Abstract
(1) Background: Clostridioides difficile infection (CDI) is associated with a high recurrence rate, and a significant proportion of patients with CDI are readmitted following discharge. We aimed to identify the risk factors for CDI-related readmission within 90 days following an index hospital stay for CDI. (2) Methods: We analyzed the electronic medical data of admitted patients in our health system over a two-year period. A multivariate logistic regression model, supplemented with bias-corrected and accelerated confidence intervals (BCa-CI), was implemented to assess the risk factors. (3) Results: A total of 1253 adult CDI index cases were included in the analysis. The readmission rate for CDI within 90 days of discharge was 11% (140/1253). The risk factors for CDI-related readmission were fluoroquinolone exposure within 90 days before the day of index CDI diagnosis (aOR: 1.58, 95% CI: 1.05–2.37), higher Elixhauser comorbidity score (aOR: 1.05, 95% CI: 1.02–1.07), and being discharged home (aOR: 1.64, 95% CI: 1.06–2.54). In contrast, a longer length of index stay (aOR: 0.97, 95% BCa-CI: 0.95–0.99) was associated with reduced odds of readmission for CDI. (4) Conclusion: More than 1 out of 10 patients were readmitted for CDI following an index hospital stay for CDI. Patients with recent previous fluoroquinolone exposure, greater overall comorbidity burden, and those discharged home are at higher risk of readmission for CDI.
1. Introduction
The clinical severity of Clostridioides difficile infection (CDI) ranges from asymptomatic colonization to severe illness that may result in death [1]. The incidence of CDI is associated with antibiotic use [2], while the severity of CDI is associated with advanced age [3]. Although CDI has mainly been considered a nosocomial infection, the incidence of CDI has become more prominent in community settings [4], with almost half of cases in the USA originating in the community [5].
Recurrent CDI occurs when symptoms reappear within 8 weeks following clearance of an initial episode of CDI [6,7]. Recurrence occurs in up to 25% of patients, with the risk of a subsequent recurrence being greater after each recurrence [8,9]. As a result, recurrence contributes to high rates of readmission and associated hospital costs [10,11,12].
In this study, we utilized patient medical data derived from an electronic medical record system of the largest hospital network in the state of Rhode Island, USA, to identify the risk factors associated with readmission for CDI within 90 days of discharge from an index hospital stay for CDI. Due to the recurrent nature of illness and high readmission rate, it is imperative to identify high-risk patients for CDI-related readmission.
2. Materials and Methods
2.1. Patient Selection
Patients admitted to Rhode Island Hospital, the Miriam Hospital, and Newport Hospital in Rhode Island between 1 January 2016 and 1 August 2018, who were at least 18 years of age at the time of admission, and had a positive C. difficile polymerase chain reaction (PCR) test result during an index stay were considered eligible for inclusion. Patients who died during the index stay or were discharged to a hospice were excluded. Patients who received fidaxomicin or did not receive inpatient treatment for CDI were also excluded from analysis.
2.2. CDI Index Case Definition
In accordance with the Infectious Disease Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA) guidelines [7], our institutional policy allows for the use of a standalone nucleic acid amplification test such as a PCR test for CDI diagnosis if patients have at least three unexplained (i.e., not due to laxative use) and unformed stools in 24 h. To ensure that positive PCR test results were due to active infection, we reviewed the medical charts to confirm that patients had diarrhea and that PCR tests were ordered by the patient care teams for the detection of C. difficile. Moreover, to verify that an index stay was not a case of CDI-related readmission, we looked back 90 days from the date of CDI diagnosis to check for previous CDI-related admissions.
2.3. Study Variables
We collected data on patient demographics such as age, race/ethnicity, and sex; index hospital stay characteristics such as length of stay, discharge disposition, and inpatient treatment regimen for CDI; patient comorbidity burden quantified by the van Walraven weighted Elixhauser Comorbidity Index score [13,14]; and prescription history of high-risk antibiotics associated with an increased risk of developing CDI.
For our analysis, age was categorized into four groups: 18–44, 45–64, 65–79, and over 80 years of age. Discharge disposition was contingent on patients being discharged home, such as home with or without home health services, or a healthcare facility, such as a long-term care facility (LTCF) or a skilled nursing facility. We also reviewed records for the prescription of high-risk antibiotics including cephalosporins, clindamycin, fluoroquinolones, penicillin, and combinations of penicillin with beta lactamase inhibitors [15,16]. A patient was considered exposed to a high-risk antibiotic if they were prescribed a high-risk antibiotic within 90 days prior to the day of index CDI diagnosis.
During the study period, vancomycin was the preferred treatment regimen for a first episode of CDI [7]. Following a medical chart review, patients were categorized as receiving either: (a) oral vancomycin only, (b) oral or intravenous (IV) metronidazole only, (c) sequential dual therapy if they received oral vancomycin and either oral/IV metronidazole with less than 24 h of overlap, or (d) concurrent dual therapy if they received oral vancomycin and either oral/IV metronidazole concurrently for 24 h or more.
In line with the Centers for Disease Control and Prevention (CDC) guidelines [17], CDI index cases were categorized into three different groups (Table A1). Community-onset healthcare facility-associated and hospital-onset cases were grouped together and further classified as healthcare associated [18].
2.4. Study Outcome
All adult patients discharged, except those discharged to a hospice, from an index hospital stay for CDI were considered at risk of readmission. Readmission was defined as an inpatient admission within 90 days of discharge, with either a primary encounter diagnosis of CDI or a positive C. difficile PCR test result on Day 1, 2, or 3 of admission. For patients who had multiple readmissions, only the first cases of readmission were included in the analysis.
During the study period, a 10-day antibiotic course was the mainstay therapy for the treatment of CDI [7]. Additionally, recurrent CDI may occur up to 8 weeks following the clearance of an initial episode of CDI. Thus, we selected a 90-day readmission window to maximize the capture of patients who required hospital readmission for CDI following discharge.
2.5. Statistical Analysis
The continuous variables were represented as medians with IQRs. Univariate tests of association between patient readmission status and patient/index hospital stay characteristics were performed using Wilcoxon rank sum tests for continuous variables and Pearson’s Chi-square test for categorical variables.
To identify the risk factors for CDI-related readmission, age, sex, race/ethnicity, CDI index case classification, length of index hospital stay, discharge disposition, Elixhauser comorbidity score, inpatient CDI treatment regimen, and exposure to individual high-risk antibiotics prior to index CDI diagnosis were included in a multivariate logistic regression model. The model estimated the adjusted odds ratios (aOR), along with 95% normal-based confidence intervals (95% CI). Bootstrapping of 1000 iterations was then performed to present bias-corrected and accelerated confidence intervals (BCa-CI). Bootstrapping is a random sampling with replacement procedure that uses the original sample to construct a bootstrap distribution that estimates the shape of the sampling distribution of a point estimate [19], such as an odds ratio [20]. BCa-CIs correct for potential bias and skewness of bootstrap distributions and offer more robust coverage than normal-based confidence intervals, since bootstrapping utilizes simulations to circumvent the assumption that the underlying distribution of the point estimate is normally distributed [21,22]. Additionally, we performed a secondary analysis to identify CDI-related readmission risk factors in which readmitted patients without a documented positive C. difficile PCR test result during readmission stay were excluded from the analysis. Statistical analyses were performed using Stata/SE 17 (StataCorp, College Station, TX, USA). Statistical significance was defined as p < 0.05.
3. Results
Between 1 January 2016 and 1 August 2018, a total of 1487 adult CDI cases were documented. A total of 1253 patients were included in the study after we excluded patients as described in Figure 1.
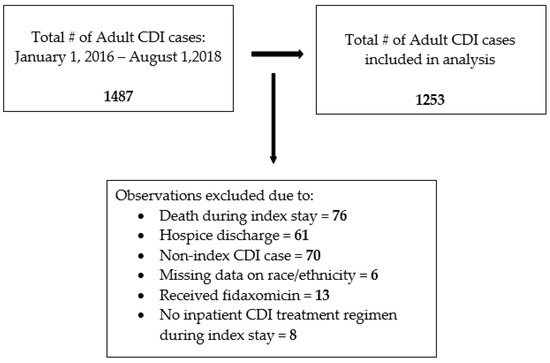
Figure 1.
Flow diagram for inclusion of CDI index cases for analysis.
As shown in Table 1, 753/1253 (60%) CDI index cases were classified as healthcare associated, based on the criteria detailed in the Methods section. Moreover, 1020/1253 (81%) patients were Non-Hispanic White, 744/1253 (59%) were discharged home, and 800/1253 (64%) were prescribed a high-risk antibiotic prior to CDI diagnosis. The median length of index stay for all patients was 7 days (IQR: 4–13).

Table 1.
Characteristics of CDI index cases.
Overall, 140/1253 (11%) patients were readmitted for CDI within 90 days of discharge. Moreover, 114/140 (81%) patients were readmitted with a positive C. difficile PCR test result, and 26/140 (19%) were readmitted with a primary diagnosis of CDI in the absence of a PCR test. Following secondary analysis in which readmitted patients without a documented positive C. difficile PCR test result during readmission stay were excluded (n = 26), a total of 114/1253 (9%) patients were readmitted within 90 days of discharge (Table S1).
In our multivariate logistic regression model (Table 2), fluoroquinolone exposure within 90 days prior to the day of index CDI diagnosis, compared with no fluoroquinolone exposure, was associated with an increased risk of readmission for CDI by 58% (aOR: 1.58, 95% CI: 1.05–2.37). No other individual high-risk antibiotic was associated with readmission for CDI. A one-unit increase in the Elixhauser comorbidity score was associated with an increased risk of readmission for CDI of 5% (aOR: 1.05, 95% CI: 1.02–1.07). Furthermore, being discharged home, compared with being discharged to a healthcare facility, was associated with an increased risk of readmission for CDI of 64% (aOR: 1.64, 95% CI: 1.06–2.54).

Table 2.
Adjusted associations of factors with CDI-related readmission.
After both bootstrapping and secondary analysis in which patients without a documented positive C. difficile PCR test result during readmission stay were excluded, fluoroquinolone exposure, higher Elixhauser comorbidity score, and being discharged home remained statistically significant risk factors for CDI-related readmission (Table 2 and Table S2, respectively). Additionally, after bootstrapping, length of index hospital stay was associated with reduced odds of readmission for CDI. Specifically, the odds of readmission for CDI decreased by 3% (aOR: 0.97, 95% BCa-CI: 0.95–0.99) for each additional day spent in the hospital during the index stay.
4. Discussion
Utilizing the largest hospital network in the state of Rhode Island, USA, we identified the risk factors for readmission for CDI following an index hospital stay for CDI. More than 1 out of 10 (11%) patients were readmitted for CDI within 90 days of discharge. From the analysis of this cohort, we found that fluoroquinolone exposure within 90 days prior to the day of index CDI diagnosis, higher Elixhauser comorbidity score, and being discharged home were independent risk factors for readmission for CDI. Notably, these three identified risk factors remained statistically significant after excluding patients who were readmitted without a documented positive C. difficile PCR test result.
Our rate of readmission (11%) is comparable to the rate reported by Psoinos et al., who analyzed a national sample of Medicare beneficiaries and found that 13% of patients were readmitted for CDI within 90 days of discharge from an index stay for CDI [23]. Moreover, our finding that fluoroquinolone exposure prior to index CDI diagnosis was a risk factor for CDI-related readmission extends the established connection between fluoroquinolone use and CDI. Additionally, our finding that being discharged home was a risk factor for CDI-related readmission is consistent with previous studies [24,25,26]. Lastly, a greater number of Elixhauser-related comorbidities [23,25] and individual comorbidities such as renal failure [24,25,27] and inflammatory bowel disease [10] are risk factors for CDI-related readmission. We found that a higher Elixhauser comorbidity score, as a marker for overall patient comorbidity burden, was a risk factor for CDI-related readmission.
Appropriate antibiotic use is critical for the proper treatment [28] and effective chemoprophylaxis [29,30] of bacterial infections. Institution-based interventions [31] and clinical patient parameters [32] can guide the proper prescription of antibiotics. During periods of severe healthcare strain, such as the ongoing novel coronavirus disease 2019 (COVID-19) pandemic, the potential consequences of misusing antibiotics are notable [33]. The significance of continued appropriate antibiotic use through antimicrobial stewardship programs is highlighted in its association with the reduced incidence of CDI [34]. In particular, the relationship between CDI and fluoroquinolones is well established, with fluoroquinolones associated with a high risk of developing CDI [15,16] and CDI recurrence [35]. A reduction in fluoroquinolone prescriptions has contributed to decreased CDI incidence in England [36] and the USA [37]. In a previous study, members of our team found that a decrease in the rate of antibiotic prescription, particularly fluoroquinolones, was associated with a decrease in the incidence of hospital-onset CDI [38]. However, if a fluoroquinolone must be prescribed, then its selection based on appropriate antimicrobial stewardship programs might minimize the risk of patients developing CDI [39]. Our study finding that fluoroquinolone exposure is a risk factor for CDI-related readmission strengthens the argument for judicious prescription of fluoroquinolones and the need for close monitoring by antimicrobial stewardship.
We found that a unit increase in the Elixhauser comorbidity score was associated with an increased risk of readmission. Comorbidity burden is associated with developing CDI [40,41] and readmission for CDI, with the risk of readmission greatest among patients with at least three Elixhauser-related comorbidities [23,25]. For this study, we opted to use a weighted Elixhauser comorbidity score that quantified the overall patient comorbidity burden [13]. Thus, our finding that a higher Elixhauser comorbidity score is a risk factor for CDI-related readmission adds utility to the established connection between comorbidities and CDI by expanding on previous studies that evaluated either individual [24] or number of comorbidities [23,25] without quantifying the burden. Our method of quantifying the comorbidity burden avoids analyzing multiple individual comorbidities, which increases the risk of overfitting the regression model, and categorizing patients based on the number of Elixhauser comorbidities, which assumes that each Elixhauser comorbidity affects the outcome equally.
Compared with community settings, healthcare settings such as LTCFs are linked with a higher prevalence of C. difficile colonization among residents [42] and increased incidence of both initial and recurrent CDI [43,44,45]. However, we found that patients discharged home are more likely to be readmitted for CDI. To explain this finding, healthcare facilities may be better equipped to follow post-discharge instructions, such as ensuring treatment adherence, and manage CDI-related symptoms before readmission is warranted [26]. Similar supportive and comprehensive follow-up monitoring should be accessible to patients who are discharged home. Costantino et al. conducted an intervention in which outpatient calls were made to patients discharged home after any-cause hospitalization [46]. Patients were asked whether they understood which signs/symptoms to be alert to, filled their outpatient prescriptions, and scheduled the necessary follow-up visits. The researchers found that the outpatient calls, particularly if conducted closer to the time of discharge, reduced the rate of all-cause 30-day readmissions [46]. Given that patients who are discharged home are at greater risk of CDI-related readmission, similar close-monitoring outpatient interventions to monitor treatment adherence and manage disease progression should be evaluated and implemented if they are effective in minimizing the rate of CDI-related readmissions.
Interestingly, we found that in our cohort, a longer length of index stay was associated with reduced odds of readmission for CDI. Thus, an extended index stay may serve as a protective factor, instead of a risk factor, for CDI-related readmission. However, CDI-related hospital stays are associated with greater financial cost [47,48], so prolonging hospital stays for patients is not practical. Therefore, the need for effective outpatient interventions that minimize the rate of CDI-related readmissions is further emphasized.
Our study offers a novel evaluation of potential CDI-related readmission risk factors not available in previous studies that evaluated the risk factors for CDI-related readmission following index stay for CDI [24,25,27]. For instance, our model accounts for the inpatient treatment regimen for CDI to assess whether the specific treatment regimen that a patient received is associated with readmission, the timing of C. difficile specimen collection to assess whether patients with community- and healthcare-associated CDI are differentially at risk for readmission, and high-risk antibiotic use prior to index CDI diagnosis to assess whether exposure to a high-risk antibiotic is associated with readmission. Moreover, a commonly reported study limitation of previous studies [23,24,25,27] that relied on administrative claim data is that using the International Classification of Disease codes to identify both CDI index and readmission cases may underestimate the number of CDI cases, resulting in missed cases. Access to patient medical charts allowed us to identify patients who had a positive C. difficile PCR test result during their index stay to maximize the number of CDI cases for analysis. For readmission cases, we relied on positive PCR test results for identification, along with primary encounter diagnoses if repeat testing was not conducted during readmission.
Since laboratory testing cannot differentiate between C. difficile colonization and active infection [49], patients may have a positive PCR test result in the absence of active infection. To confirm that a patient was an index CDI case, we performed a medical chart review to verify that patients with a positive C. difficile PCR test result during their index stay had diarrhea, which is in accordance with IDSA and SHEA guidelines [7], as described in the Methods section. However, while laboratory confirmation is recommended before treating patients for suspected recurrence [7], a positive PCR test result alone without testing for toxin production among patients readmitted with diarrhea may result in an overestimation of readmission cases.
Regarding study limitations, causality cannot be inferred from the associations described due to the retrospective nature of the study, with the objective of identifying the risk factors and not the causal mechanisms of action. In addition, we did not have access to outside records, so patients may have had a positive C. difficile PCR test result prior to readmission. Thus, we reviewed the medical chart data of all index cases readmitted within 90 days of discharge to identify readmission cases who had a principal encounter diagnosis of CDI without a documented PCR test. However, relying on primary encounter diagnoses, which are based on clinical judgement after considering patients’ symptoms and CDI history, may also result in overestimation of readmission cases, since we do not have laboratory confirmation that the causative agent of diarrhea was C. difficile. In addition, laboratory values such as serum creatinine level and leukocyte count that are markers of CDI severity [7] were not included in our model, since they were not consistently reported for patients. We also did not analyze outpatient use of proton pump inhibitors or high-risk antibiotic use during or following discharge from the index stay for CDI. Furthermore, our high-risk antibiotic data were limited to a binary categorization of either having been prescribed or not prescribed individual high-risk antibiotics during the specified period. The claim data did not provide reliable data on the number of days a patient was prescribed high-risk antibiotics, and we could not verify whether patients followed prescription orders. Lastly, our study results may have limited generalizability, since the cohort only consisted of patients from hospitals in our state.
5. Conclusions
This study identified three main risk factors for readmission for CDI: fluoroquinolone exposure within 90 days prior to the day of index CDI diagnosis, higher Elixhauser comorbidity score as a measure of overall patient comorbidity burden, and being discharged home following an index stay. Identifying high-risk patients for readmission for CDI is imperative due to the significant rate of costly CDI-related readmissions. Continued antimicrobial stewardship efforts such as close monitoring of fluoroquinolone prescription and the use of alternative agents are critical to sustaining decreased incidence of CDI, and may aid in diminishing the threat of CDI across all levels ranging from initial CDI, recurrent CDI, and inpatient readmission. Additionally, more research is needed to evaluate and implement effective outpatient interventions that minimize the rate of CDI-related readmissions, especially among high-risk patients such as those discharged home and patients with a greater overall comorbidity burden who may benefit from closer monitoring following discharge.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/pathogens11050555/s1, Table S1: Characteristics of CDI cases among patients readmitted with a PCR test; Table S2: Adjusted associations of factors with CDI-related readmissions among patients readmitted with a PCR test.
Author Contributions
G.B.: conceptualization, methodology, data curation, formal analysis, original draft preparation, and editing. F.S.: conceptualization, methodology, data curation, review, and editing. M.K.: conceptualization, review, and editing. E.K.M.: conceptualization and data curation. Q.-L.T.: conceptualization, data curation, review, and editing. I.M.Z.: conceptualization, review, and editing. E.M.: supervision, conceptualization, methodology, review, and editing. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board (or Ethics Committee) of Lifespan (protocol code #009018, 16 May 2018) for studies involving humans.
Informed Consent Statement
Patient consent was waived due to the study being a retrospective chart review.
Data Availability Statement
The datasets generated and/or analyzed during the current study are not publicly available due to HIPAA restrictions. De-identified summary data are available from the corresponding author on reasonable request.
Conflicts of Interest
The authors declare no conflict of interest.
Appendix A

Table A1.
CDI index case definitions.
Table A1.
CDI index case definitions.
| Community Associated | Specimen collected on Day 1, 2, or 3 of inpatient admission and patients were not previously discharged from an inpatient setting within 28 days of collection date |
| Community-Onset Healthcare Facility Associated | Specimen collected on Day 1, 2, or 3 of inpatient admission and patients were previously discharged from an inpatient setting within 28 days of collection date |
| Hospital-Onset | Specimen collected > 3 days after inpatient admission date |
References
- Mullish, B.H.; Williams, H.R. Clostridium difficile Infection and Antibiotic-Associated Diarrhoea. Clin. Med. 2018, 18, 237–241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Loo, V.G.; Bourgault, A.-M.; Poirier, L.; Lamothe, F.; Michaud, S.; Turgeon, N.; Toye, B.; Beaudoin, A.; Frost, E.H.; Gilca, R.; et al. Host and Pathogen Factors for Clostridium difficile Infection and Colonization. N. Engl. J. Med. 2011, 365, 1693–1703. [Google Scholar] [CrossRef] [Green Version]
- Henrich, T.J.; Krakower, D.; Bitton, A.; Yokoe, D.S. Clinical Risk Factors for Severe Clostridium difficile–Associated Disease. Emerg. Infect. Dis. 2009, 15, 415–422. [Google Scholar] [CrossRef] [PubMed]
- Ofori, E.; Ramai, D.; Dhawan, M.; Mustafa, F.; Gasperino, J.; Reddy, M. Community-Acquired Clostridium difficile: Epidemiology, Ribotype, Risk Factors, Hospital and Intensive Care Unit Outcomes, and Current and Emerging Therapies. J. Hosp. Infect. 2018, 99, 436–442. [Google Scholar] [CrossRef]
- Guh, A.Y.; Mu, Y.; Winston, L.G.; Johnston, H.; Olson, D.; Farley, M.M.; Wilson, L.E.; Holzbauer, S.M.; Phipps, E.C.; Dumyati, G.K.; et al. Trends in U.S. Burden of Clostridioides Difficile Infection and Outcomes. N. Engl. J. Med. 2020, 382, 1320–1330. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the Treatment Guidance Document for Clostridium difficile Infection. Clin. Microbiol. Infect. 2014, 20, 1–26. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2018, 66, e1–e48. [Google Scholar] [CrossRef]
- Kelly, C.P. Can We Identify Patients at High Risk of Recurrent Clostridium difficile Infection? Clin. Microbiol. Infect. Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2012, 18 (Suppl. S6), 21–27. [Google Scholar] [CrossRef] [Green Version]
- Kelly, C.P.; LaMont, J.T. Clostridium difficile--More Difficult than Ever. N. Engl. J. Med. 2008, 359, 1932–1940. [Google Scholar] [CrossRef]
- Deshpande, A.; Pant, C.; Olyaee, M.; Donskey, C.J. Hospital Readmissions Related to Clostridium difficile Infection in the United States. Am. J. Infect. Control 2018, 46, 346–347. [Google Scholar] [CrossRef]
- Chopra, T.; Neelakanta, A.; Dombecki, C.; Awali, R.A.; Sharma, S.; Kaye, K.S.; Patel, P. Burden of Clostridium difficile Infection on Hospital Readmissions and Its Potential Impact under the Hospital Readmission Reduction Program. Am. J. Infect. Control 2015, 43, 314–317. [Google Scholar] [CrossRef] [PubMed]
- Olsen, M.A.; Yan, Y.; Reske, K.A.; Zilberberg, M.; Dubberke, E.R. Impact of Clostridium difficile Recurrence on Hospital Readmissions. Am. J. Infect. Control 2015, 43, 318–322. [Google Scholar] [CrossRef] [PubMed]
- van Walraven, C.; Austin, P.C.; Jennings, A.; Quan, H.; Forster, A.J. A Modification of the Elixhauser Comorbidity Measures into a Point System for Hospital Death Using Administrative Data. Med. Care 2009, 47, 626–633. [Google Scholar] [CrossRef] [PubMed]
- Quan, H.; Sundararajan, V.; Halfon, P.; Fong, A.; Burnand, B.; Luthi, J.-C.; Saunders, L.D.; Beck, C.A.; Feasby, T.E.; Ghali, W.A. Coding Algorithms for Defining Comorbidities in ICD-9-CM and ICD-10 Administrative Data. Med. Care 2005, 43, 1130–1139. [Google Scholar] [CrossRef]
- Brown, K.A.; Khanafer, N.; Daneman, N.; Fisman, D.N. Meta-Analysis of Antibiotics and the Risk of Community-Associated Clostridium difficile Infection. Antimicrob. Agents Chemother. 2013, 57, 2326–2332. [Google Scholar] [CrossRef] [Green Version]
- Deshpande, A.; Pasupuleti, V.; Thota, P.; Pant, C.; Rolston, D.D.K.; Sferra, T.J.; Hernandez, A.V.; Donskey, C.J. Community-Associated Clostridium difficile Infection and Antibiotics: A Meta-Analysis. J. Antimicrob. Chemother. 2013, 68, 1951–1961. [Google Scholar] [CrossRef] [Green Version]
- Multidrug-Resistant Organism & Clostridioides Difficile Infection (MDRO/CDI) Module. Available online: https://www.cdc.gov/nhsn/psc/cdiff/index.html (accessed on 27 April 2022).
- Lessa, F.C.; Mu, Y.; Bamberg, W.M.; Beldavs, Z.G.; Dumyati, G.K.; Dunn, J.R.; Farley, M.M.; Holzbauer, S.M.; Meek, J.I.; Phipps, E.C.; et al. Burden of Clostridium difficile Infection in the United States. N. Engl. J. Med. 2015, 372, 825–834. [Google Scholar] [CrossRef] [Green Version]
- Kulesa, A.; Krzywinski, M.; Blainey, P.; Altman, N. Sampling Distributions and the Bootstrap. Nat. Methods 2015, 12, 477–478. [Google Scholar] [CrossRef] [Green Version]
- Bland, J.M.; Altman, D.G. The Odds Ratio. BMJ 2000, 320, 1468. [Google Scholar] [CrossRef] [Green Version]
- DiCiccio, T.J.; Efron, B. Bootstrap Confidence Intervals. Stat. Sci. 1996, 11, 189–228. [Google Scholar] [CrossRef]
- Carpenter, J.; Bithell, J. Bootstrap Confidence Intervals: When, Which, What? A Practical Guide for Medical Statisticians. Stat. Med. 2000, 19, 1141–1164. [Google Scholar] [CrossRef]
- Psoinos, C.M.; Collins, C.E.; Ayturk, M.D.; Anderson, F.A.; Santry, H.P. Post-Hospitalization Treatment Regimen and Readmission for C. Difficile Colitis in Medicare Beneficiaries. World J. Surg. 2018, 42, 246–253. [Google Scholar] [CrossRef]
- Verheyen, E.; Dalapathi, V.; Arora, S.; Patel, K.; Mankal, P.K.; Kumar, V.; Lung, E.; Kotler, D.P.; Grinspan, A. High 30-Day Readmission Rates Associated with Clostridiumdifficile Infection. Am. J. Infect. Control 2019, 47, 922–927. [Google Scholar] [CrossRef] [PubMed]
- Collins, C.E.; Ayturk, M.D.; Anderson, F.A.; Santry, H.P. Predictors and Outcomes of Readmission for Clostridium difficile in a National Sample of Medicare Beneficiaries. J. Gastrointest. Surg. 2015, 19, 88–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoffman, G.J.; Min, L.C.; Liu, H.; Marciniak, D.J.; Mody, L. Role of Post-Acute Care in Readmissions for Preexisting Healthcare-Associated Infections. J. Am. Geriatr. Soc. 2020, 68, 370–378. [Google Scholar] [CrossRef] [PubMed]
- Dinh, A.; Le Monnier, A.; Emery, C.; Alami, S.; Torreton, É.; Duburcq, A.; Barbier, F. Predictors and Burden of Hospital Readmission with Recurrent Clostridioides Difficile Infection: A French Nation-Wide Inception Cohort Study. Eur. J. Clin. Microbiol. Infect. Dis. Off. Publ. Eur. Soc. Clin. Microbiol. 2019, 38, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- The Core Elements of Hospital Antibiotic Stewardship Programs. Available online: https://www.cdc.gov/antibiotic-use/healthcare/pdfs/hospital-core-elements-H.pdf (accessed on 24 March 2022).
- Purba, A.K.R.; Setiawan, D.; Bathoorn, E.; Postma, M.J.; Dik, J.-W.H.; Friedrich, A.W. Prevention of Surgical Site Infections: A Systematic Review of Cost Analyses in the Use of Prophylactic Antibiotics. Front. Pharmacol. 2018, 9, 776. [Google Scholar] [CrossRef]
- Najjar, P.A.; Smink, D.S. Prophylactic Antibiotics and Prevention of Surgical Site Infections. Surg. Clin. N. Am. 2015, 95, 269–283. [Google Scholar] [CrossRef]
- Davey, P.; Marwick, C.A.; Scott, C.L.; Charani, E.; McNeil, K.; Brown, E.; Gould, I.M.; Ramsay, C.R.; Michie, S. Interventions to Improve Antibiotic Prescribing Practices for Hospital Inpatients. Cochrane Database Syst. Rev. 2017, 2, CD003543. [Google Scholar] [CrossRef] [Green Version]
- Zhang, K.; Xie, K.; Zhang, C.; Liang, Y.; Chen, Z.; Wang, H. C-Reactive Protein Testing to Reduce Antibiotic Prescribing for Acute Respiratory Infections in Adults: A Systematic Review and Meta-Analysis. J. Thorac. Dis. 2022, 14, 123–134. [Google Scholar] [CrossRef]
- Spigaglia, P. COVID-19 and Clostridioides Difficile Infection (CDI): Possible Implications for Elderly Patients. Anaerobe 2020, 64, 102233. [Google Scholar] [CrossRef] [PubMed]
- Baur, D.; Gladstone, B.P.; Burkert, F.; Carrara, E.; Foschi, F.; Döbele, S.; Tacconelli, E. Effect of Antibiotic Stewardship on the Incidence of Infection and Colonisation with Antibiotic-Resistant Bacteria and Clostridium difficile Infection: A Systematic Review and Meta-Analysis. Lancet Infect. Dis. 2017, 17, 990–1001. [Google Scholar] [CrossRef]
- Abou Chakra, C.N.; Pepin, J.; Sirard, S.; Valiquette, L. Risk Factors for Recurrence, Complications and Mortality in Clostridium difficile Infection: A Systematic Review. PLoS ONE 2014, 9, e98400. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dingle, K.E.; Didelot, X.; Quan, T.P.; Eyre, D.W.; Stoesser, N.; Golubchik, T.; Harding, R.M.; Wilson, D.J.; Griffiths, D.; Vaughan, A.; et al. Effects of Control Interventions on Clostridium difficile Infection in England: An Observational Study. Lancet Infect. Dis. 2017, 17, 411–421. [Google Scholar] [CrossRef] [Green Version]
- Wenzler, E.; Mulugeta, S.G.; Danziger, L.H. The Antimicrobial Stewardship Approach to Combating Clostridium difficile. Antibiot. Basel Switz. 2015, 4, 198–215. [Google Scholar] [CrossRef]
- Zacharioudakis, I.M.; Zervou, F.N.; Shehadeh, F.; Mylona, E.K.; Mylonakis, E. Association of Community Factors with Hospital-Onset Clostridioides (Clostridium) Difficile Infection: A Population Based U.S.-Wide Study. EClinicalMedicine 2019, 8, 12–19. [Google Scholar] [CrossRef] [Green Version]
- Brown, K.A.; Langford, B.; Schwartz, K.L.; Diong, C.; Garber, G.; Daneman, N. Antibiotic Prescribing Choices and Their Comparative C. Difficile Infection Risks: A Longitudinal Case-Cohort Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2021, 72, 836–844. [Google Scholar] [CrossRef] [Green Version]
- Blot, S.; Ruppé, E.; Harbarth, S.; Asehnoune, K.; Poulakou, G.; Luyt, C.-E.; Rello, J.; Klompas, M.; Depuydt, P.; Eckmann, C.; et al. Healthcare-Associated Infections in Adult Intensive Care Unit Patients: Changes in Epidemiology, Diagnosis, Prevention and Contributions of New Technologies. Intensive Crit. Care Nurs. 2022, 70, 103227. [Google Scholar] [CrossRef]
- Guh, A.Y.; Adkins, S.H.; Li, Q.; Bulens, S.N.; Farley, M.M.; Smith, Z.; Holzbauer, S.M.; Whitten, T.; Phipps, E.C.; Hancock, E.B.; et al. Risk Factors for Community-Associated Clostridium difficile Infection in Adults: A Case-Control Study. Open Forum Infect. Dis. 2017, 4, ofx171. [Google Scholar] [CrossRef] [Green Version]
- Ziakas, P.D.; Zacharioudakis, I.M.; Zervou, F.N.; Grigoras, C.; Pliakos, E.E.; Mylonakis, E. Asymptomatic Carriers of Toxigenic C. Difficile in Long-Term Care Facilities: A Meta-Analysis of Prevalence and Risk Factors. PLoS ONE 2015, 10, e0117195. [Google Scholar] [CrossRef]
- Campbell, R.J.; Giljahn, L.; Machesky, K.; Cibulskas-White, K.; Lane, L.M.; Porter, K.; Paulson, J.O.; Smith, F.W.; McDonald, L.C. Clostridium difficile Infection in Ohio Hospitals and Nursing Homes during 2006. Infect. Control Hosp. Epidemiol. 2009, 30, 526–533. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Karanika, S.; Grigoras, C.; Flokas, M.E.; Alevizakos, M.; Kinamon, T.; Kojic, E.M.; Mylonakis, E. The Attributable Burden of Clostridium difficile Infection to Long-Term Care Facilities Stay: A Clinical Study. J. Am. Geriatr. Soc. 2017, 65, 1733–1740. [Google Scholar] [CrossRef] [PubMed]
- McConeghy, K.W.; Zullo, A.R.; Lary, C.W.; Zhang, T.; Lee, Y.; Daiello, L.; Kiel, D.P.; Berry, S. Association Between Bisphosphonates and Hospitalized Clostridioides Difficile Infection Among Frail Older Adults. J. Am. Med. Dir. Assoc. 2020, 21, 688–691. [Google Scholar] [CrossRef] [PubMed]
- Costantino, M.E.; Frey, B.; Hall, B.; Painter, P. The Influence of a Postdischarge Intervention on Reducing Hospital Readmissions in a Medicare Population. Popul. Health Manag. 2013, 16, 310–316. [Google Scholar] [CrossRef] [Green Version]
- Dubberke, E.R.; Olsen, M.A. Burden of Clostridium difficile on the Healthcare System. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 2012, 55 (Suppl. S2), S88–S892. [Google Scholar] [CrossRef]
- Zhang, S.; Palazuelos-Munoz, S.; Balsells, E.M.; Nair, H.; Chit, A.; Kyaw, M.H. Cost of Hospital Management of Clostridium difficile Infection in United States-a Meta-Analysis and Modelling Study. BMC Infect. Dis. 2016, 16, 447. [Google Scholar] [CrossRef] [Green Version]
- Tsigrelis, C. Recurrent Clostridioides Difficile Infection: Recognition, Management, Prevention. Cleve. Clin. J. Med. 2020, 87, 347–359. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).