Controlled Recirculating Wet Storage Purging V. parahaemolyticus in Oysters
Abstract
:1. Introduction
2. Results
2.1. Occurrence and Accumulation of V. parahaemolyticus in Oyster
2.2. Effects of Salinities on the Reduction of Vibrio
2.3. Effects of Temperatures on the Reduction of Vibrio
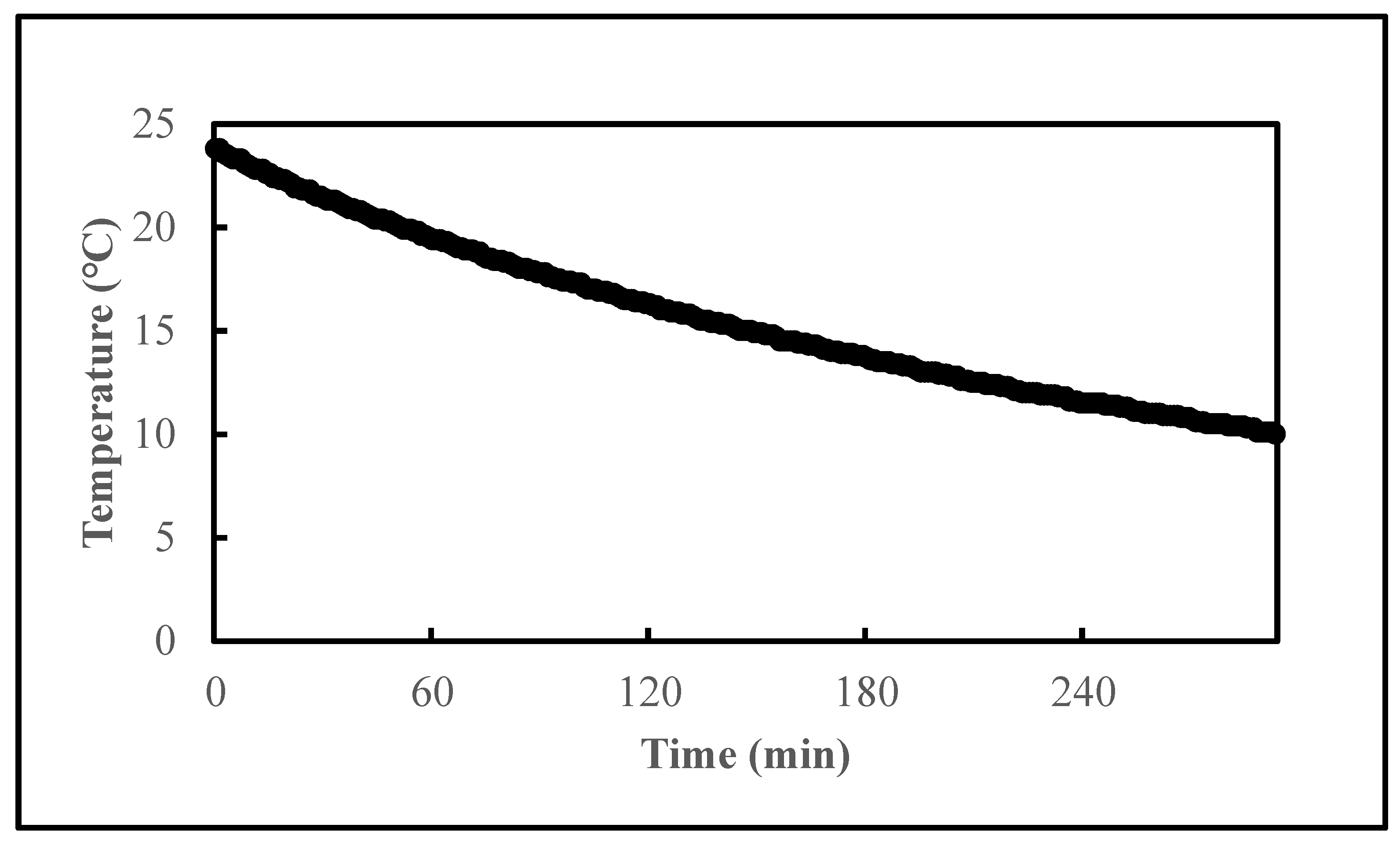
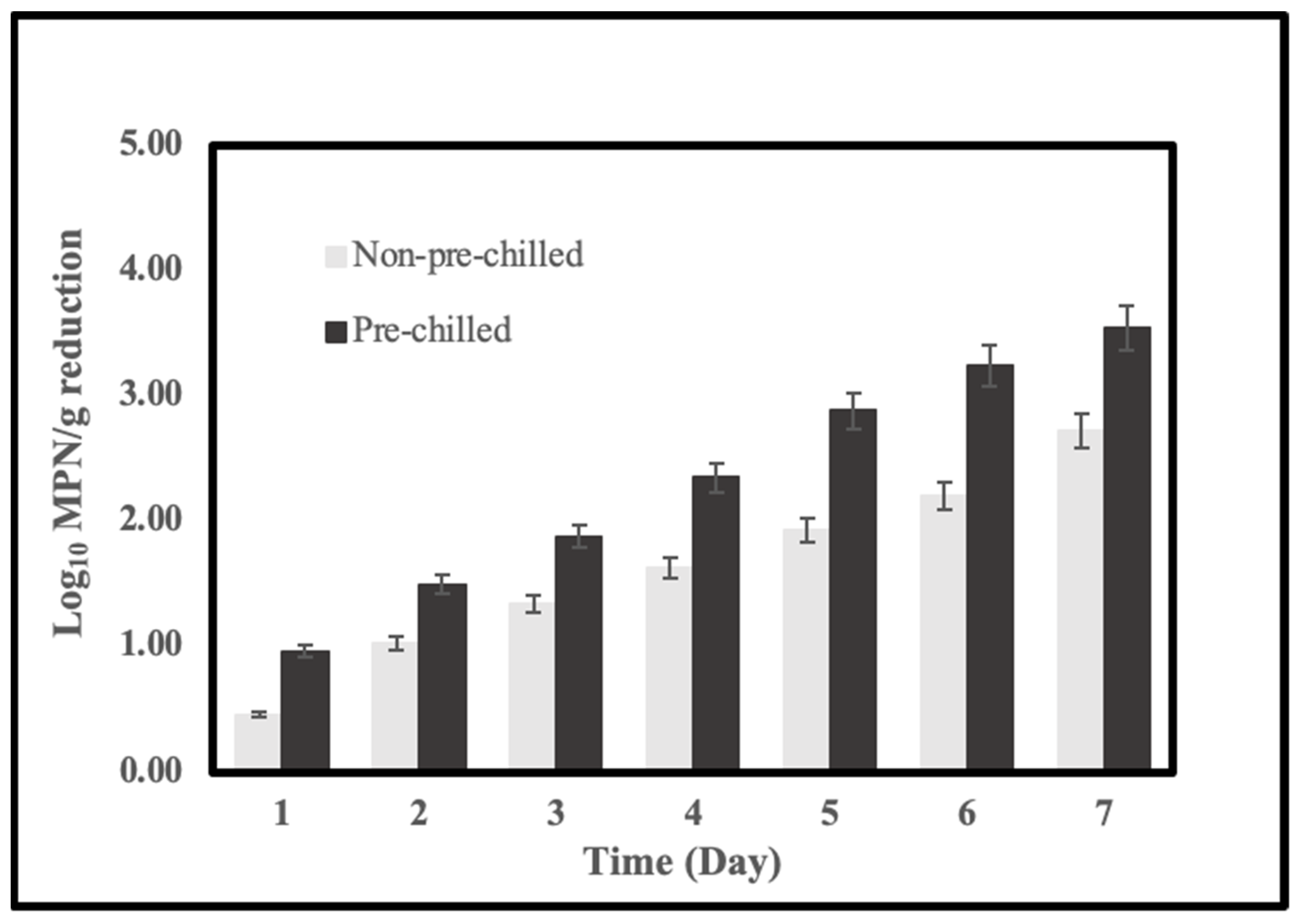
2.4. Effects of System Pre-Chilling on the Reduction of Vibrio
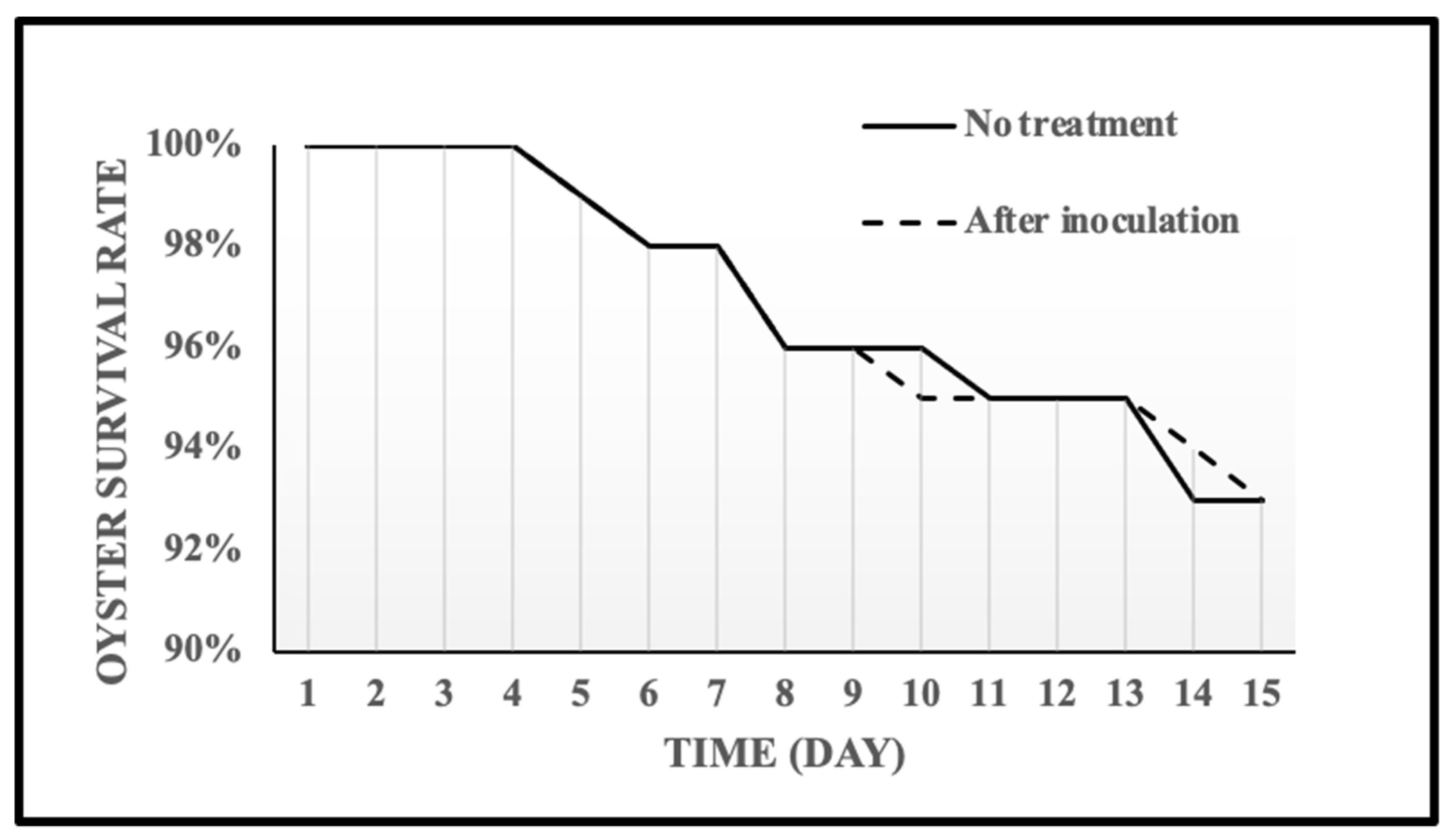
2.5. Shelf Life of the Oyster
3. Discussion
4. Materials and Methods
4.1. V. parahaemolyticus Cultures Preparation
4.2. Oyster Preparation
4.3. Accumulation of V. parahaemolyticus in Oysters
4.4. Oyster Depuration
4.5. Microbiological Analysis
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Su, Y.C.; Yang, Q.; Häse, C. Refrigerated seawater depuration for reducing Vibrio parahaemolyticus contamination in pacific oyster (Crassostrea gigas). J. Food Prot. 2010, 73, 1111–1115. [Google Scholar] [CrossRef] [PubMed]
- Phuvasate, S.; Su, Y.C. Impact of water salinity and types of oysters on depuration for reducing Vibrio parahaemolyticus in pacific oysters (Crassostrea gigas). Food Control 2013, 32, 569–573. [Google Scholar] [CrossRef]
- Center for Disease Control and Prevention (CDC). 2006. Available online: http://www.cdc.gov/mmwr/preview/mmwrhtml/mm55d807a1.htm (accessed on 18 September 2018).
- Jones, J.L.; Lydon, K.A.; Kinsey, T.P.; Friedman, B.; Curtis, M.; Schuster, R. Effects of ambient exposure, refrigeration, and icing on, Vibrio vulnificus, and, Vibrio parahaemolyticus, abundances in oysters. Inter. J. Food Microbiol. 2017, 253, 54–58. [Google Scholar] [CrossRef] [PubMed]
- Taylor, M.A.; Yu, J.W.; Howell, T.L.; Jones, S.H. Varying success of relaying to reduce Vibrio parahaemolyticus levels in oysters (Crassostrea virginica). J. Food Prot. 2018, 81, 659–669. [Google Scholar] [CrossRef] [PubMed]
- Gooch, J.A.; Depaola, A.; Bowers, J.; Marshall, D.L. Growth and survival of Vibrio parahaemolyticus in postharvest American oysters. J. Food Prot. 2002, 65, 970–974. [Google Scholar] [CrossRef] [PubMed]
- Gulnihal, O.; Chintapenta, L.K.; Amy, C.; Kenneth, H. Effects of microbial and heavy metal contaminants on environmental/ecological health and revitalization of coastal ecosystems in Delaware bay. Front. Environ. Sci. 2017, 5, 26. [Google Scholar]
- Sonja, O.; Antje, W.; Thomas, M.; Markus, K.; Sarah, R.; Gunnar, G. A polyphasic approach for the differentiation of environmental vibrio isolates from temperate waters. FEMS Microbiol. Ecol. 2015, 1, 145–162. [Google Scholar]
- Public Health Agency of Canada. 2015. Available online: http://www.phac-aspc.gc.ca/phn-asp/2015/vibrioparahaemolyticus-eng.php (accessed on 18 September 2018).
- Wang, W.; Li, M.; Li, Y. Intervention strategies for reducing Vibrio parahaemolyticus in seafood: A review. J. Food Sci. 2015, 80, 10–19. [Google Scholar] [CrossRef]
- Liu, C.; Lu, J.; Su, Y.-C. Effects of flash freezing, followed by frozen storage, on reducing Vibrio parahaemolyticus in pacific raw oysters (Crassostrea gigas). J. Food Prot. 2009, 72, 174–177. [Google Scholar] [CrossRef]
- Ma, L.; Su, Y.C. Validation of high pressure processing for inactivating Vibrio parahaemolyticus in pacific oysters (Crassostrea gigas). Int. J. Food Microbiol. 2011, 144, 469–474. [Google Scholar] [CrossRef]
- Ramos, R.J.; Miotto, M.; Squella, F.J.L.; Cirolini, A.; Ferreira, J.F.; Vieira, C.R.W. Depuration of oysters (Crassostrea gigas) contaminated with Vibrio parahaemolyticus and Vibrio vulnificus with UV light and chlorinated seawater. J. Food Prot. 2012, 75, 1501. [Google Scholar] [CrossRef] [PubMed]
- Ren, T.; Su, Y.C. Effects of electrolyzed oxidizing water treatment on reducing Vibrio parahaemolyticus and Vibrio vulnificus in raw oysters. J. Food Prot. 2006, 69, 1829–1834. [Google Scholar] [CrossRef]
- Park, S.Y.; Chung, M.S.; Ha, S.D. Combined effect of sodium hypochlorite and gamma-irradiation for the control of Vibrio vulnificus in fresh oyster and clam. LWT 2018, 91, 568–572. [Google Scholar] [CrossRef]
- Phuvasate, S.; Chen, M.H.; Su, Y.C. Reductions of vibrio parahaemolyticus in pacific oysters (Crassostrea gigas) by depuration at various temperatures. Food Microbiol. 2012, 31, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.J.; Cheney, D.; Su, Y.-C. Temperature Effects on the Depuration of Vibrio parahaemolyticus and Vibrio vulnificus from the American Oyster (Crassostrea virginica). J. Food Sci. 2009, 74, 62–66. [Google Scholar] [CrossRef] [PubMed]
- United States Food and Drug Administration. National Shellfish Sanitation Program (NSSP) Guide for the Control of Molluscan Shellfish (2019 Revision). Available online: https://www.fda.gov/media/143238/download (accessed on 10 April 2022).
- Su, Y.C.; Liu, C. Vibrio parahaemolyticus: A concern of seafood safety. Food Microbiol. 2007, 24, 549–558. [Google Scholar] [CrossRef] [PubMed]
- Shen, X.; Su, Y.C.; Liu, C.; Oscar, T.; Depola, A. Efficacy of Vibrio parahaemolyticus depuration in oysters (Crassostrea gigas). Food Microbiol. 2019, 79, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Son, N.T.; Fleet, G.H. Behavior of pathogenic bacteria in the oyster, Crassostrea commercialis, during depuration, re-laying and storage. Appl. Environ. Microbiol. 1980, 40, 994–1002. [Google Scholar] [CrossRef] [Green Version]
- Eyles, M.J.; Davey, G.R. Microbiology of commercial depuration of Sydney rock oyster, Crassostrea commercialis. J. Food Prot. 1984, 47, 703–706. [Google Scholar] [CrossRef]
- Timoney, J.F.; Abston, A. Accumulation and elimination of Escherichia coli and Salmonella typhimurium by hard clams in an in vitro system. Appl. Environ. Microbiol. 1984, 47, 986–988. [Google Scholar] [CrossRef] [Green Version]
- Parveen, S.; Hettiarachchi, K.A.; Bowers, J.C.; Jones, J.L.; Tamplin, M.L.; Mckay, R.; Beatty, W.; Brohawn, K.; Dasilva, L.V.; Depaola, A. Seasonal distribution of total and pathogenic Vibrio parahaemolyticus in chesapeake bay oysters and waters. Int. J. Food Microbiol. 2008, 128, 354–361. [Google Scholar] [CrossRef] [PubMed]
- Kaysner, C.A.; De Paola, A., Jr. Vibrio . In FDA Bacteriological Analytical Manual (BAM); 2004. Available online: https://www.fda.gov/Food/FoodScienceResearch/LaboratoryMethods/ucm070830.htm (accessed on 1 December 2021).
- Duan, J.; Su, Y.C. Comparison of a chromogenic medium with thiosulfate-citrate-bile salts-sucrose agar for detecting vibrio parahaemolyticus. J. Food Sci. 2005, 70, 125–128. [Google Scholar] [CrossRef]

| Vibrio Levels | Date | |||||
|---|---|---|---|---|---|---|
| 28 November 2017 | 12 December 2017 | 10 April 2018 | 4 June 2018 | 2 July 2018 | 31 July 2018 | |
| Before inoculation | 0.54 ± 0.16 | 0.45 ± 0.16 | 0.36 ± 0.00 | 2.44 ± 0.20 | 3.04 ± 0.15 | 3.31 ± 0.31 |
| After inoculation | 5.92 ± 0.22 | 5.41 ± 0.24 | 5.50 ± 0.28 | 5.79 ± 0.22 | 5.17 ± 0.21 | 5.79 ± 0.22 |
| Time (Day) | Salinity (ppt) | ||
|---|---|---|---|
| 15 | 20 | 25 | |
| 0 | 5.18 ± 0.00 A | 5.50 ± 0.28 A | 5.41 ± 0.24 A |
| 1 | 4.86 ± 0.19 B | 4.86 ± 0.19 B | 4.79 ± 0.22 B |
| 2 | 3.86 ± 0.19 C | 3.99 ± 0.37 C | 4.24 ± 0.12 C |
| 3 | 3.65 ± 0.30 CD | 3.75 ± 0.19 CD | 3.97 ± 0.00 CD |
| 4 | 3.30 ± 0.11 D | 3.30 ± 0.11 DE | 3.66 ± 0.00 DE |
| 5 | 2.97 ± 0.00 E | 2.97 ± 0.00 EF | 3.24 ± 0.24 EF |
| 6 | 2.53 ± 0.18 E | 2.63 ± 0.00 FG | 2.86 ± 0.19 FG |
| 7 | 2.32 ± 0.00 F | 2.36 ± 0.00 G | 2.63 ± 0.00 G |
| Total log reduction | 2.85 ± 0.00 a | 3.14 ± 0.00 b | 2.77 ± 0.12 a |
| FDA Requirement Achieved (3 log reduction) | No | Yes | Yes |
| Time (Day) | Temperature (°C) | ||
|---|---|---|---|
| 7.5 | 10 | 12.5 | |
| 0 | 5.79 ± 0.22 A | 5.50 ± 0.28 A | 5.47 ± 0.16 A |
| 1 | 5.31 ± 0.12 B | 4.86 ± 0.19 B | 4.72 ± 0.29 B |
| 2 | 4.29 ± 0.11 C | 3.99 ± 0.37 C | 4.38 ± 0.00 BC |
| 3 | 3.86 ± 0.19 D | 3.75 ± 0.19 CD | 3.86 ± 0.28 CD |
| 4 | 3.01 ± 0.06 EF | 3.30 ± 0.11 DE | 3.65 ± 0.19 DE |
| 5 | 2.77 ± 0.12 F | 2.97 ± 0.00 EF | 3.57 ± 0.30 DEF |
| 6 | 2.57 ± 0.16 F | 2.63 ± 0.00 FG | 3.24 ± 0.16 EF |
| 7 | 2.41 ± 0.05 F | 2.36 ± 0.00 G | 3.04 ± 0.12 F |
| Total log reduction | 3.38 ± 0.05 a | 3.14 ± 0.00 b | 2.44 ± 0.12 c |
| FDA Requirement Achieved (3 log reduction) | Yes | Yes | No |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mu, R.; Liu, C.; Parveen, S.; Webster, D.; Pang, J. Controlled Recirculating Wet Storage Purging V. parahaemolyticus in Oysters. Pathogens 2022, 11, 553. https://doi.org/10.3390/pathogens11050553
Mu R, Liu C, Parveen S, Webster D, Pang J. Controlled Recirculating Wet Storage Purging V. parahaemolyticus in Oysters. Pathogens. 2022; 11(5):553. https://doi.org/10.3390/pathogens11050553
Chicago/Turabian StyleMu, Ruojun, Chengchu Liu, Salina Parveen, Donald Webster, and Jie Pang. 2022. "Controlled Recirculating Wet Storage Purging V. parahaemolyticus in Oysters" Pathogens 11, no. 5: 553. https://doi.org/10.3390/pathogens11050553
APA StyleMu, R., Liu, C., Parveen, S., Webster, D., & Pang, J. (2022). Controlled Recirculating Wet Storage Purging V. parahaemolyticus in Oysters. Pathogens, 11(5), 553. https://doi.org/10.3390/pathogens11050553






