Cercariae of a Bird Schistosome Follow a Similar Emergence Pattern under Different Subarctic Conditions: First Experimental Study
Abstract
1. Introduction
2. Results
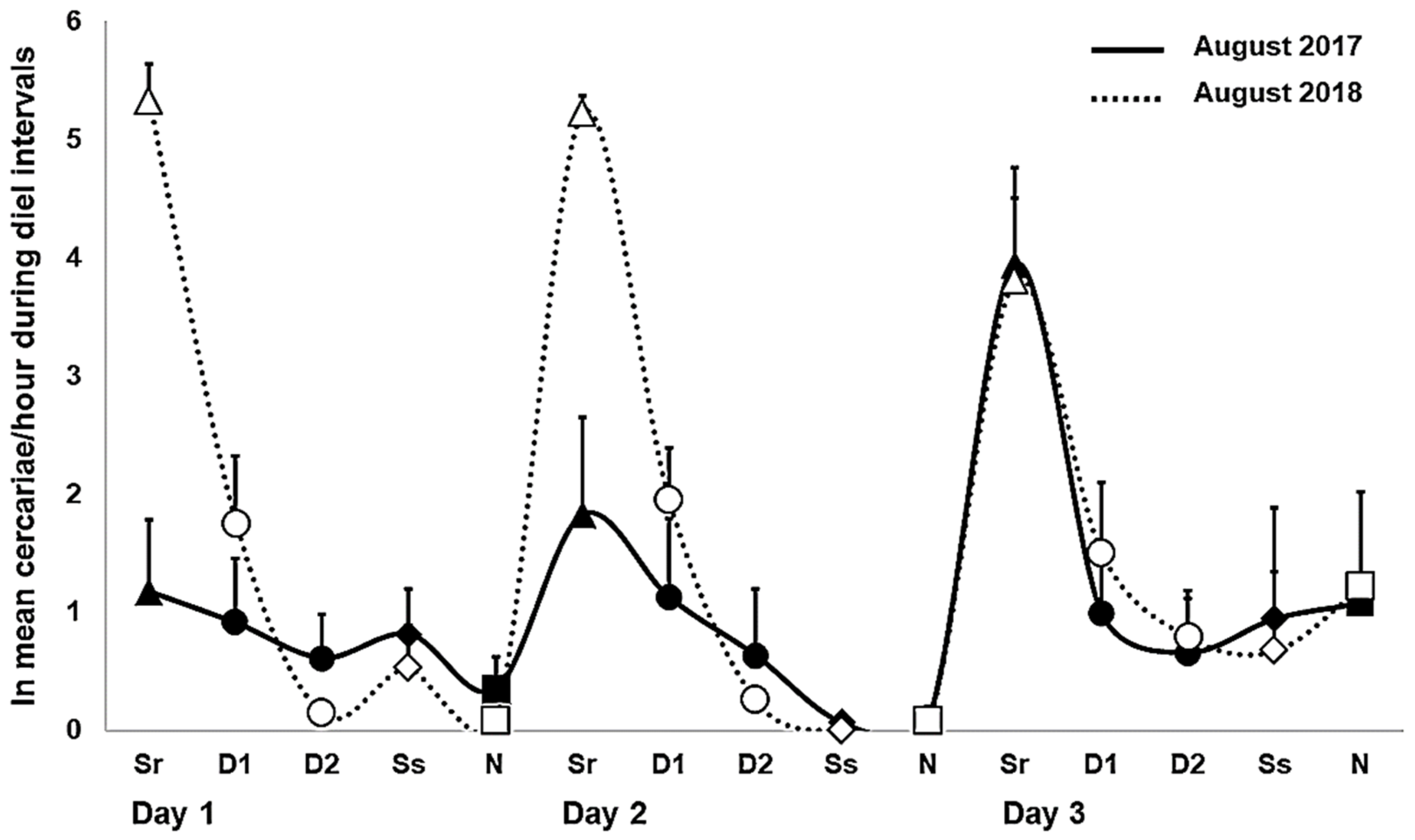
2.1. Daily Patterns in Cercarial Emergence
2.2. Daily Output in Cercarial Emergence
3. Discussion
4. Materials and Methods
4.1. Snail and Parasite Material
4.2. Experimental Setup and Monitoring of Cercarial Emergence
4.3. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A

| Season | Snail Code | Observed Number of Emerged Cercariae during Diel Intervals | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| DAY 1 | DAY 2 | DAY 3 | ||||||||||||||
| August 2016 | 0:00– 6:00 | 6:00– 12:00 | 12:00– 18:00 | 18:00– 0:00 | 12:00– 00:00 | 00:00– 12:00 | 0:00– 6:00 | 6:00– 12:00 | 12:00– 18:00 | 18:00– 0:00 | ||||||
| Field | 1 T-A16 | 238 | 26 | 12 | 1 | 14 | 16 | – a | – | – | – | |||||
| 2 T-A16 | 1263 | 4 | 10 | 18 | 0 | 1 | – | – | – | – | ||||||
| 3 T-A16 | 3 | 0 | 53 | 0 | 1 | 31 | – | – | – | – | ||||||
| 4 T-A16 | 16 | 57 | 4 | 0 | 0 | 1 | – | – | – | – | ||||||
| 5 T-A16 | 57 | 22 | 3 | 0 | 1 | 0 | – | – | – | – | ||||||
| 6 T-A16 | 57 | 22 | 6 | 1 | 2 | 57 | – | – | – | – | ||||||
| 7 T-A16 | 70 | 0 | 26 | 0 | 0 | 8 | – | – | – | – | ||||||
| Laboratory | 1 T-A16 | – a | – | – | – | – a | – | 19 | 48 | 0 | 1 | |||||
| 2 T-A16 | – | – | – | – | – | – | 96 | 18 | 2 | 1 | ||||||
| 3 T-A16 | – | – | – | – | – | – | 40 | 0 | 1 | 1 | ||||||
| 4 T-A16 | – | – | – | – | – | – | 208 | 0 | 14 | 0 | ||||||
| 5 T-A16 | – | – | – | – | – | – | 10 | 1 | 2 | 1 | ||||||
| 6 T-A16 | – | – | – | – | – | – | 112 | 9 | 6 | 2 | ||||||
| 7 T-A16 | – | – | – | – | – | – | 33 | 4 | 0 | 2 | ||||||
| August | Sunrise | Day 1 | Day 2 | Sunset | Night | Sunrise | Day 1 | Day 2 | Sunset | Night | Sunrise | Day 1 | Day 2 | Sunset | Night | |
| 2017 | 2:00– 4:30 | 4:30– 13:15 | 13:15– 22:00 | 22:00– 0:30 | 0:30– 2:00 | 2:00– 4:30 | 4:30– 13:15 | 13:15– 22:00 | 22:00– 0:30 | 0:30– 2:00 | 2:00– 4:30 | 4:30– 13:15 | 13:15– 22:00 | 22:00– 0:30 | 0:30– 2:00 | |
| Field | 1 T-A17 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | – b | ||||
| 2 T-A17 | 4 | 0 | 1 | 0 | 0 | 180 | 24 | 0 | 1 | 0 | 288 | 6 | 2 | 0 | 2 | |
| 3 T-A17 | 0 | 2 | 1 | 5 | 0 | 7 | 9 | 3 | 0 | 0 | – b | |||||
| 4 T-A17 | 14 | 156 | 64 | 18 | 1 | 1 | 0 | 0 | 0 | 0 | – b | |||||
| 5 T-A17 | 116 | 80 | 25 | 11 | 6 | 56 | 316 | 148 | 0 | 0 | 54 | 29 | 18 | 14 | 4 | |
| 6 T-A17 | 0 | 0 | 0 | 0 | 0 | – b | ||||||||||
| Laboratory | 1 T-A17 | |||||||||||||||
| 2 T-A17 | 91 | 25 | 1 | 136 | 0 | 528 | 5 | 0 | 4 | 0 | 84 | 15 | 0 | 5 | 0 | |
| 3 T-A17 | ||||||||||||||||
| 4 T-A17 | ||||||||||||||||
| 5 T-A17 | – c | |||||||||||||||
| 6 T-A17 | ||||||||||||||||
| August | Sunrise | Day 1 | Day 2 | Sunset | Night | Sunrise | Day 1 | Day 2 | Sunset | Night | Sunrise | Day 1 | Day 2 | Sunset | Night | |
| 2018 | 1:30– 4:00 | 4:00– 12:45 | 12:45– 21:30 | 21:30– 0:00 | 0:00– 1:30 | 1:30– 4:00 | 4:00– 12:45 | 12:45– 21:30 | 21:30– 0:00 | 0:00– 1:30 | 1:30– 4:00 | 4:00– 12:45 | 12:45– 21:30 | 21:30– 0:00 | 0:00– 1:30 | |
| Field | 1 T-A18 | 312 | 14 | 0 | 0 | 0 | 380 | 16 | 0 | 0 | 0 | 28 | 1 | 0 | 0 | 0 |
| 2 T-A18 | 1840 | 204 | 5 | 18 | 0 | 700 | 96 | 3 | 0 | 0 | 416 | 37 | 68 | 140 | 260 | |
| 3 T-A18 | 192 | 59 | 0 | 0 | 0 | 376 | 8 | 1 | 0 | 0 | 35 | 5 | 0 | 0 | 0 | |
| 4 T-A18 | 544 | 0 | 1 | 5 | 1 | 748 | 184 | 2 | 0 | 0 | 11 | 22 | 7 | 0 | 1 | |
| 5 T-A18 | 756 | 264 | 1 | 0 | 0 | 416 | 180 | 8 | 0 | 1 | 428 | 600 | 44 | 0 | 3 | |
| 6 T-A18 | 388 | 13 | 2 | 0 | 0 | 304 | 27 | 3 | 0 | 0 | 724 | 23 | 2 | 0 | 1 | |
| Laboratory | 9:00– 21:00 | 21:00– 9:00 | 9:00– 21:00 | 21:00– 9:00 | 9:00– 21:00 | 21:00– 9:00 | ||||||||||
| 1 T-A18 | 0 | 684 | 0 | 284 | 0 | 352 | ||||||||||
| 2 T-A18 | – c | |||||||||||||||
| 3 T-A18 | 1 | 372 | 0 | 116 | 0 | 652 | ||||||||||
| 4 T-A18 | 40 | 1564 | 4 | 1580 | – b | |||||||||||
| 5 T-A18 | 43 | 1248 | 5 | 988 | 6 | 824 | ||||||||||
| 6 T-A18 | – c | |||||||||||||||
| October | Sunrise | Day | Sunset | Night 1 | Night 2 | Sunrise | Day | Sunset | Night 1 | Night 2 | Sunrise | Day | Sunset | Night 1 | Night 2 | |
| 2018 | 7:00– 8:00 | 8:00– 17:15 | 17:15– 18:15 | 18:15– 0:40 | 0:40– 7:00 | 7:00– 8:00 | 8:00– 17:15 | 17:15– 18:15 | 18:15– 0:40 | 0:40– 7:00 | 7:00– 8:00 | 8:00– 17:15 | 17:15– 18:15 | 18:15– 0:40 | 0:40– 7:00 | |
| Field | 1 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 3 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 | 1 | 3 | 0 | 0 | 0 | 0 | |
| 4 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| 5 T-O18 | 0 | 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | |
| 6 T-O18 | 1 | 5 | 0 | 1 | 2 | 7 | 5 | 0 | 0 | 0 | 2 | 5 | 0 | 0 | 0 | |
| Laboratory | 9:00– 21:00 | 21:00– 9:00 | 9:00– 21:00 | 21:00– 9:00 | 9:00– 21:00 | 21:00– 9:00 | ||||||||||
| 1 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 2 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 3 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 4 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 5 T-O18 | 0 | 0 | 0 | 0 | 0 | 0 | ||||||||||
| 6 T-O18 | 0 | 0 | 0 | 0 | 13 | 8 | ||||||||||
| Season/Year | Snail Code | Length (mm) | Width (mm) | Double Infection Identity | Double Infection Status |
|---|---|---|---|---|---|
| August 2016 | 1 T-A16 | 14.51 | 8.91 | – | – |
| 2 T-A16 | 15.73 | 10.30 | – | – | |
| 3 T-A16 | 12.81 | 7.44 | – | – | |
| 4 T-A16 | 16.12 | 11.05 | – | – | |
| 5 T-A16 | 11.06 | 7.44 | – | – | |
| 6 T-A16 | 11.76 | 7.70 | – | – | |
| 7 T-A16 | 15.33 | 9.76 | Plagiorchis sp. | patent | |
| August 2017 | 1 T-A17 | 8.69 | 5.16 | – | – |
| 2 T-A17 | 10.45 | 8.84 | – | – | |
| 3 T-A17 | 15.36 | 12.44 | – | – | |
| 4 T-A17 | 14.76 | 12.73 | Plagiorchis sp. | prepatent | |
| 5 T-A17 | 12.81 | 10.93 | – | – | |
| 6 T-A17 | 12.77 | 10.89 | Apatemon sp. | prepatent | |
| August 2018 | 1 T-A18 | 10.57 | 6.15 | – | – |
| 2 T-A18 | 12.27 | 7.98 | – | – | |
| 3 T-A18 | 9.57 | 5.87 | – | – | |
| 4 T-A18 | 12.80 | 7.78 | – | – | |
| 5 T-A18 | 11.26 | 6.23 | – | – | |
| 6 T-A18 | 13.92 | 8.06 | – | – | |
| October 2018 | 1 T-O18 | 12.63 | 8.25 | Plagiorchis sp. | prepatent |
| 2 T-O18 | 9.17 | 5.79 | – | – | |
| 3 T-O18 | 11.11 | 6.85 | – | – | |
| 4 T-O18 | 11.17 | 6.54 | Plagiorchis sp. | prepatent | |
| 5 T-O18 | 15.11 | 8.60 | Cotylurus sp. | prepatent | |
| 6 T-O18 | 19.96 | 11.14 | Diplostomum sp. | prepatent |
| Season/Year | Type of Experiment | Experimental Day | Temperature and Light Intensity during Diel Intervals | |||||
|---|---|---|---|---|---|---|---|---|
| August 2016 | 0:00–6:00 | 6:00–12:00 | 12:00–18:00 | 18:00–0:00 | ||||
| Field | Day 1 | °C | 7.8 | 8.5 | 9.2 | 8.4 | ||
| Lx | 415 | 6962 | 13821 | 1216 | ||||
| 12:00–0:00 | 0:00–12:00 | |||||||
| Day 2 | °C | 8.8 | 7.3 | |||||
| Lx | 5803 | 2311 | ||||||
| Sunrise 2:00–4:30 | Day 1 4:33–13:15 | Day 2 13:15–22:00 | Sunset 22:00–0:30 | Night 0:30–2:00 | ||||
| Field | Day 1 | °C | 7.8 | 8.4 | 9.0 | 7.8 | 7.7 | |
| Lx | 108 | 6321 | 9107 | 9 | 0 | |||
| Day 2 | °C | 6.8 | 7.4 | 9.1 | 7.7 | 7.2 | ||
| Lx | 120 | 3448 | 6823 | 7 | 0 | |||
| 0:00–6:00 | 6:00–12:00 | 12:00–18:00 | 18:00–0:00 | |||||
| Laboratory | Day 1 | °C | 13.7 | 13.8 | 13.8 | 13.7 | ||
| Lx | 372 | 538 | 505 | 311 | ||||
| August 2017 | Sunrise 2:00–4:30 | Day 1 4:33–13:15 | Day 2 13:15–22:00 | Sunset 22:00–0:30 | Night 0:30–2:00 | |||
| Field | Day 1 | °C | 12.8 | 13.1 | 12.9 | 13.0 | 12.9 | |
| Lx | 143 | 7659 | 5871 | 37 | 0 | |||
| Day 2 | °C | 12.6 | 13.1 | 13.8 | 12.8 | 12.7 | ||
| Lx | 71 | 8370 | 13145 | 16 | 0 | |||
| Day 3 | °C | 13.0 | 12.7 | 12.5 | 13.2 | 13.1 | ||
| Lx | 153 | 11024 | 13647 | 14 | 0 | |||
| Laboratory | Day 1 | °C | 13.4 | 13.6 | 13.7 | 13.4 | 13.3 | |
| Lx | 31 | 535 | 533 | 77 | 0 | |||
| Day 2 | °C | 13.1 | 13.2 | 13.2 | 13.3 | 13.2 | ||
| Lx | 60 | 571 | 511 | 55 | 0 | |||
| Day 3 | °C | 13.1 | 13.3 | 13.3 | 13.1 | 13.1 | ||
| Lx | 27 | 553 | 491 | 27 | 0 | |||
| August 2018 | Sunrise 1:30–4:00 | Day 1 4:00–12:45 | Day 2 12:45–21:30 | Sunset 21:30–0:00 | Night 0:00–1:30 | |||
| Field | Day 1 | °C | 15.4 | 15.7 | 16.3 | 15.9 | 14.9 | |
| Lx | 30 | 13949 | 5428 | 146 | 0 | |||
| Day 2 | °C | 15.9 | 11.5 | 16.9 | 16.3 | 16.0 | ||
| Lx | 11 | 2960 | 12926 | 17 | 0 | |||
| Day 3 | °C | 14.4 | 14.5 | 13.1 | 14.1 | 14.1 | ||
| Lx | 12 | 4787 | 4591 | 63 | 0 | |||
| 9:00–21:00 | 21:00–9:00 | |||||||
| Laboratory | Day 1 | °C | 14.2 | 14.1 | ||||
| Lx | 965 | 413 | ||||||
| Day 2 | °C | 14.2 | 14.1 | |||||
| Lx | 978 | 466 | ||||||
| Day 3 | °C | 14.3 | 14.1 | |||||
| Lx | 965 | 471 | ||||||
| Sunrise 1:30–4:00 | Day 1 4:00–12:45 | Day 2 12:45–21:30 | Sunset 21:30–0:00 | Night 0:00–1:30 | ||||
| Laboratory | Day 1 | °C | 14.1 | 14.2 | 14.2 | 14.1 | 14.1 | |
| Lx | 10 | 926 | 976 | 89 | 0 | |||
| Day 2 | °C | 14.0 | 14.1 | 14.2 | 14.1 | 14.1 | ||
| Lx | 10 | 965 | 983 | 115 | 0 | |||
| Day 3 | °C | 14.1 | 14.2 | 14.2 | 14.1 | 14.0 | ||
| Lx | 9 | 954 | 967 | 150 | 0 | |||
| October 2018 | Sunrise | Day | Sunset | Night 1 | Night 2 | |||
| 7:00–8:00 | 8:00–17:15 | 17:15–18:15 | 18:15–0:40 | 0:40–7:00 | ||||
| Field | Day 1 | °C | 6.3 | 6.4 | 6.6 | 6.5 | 6.4 | |
| Lx | 236 | 2198 | 6 | 0 | 0 | |||
| Day 2 | °C | 6.3 | 6.5 | 6.6 | 6.5 | 6.3 | ||
| Lx | 109 | 1786 | 70 | 0 | 0 | |||
| Day 3 | °C | 6.5 | 6.6 | 6.3 | 6.2 | 6.5 | ||
| Lx | 43 | 2929 | 39 | 0 | 0 | |||
| 9:00–21:00 | 21:00–9:00 | |||||||
| Laboratory | Day 1 | °C | 5.8 | 5.7 | ||||
| Lx | 1367 | 161 | ||||||
| Day 2 | °C | 5.7 | 5.7 | |||||
| Lx | 1,456 | 183 | ||||||
| Day 3 | °C | 5.8 | 5.7 | |||||
| Lx | 1429 | 186 | ||||||
| Sunrise | Day | Sunset | Night 1 | Night 2 | ||||
| 7:00–8:00 | 8:00–17:15 | 17:15–18:15 | 18:15–0:40 | 0:40–7:00 | ||||
| Laboratory | Day 1 | °C | 6.0 | 5.8 | 5.6 | 5.6 | 5.7 | |
| Lx | 103 | 1954 | 102 | 0 | 0 | |||
| Day 2 | °C | 6.0 | 5.7 | 5.6 | 5.7 | 5.7 | ||
| Lx | 103 | 2095 | 176 | 0 | 0 | |||
| Day 3 | °C | 6.1 | 5.8 | 5.6 | 5.7 | 5.7 | ||
| Lx | 108 | 2075 | 35 | 0 | 0 | |||
References
- Colley, D.G.; Bustinduy, A.L.; Secor, W.E.; King, C.H. Human schistosomiasis. Lancet 2014, 383, 2253–2264. [Google Scholar] [CrossRef]
- Jamieson, B.G.M. Schistosoma: Biology, Pathology and Control; CRC Press: Boca Raton, FL, USA, 2017; p. 523. [Google Scholar] [CrossRef]
- Brant, S.V.; Loker, E.S. Molecular systematics of the avian schistosome genus Trichobilharzia (Trematoda: Schistosomatidae) in North America. J. Parasitol. 2009, 95, 941–963. [Google Scholar] [CrossRef] [PubMed]
- Soldánová, M.; Selbach, C.; Kalbe, M.; Kostadinova, A.; Sures, B. Swimmer’s itch: Etiology, impact, and risk factors in Europe. Trends Parasitol. 2013, 29, 65–74. [Google Scholar] [CrossRef] [PubMed]
- Horák, P.; Mikeš, L.; Lichtenbergová, L.; Skála, V.; Soldánová, M.; Brant, S.V. Avian schistosomes and outbreaks of cercarial dermatitis. Clin. Microbiol. Rev. 2015, 28, 165–190. [Google Scholar] [CrossRef]
- Galaktionov, K.V.; Dobrovolskij, A. The Biology and Evolution of Trematodes: An Essay on the Biology, Morphology, Life Cycles, Transmissions, and Evolution of Digenetic Trematodes; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2003; p. 592. [Google Scholar]
- Pandian, T.J. Reproduction and Development in Platyhelminthes; CRC Press: Boca Raton, FL, USA, 2020; p. 320. [Google Scholar]
- Combes, C.; Fournier, A.; Moné, H.; Théron, A. Behaviours in trematode cercariae that enhance parasite transmission: Patterns and processes. Parasitology 1994, 109, 3–13. [Google Scholar] [CrossRef]
- Joosse, J.; van Elk, R. Trichobilharzia ocellata: Physiological characterization of giant growth, glycogen depletion, and absence of reproductive activity in the intermediate snail host, Lymnaea stagnalis. Exp. Parasitol. 1986, 62, 1–13. [Google Scholar] [CrossRef]
- Gérard, C.; Théron, A. Age/size- and time-specific effects of Schistosoma mansoni on energy allocation patterns of its snail host Biomphalaria glabrata. Oecologia 1997, 112, 447–452. [Google Scholar] [CrossRef]
- Żbikowska, E.; Marszewska, A. Thermal preferences of bird schistosome snail hosts increase the risk of swimmer’s itch. J. Therm. Biol. 2018, 78, 22–26. [Google Scholar] [CrossRef]
- Skála, V.; Walker, A.J.; Horák, P. Snail defence responses to parasite infection: The Lymnaea stagnalis-Trichobilharzia szidati model. Dev. Comp. Immunol. 2020, 102, 103464. [Google Scholar] [CrossRef]
- Loker, E.S. A comparative study of the life-histories of mammalian schistosomes. Parasitology 1983, 87, 343–369. [Google Scholar] [CrossRef]
- Haas, W. Physiological analyses of host-finding behaviour in trematode cercariae: Adaptations for transmission success. Parasitology 1994, 109, S15–S29. [Google Scholar] [CrossRef] [PubMed]
- Saap, K.K.; Loker, E.S. Mechanisms underlying digenean-snail specificity: Role of miracidial attachment and host plasma factors. J. Parasitol. 2000, 86, 1012–1019. [Google Scholar] [CrossRef]
- Haas, W. Parasitic worm: Strategies of host finding, recognition and invasion. Zoology 2003, 106, 349–364. [Google Scholar] [CrossRef] [PubMed]
- Combes, C.; Bartoli, P.; Théron, A. Trematode transmission strategies. In The Behavioural Ecology of Parasites; Lewis, E.E., Campbell, J.F., Sukhdeo, M.V.K., Eds.; CABI: Wallingford, UK, 2002; pp. 1–12. [Google Scholar] [CrossRef]
- Morley, N.J. Cercariae (Platyhelminthes: Trematoda) as neglected components of zooplankton communities in freshwater habitats. Hydrobiologia 2012, 691, 7–19. [Google Scholar] [CrossRef]
- Esch, G.W.; Curtis, L.A.; Barger, M.A. A perspective on the ecology of trematode communities in snails. Parasitology 2001, 123, S57–S75. [Google Scholar] [CrossRef]
- Théron, A. Chronobiology of trematode cercarial emergence: From data recovery to epidemiological, ecological and evolutionary implications. Adv. Parasitol. 2015, 88, 123–164. [Google Scholar] [CrossRef]
- Mouahid, G.; Idris, M.A.; Verneau, O.; Théron, A.; Shaban, M.M.; Moné, H. A new chronotype of Schistosoma mansoni: Adaptive significance. Trop. Med. Int. Health 2012, 17, 727–732. [Google Scholar] [CrossRef]
- Mouahid, G.; Mintsa Nguema, R.; Al Mashikhi, K.M.; Al Yafae, S.A.; Idris, M.A.; Moné, H. Host-parasite life-histories of the diurnal vs. nocturnal chronotypes of Schistosoma mansoni: Adaptive significance. Trop. Med. Int. Health 2019, 24, 692–700. [Google Scholar] [CrossRef]
- Wang, S.R.; Zhu, Y.J.; Ge, Q.P.; Yang, M.J.; Huang, J.L.; Huang, W.Q.; Zhuge, H.X.; Lu, D.B. Effect of photoperiod change on chronobiology of cercarial emergence of Schistosoma japonicum derived from hilly and marshy regions of China. Exp. Parasitol. 2015, 159, 227–232. [Google Scholar] [CrossRef]
- Cort, W.W. Studies on schistosome dermatitis XI. Status of knowledge after more than 20 years. Am. J. Hyg. 1950, 52, 251–307. [Google Scholar]
- Neuhaus, W. Biologie und Entwicklung von Trichobilharzia szidati n. sp. (Trematoda Schistosomatidae), einem Erreger von Dermatitis bei Menschen. Z. Parasitenkd. 1952, 15, 203–266. [Google Scholar] [CrossRef] [PubMed]
- Chernogorenko, M.I.; Boryak, Y.V. The biology of cercariae of Trichobilharzia ocellata La Val., 1854. Gidrobiol. Zhurnal 1973, 9, 104–108. (In Russian) [Google Scholar]
- Anderson, P.A.; Nowosielski, J.W.; Croll, N.A. The emergence of cercariae of Trichobilharzia ocellata and its relationship to the activity of its snail host Lymnaea stagnalis. Can. J. Zool. 1976, 54, 1481–1487. [Google Scholar] [CrossRef] [PubMed]
- Appleton, C.C.; Lethbridge, R.C. Schistosome dermatitis in the Swan Estuary, Western Australia. Med. J. Aust. 1979, 1, 141–145. [Google Scholar] [CrossRef]
- Sluiters, J.E.; Brussaard-Wust, C.M.; Meuleman, E.A. The relationship between miracidial dose, production of cercariae, and reproductive activity of the host in the combination Trichobilharzia ocellata and Lymnaea stagnalis. Z. Parasitenkd. 1980, 63, 13–26. [Google Scholar] [CrossRef]
- Leighton, B.J.; Zervos, S.; Webster, J.M. Ecological factors in schistosome transmission, and an environmentally benign method for controlling snails in a recreational lake with a record of schistosome dermatitis. Parasitol. Int. 2000, 49, 9–17. [Google Scholar] [CrossRef]
- Soldánová, M.; Selbach, C.; Sures, B. The early worm catches the bird? Productivity and patterns of Trichobilharzia szidati cercarial emission from Lymnaea stagnalis. PLoS ONE 2016, 11, e0149678. [Google Scholar] [CrossRef]
- Rudko, S.P.; Reimink, R.L.; Froelich, K.; Gordy, M.A.; Blankespoor, C.L.; Hanington, P.C. Use of qPCR-based cercariometry to assess swimmer’s itch in recreational lakes. Ecohealth 2018, 15, 827–839. [Google Scholar] [CrossRef]
- Al-Jubury, A.; Kania, P.; Bygum, A.; Buchmann, K. Temperature and light effects on Trichobilharzia szidati cercariae with implications for a risk analysis. Acta. Vet. Scand. 2020, 62, 54. [Google Scholar] [CrossRef]
- Oyarzún-Ruiz, P.; Thomas, P.; Santodomingo, A.; Collado, G.; Muñoz, P.; Moreno, L. Morphological, behavioral, and molecular characterization of avian schistosomes (Digenea: Schistosomatidae) in the native snail Chilina dombeyana (Chilinidae) from Southern Chile. Pathogens 2022, 11, 332. [Google Scholar] [CrossRef]
- Rojo-Vazquez, F.A.; Simon-Martin, F. Algunos aspectos de la biología de las cercarias de Trichobilharzia sp. del Rio Canedo (Provinciade Salamanca, Espana). Rev. Iber. Parasitol. 1985, 45, 141–148. (In Spanish) [Google Scholar]
- Rind, S. Three ocellate schistosome cercariae (Trematoda: Schistosomatidae) in Gyraulus corinna, with reference to Cercaria longicauda MacFarlane, 1944 in Lymnaea tomentosa. N. Z. J. Zool. 1991, 18, 53–62. [Google Scholar] [CrossRef]
- Shakarbayev, U.A.; Akramova, F.D.; Norkobilov, B.T.; Azimov, D.A. Cercariae of trematodes of mollusks (Gastropoda, Pulmonates) in reservoirs of Uzbekistan. Pharma Innov. J. 2020, 9, 607–611. [Google Scholar]
- Shostak, A.W.; Esch, G.W. Photocycle-dependent emergence by cercariae of Halipegus occidualis from Helisoma anceps, with special reference to cercarial emergence patterns as adaptations for transmission. J. Parasitol. 1990, 76, 790–795. [Google Scholar] [CrossRef]
- Poulin, R. Global warming and temperature-mediated increases in cercarial emergence in trematode parasites. Parasitology 2006, 132, 143–151. [Google Scholar] [CrossRef] [PubMed]
- Morley, N.J.; Lewis, J.W. Thermodynamics of cercarial development and emergence in trematodes. Parasitology 2013, 140, 1211–1224. [Google Scholar] [CrossRef] [PubMed]
- Selbach, C.; Poulin, R. Some like it hotter: Trematode transmission under changing temperature conditions. Oecologia 2020, 194, 745–755. [Google Scholar] [CrossRef]
- Wolmarans, C.T.; de Kock, K.N.; Strauss, H.D.; Bornman, M. Daily emergence of Schistosoma mansoni and S. haematobium cercariae from naturally infected snails under field conditions. J. Helminthol. 2002, 76, 273–277. [Google Scholar] [CrossRef]
- Lu, D.B.; Wang, T.P.; Rudge, J.W.; Donnelly, C.A.; Fang, G.R.; Webster, J.P. Evolution in a multi-host parasite: Chronobiological circadian rhythm and population genetics of Schistosoma japonicum cercariae indicates contrasting definitive host reservoirs by habitat. Int. J. Parasitol. 2009, 39, 1581–1588. [Google Scholar] [CrossRef]
- Skírnisson, K.; Aldhoun, J.A.; Kolářová, L. A review on swimmer’s itch and the occurrence of bird schistosomes in Iceland. J. Helminthol. 2009, 83, 165–171. [Google Scholar] [CrossRef]
- Nikolaev, K.E.; Levakin, I.A.; Galaktionov, K.V. A month for the mission: Using a sentinel approach to determine the transmission window of digenean cercariae in the subarctic White Sea. J. Helminthol. 2021, 95, e50. [Google Scholar] [CrossRef] [PubMed]
- Galaktionov, K.V. Patterns and processes influencing helminth parasites of Arctic coastal communities during climate change. J. Helminthol. 2017, 91, 387–408. [Google Scholar] [CrossRef] [PubMed]
- Brassard, P.; Curtis, M.A.; Rau, M.E. Seasonality of Diplostomum spathaceum (Trematoda, Strigeidae) transmission to brook trout (Salvelinus fontinalis) in northern Quebec, Canada. Can. J. Zool. 1982, 60, 2258–2263. [Google Scholar] [CrossRef]
- Prokofiev, V.V.; Galaktionov, K.V.; Levakin, I.A.; Nikolaev, K.E. Light or temperature? What regulates the emergency of trematode cercariae from the molluscan hosts and how it is done. Parazitologiya 2020, 54, 179–197. (In Russian) [Google Scholar]
- Larsen, A.H.; Bresciani, J.; Buchmann, K. Increasing frequency of cercarial dermatitis at higher latitudes. Acta Parasitol. 2004, 49, 217–221. [Google Scholar]
- Aldhoun, J.A.; Faltýnková, A.; Karvonen, A.; Horák, P. Schistosomes in the North: A unique finding from a prosobranch snail using molecular tools. Parasitol. Int. 2009, 58, 314–317. [Google Scholar] [CrossRef]
- Soleng, A.; Mehl, R. Geographical distribution of cercarial dermatitis in Norway. J. Helminthol. 2011, 85, 345–352. [Google Scholar] [CrossRef]
- Gordy, M.A.; Cobb, T.P.; Hanington, P.C. Swimmer’s itch in Canada: A look at the past and survey of the present to plan for the future. Environ. Health 2018, 17, 73. [Google Scholar] [CrossRef]
- Gordy, M.A.; Hanington, P.C. A fine-scale phylogenetic assessment of digenean trematodes in central Alberta reveals we have yet to uncover their total diversity. Ecol. Evol. 2019, 9, 3153–3238. [Google Scholar] [CrossRef]
- Soldánová, M.; Georgieva, S.; Roháčová, J.; Knudsen, R.; Kuhn, J.A.; Henriksen, E.H.; Siwertsson, A.; Shaw, J.C.; Kuris, A.M.; Amundsen, P.-A.; et al. Molecular analyses reveal high species diversity of trematodes in a sub-Arctic lake. Int. J. Parasitol. 2017, 47, 327–345. [Google Scholar] [CrossRef]
- Born-Torrijos, A.; Paterson, R.A.; van Beest, G.S.; Schwelm, J.; Vyhlídalová, T.; Henriksen, E.H.; Knudsen, R.; Kristoffersen, R.; Amundsen, P.-A.; Soldánová, M. Temperature does not influence functional response of amphipods consuming different trematode prey. Parasitol. Res. 2020, 119, 4271–4276. [Google Scholar] [CrossRef] [PubMed]
- Born-Torrijos, A.; Paterson, R.A.; van Beest, G.S.; Vyhlídalová, T.; Henriksen, E.H.; Knudsen, R.; Kristoffersen, R.; Amundsen, P.-A.; Soldánová, M. Cercarial behaviour alters the consumer functional response of three-spined sticklebacks. J. Anim. Ecol. 2021, 90, 978–988. [Google Scholar] [CrossRef] [PubMed]
- Born-Torrijos, A.; van Beest, G.S.; Vyhlídalová, T.; Knudsen, R.; Kristoffersen, R.; Amundsen, P.; Thieltges, D.W.; Soldánová, M. Taxa-specific activity loss and mortality patterns in freshwater trematode cercariae under subarctic conditions. Parasitology 2022, 149, 457–468. [Google Scholar] [CrossRef] [PubMed]
- Soldánová, M.; Kundid, P.; Scholz, T.; Kristoffersen, R.; Knudsen, R. Somatic dimorphism in cercariae of a bird schistosome. Pathogens 2022, 11, 290. [Google Scholar] [CrossRef] [PubMed]
- Marcogliese, D.J. Implications of climate change for parasitism of animals in the aquatic environment. Can. J. Zool. 2001, 79, 1331–1352. [Google Scholar] [CrossRef]
- Kutz, S.J.; Jenkins, E.J.; Veitch, A.M.; Ducrocq, J.; Polley, L.; Elkin, B.; Lair, S. The Arctic as a model for anticipating, preventing, and mitigating climate change impacts on host-parasite interactions. Vet. Parasitol. 2009, 163, 217–228. [Google Scholar] [CrossRef]
- Mas-Coma, S.; Valero, A.V.; Bargues, M.D. Climate change effects on trematodiases, with emphasis on zoonotic fascioliasis and schistosomiasis. Vet. Parasitol. 2009, 163, 264–280. [Google Scholar] [CrossRef]
- Hoberg, E.P.; Cook, J.A.; Agosta, S.J.; Boeger, W.; Galbreath, K.E.; Laaksonen, S.; Kutz, S.J.; Brooks, D.R. Arctic systems in the Quaternary: Ecological collision, faunal mosaics and the consequences of a wobbling climate. J. Helminthol. 2017, 91, 409–421. [Google Scholar] [CrossRef]
- Lashaki, E.K.; Teshnizi, S.H.; Gholami, S.; Fakhar, M.; Brant, S.V.; Dodangeh, S. Global prevalence status of avian schistosomes: A systematic review with meta-analysis. Parasite Epidemiol. Control 2020, 9, e00142. [Google Scholar] [CrossRef]
- Prokofiev, V.V.; Galaktionov, K.V.; Levakin, I.A. Patterns of parasite transmission in polar seas: Daily rhythms of cercarial emergence from intertidal snails. J. Sea Res. 2016, 113, 85–98. [Google Scholar] [CrossRef]
- Vyhlídalová, T.; Soldánová, M. Species-specific patterns in cercarial emergence of Diplostomum spp. from snails Radix lagotis. Int. J. Parasitol. 2020, 50, 1177–1188. [Google Scholar] [CrossRef] [PubMed]
- Anderson, R.M.; May, R.M. Prevalence of schistosome infections within molluscan populations: Observed patterns and theoretical predictions. Parasitology 1979, 79, 63–94. [Google Scholar] [CrossRef] [PubMed]
- N’Goran, E.; Brémond, P.; Sellin, E.; Sellin, B.; Théron, A. Intraspecific diversity of Schistosoma haematobium in west Africa: Chronobiology of cercarial emergence. Acta Trop. 1997, 66, 35–44. [Google Scholar] [CrossRef]
- Noda, S.; Sato, K.; Katsumata, T.; Nojima, H.; Muhoho, N.D. The influence of shadowing on emergence of Schistosoma haematobium during day time. Jpn. J. Parasitol. 1986, 35, 249.e251. [Google Scholar]
- Arp, C.D.; Jones, B.M.; Whitman, M.; Larsen, A.; Urban, F.E. Lake temperature and ice cover regimes in the Alaskan Subarctic and Arctic: Integrated monitoring, remote sensing, and modeling. J. Am. Water Resour. Assoc. 2010, 46, 777–791. [Google Scholar] [CrossRef]
- Thieltges, D.W.; Rick, J. Effect of temperature on emergence, survival and infectivity of cercariae of the marine trematode Renicola roscovita (Digenea: Renicolidae). Dis. Aquat. Org. 2006, 73, 63–68. [Google Scholar]
- Thieltges, D.W.; Jensen, K.T.; Poulin, R. The role of biotic factors in the transmission of free-living endohelminth stages. Parasitology 2008, 135, 407–426. [Google Scholar] [CrossRef]
- Johnson, P.T.J.; Dobson, A.; Lafferty, K.D.; Marcogliese, D.J.; Memmott, J.; Orlofske, S.A.; Poulin, R.; Thieltges, D.W. When parasites become prey: Ecological and epidemiological significance of eating parasites. Trends Ecol. Evol. 2010, 25, 362–371. [Google Scholar] [CrossRef]
- Koprivnikar, J. The enemy of my enemy is my friend: Consumption of parasite infectious stages benefits hosts and predators depending on transmission mode. J. Anim. Ecol. 2022, 91, 4–7. [Google Scholar] [CrossRef]
- Morley, N.J.; Adam, M.E.; Lewis, J.W. The effects of host size and temperature on the emergence of Echinoparyphium recurvatum cercariae from Lymnaea peregra under natural light conditions. J. Helminthol. 2010, 84, 317–326. [Google Scholar] [CrossRef]
- Graham, L.A. Effect of snail size on the prevalence and intensity of avian schistosome infection: Relating laboratory to field studies. J. Parasitol. 2003, 89, 458–463. [Google Scholar] [CrossRef]
- Lim, H.K.; Heyneman, D. Intramolluscan inter-trematode antagonism: A review of factors influencing the host-parasite system and its possible role in biological control. Adv. Parasitol. 1972, 10, 191–268. [Google Scholar] [CrossRef] [PubMed]
- McCarthy, A.M. Photoperiodic cercarial emergence patterns of the digeneans Echinoparyphium recurvatum and Plagiorchis sp. from a mixed infection in Lymnaea peregra. J. Helminthol. 1999, 73, 59–62. [Google Scholar] [CrossRef]
- Soldánová, M.; Kuris, A.M.; Scholz, T.; Lafferty, K.D. The role of spatial and temporal heterogeneity and competition in structuring trematode communities in the great pond snail, Lymnaea stagnalis (L.). J. Parasitol. 2012, 98, 460–471. [Google Scholar] [CrossRef]
- Massoud, J. The effect of variation in miracidial exposure dose on laboratory infections of Ornithobilharzia turkestanicum in Lymnaea gedrosiana. J. Helminthol. 1974, 4, 139–144. [Google Scholar] [CrossRef]
- Seppälä, O.; Liljeroos, K.; Karvonen, A.; Jokela, J. Host condition as a constraint for parasite reproduction. Oikos 2008, 117, 749–753. [Google Scholar] [CrossRef]
- Berkhout, B.W.; Lloyd, M.M.; Poulin, R.; Studer, A. Variation among genotypes in response to increasing temperature in a marine parasite: Evolutionary potential in the face of global warming? Int. J. Parasitol. 2014, 44, 1019–1027. [Google Scholar] [CrossRef]
- Klemetsen, A.; Knudsen, R. Diversity and abundance of water birds in a subarctic lake during three decades. Fauna Norv. 2013, 33, 21–27. [Google Scholar] [CrossRef][Green Version]
- Sayler, R.D.; Afton, A.D. Ecological aspects of common goldeneyes Bucephala clangula wintering on the upper Mississippi River. Ornis Scand. 1981, 12, 99–108. [Google Scholar] [CrossRef]
- Danell, K.; Sjöberg, K. Seasonal and diel changes in the feeding behaviour of some dabbling duck species on a breeding lake in northern Sweden. Ornis Scand. 1982, 13, 129–134. [Google Scholar] [CrossRef]
- Amundsen, P.-A.; Primicerio, R.; Smalås, A.; Henriksen, E.H.; Knudsen, R.; Kristoffersen, R.; Klemetsen, A. Long-term ecological studies in northern lakes - challenges, experiences, and accomplishments. Limnol. Oceanogr. 2019, 64, S11–S21. [Google Scholar] [CrossRef]
- Jouet, D.; Skírnisson, K.; Kolářová, L.; Ferté, H. Molecular diversity of Trichobilharzia franki in two intermediate hosts (Radix auricularia and Radix peregra): A complex of species. Infect. Genet. Evol. 2010, 10, 1218–1227. [Google Scholar] [CrossRef] [PubMed]
- WMO (World Meteorological Organization). 2018 Annual Report: WMO for the the Twenty-First Century; WMO: Geneva, Switzerland, 2018; p. 24. [Google Scholar]

| Snail Samples | 2016 | 2016 | 2017 | 2017 | 2018 | 2018 | ||
|---|---|---|---|---|---|---|---|---|
| August | October | August | October | August | October | |||
| No. of examined snails | 286 | 347 | 434 | 577 | 725 | 689 | ||
| No. of infected snails with trematodes (overall prevalence, %) | 67 (23.4) | 72 (20.8) | 150 (34.6) | 153 (26.5) | 235 (32.4) | 144 (20.9) | ||
| No. of infected snails with patent and prepatent Trichobilharzia (prevalence, %) | 7 (2.5) | 0 | 35 (8.1) | 9 (1.6) | 34 (4.7) | 14 (2.0) | ||
| No. of infected snails with patent Trichobilharzia | 7 | 0 | 22 | 0 | 28 | 8 | ||
| Experimental Setup | August 2016 | August 2017 | August 2018 | October 2018 | ||||
| Field | Laboratory | Field | Laboratory | Field | Laboratory | Field | Laboratory | |
| No. of experimental days | 2 | 1 | 3 | 3 | 3 | 3 | 3 | 3 |
| No. of diel intervals monitored per 24 h | 4 and 2 a | 4 | 5 | 5 | 5 | 2 | 5 | 2 |
| No. of experimental snails, initial and [survived] | 7 [7] | 7 [7] | 6 [2] | 1 [1] | 6 [6] | 4 [3] | 6 [6] | 6 [6] |
| Water temperature, mean (range, °C) | 8.3 (6.6–9.9) | 13.8 (13.7–14.1) | 13.0 (10.7–14.8) | 13.4 (13.0–14.1) | 14.8 (8.5–17.7) | 14.2 (12.5–15.9) | 6.4 (5.9–6.8) | 5.7 (5.5–6.5) |
| Water illumination, mean (range, Lx) | 4856 (0–49,600) | 437 (0–538) | 7250 (0–88,178) | 397 (0–742) | 5433 (0–74,400) | 710 (0–1044) | 903 (0–7578) | 796 (0–2239) |
| Daily Output Rates of Cercariae | ||||||||
| Total no. from initial and [surviving] snails | 2101 | 631 | 1668 (1385) | 894 | 10,928 | 8763 (5575) | 38 | 21 |
| Range from initial and [surviving] snails | 1–1295 | 14–222 | 0–520 (5–520) | 104–537 | 29–2067 | 116–1604 (116–1291) | 0–12 | 0–21 |
| Mean ± SD from initial and [surviving] snails | 150 ± 337 | 90 ± 72 | 128 ± 164 [231 ± 175] | 298 | 607 ± 496 | 797 ± 519 [619 ± 377] | 2 ± 4 | 1 ± 5 |
| Season/Type of Experiment | Experimental Day | Observed Number of Emerged Cercariae (Mean) during Diel Intervals | ||||
|---|---|---|---|---|---|---|
| August 2016 | 0:00–6:00 | 6:00–12:00 | 12:00–18:00 | 18:00–0:00 | ||
| Field | Day 1 (n a = 7) | 1704 (243) | 131 (19) | 114 (16) | 20 (3) | |
| 12:00–0:00 | 0:00–12:00 | |||||
| Day 2 (n = 7) | 114 (16) | 18 (3) | ||||
| 0:00–6:00 | 6:00–12:00 | 12:00–18:00 | 18:00–0:00 | |||
| Laboratory | Day 1 (n = 7) | 518 (74) | >80 (11) | >25 (4) | >8 (1) | > |
| August | Sunrise | Day 1 | Day 2 | Sunset | Night | |
| 2017 | 2:00–4:30 | 4:33–13:15 | 13:15–22:00 | 22:00–0:30 | 0:30–2:00 | |
| Field | Day 1 (n = 6) | 135 (23) | 238 (40) | 91 (15) | 34 (6) | 7 (1) |
| Day 2 (n = 5) | 244 (49) | 349 (70) | 151 (30) | 1 (0.2) | 1 (0.2) | |
| Day 3 (n = 2) | 342 (171) | 35 (18) | 20 (10) | 14 (7) | 6 (3) | |
| Laboratory | Day 1 (n = 1) | 91 | 25 | 1 | 136 | 0 |
| Day 2 (n = 1) | 528 | 5 | 0 | 4 | 0 | |
| Day 3 (n = 1) | 84 | 15 | 0 | 5 | 0 | |
| August | Sunrise | Day 1 | Day 2 | Sunset | Night | |
| 2018 | 1:30–4:00 | 4:00–12:45 | 12:45–21:30 | 21:30–0:00 | 0:00–1:30 | |
| Field | Day 1 (n = 6) | 4032 (672) | 554 (92) | 9 (2) | 23 (4) | 1 (0.2) |
| Day 2 (n = 6) | 2924 (487) | 511 (85) | 17 (3) | 0 (0) | 1 (0.2) | |
| Day 3 (n = 6) | 1642 (247) | 688 (115) | 121(20) | 140 (23) | 265 (44) | |
| 9:00–21:00 | 21:00–9:00 | |||||
| Laboratory | Day 1 (n = 4) | 84 (21) | 3868 (967) | |||
| Day 2 (n = 4) | 9 (2) | 2968 (742) | ||||
| Day 3 (n = 3) | 6 (2) | 1828 (609) | ||||
| October | Sunrise | Day | Sunset | Night 1 | Night 2 | |
| 2018 | 7:00–8:00 | 8:00–17:15 | 17:15–18:15 | 18:15–0:40 | 0:40–7:00 | |
| Field | Day 1 (n = 6) | 1 (0.2) | 7 (1) | 0 (0) | 1 (0.2) | 2 (0.3) |
| Day 2 (n = 6) | 7 (1) | 6 (1) | 1 (0.2) | 1 (0.2) | 1 (0.2) | |
| Day 3 (n = 6) | 6 (1) | 5 (1) | 0 (0) | 0 (0) | 0 (0) | |
| 9:00–21:00 | 21:00–9:00 | |||||
| Laboratory | Day 1 (n = 6) | 0 (0) | 0 (0) | |||
| Day 2 (n = 6) | 0 (0) | 0 (0) | ||||
| Day 3 (n = 6) | 13 (2) | 8 (1) | ||||
| Season/ Experimental Dday | No. of Snails | Temperature | Light Intensity | Snail Length | |||
|---|---|---|---|---|---|---|---|
| ra | pb | r | p | r | p | ||
| August 2016 | |||||||
| Day 1 | 6 | −0.680 | 0.320 | −0.317 | 0.683 | 0.463 | 0.296 |
| Day 2 | 6 | – c | – | – c | – | −0.358 | 0.430 |
| Pooled data | 6 | – c | – | – c | – | 0.382 | 0.397 |
| August 2017 | |||||||
| Day 1 | 6 | −0.164 | 0.793 | 0.080 | 0.897 | 0.486 | 0.329 |
| Day 2 | 5 | 0.042 | 0.947 | 0.265 | 0.667 | −0.004 | 0.995 |
| Day 3 | 2 | 0.416 | 0.486 | −0.495 | 0.397 | – d | – |
| Pooled data | 6 | 0.145 | 0.606 | −0.103 | 0.715 | 0.251 | 0.632 |
| August 2018 | |||||||
| Day 1 | 6 | −0.224 | 0.717 | −0.012 | 0.985 | 0.329 | 0.525 |
| Day 2 | 6 | −0.276 | 0.653 | −0.295 | 0.630 | 0.233 | 0.657 |
| Day 3 | 6 | 0.713 | 0.176 | −0.650 | 0.235 | 0.497 | 0.316 |
| Pooled data | 6 | −0.116 | 0.717 | −0.281 | 0.352 | 0.511 | 0.301 |
| October 2018 | |||||||
| Day 1 | 6 | −0.873 | 0.053 | 0.510 | 0.380 | 0.958 | 0.003 |
| Day 2 | 6 | −0.401 | 0.503 | −0.149 | 0.811 | 0.790 | 0.062 |
| Day 3 | 6 | 0.406 | 0.498 | −0.119 | 0.849 | 0.740 | 0.092 |
| Pooled data | 6 | −0.018 | 0.948 | −0.080 | 0.776 | 0.844 | 0.034 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Soldánová, M.; Born-Torrijos, A.; Kristoffersen, R.; Knudsen, R.; Amundsen, P.-A.; Scholz, T. Cercariae of a Bird Schistosome Follow a Similar Emergence Pattern under Different Subarctic Conditions: First Experimental Study. Pathogens 2022, 11, 647. https://doi.org/10.3390/pathogens11060647
Soldánová M, Born-Torrijos A, Kristoffersen R, Knudsen R, Amundsen P-A, Scholz T. Cercariae of a Bird Schistosome Follow a Similar Emergence Pattern under Different Subarctic Conditions: First Experimental Study. Pathogens. 2022; 11(6):647. https://doi.org/10.3390/pathogens11060647
Chicago/Turabian StyleSoldánová, Miroslava, Ana Born-Torrijos, Roar Kristoffersen, Rune Knudsen, Per-Arne Amundsen, and Tomáš Scholz. 2022. "Cercariae of a Bird Schistosome Follow a Similar Emergence Pattern under Different Subarctic Conditions: First Experimental Study" Pathogens 11, no. 6: 647. https://doi.org/10.3390/pathogens11060647
APA StyleSoldánová, M., Born-Torrijos, A., Kristoffersen, R., Knudsen, R., Amundsen, P.-A., & Scholz, T. (2022). Cercariae of a Bird Schistosome Follow a Similar Emergence Pattern under Different Subarctic Conditions: First Experimental Study. Pathogens, 11(6), 647. https://doi.org/10.3390/pathogens11060647