Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles
Abstract
:1. Introduction
2. Results
2.1. Identification of E. coli
2.2. Detection of Virulence-Associated Genes
2.3. Antibiotic Susceptibility Testing
2.4. Detection of Resistance Genes
2.5. Determination of E. coli Phylogenetic Groups
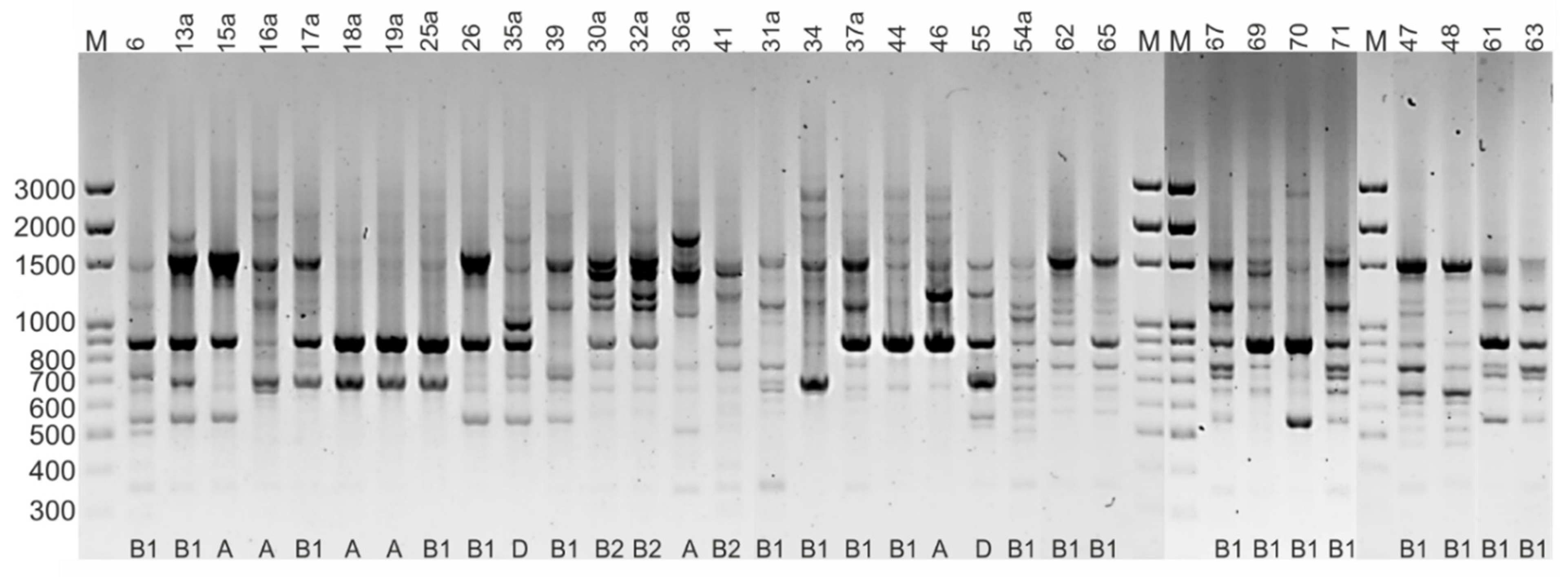
2.6. Rep-PCR Fingerprinting
3. Discussion
4. Materials and Methods
4.1. Collection of Faecal Samples
4.2. E. coli Isolation
4.3. Identification of E. coli
4.4. DNA Extraction
4.5. Detection of Virulence Genes
4.6. Antimicrobial Susceptibility Testing
4.7. Detection of Resistance Genes
4.8. Determination of E. coli Phylogenetic Groups
4.9. Rep-PCR Fingerprinting
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FEDIAF (The European Pet Food Industry). FEDIAF Annual Report 2021. p. 44, 50. Available online: https://www.fediaf.org/annual-report.html (accessed on 20 January 2022).
- Valdez, J.W. Using Google Trends to Determine Current, Past, and Future Trends in the Reptile Pet Trade. Animals 2021, 11, 676. [Google Scholar] [CrossRef]
- Ramos, C.P.; Santana, J.A.; Morcatti Coura, F.; Xavier, R.G.C.; Leal, C.A.G.; Oliveira Junior, C.A.; Heinemann, M.B.; Lage, A.P.; Lobato, F.C.F.; Silva, R.O.S. Identification and Characterization of Escherichia coli, Salmonella Spp., Clostridium perfringens, and C. difficile Isolates from Reptiles in Brazil. Biomed. Res. Int. 2019, 2019, 9530732. [Google Scholar] [CrossRef] [Green Version]
- Książczyk, M.; Dudek, B.; Kuczkowski, M.; O’Hara, R.; Korzekwa, K.; Wzorek, A.; Korzeniowska-Kowal, A.; Upton, M.; Junka, A.; Wieliczko, A.; et al. The Phylogenetic Structure of Reptile, Avian and Uropathogenic Escherichia coli with Particular Reference to Extraintestinal Pathotypes. Int. J. Mol. Sci. 2021, 22, 1192. [Google Scholar] [CrossRef]
- Lindstedt, B.A.; Finton, M.D.; Porcellato, D.; Brandal, L.T. High frequency of hybrid Escherichia coli strains with combined Intestinal Pathogenic Escherichia coli (IPEC) and Extraintestinal Pathogenic Escherichia coli (ExPEC) virulence factors isolated from human faecal samples. BMC Infect. Dis. 2018, 18, 544. [Google Scholar] [CrossRef]
- Wasiński, B. Extra-intestinal pathogenic Escherichia coli—Threat connected with food-borne infections. Ann. Agric. Environ. Med. 2019, 26, 532–537. [Google Scholar] [CrossRef]
- García, A.; Fox, J.G. A One Health Perspective for Defining and Deciphering Escherichia coli Pathogenic Potential in Multiple Hosts. Comp. Med. 2021, 71, 3–45. [Google Scholar] [CrossRef]
- Rodriguez-Siek, K.E.; Giddings, C.W.; Doetkott, C.; Johnson, T.J.; Fakhr, M.K.; Nolan, L.K. Comparison of Escherichia coli isolates implicated in human urinary tract infection and avian colibacillosis. Microbiology 2005, 151, 2097–2110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gomes, T.A.; Elias, W.P.; Scaletsky, I.C.; Guth, B.E.; Rodrigues, J.F.; Piazza, R.M.; Ferreira, L.C.; Martinez, M.B. Diarrheagenic Escherichia coli. Braz. J. Microbiol. 2016, 47, 3–30. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista-Trujillo, G.U.; Gutiérrez-Miceli, F.A.; Mandujano-García, L.; Oliva-Llaven, M.A.; Ibarra-Martínez, C.; Mendoza-Nazar, P.; Ruiz-Sesma, B.; Tejeda-Cruz, C.; Pérez-Vázquez, L.C.; Pérez-Batrez, J.E.; et al. Captive Green Iguana Carries Diarrheagenic Escherichia coli Pathotypes. Front. Vet. Sci. 2020, 7, 99. [Google Scholar] [CrossRef]
- Gopee, N.V.; Adesiyun, A.A.; Caesar, K. A longitudinal study of Escherichia coli strains isolated from captive mammals, birds, and reptiles in Trinidad. J. Zoo Wildl. Med. Off. Publ. Am. Assoc. Zoo Vet. 2000, 31, 353–360. [Google Scholar] [CrossRef]
- Sylvester, W.R.B.; Amadi, V.; Hegamin-Younger, C.; Pinckey, R.; Macpherson, C.N.L.; Mckibben, J.S.; Bruhl-Day, R.; John-Sylvester, K.D.; Hariharan, H. Occurrence of Antibiotic Resistant Escherichia coli in Green Iguanas (Iguana Iguana) in Grenada, West Indies. Int. J. Vet. Med. Res. Rep. 2014, 2014, 260412. [Google Scholar] [CrossRef]
- Unger, F.; Eisenberg, T.; Prenger-Berninghoff, E.; Leidner, U.; Ludwig, M.L.; Rothe, M.; Semmler, T.; Ewers, C. Imported reptiles as a risk factor for the global distribution of Escherichia coli harbouring the colistin resistance gene mcr-1. Int. J. Antimicrob. Agents 2017, 49, 122–123. [Google Scholar] [CrossRef] [PubMed]
- Nowaczek, A.; Dec, M.; Stępień-Pyśniak, D.; Urban-Chmiel, R.; Marek, A.; Różański, P. Antibiotic Resistance and Virulence Profiles of Escherichia coli Strains Isolated from Wild Birds in Poland. Pathogens 2021, 10, 1059. [Google Scholar] [CrossRef] [PubMed]
- Turchi, B.; Dec, M.; Bertelloni, F.; Winiarczyk, S.; Gnat, S.; Bresciani, F.; Viviani, F.; Cerri, D.; Fratini, F. Antibiotic Susceptibility and Virulence Factors in Escherichia coli from Sympatric Wildlife of the Apuan Alps Regional Park (Tuscany, Italy). Microb. Drug Resist. 2019, 25, 772–780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tiseo, K.; Huber, L.; Gilbert, M.; Robinson, T.P.; Van Boeckel, T.P. Global Trends in Antimicrobial Use in Food Animals from 2017 to 2030. Antibiotics 2020, 9, 918. [Google Scholar] [CrossRef]
- Zając, M.; Skarżyńska, M.; Lalak, A.; Kwit, R.; Śmiałowska-Węglińska, A.; Pasim, P.; Szulowski, K.; Wasyl, D. Salmonella in Captive Reptiles and Their Environment-Can We Tame the Dragon? Microorganisms 2021, 9, 1012. [Google Scholar] [CrossRef]
- Arnafia, W.; Ningrum, S.G.; Adji, R.S.; Lukman, D.W.; Pasaribu, F.H.; Wibawan, I.W.T. Isolation of Salmonella from reptiles in pet shop and its susceptibility to antibiotics in Indonesia. Hum. Vet. Med. 2016, 8, 177–181. [Google Scholar]
- Bertelloni, F.; Chemaly, M.; Cerri, D.; Gall, F.L.; Ebani, V.V. Salmonella infection in healthy pet reptiles: Bacteriological isolation and study of some pathogenic characters. Acta Microbiol. Immunol. Hung. 2016, 63, 203–216. [Google Scholar] [CrossRef] [Green Version]
- Mazurek, J.; Pusz, P.; Bok, E.; Stosik, M.; Baldy-Chudzik, K. The phenotypic and genotypic characteristics of antibiotic resistance in Escherichia coli populations isolated from farm animals with different exposure to antimicrobial agents. Pol. J. Microbiol. 2013, 62, 173–179. [Google Scholar] [CrossRef]
- Jurado-Rabadán, S.; de la Fuente, R.; Ruiz-Santa-Quiteria, J.A.; Orden, J.A.; de Vries, L.E.; Agersø, Y. Detection and linkage to mobile genetic elements of tetracycline resistance gene tet(M) in Escherichia coli isolates from pigs. BMC Vet. Res. 2014, 10, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dec, M.; Stępień-Pyśniak, D.; Gnat, S.; Fratini, F.; Urban-Chmiel, R.; Cerri, D.; Winiarczyk, S.; Turchi, B. Antibiotic Susceptibility and Virulence Genes in Enterococcus Isolates from Wild Mammals Living in Tuscany, Italy. Microb. Drug Resist. 2020, 26, 505–519. [Google Scholar] [CrossRef]
- Dec, M.; Nowaczek, A.; Stępień-Pyśniak, D.; Wawrzykowski, J.; Urban-Chmiel, R. Identification and antibiotic susceptibility of lactobacilli isolated from turkeys. BMC Microbiol. 2018, 18, 168. [Google Scholar] [CrossRef] [PubMed]
- Ur Rahman, S.; Ali, T.; Ali, I.; Khan, N.A.; Han, B.; Gao, J. The Growing Genetic and Functional Diversity of Extended Spectrum Beta-Lactamases. Biomed. Res. Int. 2018, 2018, 9519718. [Google Scholar] [CrossRef] [PubMed]
- Paterson, D.L.; Bonomo, R.A. Extended-spectrum beta-lactamases: A clinical update. Clin. Microbiol. Rev. 2005, 18, 657–686. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Sun, Q.; Zhao, L. Virulence Factors and Antibiotic Resistance of Avian Pathogenic Escherichia Coli in Eastern China. J. Vet. Res. 2019, 63, 317–320. [Google Scholar] [CrossRef] [Green Version]
- Gordon, D.M.; Cowling, A. The distribution and genetic structure of Escherichia coli in Australian vertebrates: Host and geographic effects. Microbiology 2003, 149, 3575–3586. [Google Scholar] [CrossRef] [Green Version]
- Godambe, L.P.; Bandekar, J.; Shashidhar, R. Species specific PCR based detection of Escherichia coli from Indian foods. 3 Biotech 2017, 7, 130. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ewers, C.; Janssen, T.; Kiessling, S.; Philipp, H.C.; Wieler, L.H. Rapid detection of virulence-associated genes in avian pathogenic Escherichia coli by multiplex polymerase chain reaction. Avian Dis. 2005, 49, 269–273. [Google Scholar] [CrossRef]
- Tóth, I.; Hérault, F.; Beutin, L.; Oswald, E. Production of cytolethal distending toxins by pathogenic Escherichia coli strains isolated from human and animal sources: Establishment of the existence of a new cdt variant (Type IV). J. Clin. Microbiol. 2003, 41, 4285–4291. [Google Scholar] [CrossRef] [Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 28th ed.; CLSI Supplement M100; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2018. [Google Scholar]
- EUCAST. The European Committee on Antimicrobial Susceptibility Testing. Breakpoint Tables for Interpretation of MICs and Zone Diameters. Version 11.0. 2021. Available online: http://www.eucast.org (accessed on 10 December 2021).
- Feng, Y.; Mannion, A.; Madden, C.M.; Swennes, A.G.; Townes, C.; Byrd, C.; Marini, R.P.; Fox, J.G. Cytotoxic Escherichia coli strains encoding colibactin and cytotoxic necrotizing factor (CNF) colonize laboratory macaques. Gut Pathog. 2017, 9, 71. [Google Scholar] [CrossRef]
- Cruz-Soto, A.S.; Toro-Castillo, V.; Munguía-Magdaleno, C.O.; Torres-Flores, J.E.; Flores-Pantoja, L.E.; Loeza-Lara, P.D.; Jiménez-Mejía, R. Genetic relationships, biofilm formation, motility and virulence of Escherichia coli isolated from bovine mastitis. Rev. Mex. Cienc. Pecu. 2020, 11, 167–182. [Google Scholar] [CrossRef]
- Fang, H.; Ataker, F.; Hedin, G.; Dornbusch, K. Molecular epidemiology of extended-spectrum beta-lactamases among Escherichia coli isolates collected in a Swedish hospital and its associated health care facilities from 2001 to 2006. J. Clin. Microbiol. 2008, 46, 707–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kozak, G.K.; Boerlin, P.; Janecko, N.; Reid-Smith, R.J.; Jardine, C. Antimicrobial resistance in Escherichia coli isolates from swine and wild small mammals in the proximity of swine farms and in natural environments in Ontario, Canada. Appl. Environ. Microbiol. 2009, 75, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grape, M.; Motakefi, A.; Pavuluri, S.; Kahlmeter, G. Standard and real-time multiplex PCR methods for detection of trimethoprim resistance dfr genes in large collections of bacteria. Clin. Microbiol. Infect. 2007, 13, 1112–1118. [Google Scholar] [CrossRef] [Green Version]


| Group of Reptiles | Number of Samples | Species (Number of Samples) | Diet Group | Number of E. coli Isolates (%) |
|---|---|---|---|---|
| Snakes | 31 | Pantheropsis guttatus (14) Python regius (3) Boa constrictor (4) Lampropeltis triangulum (4) Morelia pilota (1) Orthrophis teaniurus (5) | Carnivore Carnivore Carnivore Carnivore Carnivore Carnivore | 7 (50) 3 (100) 3 (75) 2 (50) 1 (100) 0 |
| Lizards | 18 | Pogona vitticeps (6) Iguana iguana (4) Eublepharis macularius (3) Furcifer pardali (5) | Omnivore Herbivore Carnivore Omnivore | 5 (83) 2 (50) 0 1 (20) |
| Turtles | 18 | Testudo horsfieldii (8) Testudo hermanni (9) Chelonoidis carbonaria (1) | Herbivore Herbivore Herbivore | 5 (62.5) 2 (22) 1 (100) |
| Total: | 67 | 32 (47.8) |
| Isolate | Species | Phylogenetic Group | Antibiotic Phenotype Pattern (Including Resistant and Intermediate Strains) a | Resistance Genes | Virulence Genes |
|---|---|---|---|---|---|
| 6 | Corn snake (Pantheropsis guttatus) | B1 | AMP-AK-S-CIP | - | traT |
| 13a | Ball python (Python regius) | B1 | AMP | - | irp-2, iucD, traT, fyuA |
| 15a | Ball python (Python regius) | A | - | - | - |
| 16a | Corn snake (Pantheropsis guttatus) | A | AMP-AMC-AK-S | - | pic, astA |
| 26 | Boa constrictor snake (Boa constrictor) | B1 | S | - | - |
| 30a | Corn snake (Pantheropsis guttatus) | B2 | S | - | irp-2, vat, traT, fyuA |
| 31a | Corn snake (Pantheropsis guttatus) | B1 | S | aadA | traT |
| 32a | Milk snake (Lampropeltis triangulum) | B2 | S | - | irp-2, vat, traT, fyuA |
| 34 | Ball python (Python regius) | B1 | S | - | traT |
| 35a | Boa constrictor snake (Boa constrictor) | D | S | - | vat |
| 36a | Boa constrictor snake (Boa constrictor) | A | - | - | traT |
| 37a | Corn snake (Pantheropsis guttatus) | B1 | CN-AK-S-T-Wb | - | - |
| 39 | Corn snake (Pantheropsis guttatus) | B1 | - | - | |
| 41 | Milk snake (Lampropeltis triangulum) | B2 | S | - | pic, irp-2, traT, fyuA |
| 47 | Corn snake (Pantheropsis guttatus) | B1 | S | - | - |
| 48 | Carpet python (Morelia spilota) | B1 | S | - | traT, fyuA |
| 54a | Panther chameleon (Furcifer pardalis) | B1 | S | - | - |
| 46 | Central bearded dragon (Pogona vitticeps) | A | S-T | tetA | - |
| 55 | Central bearded dragon (Pogona vitticeps) | D | CN-AK-S-T-CIP | tetB, tetM | irp-2, fyuA |
| 61 | Central bearded dragon (Pogona vitticeps) | B1 | AMP-S | blaTEM | traT |
| 62 | Central bearded dragon (Pogona vitticeps) | B1 | S | - | - |
| 63 | Central bearded dragon (Pogona vitticeps) | B1 | S | - | - |
| 44 | Green iguana (Iguana iguana) | B1 | S | - | traT |
| 65 | Green iguana (Iguana iguana) | B1 | AMP-S | - | - |
| 17a | Steppe tortoise (Testudo horsfieldii) | B1 | - | - | traT |
| 18a | Steppe tortoise (Testudo horsfieldii) | A | S | - | fyuA |
| 19a | Steppe tortoise (Testudo horsfieldii) | A | - | - | - |
| 25a | Red-footed tortoise (Chelonoidis carbonaria) | B1 | AK-S | - | - |
| 67 | Greek tortoise (Testudo hermanni) | B1 | S | - | - |
| 69 | Steppe tortoise (Testudo horsfieldii) | B1 | S | - | - |
| 70 | Steppe tortoise (Testudo horsfieldii) | B1 | S | - | - |
| 71 | Greek tortoise (Testudo hermanni) | B1 | S | - | - |
| Detected Genes | Annealing Temperature (°C) | Reference | |
|---|---|---|---|
| Multiplex I | stx1, stx2, hylA, eaeA, saa | 65 (10 cycles) then 62 (20 cycles) | [14] |
| Multiplex II | ecsV, ent, bfpB, invE, astA, aggR, pic, ipaH, elt, estIa, estIb | 62 | [14] |
| Multiplex III | ompT, iutA | 63 | [14] |
| Multiplex IV | tsh, pap-C, iss, irp-2 | 57 | [29] |
| Multiplex V | iucD, vat | 54 | |
| Uniplex I | cva/cvi | 58 | |
| Uniplex II | cnf | 55 | [30] |
| Uniplex III | kpsII | 56 | [4] |
| Uniplex IV | traT | 59 | |
| Uniplex V | fyuA | 59 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dec, M.; Stepien-Pysniak, D.; Szczepaniak, K.; Turchi, B.; Urban-Chmiel, R. Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles. Pathogens 2022, 11, 127. https://doi.org/10.3390/pathogens11020127
Dec M, Stepien-Pysniak D, Szczepaniak K, Turchi B, Urban-Chmiel R. Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles. Pathogens. 2022; 11(2):127. https://doi.org/10.3390/pathogens11020127
Chicago/Turabian StyleDec, Marta, Dagmara Stepien-Pysniak, Klaudiusz Szczepaniak, Barbara Turchi, and Renata Urban-Chmiel. 2022. "Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles" Pathogens 11, no. 2: 127. https://doi.org/10.3390/pathogens11020127
APA StyleDec, M., Stepien-Pysniak, D., Szczepaniak, K., Turchi, B., & Urban-Chmiel, R. (2022). Virulence Profiles and Antibiotic Susceptibility of Escherichia coli Strains from Pet Reptiles. Pathogens, 11(2), 127. https://doi.org/10.3390/pathogens11020127







