Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Population
2.2. Serological Testing and Imaging Procedures
2.3. Data Evaluation
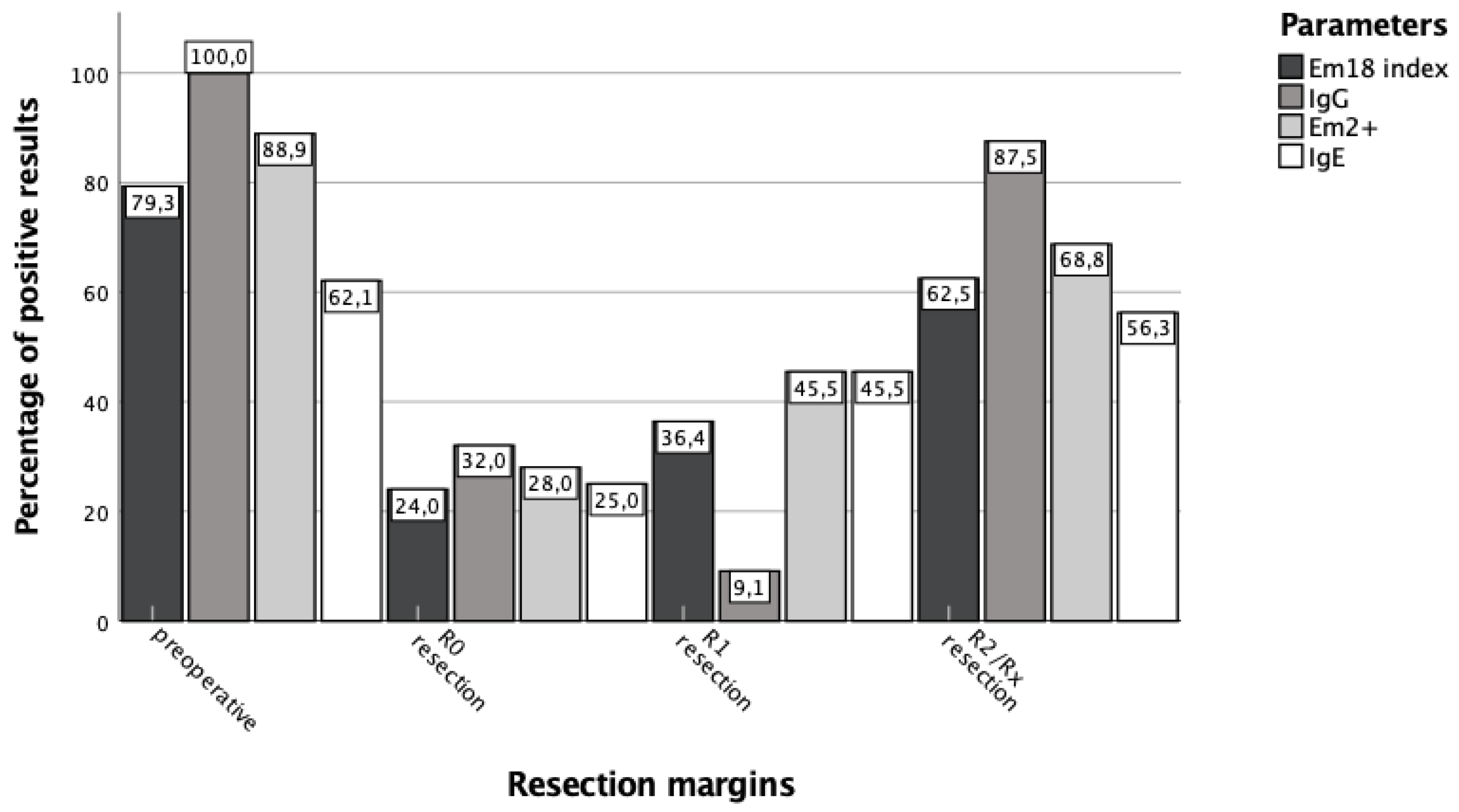
3. Results
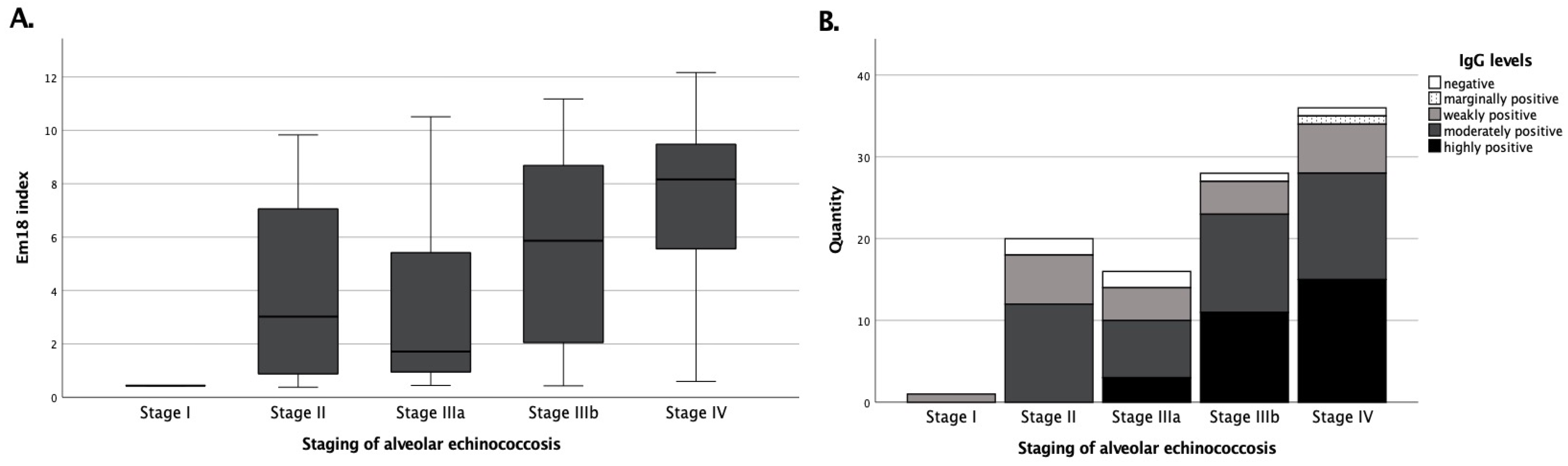
3.1. recEm18 Improves the IgG-Based Serodiagnosis of Metastasis-like AE Patterns
3.2. Echinococcus IgG and recEm18 Correlate with Tracer Uptake, Size, and Parasitic Lesion Number
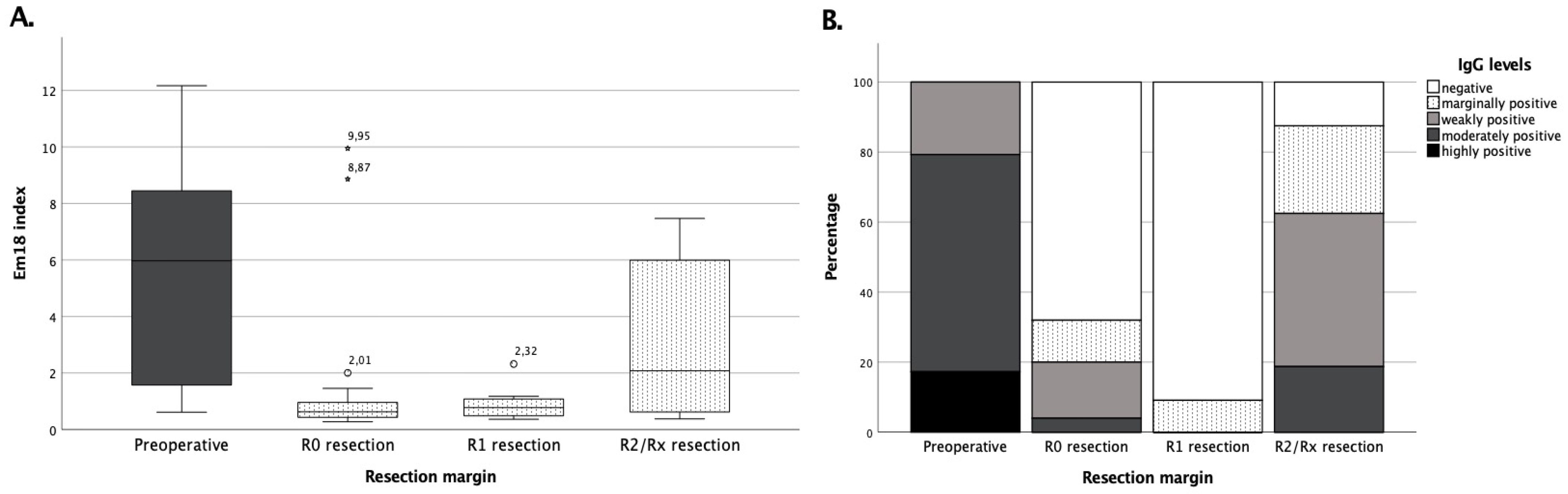
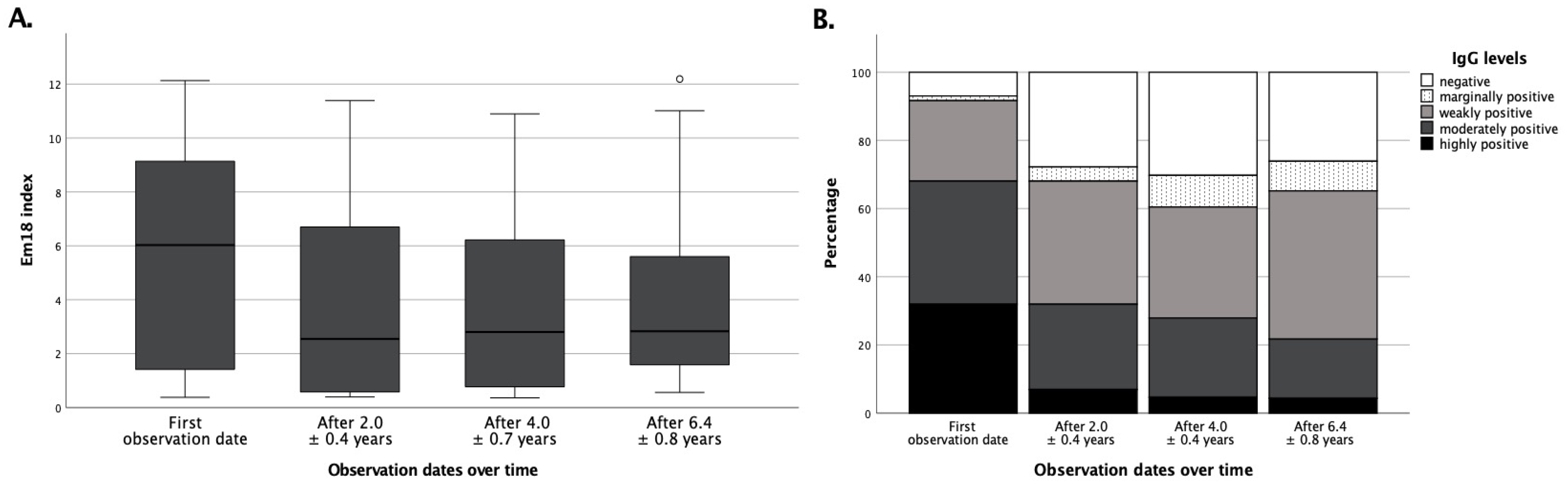
3.3. Surgical Treatment Success Is Best Reflected by IgG and RecEm18 Follow-Ups
3.4. Pharmacological Treatment Success Is Not Sufficiently Reflected by Serological Means Alone
3.5. Use of Echinococcus IgG and Em2+ Is Reasonable for Initial AE Serodiagnosis
4. Discussion
4.1. Echinococcus IgG and recEm18 Are Robust Tools for Serodiagnosis of AE
4.2. Correlation of RecEm18 and PET-CTI Tracer Uptake May Offer Alternative Follow-Up Strategies
4.3. Serological Follow-Ups Are Useful after Surgical Treatment, but Limited for Pharmacotherapy
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Woolsey, I.D.; Miller, A.L. Echinococcus granulosus sensu lato and Echinococcus multilocularis: A review. Res. Vet. Sci. 2021, 135, 517–522. [Google Scholar] [CrossRef]
- McManus, D.P.; Zhang, W.; Li, J.; Bartley, P.B. Echinococcosis. Lancet 2003, 362, 1295–1304. [Google Scholar] [CrossRef]
- Gruener, B.; Kern, P.; Mayer, B.; Graeter, T.; Hillenbrand, A.; Barth, T.E.F.; Muche, R.; Henne-Bruns, D.; Kratzer, W.; Kern, P. Comprehensive diagnosis and treatment of alveolar echinococcosis: A single-center, long-term observational study of 312 patients in Germany. GMS Infect. Dis. 2017, 5, Doc01. [Google Scholar] [CrossRef]
- Kern, P.; Wen, H.; Sato, N.; Vuitton, D.A.; Gruener, B.; Shao, Y.; Delabrousse, E.; Kratzer, W.; Bresson-Hadni, S. WHO classification of alveolar echinococcosis: Principles and application. Parasitol. Int. 2006, 55, S283–S287. [Google Scholar] [CrossRef]
- Reinehr, M.; Micheloud, C.; Grimm, F.; Kronenberg, P.A.; Grimm, J.; Beck, A.; Nell, J.; Meyer zu Schwabedissen, C.; Furrer, E.; Muellhaupt, B.; et al. Pathology of echinococcosis: A morphologic and immunohistochemical study on 138 specimens with focus on the differential diagnosis between cystic and alveolar Echinococcosis. Am. J. Surg. Pathol. 2020, 44, 43–54. [Google Scholar] [CrossRef]
- Lightowlers, M.W.; Gasser, R.B.; Hemphill, A.; Romig, T.; Tamarozzi, F.; Deplazes, P.; Torgerson, P.R.; Garcia, H.H.; Kern, P. Advances in the treatment, diagnosis, control and scientific understanding of taeniid cestode parasite infections over the past 50 years. Int. J. Parasitol. 2021, 51, 1167–1192. [Google Scholar] [CrossRef]
- Yangdan, C.R.; Wang, C.; Zhang, L.Q.; Ren, B.; Fan, H.N.; Lu, M.D. Recent advances in ultrasound in the diagnosis and evaluation of the activity of hepatic alveolar echinococcosis. Parasitol. Res. 2021, 120, 3077–3082. [Google Scholar] [CrossRef]
- Brunetti, E.; Kern, P.; Vuitton, D.A. Expert consensus for the diagnosis and treatment of cystic and alveolar echinococcosis in humans. Acta Trop. 2010, 114, 1–16. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Silva, R.; Fittipaldo, V.A.; Buonfrate, D.; Gottstein, B.; Siles-Lucas, M. Serology for the diagnosis of human hepatic cystic echinococcosis and its relation with cyst staging: A systematic review of the literature with meta-analysis. PLoS Negl. Trop. Dis. 2021, 15, e0009370. [Google Scholar] [CrossRef] [PubMed]
- Salm, L.A.; Lachenmayer, A.; Perrodin, S.F.; Candinas, D.; Beldi, G. Surgical treatment strategies for hepatic alveolar echinococcosis. Food Waterborne Parasitol. 2019, 15, e00050. [Google Scholar] [CrossRef] [PubMed]
- Tamarozzi, F.; Nicoletti, G.J.; Neumayr, A.; Brunetti, E. Acceptance of standardized ultrasound classification, use of albendazole, and long-term follow-up in clinical management of cystic echinococcosis: A systematic review. Curr. Opin. Infect. Dis. 2014, 27, 425–431. [Google Scholar] [CrossRef]
- Bresson-Hadni, S.; Spahr, L.; Chappuis, F. Hepatic alveolar echinococcosis. Semin. Liver Dis. 2021, 41, 393–408. [Google Scholar] [CrossRef]
- Kratzer, W.; Gruener, B.; Kaltenbach, T.E.; Ansari-Bitzenberger, S.; Kern, P.; Fuchs, M.; Mason, R.A.; Barth, T.F.E.; Haenle, M.M.; Hillenbrand, A.; et al. Proposal of an ultrasonographic classification for hepatic alveolar echinococcosis: Echinococcosis multilocularis Ulm classification-ultrasound. World J. Gastroenterol. 2015, 21, 12392–12402. [Google Scholar] [CrossRef]
- Bi, X.J.; Shao, Y.M.; Li, L.; Wang, Y.; Zhang, C.S.; Lue, G.D.; Aji, T.; Li, J.; Zhang, W.B.; Wen, H.; et al. Evaluation of the diagnostic value of the immunoblotting and ELISA tests using recombinant Em18 antigen in human alveolar echinococcosis from Xingjiang China. Exp. Ther. Med. 2018, 16, 3155–3160. [Google Scholar] [CrossRef]
- Gottstein, B.; Lachenmayer, A.; Beldi, G.; Wang, J.; Merkle, B.; Vu, X.L.; Kurath, U.; Mueller, N. Diagnostic and follow-up performance of serological tests for different forms/courses of alveolar echinococcosis. Food Waterborne Parasitol. 2019, 16, e00055. [Google Scholar] [CrossRef]
- Ishikawa, Y.; Sako, Y.; Itoh, S.; Ohtake, T.; Kohgo, Y.; Matsuno, T.; Ohsaki, Y.; Miyokawa, N.; Nakao, M.; Nakaya, K.; et al. Serological monitoring of progression of alveolar echinococcosis with multiorgan involvement by use of recombinant Em18. J. Clin. Microbiol. 2009, 47, 3191–3196. [Google Scholar] [CrossRef] [Green Version]
- Tappe, D.; Frosch, M.; Sako, Y.; Itoh, S.; Gruener, B.; Reuter, S.; Nakao, M.; Ito, A.; Kern, P. Close relationship between clinical regression and specific serology in the follow-up of patients with alveolar echinococcosis in different clinical stages. Am. J. Trop. Med. Hyg. 2009, 80, 792–797. [Google Scholar] [CrossRef]
- Tappe, D.; Sako, Y.; Itoh, S.; Frosch, M.; Gruener, B.; Kern, P.; Ito, A. Immunoglobulin G subclass responses to recombinant Em18 in the follow-up of patients with alveolar echinococcosis in different clinical stages. Clin. Vaccine Immunol. 2010, 17, 944–948. [Google Scholar] [CrossRef] [Green Version]
- Sulima, M.; Szostakowska, B.; Nahorski, W.; Sikorska, K.; Wołyniec, W.; Wąż, P. The usefulness of commercially available serological tests in the diagnosis and monitoring of treatment in patients with alveolar echinococcosis. Clin. Exp. Hepatol. 2019, 5, 327–333. [Google Scholar] [CrossRef]
- Bresson-Hadni, S.; Delabrousse, E.; Blagosklonov, O.; Bartholomot, B.; Koch, S.; Miguet, J.P.; Mantion, G.A.; Vuitton, D.A. Imaging aspects and non-surgical interventional treatment in human alveolar echinococcosis. Parasitol. Int. 2006, 55, S267–S272. [Google Scholar] [CrossRef]
- Husmann, L.; Muehlematter, U.J.; Grimm, F.; Ledergerber, B.; Messerli, M.; Kudura, K.; Gruenig, H.; Muellhaupt, B.; Hasse, B.; Huellner, M.W. PET/CT helps to determine treatment duration in patients with resected as well as inoperable alveolar echinococcosis. Parasitol. Int. 2021, 83, 102356. [Google Scholar] [CrossRef]
- Liu, W.; Delabrousse, E.; Blagosklonov, O.; Wang, J.; Zeng, H.; Jiang, Y.; Wang, J.; Qin, Y.; Vuitton, D.A.; Wen, H. Innovation in hepatic alveolar echinococcosis imaging: Best use of old tools, and necessary evaluation of new ones. Parasite 2014, 21, 74. [Google Scholar] [CrossRef]
- Ammann, R.W.; Stumpe, K.D.; Grimm, F.; Deplazes, P.; Huber, S.; Bertogg, K.; Fischer, D.R.; Muellhaupt, B. Outcome after discontinuing long-term benzimidazole treatment in 11 patients with non-resectable alveolar echinococcosis with negative FDG-PET/CT and anti-EmII/3-10 serology. PLoS Negl. Trop. Dis. 2015, 9, e0003964. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.D.; Wilmore, J.R.; Allman, D. Cellular dynamics of memory B cell populations: IgM+ and IgG+ memory B cells persist indefinitely as quiescent cells. J. Immunol. 2015, 195, 4753–4759. [Google Scholar] [CrossRef] [Green Version]
- Gottstein, B.; Tschudi, K.; Eckert, J.; Ammann, R. Em2-ELISA for the follow-up of alveolar echinococcosis after complete surgical resection of liver lesions. Trans. R. Soc. Trop. Med. Hyg. 1989, 83, 389–393. [Google Scholar] [CrossRef] [Green Version]
- Ammann, R.W.; Renner, E.C.; Gottstein, B.; Grimm, F.; Eckert, J.; Renner, E.L.; Swiss Echinococcosis Study Group. Immunosurveillance of alveolar echinococcosis by specific humoral and cellular immune tests: Long-term analysis of the Swiss chemotherapy trial (1976–2001). J. Hepatol. 2004, 41, 551–559. [Google Scholar] [CrossRef]
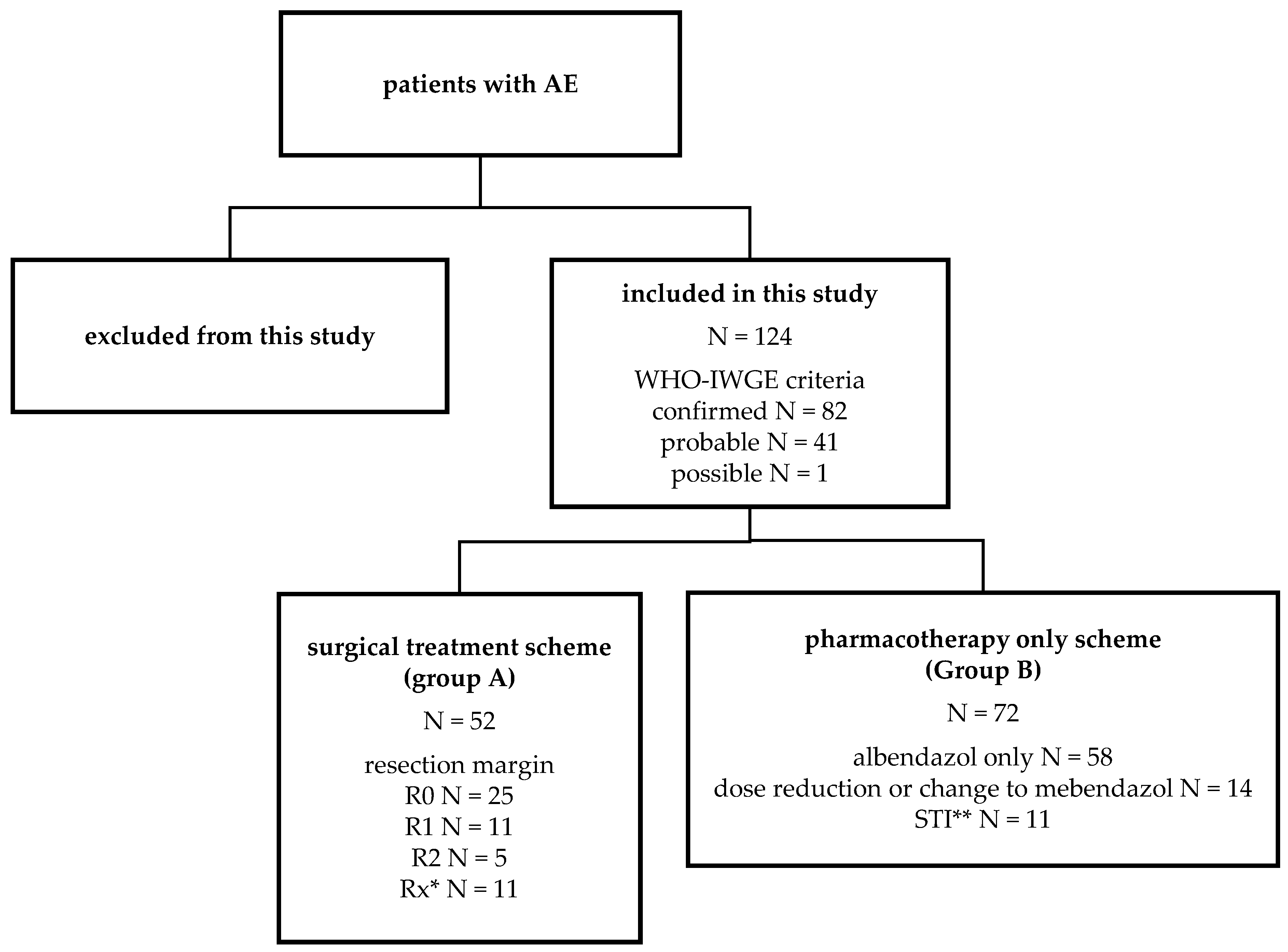
| Group A Surgical Treatment Scheme | Group B Pharmacotherapy only Scheme | Group C Patients with Preoperative Serology | |
|---|---|---|---|
| N (% of total) | 52 (41.9%) | 72 (58.1%) | 101 (81.5%) |
| age at the end of follow-up range | 50.8 ± 17.3 years 23.0–82.8 | 66.4 ± 14.3 years 22.8–89.0 | 60.8 ± 17.4 years 22.8–89.0 |
| stage * (% of group) I II IIIa IIIb IV | 1 (1.9%) 17 (32.7%) 4 (7.7%) 14 (26.9%) 16 (30.8%) | 1 (1.4%) 7 (9.7%) 14 (19.4%) 18 (25.0%) 32 (44.4%) | 1 (1.0%) 20 (19.8%) 16 (15.8%) 28 (27.7%) 36 (35.6%) |
| outcome ** cured chronically stable without medication chronically stable with medication progression recurrence follow-up incomplete | 24 (46.2%) 16 (30.8%) 10 (19.2%) 1 (1.9%) 0 (0%) 1 (1.9%) | - 8 (11.1%) 57 (79.2%) 7 (9.7%) 0 (0%) - | 19 (18.8%) 14 (13.9%) 60 (59.4%) 7 (6.9%) 0 (0%) 1 (1.0%) |
| Negative | Positive | |
|---|---|---|
| recEm18 index | 12 (20.3%) | 47 (79.7%) |
| Echinococcus IgG | 4 (6.8%) | 55 (93.2%) |
| Em2+ | 12 (20.3%) | 47 (79.7%) |
| total IgE | 20 (33.9%) | 39 (66.1%) |
| Tracer Uptake in PET-CTI | Size of Parasitic Lesions | Number of Parasitic Lesions | Initial Staging | Patient Outcome | |||
|---|---|---|---|---|---|---|---|
| Spearman-Rho | recEm18 index | K * | 0.660 ** | 0.711 ** | 0.227 ** | 0.258 ** | 0.527 ** |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ||
| N | 314 | 368 | 368 | 101 | 123 | ||
| Echinococcus IgG | K * | 0.670 ** | 0.697 ** | 0.287 ** | 0.321 ** | 0.502 ** | |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ||
| N | 313 | 367 | 367 | 101 | 123 | ||
| Em2+ | K * | 0.556 ** | 0.580 ** | 0.184 ** | 0.268 ** | 0.480 ** | |
| p-value | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | p < 0.05 | ||
| N | 312 | 366 | 366 | 101 | 123 | ||
| total IgE | K * | 0.394 ** | 0.476 ** | 0.087 | 0.186 ** | 0.272 ** | |
| p-value | p < 0.05 | p < 0.05 | p = 0.05 | p < 0.05 | p < 0.05 | ||
| N | 307 | 360 | 360 | 101 | 123 | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hotz, J.F.; Peters, L.; Kapp-Schwörer, S.; Theis, F.; Eberhardt, N.; Essig, A.; Grüner, B.; Hagemann, J.B. Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG. Pathogens 2022, 11, 239. https://doi.org/10.3390/pathogens11020239
Hotz JF, Peters L, Kapp-Schwörer S, Theis F, Eberhardt N, Essig A, Grüner B, Hagemann JB. Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG. Pathogens. 2022; 11(2):239. https://doi.org/10.3390/pathogens11020239
Chicago/Turabian StyleHotz, Julian Frederic, Lynn Peters, Silke Kapp-Schwörer, Frauke Theis, Nina Eberhardt, Andreas Essig, Beate Grüner, and Jürgen Benjamin Hagemann. 2022. "Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG" Pathogens 11, no. 2: 239. https://doi.org/10.3390/pathogens11020239
APA StyleHotz, J. F., Peters, L., Kapp-Schwörer, S., Theis, F., Eberhardt, N., Essig, A., Grüner, B., & Hagemann, J. B. (2022). Evaluation of Serological Markers in Alveolar Echinococcosis Emphasizing the Correlation of PET-CTI Tracer Uptake with RecEm18 and Echinococcus-Specific IgG. Pathogens, 11(2), 239. https://doi.org/10.3390/pathogens11020239






