Trash to Treasure: How Insect Protein and Waste Containers Can Improve the Environmental Footprint of Mosquito Egg Releases
Abstract
:1. Introduction
2. Materials and Methods
2.1. Mosquito Strains and Maintenance
2.2. Mosquito Rearing
2.2.1. Larval Diet
2.2.2. Four-Diet Comparison
2.2.3. Test of Food and Eggs Inside Capsules
2.2.4. Waste Container Comparison
2.3. Hatch Rate
2.4. Emergence Rate
2.5. Wing Length
2.6. Wolbachia Density
2.7. Statistical Analysis
2.8. Kiribati Case Study
2.8.1. Kiribati Waste Container Field Trial
2.8.2. Cost Analysis
3. Results
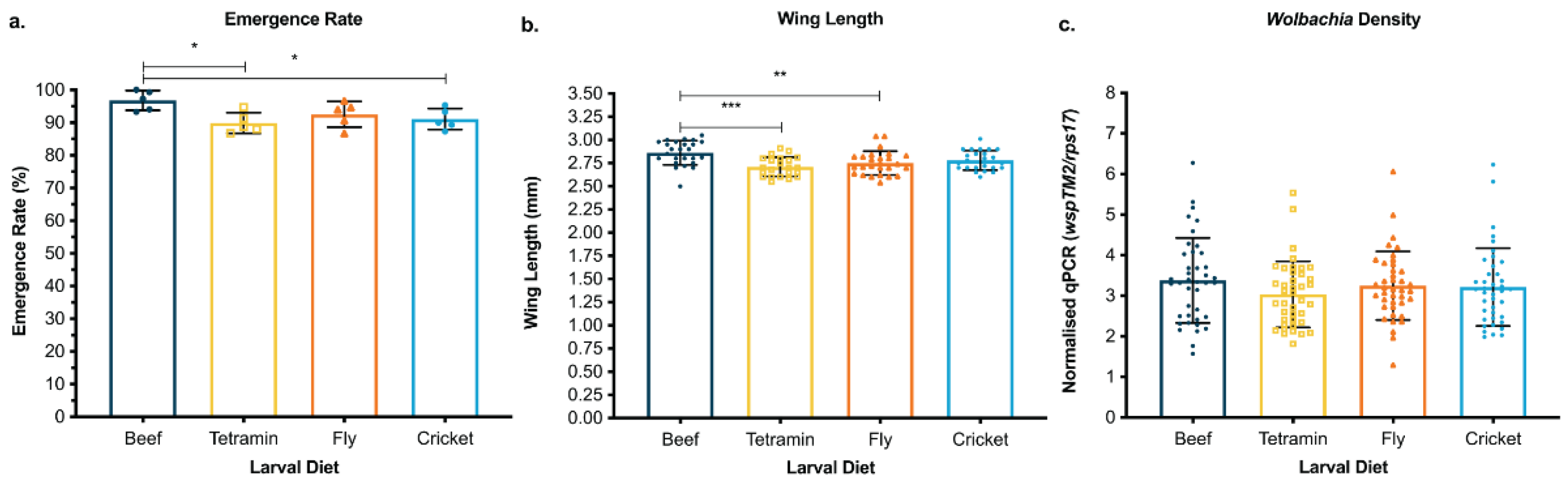
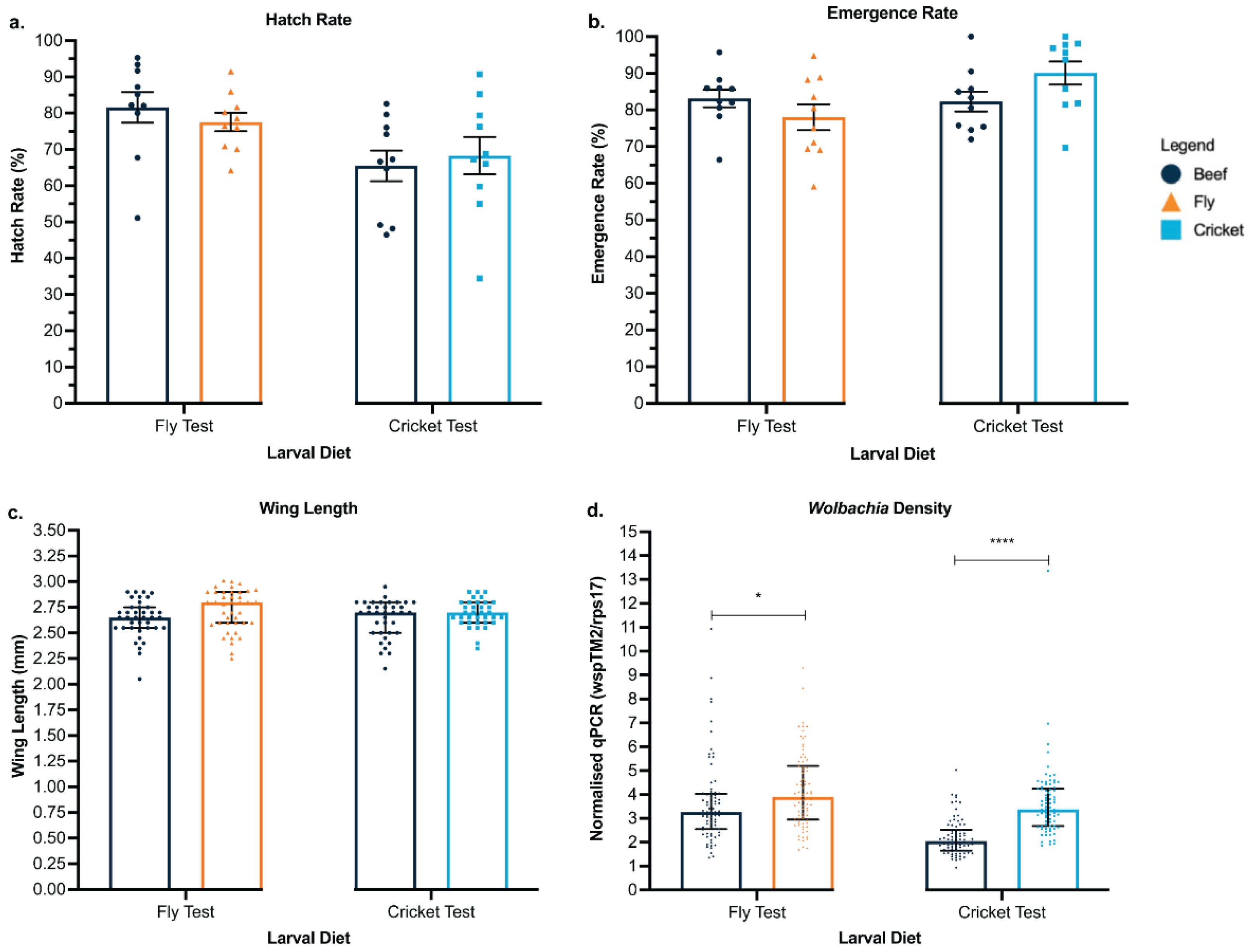
3.1. Cricket and Black Soldier Fly Meal Offer Viable and Environmentally Friendly Protein Sources for Mosquito Larval Diet
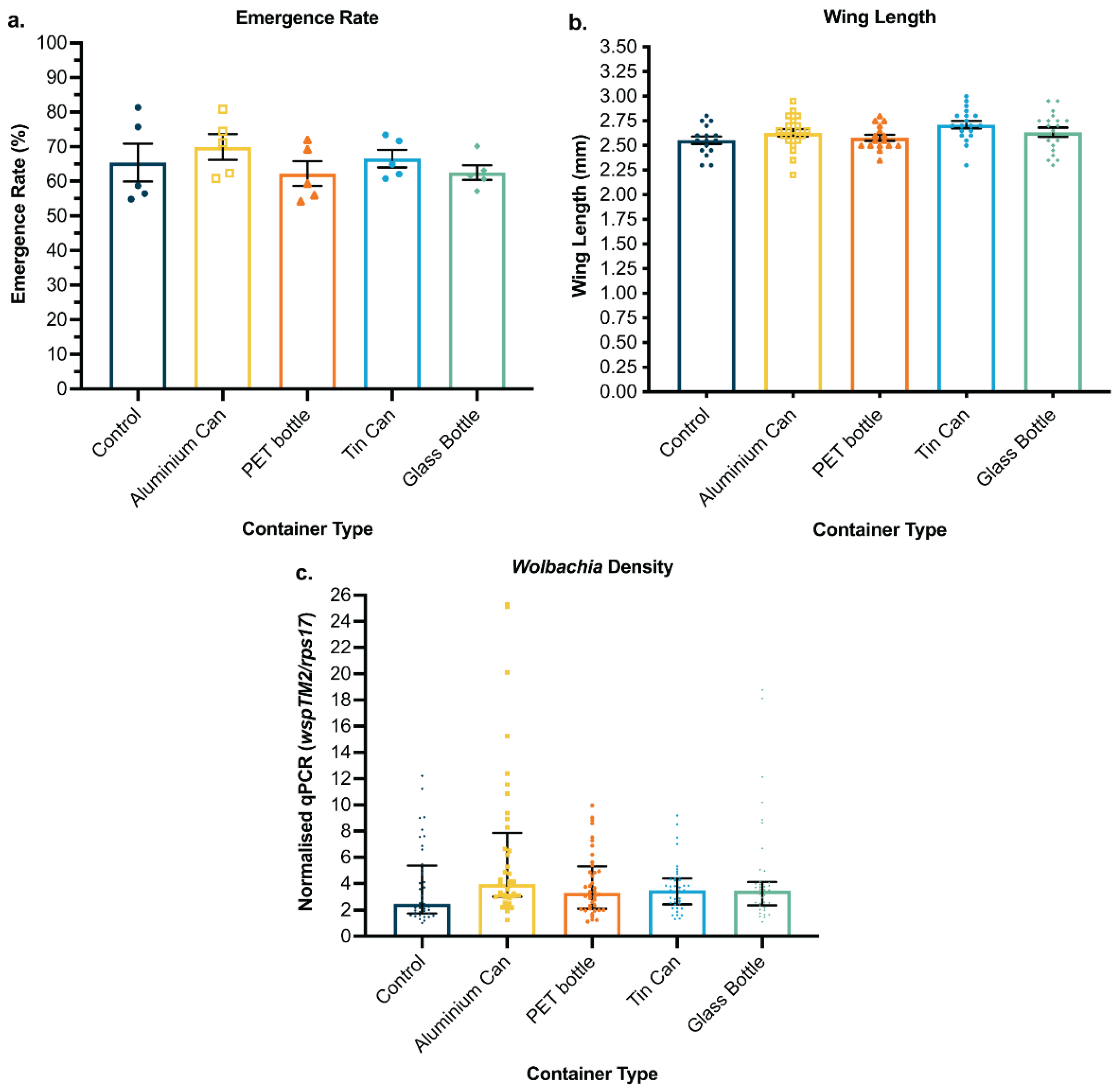
3.2. Waste Containers Support Successful Aquatic Development
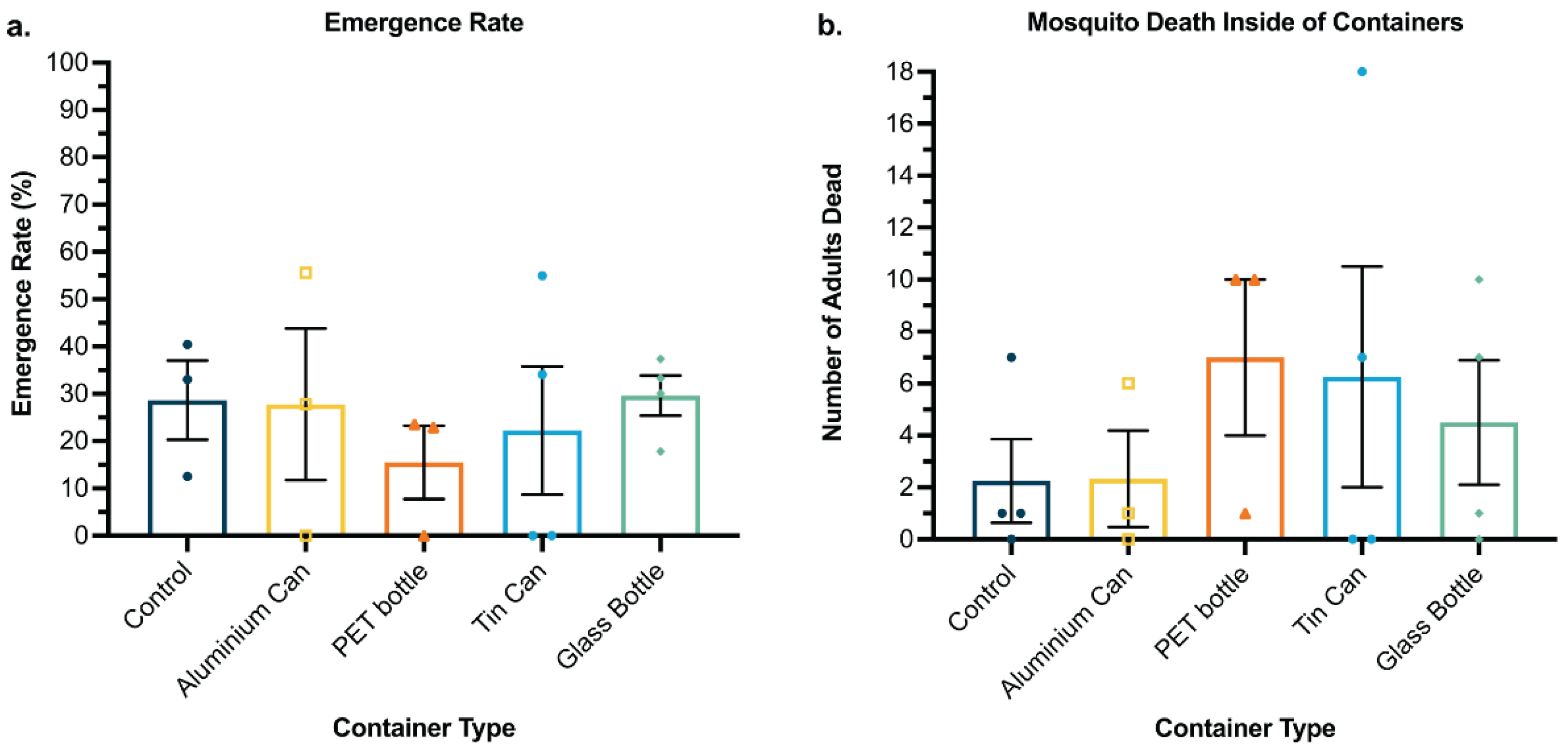
3.3. Kiribati Case Study: Waste Containers Tested in the Field Performed Similarly to Control Containers
3.4. Cost Analysis Reveals Significant Cost Reductions and Environmental Benefits through Waste Container Release Method
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Brady, O.J.; Gething, P.W.; Bhatt, S.; Messina, J.P.; Brownstein, J.S.; Hoen, A.G.; Moyes, C.L.; Farlow, A.W.; Scott, T.W.; Hay, S.I. Refining the Global Spatial Limits of Dengue Virus Transmission by Evidence-Based Consensus. PLoS Negl. Trop. Dis. 2012, 6, e1760. [Google Scholar] [CrossRef]
- da Oliveira, L.N.S.; Itria, A.; Lima, E.C. Cost of Illness and Program of Dengue: A Systematic Review. PLoS ONE 2019, 14, e0211401. [Google Scholar] [CrossRef] [Green Version]
- WHO. Dengue and Sever Dengue Fact Sheet; World Health Organisation: Geneva, Switzerland, 2021. [Google Scholar]
- Bhatt, S.; Gething, P.W.; Brady, O.J.; Messina, J.P.; Farlow, A.W.; Moyes, C.L.; Drake, J.M.; Brownstein, J.S.; Hoen, A.G.; Sankoh, O.; et al. The Global Distribution and Burden of Dengue. Nature 2013, 496, 504–507. [Google Scholar] [CrossRef]
- Martina, B.E.; Barzon, L.; Pijlman, G.P.; de la Fuente, J.; Rizzoli, A.; Wammes, L.J.; Takken, W.; van Rij, R.P.; Papa, A. Human to Human Transmission of Arthropod-Borne Pathogens. Curr. Opin. Virol. 2017, 22, 13–21. [Google Scholar] [CrossRef] [PubMed]
- Souza-Neto, J.A.; Powell, J.R.; Bonizzoni, M. Aedes aegypti Vector Competence Studies: A Review. Infect. Genet. Evol. 2019, 67, 191–209. [Google Scholar] [CrossRef] [PubMed]
- Michael, B.; Deen, J.; Buchy, P.; Gubler, D.; Harris, E.; Homach, J. World Health Organization Dengue Guidelines for Diagnosis, Treatment, Prevention, and Control; WHO Publisher: Geneva, Switzerland, 2009; p. 3. [Google Scholar]
- Messina, J.P.; Brady, O.J.; Pigott, D.M.; Golding, N.; Kraemer, M.U.G.; Scott, T.W.; Wint, G.R.W.; Smith, D.L.; Hay, S.I. The Many Projected Futures of Dengue. Nat. Rev. Microbiol. 2015, 13, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Moreira, L.A.; Iturbe-Ormaetxe, I.; Jeffery, J.A.; Lu, G.; Pyke, A.T.; Hedges, L.M.; Rocha, B.C.; Hall-Mendelin, S.; Day, A.; Riegler, M.; et al. A Wolbachia Symbiont in Aedes aegypti Limits Infection with Dengue, Chikungunya, and Plasmodium. Cell 2009, 139, 1268–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aliota, M.T.; Peinado, S.A.; Velez, I.D.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Zika Virus by Aedes aegypti. Sci. Rep. 2016, 6, 28792. [Google Scholar] [CrossRef]
- Aliota, M.T.; Walker, E.C.; Uribe Yepes, A.; Dario Velez, I.; Christensen, B.M.; Osorio, J.E. The wMel Strain of Wolbachia Reduces Transmission of Chikungunya Virus in Aedes aegypti. PLoS Negl. Trop. Dis. 2016, 10, e0004677. [Google Scholar] [CrossRef] [Green Version]
- van den Hurk, A.F.; Hall-Mendelin, S.; Pyke, A.T.; Frentiu, F.D.; McElroy, K.; Day, A.; Higgs, S.; O’Neill, S.L. Impact of Wolbachia on Infection with Chikungunya and Yellow Fever Viruses in the Mosquito Vector Aedes aegypti. PLoS Negl. Trop. Dis. 2012, 6, e1892. [Google Scholar] [CrossRef] [Green Version]
- Hoffmann, A.A.; Montgomery, B.L.; Popovici, J.; Iturbe-Ormaetxe, I.; Johnson, P.H.; Muzzi, F.; Greenfield, M.; Durkan, M.; Leong, Y.S.; Dong, Y.; et al. Successful Establishment of Wolbachia in Aedes Populations to Suppress Dengue Transmission. Nature 2011, 476, 454. [Google Scholar] [CrossRef] [PubMed]
- Ryan, P.A.; Turley, A.P.; Wilson, G.; Hurst, T.P.; Retzki, K.; Brown-Kenyon, J.; Hodgson, L.; Kenny, N.; Cook, H.; Montgomery, B.L.; et al. Establishment of wMel Wolbachia in Aedes aegypti Mosquitoes and Reduction of Local Dengue Transmission in Cairns and Surrounding Locations in Northern Queensland, Australia. Gates Open Res. 2019, 3, 1547. [Google Scholar] [CrossRef] [PubMed]
- Utarini, A.; Indriani, C.; Ahmad, R.A.; Tantowijoyo, W.; Arguni, E.; Ansari, M.R.; Supriyati, E.; Wardana, D.S.; Meitika, Y.; Ernesia, I.; et al. Efficacy of Wolbachia-Infected Mosquito Deployments for the Control of Dengue. N. Engl. J. Med. 2021, 384, 2177–2186. [Google Scholar] [CrossRef] [PubMed]
- Harris, A.F.; Nimmo, D.; McKemey, A.R.; Kelly, N.; Scaife, S.; Donnelly, C.A.; Beech, C.; Petrie, W.D.; Alphey, L. Field Performance of Engineered Male Mosquitoes. Nat. Biotechnol. 2011, 29, 1034–1037. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, T.H.; Nguyen, H.L.; Nguyen, T.Y.; Vu, S.N.; Tran, N.D.; Le, T.N.; Vien, Q.M.; Bui, T.C.; Le, H.T.; Kutcher, S.; et al. Field Evaluation of the Establishment Potential of wMelPop Wolbachia in Australia and Vietnam for Dengue Control. Parasit. Vectors 2015, 8, 563. [Google Scholar] [CrossRef] [Green Version]
- Nazni, W.A.; Hoffmann, A.A.; NoorAfizah, A.; Cheong, Y.L.; Mancini, M.V.; Golding, N.; Kamarul, G.M.R.; Arif, M.A.K.; Thohir, H.; NurSyamimi, H.; et al. Establishment of Wolbachia Strain wAlbB in Malaysian Populations of Aedes eegypti for Dengue Control. Curr. Biol. 2019, 29, 4241–4248.e5. [Google Scholar] [CrossRef] [Green Version]
- Tantowijoyo, W.; Andari, B.; Arguni, E.; Budiwati, N.; Nurhayati, I.; Fitriana, I.; Ernesia, I.; Daniwijaya, E.W.; Supriyati, E.; Yusdiana, D.H.; et al. Stable Establishment of wMel Wolbachia in Aedes aegypti Populations in Yogyakarta, Indonesia. PLoS Negl. Trop. Dis. 2020, 14, e0008157. [Google Scholar] [CrossRef]
- O’Neill, S.L.; Ryan, P.A.; Turley, A.P.; Wilson, G.; Retzki, K.; Iturbe-Ormaetxe, I.; Dong, Y.; Kenny, N.; Paton, C.J.; Ritchie, S.A.; et al. Scaled Deployment of Wolbachia to Protect the Community from Dengue and Other Aedes Transmitted Arboviruses. Gates Open Res. 2018, 2, 36. [Google Scholar] [CrossRef]
- Puggioli, A.; Balestrino, F.; Damiens, D.; Lees, R.S.; Soliban, S.M.; Madakacherry, O.; Dindo, M.L.; Bellini, R.; Gilles, J.R.L. Efficiency of Three Diets for Larval Development in Mass Rearing Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 2013, 50, 819–825. [Google Scholar] [CrossRef] [Green Version]
- Halloran, A.; Hanboonsong, Y.; Roos, N.; Bruun, S. Life Cycle Assessment of Cricket Farming in North-Eastern Thailand. J. Clean. Prod. 2017, 156, 83–94. [Google Scholar] [CrossRef]
- Smetana, S.; Mathys, A.; Knoch, A.; Heinz, V. Meat Alternatives: Life Cycle Assessment of Most Known Meat Substitutes. Int. J. Life Cycle Assess. 2015, 20, 1254–1267. [Google Scholar] [CrossRef]
- Oonincx, D.G.A.B.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An Exploration on Greenhouse Gas and Ammonia Production by Insect Species Suitable for Animal or Human Consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- van Huis, A.; Van Itterbeeck, J.; Klunder, H.; Mertens, E.; Afton, H.; Muir, G.; Vantomme, P. Edible Insects: Future Prospects for Food and Feed Security; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Shumo, M.; Osuga, I.M.; Khamis, F.M.; Tanga, C.M.; Fiaboe, K.K.M.; Subramanian, S.; Ekesi, S.; van Huis, A.; Borgemeister, C. The Nutritive Value of Black Soldier Fly Larvae Reared on Common Organic Waste Streams in Kenya. Sci. Rep. 2019, 9, 10110. [Google Scholar] [CrossRef] [PubMed]
- Carolyne, K.; John, N.K.; Samuel, I.; Nanna, R. Use of House Cricket to Address Food Security in Kenya: Nutrient and Chitin Composition of Farmed Crickets as Influenced by Age. Afr. J. Agric. Res. 2017, 12, 3189–3197. [Google Scholar] [CrossRef] [Green Version]
- Albert, H. Just Add Water’ GM Mosquitoes Suppress Wild Population by 95%. Labiotech 2020. Available online: https://www.labiotech.eu/trends-news/oxitec-mosquito-dengue/ (accessed on 20 February 2022).
- Just Add Water: Oxitec’s New Friendly Mosquito Mini-Capsule Technology Rapidly Suppresses 95% of Disease-Spreading Aedes aegypti in Brazil Trial. M2 Presswire 2020. Available online: https://www.oxitec.com/en/news/oxitecs-friendly-mosquito-technology-receives-us-epa-approval-for-pilot-projects-in-us (accessed on 20 February 2022).
- Chang, S. Wolbachia Capsules to Aid Mosquito Fight. Fiji Sun 2018. Available online: https://fijisun.com.fj/2018/10/21/wolbachia-capsules-to-aid-mosquito-fight/ (accessed on 20 January 2022).
- Krystosik, A.; Njoroge, G.; Odhiambo, L.; Forsyth, J.E.; Mutuku, F.; LaBeaud, A.D. Solid Wastes Provide Breeding Sites, Burrows, and Food for Biological Disease Vectors, and Urban Zoonotic Reservoirs: A Call to Action for Solutions-Based Research. Front. Public Health 2019, 7, 405. [Google Scholar] [CrossRef] [Green Version]
- Cruvinel, V.R.N.; Zolnikov, T.R.; Takashi Obara, M.; de Oliveira, V.T.L.; Vianna, E.N.; do Santos, F.S.G.; de Oliveira, K.C.; Scott, J.A. Vector-Borne Diseases in Waste Pickers in Brasilia, Brazil. Waste Manag. 2020, 105, 223–232. [Google Scholar] [CrossRef]
- Quintero, J.; Brochero, H.; Manrique-Saide, P.; Barrera-Pérez, M.; Basso, C.; Romero, S.; Caprara, A.; De Lima Cunha, J.C.; Beltrán-Ayala, E.; Mitchell-Foster, K.; et al. Ecological, Biological and Social Dimensions of Dengue Vector Breeding in Five Urban Settings of Latin America: A Multi-Country Study. BMC Infect. Dis. 2014, 14, 38. [Google Scholar] [CrossRef] [Green Version]
- Ullah, M.B.; Mridha, M.K.; Arnold, C.D.; Matias, S.L.; Khan, M.S.A.; Siddiqui, Z.; Hossain, M.; Paul, R.R.; Dewey, K.G. Factors Associated with Diarrhea and Acute Respiratory Infection in Children under Two Years of Age in Rural Bangladesh. BMC Pediatr. 2019, 19, 386. [Google Scholar] [CrossRef] [PubMed]
- Secretariat of the Pacific Regional Environment Programme (SPREP). Improved Waste Management in Kiribati: A Case Study; Secretariat of the Pacific Regional Environment Programme (SPREP): Apia, Samoa, 2021. [Google Scholar]
- Kiribati to Carry Out Considerable Efforts in Sound Management of Chemicals and Waste. Available online: https://www.unep.org/news-and-stories/story/kiribati-carry-out-considerable-efforts-sound-management-chemicals-and-waste (accessed on 10 August 2021).
- Niemi, M.; Carvan, A.; Williams, S. Kiribati Solid Waste Management Evaluation Report; New Zealand Ministry of Foreign Affairs and Trade: Wellington, New Zealand, 2019. [Google Scholar]
- Walker, T.; Johnson, P.H.; Moreira, L.A.; Iturbe-Ormaetxe, I.; Frentiu, F.D.; McMeniman, C.J.; Leong, Y.S.; Dong, Y.; Axford, J.; Kriesner, P.; et al. The wMel Wolbachia Strain Blocks Dengue and Invades Caged Aedes aegypti Populations. Nature 2011, 476, 450. [Google Scholar] [CrossRef] [PubMed]
- Ross, P.A.; Axford, J.K.; Richardson, K.M.; Endersby-Harshman, N.M.; Hoffmann, A.A. Maintaining Aedes aegypti Mosquitoes Infected with Wolbachia. J. Vis. Exp. 2017, 126, e56124. [Google Scholar] [CrossRef]
- Xi, Z.; Khoo, C.C.H.; Dobson, S.L. Wolbachia Establishment and Invasion in an Aedes aegypti Laboratory Population. Science 2005, 310, 326–328. [Google Scholar] [CrossRef] [Green Version]
- Packer, M.J.; Corbet, P.S. Size Variation and Reproductive Success of Female Aedes Punctor (Diptera: Culicidae). Ecol. Entomol. 1989, 14, 297–309. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of Relative Gene Expression Data Using Real-Time Quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]
- Yeap, H.L.; Axford, J.K.; Popovici, J.; Endersby, N.M.; Iturbe-Ormaetxe, I.; Ritchie, S.A.; Hoffmann, A.A. Assessing Quality of Life-Shortening Wolbachia-Infected Aedes aegypti Mosquitoes in the Field Based on Capture Rates and Morphometric Assessments. Parasit. Vectors 2014, 7, 58. [Google Scholar] [CrossRef] [Green Version]
- Frentiu, F.D.; Zakir, T.; Walker, T.; Popovici, J.; Pyke, A.T.; van den Hurk, A.; McGraw, E.A.; O’Neill, S.L. Limited Dengue Virus Replication in Field-Collected Aedes Aegypti Mosquitoes Infected with Wolbachia. PLoS Negl. Trop. Dis. 2014, 8, e2688. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.S.; Wilk, M.B. An Analysis of Variance Test for Normality (Complete Samples). Biometrika 1965, 52, 591–611. [Google Scholar] [CrossRef]
- Kruskal, W.H.; Wallis, W.A. Use of Ranks in One-Criterion Variance Analysis. J. Am. Stat. Assoc. 1952, 47, 583–621. [Google Scholar] [CrossRef]
- Crawley, M.J. Proportion Data. In The R Book; John Wiley & Sons: Hoboken, NJ, USA, 2012; pp. 628–649. [Google Scholar]
- Mann, H.B.; Whitney, D.R. On a Test of Whether One of Two Random Variables Is Stochastically Larger than the Other. Ann. Math. Stat. 1947, 18, 50–60. [Google Scholar] [CrossRef]
- Girden, E.R. Quantitative applications in the social sciences. In ANOVA: Repeated Measures; Sage University papers; Sage: Newbury Park, CA, USA, 1992; Volume 84, p. 77. [Google Scholar]
- Haynes, W. Tukey’s Test. In Encyclopedia of Systems Biology; Dubitzky, W., Wolkenhauer, O., Cho, K.-H., Yokota, H., Eds.; Springer: New York, NY, USA, 2013; pp. 2303–2304. ISBN 9781441998637. [Google Scholar]
- Yeap, H.L.; Endersby, N.M.; Johnson, P.H.; Ritchie, S.A.; Hoffmann, A.A. Body Size and Wing Shape Measurements as Quality Indicators of Aedes Aegypti Mosquitoes Destined for Field Release. Am. J. Trop. Med. Hyg. 2013, 89, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Whincup, T. The Traditional Meeting House of Kiribati: ‘A Tale of Two Islands’. Int. J. Res. Into Isl. Cult. 2010, 4, 18. [Google Scholar]
- Gesto, J.S.M.; Ribeiro, G.S.; Rocha, M.N.; Dias, F.B.S.; Peixoto, J.; Carvalho, F.D.; Pereira, T.N.; Moreira, L.A. Reduced Competence to Arboviruses Following the Sustainable Invasion of Wolbachia into Native Aedes aegypti from Southeastern Brazil. Sci. Rep. 2021, 11, 10039. [Google Scholar] [CrossRef]
- FAO. Global Livestock Environmental Assessment Model; Food and Agriculture Organisation of the United Nations: Rome, Italy, 2018. [Google Scholar]
- Ross, P.A.; Ritchie, S.A.; Axford, J.K.; Hoffmann, A.A. Loss of Cytoplasmic Incompatibility in Wolbachia-Infected Aedes Aegypti under Field Conditions. PLoS Negl. Trop. Dis. 2019, 13, e0007357. [Google Scholar] [CrossRef]
- Ritchie, S.A.; van den Hurk, A.F.; Smout, M.J.; Staunton, K.M.; Hoffmann, A.A. Mission Accomplished? We Need a Guide to the “Post Release” World of Wolbachia for Aedes-Borne Disease Control. Trends Parasitol. 2018, 34, 217–226. [Google Scholar] [CrossRef]
- Indian Legislative Department. The Punjab Prohibition of Cow Slaughter Act, 1955; PRS Legislative Research: New Delhi, India, 1956. [Google Scholar]
- Legal Status of Edible Insects in Some Countries. Available online: https://www.bugsolutely.com/legal-status-edible-insects/ (accessed on 22 November 2021).
- Government of Kiribati. Kiribati Development Plan 2016–2019; Government of Kiribati: Tarawa, Kiribati, 2015.
- Woodruf, A. Solid Waste Management in the Pacific; Asian Development Bank: Mandaluyong, Philippines, 2014. [Google Scholar]
- Dengue and Chikungunya Cases on the Decline in Kiribati. Available online: https://reliefweb.int/report/kiribati/dengue-and-chikungunya-cases-decline-kiribati (accessed on 20 February 2022).
- Farnesi, L.C.; Belinato, T.A.; Gesto, J.S.M.; Martins, A.J.; Bruno, R.V.; Moreira, L.A. Embryonic Development and Egg Viability of wMel-Infected Aedes Aegypti. Parasit. Vectors 2019, 12, 211. [Google Scholar] [CrossRef]
- Low Cost Solar Way to Provide Clean Water in Kiribati; Secretariat of the Pacific Community (SPC): Noumea, New Caledonia, 2018; Available online: https://www.spc.int/updates/blog/2018/01/low-cost-solar-way-to-provide-clean-water-in-kiribati (accessed on 20 February 2022).
- Solar Disinfection (SODIS) in Kiribati; Secretariat of the Pacific Community (SPC): Noumea, New Caledonia, 2014; Available online: http://ccprojects.gsd.spc.int/wp-content/uploads/2016/07/11.-SODIS-factsheet.pdf (accessed on 20 February 2022).
- Thirapatsakun, L.; Tauthong, P.; Phanthumachinda, B. Others Surface Preferences for Oviposition of Aedes aegypti in Thailand. Southeast Asian J. Trop. Med. Public Health 1981, 12, 209–212. [Google Scholar]
| Strategy | Container | Source | Distribution Method |
|---|---|---|---|
| 1 | Aluminum can | Recycling facility | Community |
| 2 | Cardboard MRC | Detpak, imported into Kiribati | Community |
| 3 | Aluminum can | Recycling facility | Staff |
| 4 | Cardboard MRC | Detpak, imported into Kiribati | Staff |
| Strategy 1 | Strategy 2 | Strategy 3 | Strategy 4 | |
|---|---|---|---|---|
| Method of distributing container | Community distribution | Community distribution | Staff-led distribution | Staff-led distribution |
| Materials used | Aluminum can | Cardboard + PET lining | Aluminium can | Cardboard + PET lining |
| Mosquito release rounds | 5 | 5 | 5 | 5 |
| Duration | 10-week deployment | 10-week deployment | 10-week deployment | 10-week deployment |
| Projected total number of mosquitoes released over 5 release rounds | 807,429 | 807,429 | 1,229,513 | 1,229,513 |
| Total cost of sourcing and preparing containers | $568.80 | $21,684.00 | $568.80 | $21,684.00 |
| Total staff salary cost | $5078.00 | $7200.00 | $120,758.00 | $168,000.00 |
| Total deployment cost | $7896 | $38,387 | $153,150 | $233,768 |
| Deployment cost per person | $0.14 | $0.68 | $2.72 | $4.15 |
| Supplier availability | Collect from recycling center | Collect from communities | Collect from recycling center | Import from overseas |
| Recyclable in Kiribati | Yes | No | Yes | No |
| Reusable | Yes | No | Yes | No |
| Require addition of labels | Yes | No | Yes | No |
| Environmental impact | Neutral– positive | Negative | Neutral– positive | Negative |
| Local supplier | Yes | No | Yes | No |
| Mosquito release quality | Low | Low | Medium | Medium |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Allman, M.J.; Slack, A.J.; Abello, N.P.; Lin, Y.-H.; O’Neill, S.L.; Robinson, A.J.; Flores, H.A.; Joubert, D.A. Trash to Treasure: How Insect Protein and Waste Containers Can Improve the Environmental Footprint of Mosquito Egg Releases. Pathogens 2022, 11, 373. https://doi.org/10.3390/pathogens11030373
Allman MJ, Slack AJ, Abello NP, Lin Y-H, O’Neill SL, Robinson AJ, Flores HA, Joubert DA. Trash to Treasure: How Insect Protein and Waste Containers Can Improve the Environmental Footprint of Mosquito Egg Releases. Pathogens. 2022; 11(3):373. https://doi.org/10.3390/pathogens11030373
Chicago/Turabian StyleAllman, Megan J., Aidan J. Slack, Nigel P. Abello, Ya-Hsun Lin, Scott L. O’Neill, Andrea J. Robinson, Heather A. Flores, and D. Albert Joubert. 2022. "Trash to Treasure: How Insect Protein and Waste Containers Can Improve the Environmental Footprint of Mosquito Egg Releases" Pathogens 11, no. 3: 373. https://doi.org/10.3390/pathogens11030373
APA StyleAllman, M. J., Slack, A. J., Abello, N. P., Lin, Y.-H., O’Neill, S. L., Robinson, A. J., Flores, H. A., & Joubert, D. A. (2022). Trash to Treasure: How Insect Protein and Waste Containers Can Improve the Environmental Footprint of Mosquito Egg Releases. Pathogens, 11(3), 373. https://doi.org/10.3390/pathogens11030373







