHIT Consortium; Arumugam, M.; Raes, J.; Pelletier, E.; Le Paslier, D.; Yamada, T.; Mende, D.R.; Fernandes, G.R.; Tap, J.; Bruls, T.; et al. Enterotypes of the human gut microbiome. Nature 2011, 473, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Costea, P.I.; Hildebrand, F.; Arumugam, M.; Bäckhed, F.; Blaser, M.J.; Bushman, F.D.; de Vos, W.M.; Ehrlich, S.D.; Fraser, C.M.; Hattori, M.; et al. Enterotypes in the landscape of gut microbial community composition. Nat. Microbiol. 2018, 3, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Gorvitovskaia, A.; Holmes, S.P.; Huse, S.M. Interpreting Prevotella and Bacteroides as biomarkers of diet and lifestyle. Microbiome 2016, 4, 15. [Google Scholar] [CrossRef] [PubMed]
- Seekatz, A.M.; Young, V.B. Clostridium difficile and the microbiota. J. Clin. Investig. 2014, 124, 4182–4189. [Google Scholar] [CrossRef] [PubMed]
- Ross, C.L.; Spinler, J.K.; Savidge, T.C. Structural and functional changes within the gut microbiota and susceptibility to Clostridium difficile infection. Anaerobe 2016, 41, 37–43. [Google Scholar] [CrossRef]
- Jackson, M.A.; Goodrich, J.K.; Maxan, M.-E.; Freedberg, D.E.; Abrams, J.A.; Poole, A.C.; Sutter, J.L.; Welter, D.; Ley, R.E.; Bell, J.T.; et al. Proton pump inhibitors alter the composition of the gut microbiota. Gut 2016, 65, 749–756. [Google Scholar] [CrossRef]
- Imhann, F.; Bonder, M.J.; Vich Vila, A.; Fu, J.; Mujagic, Z.; Vork, L.; Tigchelaar, E.F.; Jankipersadsing, S.A.; Cenit, M.C.; Harmsen, H.J.M.; et al. Proton pump inhibitors affect the gut microbiome. Gut 2016, 65, 740–748. [Google Scholar] [CrossRef]
- Freedberg, D.E.; Toussaint, N.C.; Chen, S.P.; Ratner, A.J.; Whittier, S.; Wang, T.C.; Wang, H.H.; Abrams, J.A. Proton pump inhibitors alter specific taxa in the human gastrointestinal microbiome: A crossover trial. Gastroenterology 2015, 149, 883–885.e9. [Google Scholar] [CrossRef]
- Vich Vila, A.; Collij, V.; Sanna, S.; Sinha, T.; Imhann, F.; Bourgonje, A.R.; Mujagic, Z.; Jonkers, D.M.A.E.; Masclee, A.A.M.; Fu, J.; et al. Impact of commonly used drugs on the composition and metabolic function of the gut microbiota. Nat. Commun. 2020, 11, 362. [Google Scholar] [CrossRef]
- The ANTICIPATE Study Group; Berkell, M.; Mysara, M.; Xavier, B.B.; van Werkhoven, C.H.; Monsieurs, P.; Lammens, C.; Ducher, A.; Vehreschild, M.J.G.T.; Goossens, H.; et al. Microbiota-based markers predictive of development of Clostridioides difficile infection. Nat. Commun. 2021, 12, 2241. [Google Scholar] [CrossRef]
- Vakili, B.; Fateh, A.; Asadzadeh Aghdaei, H.; Sotoodehnejadnematalahi, F.; Siadat, S.D. Intestinal microbiota in elderly inpatients with Clostridioides difficile infection. IDR 2020, 13, 2723–2731. [Google Scholar] [CrossRef] [PubMed]
- Milani, C.; Ticinesi, A.; Gerritsen, J.; Nouvenne, A.; Lugli, G.A.; Mancabelli, L.; Turroni, F.; Duranti, S.; Mangifesta, M.; Viappiani, A.; et al. Gut Microbiota composition and Clostridium difficile infection in hospitalized elderly individuals: A metagenomic study. Sci. Rep. 2016, 6, 25945. [Google Scholar] [CrossRef] [PubMed]
- Winter, S.E.; Lopez, C.A.; Bäumler, A.J. The dynamics of gut-associated microbial communities during inflammation. EMBO Rep. 2013, 14, 319–327. [Google Scholar] [CrossRef] [PubMed]
- Lupp, C.; Robertson, M.L.; Wickham, M.E.; Sekirov, I.; Champion, O.L.; Gaynor, E.C.; Finlay, B.B. Host-mediated inflammation disrupts the intestinal microbiota and promotes the overgrowth of Enterobacteriaceae. Cell Host Microbe 2007, 2, 119–129. [Google Scholar] [CrossRef]
- Mahnic, A.; Breskvar, M.; Dzeroski, S.; Skok, P.; Pintar, S.; Rupnik, M. Distinct types of gut microbiota dysbiosis in hospitalized gastroenterological patients are disease non-related and characterized with the predominance of either Enterobacteriaceae or Enterococcus. Front. Microbiol. 2020, 11, 120. [Google Scholar] [CrossRef]
- Pascal, V.; Pozuelo, M.; Borruel, N.; Casellas, F.; Campos, D.; Santiago, A.; Martinez, X.; Varela, E.; Sarrabayrouse, G.; Machiels, K.; et al. A Microbial signature for crohn’s disease. Gut 2017, 66, 813–822. [Google Scholar] [CrossRef]
- Zhang, L.; Dong, D.; Jiang, C.; Li, Z.; Wang, X.; Peng, Y. Insight into alteration of gut microbiota in clostridium difficile infection and asymptomatic C. difficile colonization. Anaerobe 2015, 34, 1–7. [Google Scholar] [CrossRef]
- Han, S.-H.; Yi, J.; Kim, J.-H.; Lee, S.; Moon, H.-W. Composition of gut microbiota in patients with toxigenic Clostridioides (Clostridium) difficile: Comparison between subgroups according to clinical criteria and toxin gene load. PLoS ONE 2019, 14, e0212626. [Google Scholar] [CrossRef]
- Rodriguez, C.; Taminiau, B.; Korsak, N.; Avesani, V.; Van Broeck, J.; Brach, P.; Delmée, M.; Daube, G. Longitudinal survey of Clostridium difficile presence and gut microbiota composition in a belgian nursing home. BMC Microbiol. 2016, 16, 229. [Google Scholar] [CrossRef]
- Jeon, Y.D.; Ann, H.W.; Lee, W.J.; Kim, J.H.; Seong, H.; Kim, J.H.; Ahn, J.Y.; Jeong, S.J.; Ku, N.S.; Yeom, J.S.; et al. Characteristics of faecal microbiota in korean patients with Clostridioides difficile-associated diarrhea. Infect. Chemother. 2019, 51, 365–375. [Google Scholar] [CrossRef] [PubMed]
- Amrane, S.; Hocquart, M.; Afouda, P.; Kuete, E.; Pham, T.-P.-T.; Dione, N.; Ngom, I.I.; Valles, C.; Bachar, D.; Raoult, D.; et al. Metagenomic and culturomic analysis of gut microbiota dysbiosis during Clostridium difficile infection. Sci. Rep. 2019, 9, 12807. [Google Scholar] [CrossRef]
- Gu, S.; Chen, Y.; Zhang, X.; Lu, H.; Lv, T.; Shen, P.; Lv, L.; Zheng, B.; Jiang, X.; Li, L. Identification of key taxa that favor intestinal colonization of Clostridium difficile in an adult chinese population. Microbes Infect. 2016, 18, 30–38. [Google Scholar] [CrossRef]
- Hernandez, D.R. Gut check: In vitro diagnostics for gut microbiome analysis. Clin. Microbiol. Newsl. 2019, 41, 57–62. [Google Scholar] [CrossRef]
- Sangster, W.; Hegarty, J.P.; Schieffer, K.M.; Wright, J.R.; Hackman, J.; Toole, D.R.; Lamendella, R.; Stewart, D.B. Bacterial and fungal microbiota changes distinguish C. difficile infection from other forms of diarrhea: Results of a prospective inpatient study. Front. Microbiol. 2016, 7, 789. [Google Scholar] [CrossRef] [PubMed]
- Stewart, D.B.; Wright, J.R.; Fowler, M.; McLimans, C.J.; Tokarev, V.; Amaniera, I.; Baker, O.; Wong, H.-T.; Brabec, J.; Drucker, R.; et al. Integrated meta-omics reveals a fungus-associated bacteriome and distinct functional pathways in Clostridioides difficile infection. mSphere 2019, 4, e00454-19. [Google Scholar] [CrossRef]
- Kim, J.; Cho, Y.; Seo, M.-R.; Bae, M.H.; Kim, B.; Rho, M.; Pai, H. Quantitative characterization of Clostridioides difficile population in the gut microbiome of patients with C. difficile infection and their association with clinical factors. Sci. Rep. 2020, 10, 17608. [Google Scholar] [CrossRef]
- Vakili, B.; Fateh, A.; Asadzadeh Aghdaei, H.; Sotoodehnejadnematalahi, F.; Siadat, S.D. Characterization of gut microbiota in hospitalized patients with Clostridioides difficile infection. Curr. Microbiol. 2020, 77, 1673–1680. [Google Scholar] [CrossRef]
- Lamendella, R.; Wright, J.R.; Hackman, J.; McLimans, C.; Toole, D.R.; Bernard Rubio, W.; Drucker, R.; Wong, H.T.; Sabey, K.; Hegarty, J.P.; et al. Antibiotic treatments for Clostridium difficile infection are associated with distinct bacterial and fungal community structures. mSphere 2018, 3, e00572-17. [Google Scholar] [CrossRef]
- Rojo, D.; Gosalbes, M.J.; Ferrari, R.; Pérez-Cobas, A.E.; Hernández, E.; Oltra, R.; Buesa, J.; Latorre, A.; Barbas, C.; Ferrer, M.; et al. Clostridium difficile heterogeneously impacts intestinal community architecture but drives stable metabolome responses. ISME J. 2015, 9, 2206–2220. [Google Scholar] [CrossRef]
- Antharam, V.C.; Li, E.C.; Ishmael, A.; Sharma, A.; Mai, V.; Rand, K.H.; Wang, G.P. Intestinal dysbiosis and depletion of butyrogenic bacteria in Clostridium difficile infection and nosocomial diarrhea. J. Clin. Microbiol. 2013, 51, 2884–2892. [Google Scholar] [CrossRef] [PubMed]
- Theriot, C.M.; Koenigsknecht, M.J.; Carlson, P.E.; Hatton, G.E.; Nelson, A.M.; Li, B.; Huffnagle, G.B.; Li, J.Z.; Young, V.B. Antibiotic-induced shifts in the mouse gut microbiome and metabolome increase susceptibility to Clostridium difficile infection. Nat. Commun. 2014, 5, 3114. [Google Scholar] [CrossRef] [PubMed]
- Buffie, C.G.; Bucci, V.; Stein, R.R.; McKenney, P.T.; Ling, L.; Gobourne, A.; No, D.; Liu, H.; Kinnebrew, M.; Viale, A.; et al. Precision microbiome reconstitution restores bile acid mediated resistance to Clostridium difficile. Nature 2015, 517, 205–208. [Google Scholar] [CrossRef] [PubMed]
- Araos, R.; Andreatos, N.; Ugalde, J.; Mitchell, S.; Mylonakis, E.; D’Agata, E.M.C. Fecal microbiome among nursing home residents with advanced dementia and Clostridium difficile. Dig. Dis. Sci. 2018, 63, 1525–1531. [Google Scholar] [CrossRef]
- Schubert, A.M.; Rogers, M.A.M.; Ring, C.; Mogle, J.; Petrosino, J.P.; Young, V.B.; Aronoff, D.M.; Schloss, P.D. Microbiome data distinguish patients with Clostridium difficile infection and non-C. difficile-associated diarrhea from healthy controls. mBio 2014, 5, e01021-14. [Google Scholar] [CrossRef] [PubMed]
- Hernández, M.; de Frutos, M.; Rodríguez-Lázaro, D.; López-Urrutia, L.; Quijada, N.M.; Eiros, J.M. Fecal microbiota of toxigenic Clostridioides difficile-associated diarrhea. Front. Microbiol. 2019, 9, 3331. [Google Scholar] [CrossRef]
- Pakpour, S.; Bhanvadia, A.; Zhu, R.; Amarnani, A.; Gibbons, S.M.; Gurry, T.; Alm, E.J.; Martello, L.A. Identifying predictive features of Clostridium difficile infection recurrence before, during, and after primary antibiotic treatment. Microbiome 2017, 5, 148. [Google Scholar] [CrossRef]
- Khanna, S.; Montassier, E.; Schmidt, B.; Patel, R.; Knights, D.; Pardi, D.S.; Kashyap, P.C. Gut microbiome predictors of treatment response and recurrence in primary Clostridium difficile infection. Aliment. Pharmacol. Ther. 2016, 44, 715–727. [Google Scholar] [CrossRef]
- Bakker, G.J.; Nieuwdorp, M. Fecal microbiota transplantation: Therapeutic potential for a multitude of diseases beyond Clostridium difficile. Microbiol. Spectr. 2017, 5. [Google Scholar] [CrossRef]
- Wortelboer, K.; Nieuwdorp, M.; Herrema, H. Fecal microbiota transplantation beyond Clostridioides difficile infections. EBioMedicine 2019, 44, 716–729. [Google Scholar] [CrossRef]
- Kim, K.O.; Gluck, M. Fecal microbiota transplantation: An update on clinical practice. Clin. Endosc. 2019, 52, 137–143. [Google Scholar] [CrossRef] [PubMed]
- Owens, C.; Broussard, E.; Surawicz, C. Fecal microbiota transplantation and donor standardization. Trends Microbiol. 2013, 21, 443–445. [Google Scholar] [CrossRef] [PubMed]
- Juul, F.E.; Garborg, K.; Bretthauer, M.; Skudal, H.; Øines, M.N.; Wiig, H.; Rose, Ø.; Seip, B.; Lamont, J.T.; Midtvedt, T.; et al. Fecal microbiota transplantation for primary Clostridium difficile infection. N. Engl. J. Med. 2018, 378, 2535–2536. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Khoruts, A.; Staley, C.; Sadowsky, M.J.; Abd, M.; Alani, M.; Bakow, B.; Curran, P.; McKenney, J.; Tisch, A.; et al. Effect of fecal microbiota transplantation on recurrence in multiply recurrent Clostridium difficile infection: A randomized trial. Ann. Intern. Med. 2016, 165, 609. [Google Scholar] [CrossRef]
- Staley, C.; Kaiser, T.; Vaughn, B.P.; Graiziger, C.T.; Hamilton, M.J.; Rehman, T.U.; Song, K.; Khoruts, A.; Sadowsky, M.J. Predicting recurrence of Clostridium difficile infection following encapsulated fecal microbiota transplantation. Microbiome 2018, 6, 166. [Google Scholar] [CrossRef]
- Haifer, C.; Paramsothy, S.; Borody, T.J.; Clancy, A.; Leong, R.W.; Kaakoush, N.O. Long-term bacterial and fungal dynamics following oral lyophilized fecal microbiota transplantation in Clostridioides difficile infection. mSystems 2021, 6, e00905-20. [Google Scholar] [CrossRef]
- Girotra, M.; Garg, S.; Anand, R.; Song, Y.; Dutta, S.K. Fecal microbiota transplantation for recurrent Clostridium difficile infection in the elderly: Long-term outcomes and microbiota changes. Dig. Dis. Sci. 2016, 61, 3007–3015. [Google Scholar] [CrossRef]
- Song, Y.; Garg, S.; Girotra, M.; Maddox, C.; von Rosenvinge, E.C.; Dutta, A.; Dutta, S.; Fricke, W.F. Microbiota dynamics in patients treated with fecal microbiota transplantation for recurrent Clostridium difficile infection. PLoS ONE 2013, 8, e81330. [Google Scholar] [CrossRef]
- Friedman-Korn, T.; Livovsky, D.M.; Maharshak, N.; Aviv Cohen, N.; Paz, K.; Bar-Gil Shitrit, A.; Goldin, E.; Koslowsky, B. Fecal transplantation for treatment of Clostridium difficile infection in elderly and debilitated patients. Dig. Dis. Sci. 2018, 63, 198–203. [Google Scholar] [CrossRef]
- Nicholson, M.R.; Mitchell, P.D.; Alexander, E.; Ballal, S.; Bartlett, M.; Becker, P.; Davidovics, Z.; Docktor, M.; Dole, M.; Felix, G.; et al. Efficacy of fecal microbiota transplantation for Clostridium difficile infection in children. Clin. Gastroenterol. Hepatol. 2020, 18, 612–619.e1. [Google Scholar] [CrossRef]
- Shen, N.T.; Maw, A.; Tmanova, L.L.; Pino, A.; Ancy, K.; Crawford, C.V.; Simon, M.S.; Evans, A.T. Timely use of probiotics in hospitalized adults prevents Clostridium difficile infection: A systematic review with meta-regression analysis. Gastroenterology 2017, 152, 1889–1900.e9. [Google Scholar] [CrossRef] [PubMed]
- Seto, C.T.; Jeraldo, P.; Orenstein, R.; Chia, N.; DiBaise, J.K. Prolonged use of a proton pump inhibitor reduces microbial diversity: Implications for Clostridium difficile susceptibility. Microbiome 2014, 2, 42. [Google Scholar] [CrossRef] [PubMed]
- Björkqvist, O.; Rangel, I.; Serrander, L.; Magnusson, C.; Halfvarson, J.; Norén, T.; Bergman-Jungeström, M. Faecalibacterium prausnitzii increases following fecal microbiota transplantation in recurrent Clostridioides difficile infection. PLoS ONE 2021, 16, e0249861. [Google Scholar] [CrossRef] [PubMed]
- Amrane, S.; Bachar, D.; Lagier, J.C.; Raoult, D. Clostridium scindens is present in the gut microbiota during Clostridium difficile infection: A metagenomic and culturomic analysis. J. Clin. Microbiol 2018, 56, e01663-17. [Google Scholar] [CrossRef]
- Parker, B.J.; Wearsch, P.A.; Veloo, A.C.M.; Rodriguez-Palacios, A. The genus Alistipes: Gut bacteria with emerging implications to inflammation, cancer, and mental health. Front. Immunol. 2020, 11, 906. [Google Scholar] [CrossRef] [PubMed]
- Cani, P.D.; de Vos, W.M. Next-generation beneficial microbes: The case of Akkermansia muciniphila. Front. Microbiol. 2017, 8, 1765. [Google Scholar] [CrossRef] [PubMed]
- Roshanravan, N.; Bastani, S.; Tutunchi, H.; Kafil, B.; Nikpayam, O.; Mesri Alamdari, N.; Hadi, A.; Sotoudeh, S.; Ghaffari, S.; Ostadrahimi, A. A Comprehensive systematic review of the effectiveness of Akkermansia muciniphila, a member of the gut microbiome, for the management of obesity and associated metabolic disorders. Arch. Physiol. Biochem. 2021. [Google Scholar] [CrossRef]
- Engevik, M.A. Mucin-degrading microbes release monosaccharides that chemoattract Clostridioides difficile and facilitate colonization of the human intestinal mucus layer. ACS Infect. Dis. 2021, 7, 1126–1142. [Google Scholar] [CrossRef]
- Krawczyk, B.; Wityk, P.; Gałęcka, M.; Michalik, M. The Many Faces of Enterococcus Spp.—Commensal, probiotic and opportunistic pathogen. Microorganisms 2021, 9, 1900. [Google Scholar] [CrossRef]
- Franz, C.M.A.P.; Huch, M.; Abriouel, H.; Holzapfel, W.; Gálvez, A. Enterococci as probiotics and their implications in food safety. Int. J. Food Microbiol. 2011, 151, 125–140. [Google Scholar] [CrossRef]
- García-Díez, J.; Saraiva, C. Use of starter cultures in foods from animal origin to improve their safety. IJERPH 2021, 18, 2544. [Google Scholar] [CrossRef] [PubMed]
- Gaca, A.O.; Lemos, J.A. Adaptation to adversity: The intermingling of stress tolerance and pathogenesis in Enterococci. Microbiol. Mol. Biol. Rev. 2019, 83, e00008-19. [Google Scholar] [CrossRef] [PubMed]
- Romyasamit, C.; Thatrimontrichai, A.; Aroonkesorn, A.; Chanket, W.; Ingviya, N.; Saengsuwan, P.; Singkhamanan, K. Enterococcus faecalis isolated from infant feces inhibits toxigenic Clostridioides (Clostridium) difficile. Front. Pediatr. 2020, 8, 572633. [Google Scholar] [CrossRef] [PubMed]
- Ticer, T.; Engevik, M. Klebsiella pneumoniae in the colonic mucus layer influences Clostridioides difficile pathogenesis. Am. J. Pathol. 2021, 192. [Google Scholar] [CrossRef]
- Kelly, C.P.; Kyne, L. The host immune response to Clostridium difficile. J. Med. Microbiol. 2011, 60, 1070–1079. [Google Scholar] [CrossRef]
- Kociolek, L.K.; Espinosa, R.O.; Gerding, D.N.; Hauser, A.R.; Ozer, E.A.; Budz, M.; Balaji, A.; Chen, X.; Tanz, R.R.; Yalcinkaya, N.; et al. Natural Clostridioides difficile toxin immunization in colonized infants. Clin. Infect. Dis. 2020, 70, 2095–2102. [Google Scholar] [CrossRef]
- Kyne, L.; Warny, M.; Qamar, A.; Kelly, C.P. Asymptomatic carriage of Clostridium difficile and serum levels of IgG antibody against toxin A. N. Engl. J. Med. 2000, 342, 390–397. [Google Scholar] [CrossRef]
| Analysis Method | Study | Study Group | Diversity | Increase in CDI Group Compared with Control Group | Decrease in CDI Group Compared with Control Group |
|---|---|---|---|---|---|
| 16S rRNA gene amplicon analysis a Illumina | [54] | CDI group (n = 15) Control group (n = 669) Mean age: 70 y Location: Europa | The alpha diversity (Chao1 and Shannon index) was lower in the CDI group. * | Enterococcus spp. * | Bifidobacterium spp., Blautia spp. Faecalibacterium spp., Bacteroides spp. and Prevotella spp. |
| qPCR | [71] | CDI group (n = 28; 79 y) Control group (n = 56; 75 y) Country: Iran | NR | A. muciniphila *, Lactobacillus spp. * and Escherichia coli * | Bacteroides spp. *, Bifidobacterium spp.* and F. prausnitzii * |
| 16S rRNA gene amplicon analysis a Illumina HiSeq | [70] | CDI group (n = 26; 66.5 y) Control group (n = 61) | The alpha diversity (Shannon index) was lower in the CDI group. * | Enterococcus *, Lactobacillus *, Escherichia * and Klebsiella *. | Bifidobacterium *, Ruminococcus *, Eubacterium *, Faecalibacterium spp.*, Prevotella *, Blautia *, Collinsella *, Dorea *, Alistipes *, Lachnospiraceae * and Coprococcus * (p < 0.05) |
| 16S rRNA gene amplicon analysis a MiSeq Illumina | [65] | CDI group (n = 11; 70.81 ± 20.1 y) Control group (n = 8; 18 to 45 y) Country: France | The alpha diversity (Shannon index) was lower in the CDI group. * | Proteobacteria *ns | Firmicute *ns Actinobacteria *ns Bacteroidetes * |
| 454 pyrosequencing analysis of bacteria e 454 GS FLX Titanium Sequencing System (Roche) | [64] | CDI group (n = 24, 64.8 ± 15.7 y) Control group (n = 13, 49.2 ± 11.5 y). Country: Korea | The richness (Chao1 index; 283.3 vs. 642.9) and the alpha diversity (Shannon index; 3.6 vs. 4.5) were lower in the CDI group. * | Proteobacteria *ns Bacteroidetes *ns Verrucomicrobia Bacteroides, Streptococcus spp., and Escherichia | Actinobacteria * Blautia, Bifidobacterium, Faecalibacterium, Collinsela, Dorea, Eubacterium, Fusicatenibacter, Prevotella, Roseburia, Subdoligranum, Ruminococcus, Clostridium, Catenibacterium, Dialister and Anaerostipes |
| 16S rRNA gene amplicon analysis Metatranscriptomic Illumina Hiseq4000 platform | [69] | CDI group (n = 18, 65,3 ± 17 y) Control group (n = 31, 60 ± 18 y) Country: United States | A lower average species evenness was observed in the CDI group with Heip’s evenness (93.4 ± 23.1 in the CDI group vs. 121.8 ± 58.2 in the control group). *ns | Metagenetic Increases in the proportions of Clostridiaceae, Peptostreptoccocaceae, and Enterococcus Metatranscriptomic Increases in the proportions of Clostridioides difficile, E. coli, unclassified Peptostreptococcaceae, and Enterobacteriaceae | Metagenetic Decreases in the proportions of Faecalibacterium and Collinsella Metatranscriptomic Decreases in the proportions of A. municiniphila, Faecalibacterium prausnitzii, Coprococcus, Alistipes shahii, Collinsella and Verrucomicrobiaceae * |
| 16S rRNA gene amplicon analysis b MiSeq technology Illumina | [68] | CDI group (n = 13; 55.5 ± 20.5 y) Control group (n = 13; 51.2 ± 16.6 y) | No difference was observed in richness or evenness between groups. *ns | Enterobaceriaceae * Peptostreptococcaceae * A. muciniphila * | Bacteroidales Clostridales |
| 16S rRNA gene amplicon analysis c Ion Chez System and Ion S5 L system | [62] | tcdB-positive group (n = 79, 62.5 ± 19.9 y) divided into two groups: CDI group (n = 58) and colonized group (n = 21). Control group (n = 20, 62.2 ± 14.4 y). Country: Korea | The richness (Chao1 index; 60 vs. 95) was lower in the tcdB group. * | Proteobacteria *, Enterobacteriaceae*, Porphyromonadaceae *, Enterococcaceae * Parabacteroides*, Enterococcus *, Veillonella *, Klebsiella * and Akkermansia * | Lachnospiraceae *, Ruminococcaceae *, Prevotellaceae * Prevotella, Phascolarctobacterium, Haemophilus, Lachnospira, Coprococcus, Dialister, Butyricimonas, Catenibacterium, Faecalibacterium, Paraprevotella, Odoribacter and Anaerostipes |
| Pyrosequencing e Roche GS Junior | [63] | Control group (n = 94) CA group (n = 24) Mean age: 78.66 y | No difference in diversity between the groups. | Blautia Flavonifractor Lachnospiraceae_unclassified | Akkermansia |
| Pyrosequencing d Illumina MiSeq | [77] | CA group (n = 7) Control group (n = 25) Mean age: 89.3 y Country: United States. | No difference in the alpha diversity or beta diversity (Shannon index: 3.47 in the CA group vs. 3.12 in the control group). | Firmicutes, Actinobacteria Akkermansia spp., Dermabacter spp., Romboutsia spp., Meiothermus spp., Peptoclostridium spp., Ruminococcaceae UGC 009 * | Bacteroidetes, Proteobacteria |
| Pyrosequencing a Roche 454 GS FLX and sequencer | [61] | CDI group (n = 8, 58.9 ± 22.2 y) CA group (n = 8, 60.5 ± 20.8 y) Control group (n = 9, 60.8 ± 16.02) Country: China | Reductions in richness (Chao) *ns and diversity (Simpson and Shannon indexes) * in the CDI and CD+ groups. | Proteobacteria Fusobacteria Clostridium cluster XI Parabacteroides Escherichia/Shigella Klebsiella Enterococcus Veillonella Lactobacillus | Bacteroidetess and Firmicutes. Prevotella, Bacteroides, Faecalibacterium, Coprococcus, Roseburia |
| Pyrosequencing d | [17] | Control group (n = 252) CA group (n = 22) Age: 65 y Country: Ireland | NR | Bacteroidaceae Ruminococcaceae Clostridiaceceae Erysipelothrichaceae Aerococacceae (one patient) Flavobacteriaceae (one patient) | Enterococcaceae Prevotellaceae Leuconostocacceae Spirochaetaceae |
| 16S rRNA gene amplicon analysis b Illumina MiSeq sequencer | [56] | CDI group (n = 25, 82.9 ± 8.5) AB group (n = 29, 84.2 ± 8.1) Control group (n = 30, 82.3 ± 6.8). Country: Italy | Decreased diversity index (Chao1 and Shannon index) in the CDI group compared to the AB group. | Klebsiella *, Escherichia/shigella *, Sutterella, Enterococcus *, Citrobacter, Veillonella, Proteus, Morganella, Hafnia, Corynebacterium, Staphylococcus | Faecalibacterium, Bifidobacterium, Akkermansia, Bacteroides *, Lachnospira *, Alistipes * |
| 16S rRNA gene amplicon analysis b QMiSeq Illumina | [66] | CDI group (n = 15; 61 y). Diarrhea group (n = 18; 56.5 y). Control group (n = 25, 58 y) Country: China | Decreased alpha diversity (Shannon index) and richness (Chao1) in the CDI group and diarrhea group. * | Proteobacteria *, Enterococcaceae, Streptococcaceae, Lactobacillaceae and Peptostreptococcaceae *ns Clostridium spp.*ns Actimnomyces and Rothiabacterium Escherichia/Shigella, Klebsiella, Proteus and Providencia | Firmicutes and Bacteroidetes * Lachnospiraceae.* Blautia–Lachnospiraceae_incertae_sedis, Roseburia and Dorea Ruminococcaceae.* Faecalibacterium, Clostridium IV, Oscillospira and Ruminococcus |
| 454-pyrosequencing f FLX Titanium platform | [73] | All samples with diarrhea CDI–toxins group (71 ± 15 y) CDI–without toxins group (66.3 ± 27 y) Control group (52.0 ± 13 y) Country: Spain | NR | Bacteroides Parabacteroides Faecalibacterium Clostridium XIVa Clostridium cluster XI CDI group with and without toxins: Phascolarctobacterium, Enterococcus, Clostridium XI cluster, flavonifractor, and Erysipelotrichaceae incertea sedis * CDI group with toxins: Enterococcus, Clostridium XI cluster, and Erysipelotrichaceae incerteae sedis | CDI group with and without toxins: Blautia, Holdemania, Enterobacteriaceae, and Veillonellaceae * CDI group with toxins: Fusobacterium, Prevotella, Vovibrio, and Dialister |
| 454-Pyrosequencing g | [78] | CDI group (n = 94; 55.9 ± 18.3) CDN (=C. difficile-negative nosocomial diarrhea) group (n = 89; 58.7 ± 14.9) Control group (n = 155; 52.2 ± 21.5) | Reduction in the diversity (inverse Simpson index) * in the CDI and CDN groups compared with the control group. | Enterococcus * Lachnospiraceae * Erysipelotrichaceae * | Bacteroides species * |
| Pyrosequencing e | [74] | CDI group (n = 39; 54.7 ± 20.1) CDN (= C. difficile-negative nosocomial diarrhea) group (n = 36; 54.6 ± 20.0) Control group (n = 40; 60.9 ± 9.1) | Reduction in the diversity (Shannon index) * in the CDI and CDN groups compared with the control group. | Bacteroides Veillonella Enterococcus Lactobacillus Fusobacterium | Firmicutes Lachnospiraceae and Ruminococcaceae Blautia * Pseudobutyrivibrio, Roseburia, Faecalibacterium, Anaerostipes, Subdoligranulum, Ruminococcus, Streptococcus, Dorea * and Coprococcus. |
| 16S rRNA gene amplicon analysis a MiSeq technology Illumina | [79] | CDI group (n = 57; 69.5 y): GDH+ and TcdB No control group Country: Spain | The richness (Chao1) was 134.32 and the alpha-diversity (Shannon index) was 4.01. | Bacteroides (46.51%), Firmicutes (34.70%), Proteobacteria (13.49%). Bacteroidaceae (31.01%). Enterobacteriaceae (9.82%), Lachnospiraceae (9.33%), Tannerllaceae (6.16%) and Ruminococcaceae (5.64%) | |
| 16S rRNA gene amplicon analysis d MiSeq technology Illumina | [80] | CDI group (n = 31; 64.0 y). Three periods: pretreatment ATB, two days after treatment, seven days after treatment or discharge. No control group | The alpha diversity (Shannon index) was lower with CDI pretreatment in the recurrent group. * | Veillonella dispar * predictor of recurrence. | / |
| 16S rRNA gene amplicon analysis d Illumina MiSeq sequencer | [81] | CDI group (n = 88; 52.7 y) G1 (ATB responder) G2 (non-ATB responder) G3 (recurrent CDI) G4 (non-recurrent CDI) No control group (other studies) | Decreased alpha diversity (Chao1 index) in the CDI group than in the control group. | G1 Ruminococcaceae; Rikenellaceae; Clostridiaceae; Bacteroides; Faecalibacterium; Rothia G2 Clostridiaceae, Lachnospiraceae, Blautia, Coprococcus, Streptococcus, Bifidobacterium, Ruminococcus and Actinomyces. G3 with recurrent CDI Veillonella, Enterobacteriaceae, Streptococci, Parabacteroides, and Lachnospiraceae | / |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Martinez, E.; Taminiau, B.; Rodriguez, C.; Daube, G. Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection. Pathogens 2022, 11, 781. https://doi.org/10.3390/pathogens11070781
Martinez E, Taminiau B, Rodriguez C, Daube G. Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection. Pathogens. 2022; 11(7):781. https://doi.org/10.3390/pathogens11070781
Chicago/Turabian StyleMartinez, Elisa, Bernard Taminiau, Cristina Rodriguez, and Georges Daube. 2022. "Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection" Pathogens 11, no. 7: 781. https://doi.org/10.3390/pathogens11070781
APA StyleMartinez, E., Taminiau, B., Rodriguez, C., & Daube, G. (2022). Gut Microbiota Composition Associated with Clostridioides difficile Colonization and Infection. Pathogens, 11(7), 781. https://doi.org/10.3390/pathogens11070781






