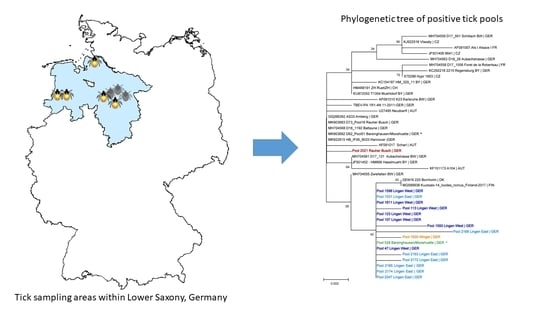
New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks
Abstract
1. Introduction
2. Results
2.1. TBEV Detection
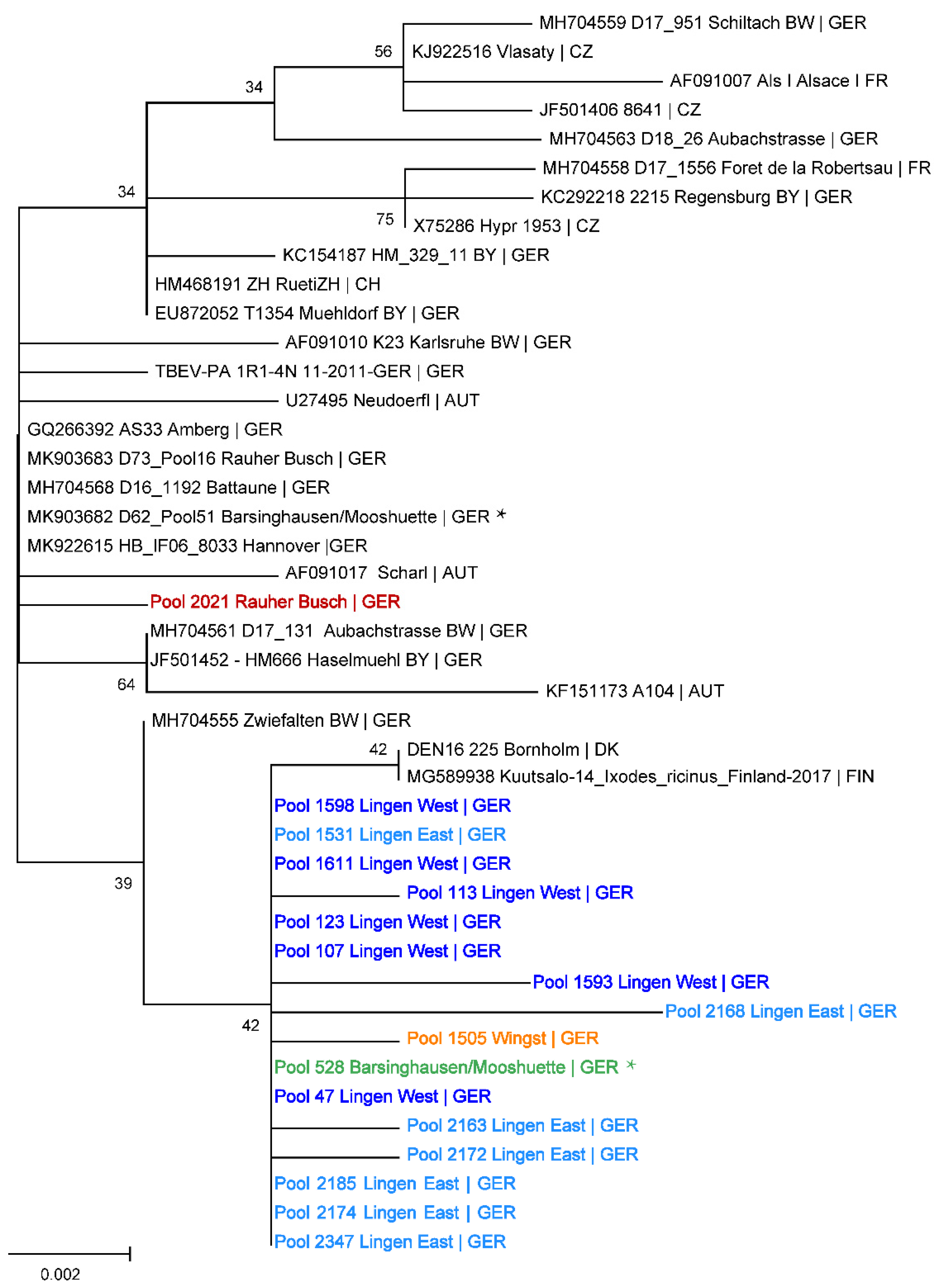
2.2. Sequencing of the Viral E Gene and Phylogenetic Analysis
2.3. Virus Cultivation
3. Discussion
4. Materials and Methods
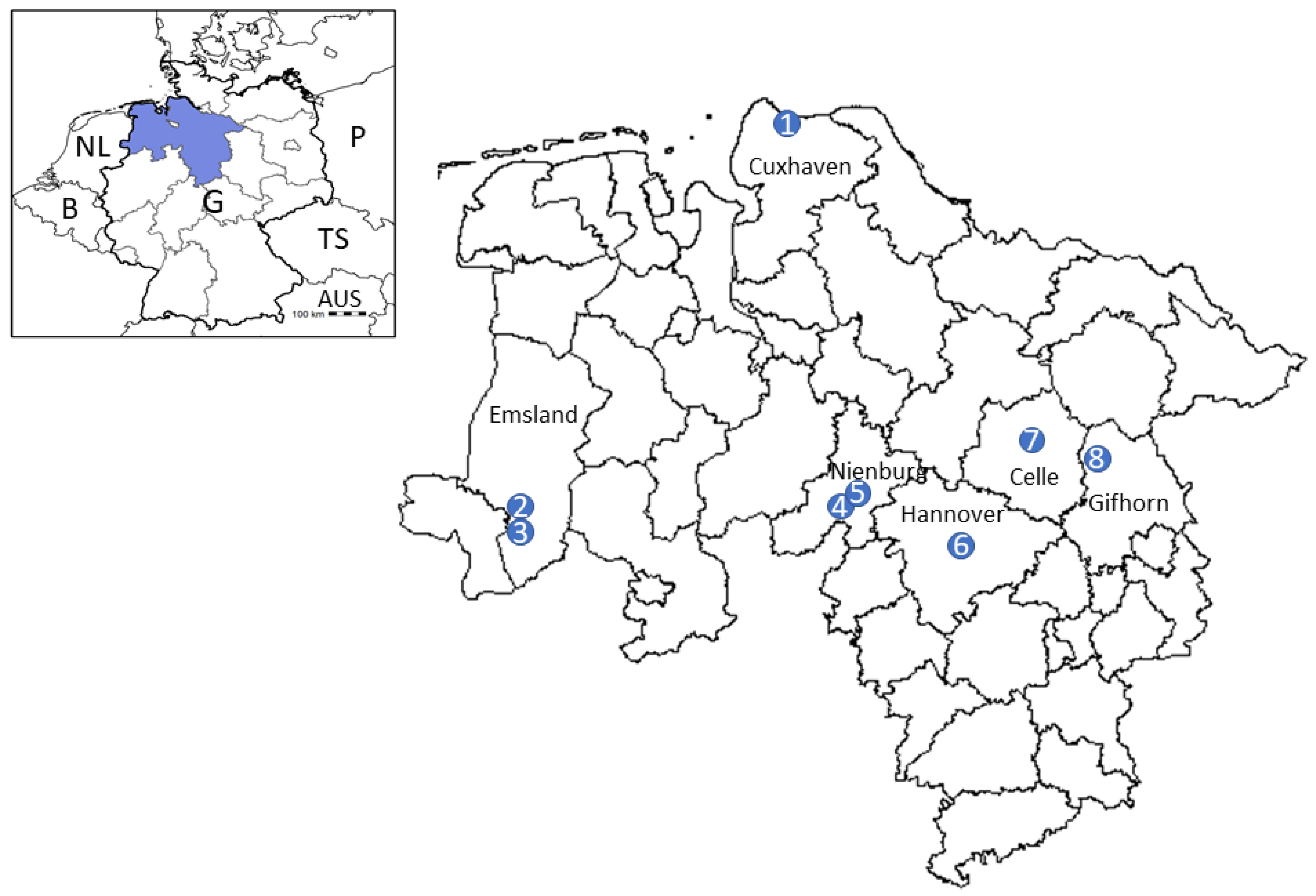
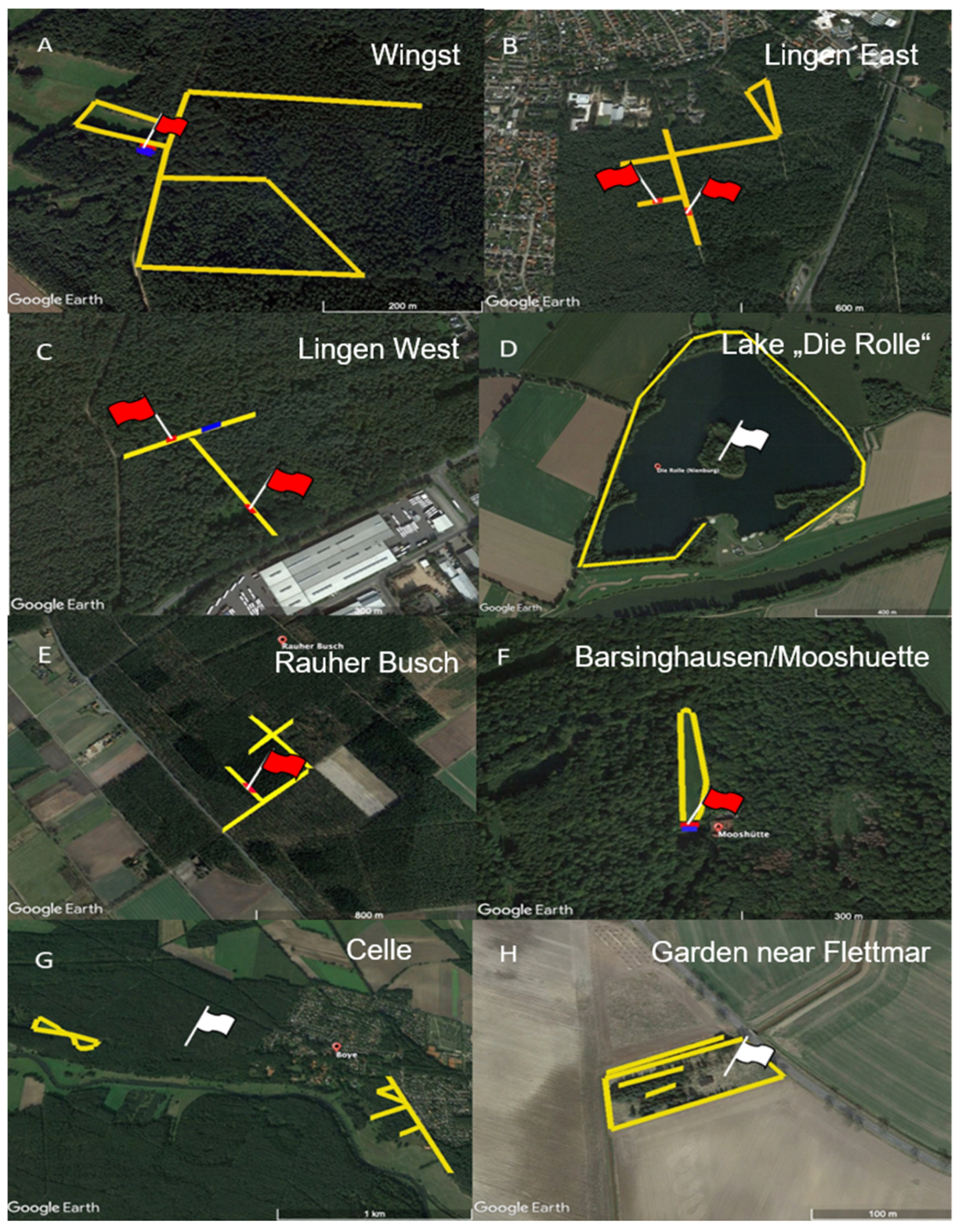
4.1. Tick Sampling
4.2. RNA-Isolation and RT-qPCR
4.3. Amplification and Sequencing of the Viral E Gene
4.4. Phylogenetic Analysis
4.5. Virus Cultivation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pfeffer, M.; Schmuck, H.M.; Leschnik, M. TBE in animals. In The TBE Book; Global Health Press Pte Ltd.: Singapore, 2020; p. 105. [Google Scholar]
- Kozlova, I.V.; Demina, T.V.; Tkachev, S.E.; Doroshchenko, E.K.; Lisak, O.V.; Verkhozina, M.M.; Karan, L.S.; Paramonov, A.; Suntsova, O.; Chernoivanova, O. Characteristics of the Baikal subtype of tick-borne encephalitis virus circulating in Eastern Siberia. Acta Biomed. Sci. 2018, 3, 53–60. [Google Scholar] [CrossRef]
- Dai, X.; Shang, G.; Lu, S.; Yang, J.; Xu, J. A new subtype of eastern tick-borne encephalitis virus discovered in Qinghai-Tibet Plateau, China. Emerg. Microbes Infect. 2018, 7, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Ruzek, D.; Avšič Županc, T.; Borde, J.; Chrdle, A.; Eyer, L.; Karganova, G.; Kholodilov, I.; Knap, N.; Kozlovskaya, L.; Matveev, A.; et al. Tick-borne encephalitis in Europe and Russia: Review of pathogenesis, clinical features, therapy, and vaccines. Antivir. Res. 2019, 164, 23–51. [Google Scholar] [CrossRef] [PubMed]
- Kaiser, R. Tick-borne encephalitis (TBE) in Germany and clinical course of the disease. Int. J. Med. Microbiol. Suppl. 2002, 291, 58–61. [Google Scholar] [CrossRef]
- Hellenbrand, W.; Kreusch, T.; Böhmer, M.M.; Wagner-Wiening, C.; Dobler, G.; Wichmann, O.; Altmann, D. Epidemiology of tick-borne encephalitis (TBE) in Germany, 2001–2018. Pathogens 2019, 8, 42. [Google Scholar] [CrossRef]
- Balogh, Z.; Egyed, L.; Ferenczi, E.; Bán, E.; Szomor, K.N.; Takács, M.; Berencsi, G. Experimental infection of goats with tick-borne encephalitis virus and the possibilities to prevent virus transmission by raw goat milk. Intervirology 2012, 55, 194–200. [Google Scholar] [CrossRef]
- Ličková, M.; Fumačová Havlíková, S.; Sláviková, M.; Slovák, M.; Drexler, J.F.; Klempa, B. Dermacentor reticulatus is a vector of tick-borne encephalitis virus. Ticks Tick Borne Dis. 2020, 11, 101414. [Google Scholar] [CrossRef]
- Gritsun, T.S.; Lashkevich, V.A.; Gould, E.A. Tick-borne encephalitis. Antivir. Res. 2003, 57, 129–146. [Google Scholar] [CrossRef]
- Dobler, G.; Hufert, F.; Pfeffer, M.; Essbauer, S. Tick-borne encephalitis: From microfocus to human disease. In Progress Parasitology; Mehlhorn, H., Ed.; Springer: Berlin/Heidelberg, Germany, 2011; pp. 323–331. [Google Scholar]
- Boelke, M.; Bestehorn, M.; Marchwald, B.; Kubinski, M.; Liebig, K.; Glanz, J.; Schulz, C.; Dobler, G.; Monazahian, M.; Becker, S.C. First isolation and phylogenetic analyses of tick-borne encephalitis virus in Lower Saxony, Germany. Viruses 2019, 11, 462. [Google Scholar] [CrossRef]
- Paulsen, K.M.; Pedersen, B.N.; Soleng, A.; Okbaldet, Y.B.; Pettersson, J.H.O.; Dudman, S.G.; Ottesen, P.; Vik, I.S.S.; Vainio, K.; Andreassen, Å. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks from three islands in north-western Norway. APMIS Suppl. 2015, 123, 759–764. [Google Scholar] [CrossRef]
- Borde, J.P.; Kaier, K.; Hehn, P.; Matzarakis, A.; Frey, S.; Bestehorn, M.; Dobler, G.; Chitimia-Dobler, L. The complex interplay of climate, TBEV vector dynamics and TBEV infection rates in ticks—Monitoring a natural TBEV focus in Germany, 2009–2018. PLoS ONE 2021, 16, e0244668. [Google Scholar] [CrossRef]
- Pettersson, J.H.; Golovljova, I.; Vene, S.; Jaenson, T.G. Prevalence of tick-borne encephalitis virus in Ixodes ricinus ticks in northern Europe with particular reference to Southern Sweden. Parasit. Vectors 2014, 7, 102. [Google Scholar] [CrossRef] [PubMed]
- Wójcik-Fatla, A.; Cisak, E.; Zając, V.; Zwoliński, J.; Dutkiewicz, J. Prevalence of tick-borne encephalitis virus in Ixodes ricinus and Dermacentor reticulatus ticks collected from the Lublin region (eastern Poland). Ticks Tick Borne Dis. 2011, 2, 16–19. [Google Scholar] [CrossRef]
- Frimmel, S.; Krienke, A.; Riebold, D.; Loebermann, M.; Littmann, M.; Fiedler, K.; Klaus, C.; Süss, J.; Reisinger, E.C. Tick-borne encephalitis virus habitats in north east Germany: Reemergence of TBEV in ticks after 15 years of inactivity. Biomed. Res. Int. 2014, 2014, 308371. [Google Scholar] [CrossRef]
- Mikryukova, T.P.; Moskvitina, N.S.; Kononova, Y.V.; Korobitsyn, I.G.; Kartashov, M.Y.; Tyuten’kov, O.Y.; Protopopova, E.V.; Romanenko, V.N.; Chausov, E.V.; Gashkov, S.I.; et al. Surveillance of tick-borne encephalitis virus in wild birds and ticks in Tomsk city and its suburbs (Western Siberia). Ticks Tick Borne Dis. 2014, 5, 145–151. [Google Scholar] [CrossRef] [PubMed]
- Klaus, C.; Hoffmann, B.; Hering, U.; Mielke, B.; Sachse, K.; Beer, M.; Süss, J. Tick-borne encephalitis (TBE) virus prevalence and virus genome characterization in field-collected ticks (Ixodes ricinus) from risk, non-risk and former risk areas of TBE, and in ticks removed from humans in Germany. Clin. Microbiol. Infect. 2010, 16, 238–244. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Velay, A.; Solis, M.; Kack-Kack, W.; Gantner, P.; Maquart, M.; Martinot, M.; Augereau, O.; De Briel, D.; Kieffer, P.; Lohmann, C.; et al. A new hot spot for tick-borne encephalitis (TBE): A marked increase of TBE cases in France in 2016. Ticks Tick Borne Dis. 2018, 9, 120–125. [Google Scholar] [CrossRef]
- Ocias, L.F.; Waldeck, M.; Hallén, I.; Nørgaard, M.; Krogfelt, K.A. Transnational exchange of surveillance data reveals previously unrecognized TBEV microfocus. Eur. J. Public Health 2019, 29, 631–633. [Google Scholar] [CrossRef]
- Holding, M.; Dowall, S.; Hewson, R. Detection of tick-borne encephalitis virus in the UK. Lancet 2020, 395, 411. [Google Scholar] [CrossRef]
- Robert-Koch-Institut. Epidemiologisches Bulletin 9/2021; Robert-Koch-Institut: Berlin/Wernigerode, Germany, 2021; Volume 9, pp. 3–20. [Google Scholar]
- Robert-Koch-Institut. Epidemiologisches Bulletin 8/2020; Robert-Koch-Institut: Berlin/Wernigerode, Germany, 2020; Volume 8, pp. 3–19. [Google Scholar]
- Robert-Koch-Institut. Epidemiologisches Bulletin 17/2018; Robert-Koch-Institut: Berlin/Wernigerode, Germany, 2018; Volume 17, pp. 161–173. [Google Scholar]
- Robert-Koch-Insitut. Epidemiologisches Bulletin 7/2019; Robert-Koch-Institut: Berlin/Wernigerode, Germany, 2019; Volume 7, pp. 57–70. [Google Scholar]
- Walter, M.; Vogelgesang, J.R.; Rubel, F.; Brugger, K. Tick-borne encephalitis virus and its European distribution in ticks and endothermic mammals. Microorganisms 2020, 8, 1065. [Google Scholar] [CrossRef]
- Dobler, G.; Gniel, D.; Petermann, R.; Pfeffer, M. Epidemiology and distribution of tick-borne encephalitis. Wien. Med. Wochenschr. 2012, 162, 230–238. [Google Scholar] [CrossRef] [PubMed]
- Schley, K.; Malerczyk, C.; Beier, D.; Schiffner-Rohe, J.; von Eiff, C.; Häckl, D.; Süß, J. Vaccination rate and adherence of tick-borne encephalitis vaccination in Germany. Vaccine 2021, 39, 830–838. [Google Scholar] [CrossRef] [PubMed]
- Timmerberg, C. Untersuchungen zum Vorkommen von Frühsommermeningoencephalitis-Viren, Borrelia burgdorferi sensu lato, Anaplasma phygozytophilum in Zeckenpopulationen und Untersuchung zur Antikörperprävalenz gegen Frühsommermeningoenzephalitis-Viren in der Bevölkerung der Region Wingst/Cuxhaven. Doctoral Thesis, Georg-August-Universität zu Göttingen, Göttingen, Germany, 2011. [Google Scholar]
- Jaenson, T.G.T.; Petersson, E.H.; Jaenson, D.G.E.; Kindberg, J.; Pettersson, J.H.O.; Hjertqvist, M.; Medlock, J.M.; Bengtsson, H. The importance of wildlife in the ecology and epidemiology of the TBE virus in Sweden: Incidence of human TBE correlates with abundance of deer and hares. Parasit. Vectors 2018, 11, 477. [Google Scholar] [CrossRef] [PubMed]
- Bestehorn, M.; Weigold, S.; Kern, W.V.; Chitimia-Dobler, L.; Mackenstedt, U.; Dobler, G.; Borde, J.P. Phylogenetics of tick-borne encephalitis virus in endemic foci in the upper Rhine region in France and Germany. PLoS ONE 2018, 13, e0204790. [Google Scholar] [CrossRef] [PubMed]
- Agergaard, C.N.; Rosenstierne, M.W.; Bødker, R.; Rasmussen, M.; Andersen, P.H.S.; Fomsgaard, A. New tick-borne encephalitis virus hot spot in Northern Zealand, Denmark, October 2019. Euro. Surveill. 2019, 24, 1900639. [Google Scholar] [CrossRef] [PubMed]
- Süss, J.; Béziat, P.; Rohr, H.P.; Treib, J.; Haass, A. Detection of the tick-borne encephalitis virus (TBEV) in ticks in several federal “Länder” of Germany by means of the polymerase chain reaction (PCR)—Characterization of the virus. Infection 1996, 24, 403–404. [Google Scholar] [CrossRef] [PubMed]
- Deutscher Wetterdienst. Deutschlandwetter im April 2021; Deutscher Wetterdienst: Offenbach, Germany, 2021.
- Süss, J. Tick-borne encephalitis 2010: Epidemiology, risk areas, and virus strains in Europe and Asia—An overview. Ticks Tick Borne Dis. 2011, 2, 2–15. [Google Scholar] [CrossRef] [PubMed]
- Asghar, N.; Pettersson, J.H.O.; Dinnetz, P.; Andreassen, Å.; Johansson, M. Deep sequencing analysis of tick-borne encephalitis virus from questing ticks at natural foci reveals similarities between quasispecies pools of the virus. J. Med. Virol. 2017, 98, 413–421. [Google Scholar] [CrossRef] [PubMed]
- Waldenström, J.; Lundkvist, A.; Falk, K.I.; Garpmo, U.; Bergström, S.; Lindegren, G.; Sjöstedt, A.; Mejlon, H.; Fransson, T.; Haemig, P.D.; et al. Migrating birds and tickborne encephalitis virus. Emerg. Infect. Dis. 2007, 13, 1215–1218. [Google Scholar] [CrossRef]
- Chitimia-Dobler, L.; Lemhöfer, G.; Król, N.; Bestehorn, M.; Dobler, G.; Pfeffer, M. Repeated isolation of tick-borne encephalitis virus from adult Dermacentor reticulatus ticks in an endemic area in Germany. Parasit. Vectors 2019, 12, 90. [Google Scholar] [CrossRef] [PubMed]
- Drehmann, M.; Springer, A.; Lindau, A.; Fachet, K.; Mai, S.; Thoma, D.; Schneider, C.R.; Chitimia-Dobler, L.; Bröker, M.; Dobler, G.; et al. The spatial distribution of Dermacentor ticks (Ixodidae) in Germany—Evidence of a continuing spread of Dermacentor reticulatus. Front. Vet. Sci. 2020, 7, 661. [Google Scholar] [CrossRef]
- Estrada-Peña, A.; Mihalca, A.D.; Petney, T.N. Ticks of Europe and North Africa; Springer: Cham, Switzerland, 2017; p. 404. [Google Scholar]
- Schwaiger, M.; Cassinotti, P. Development of a quantitative real-time RT-PCR assay with internal control for the laboratory detection of tick borne encephalitis virus (TBEV) RNA. J. Clin. Virol. 2003, 27, 136–145. [Google Scholar] [CrossRef]
- Kupča, A.M.; Essbauer, S.; Zoeller, G.; de Mendonça, P.G.; Brey, R.; Rinder, M.; Pfister, K.; Spiegel, M.; Doerrbecker, B.; Pfeffer, M.; et al. Isolation and molecular characterization of a tick-borne encephalitis virus strain from a new tick-borne encephalitis focus with severe cases in Bavaria, Germany. Ticks Tick Borne Dis. 2010, 1, 44–51. [Google Scholar] [CrossRef]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Mol. Biol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
| Location | District | 2020 | 2021 | Total | ||||
|---|---|---|---|---|---|---|---|---|
| Total | Nymphs | Adult Ticks | Total | Nymphs | Adult Ticks | |||
| Wingst 1 | Cuxhaven | 2902 | 2441 | 461 | 691 | 555 | 136 | 3593 |
| Lingen East 2 | Emsland | 1700 | 1486 | 214 | 522 | 432 | 90 | 2222 |
| Lingen West 1 | Emsland | 2652 | 1880 | 772 | 484 | 465 | 19 | 3136 |
| Lake “Die Rolle” 2 | Nienburg | 862 | 645 | 217 | 145 | 90 | 55 | 1007 |
| Rauher Busch 1 | Nienburg | 3124 | 2856 | 268 | 880 | 718 | 162 | 4004 |
| Barsinghausen/Mooshuette 1 | Hannover | 1856 | 1446 | 410 | 319 | 209 | 110 | 2175 |
| Celle 2 | Celle | 3160 | 2405 | 701 | 636 | 140 | 496 | 3742 |
| Garden near Flettmar 2 | Gifhorn | 163 | 55 | 108 | 14 | 9 | 5 | 177 |
| Location | District | No. of Collected Ticks | No. of Pools | Positive Pools (Nymphs/Adults) | Positive Pools in 2020 (Nymphs/Adults) | Positive Pools in 2021 (Nymphs/Adults) | Total MIR Nymphs (%) | Total MIR Adults (%) |
|---|---|---|---|---|---|---|---|---|
| Wingst 2 | Cuxhaven | 3593 | 401 | 1 (1/0) | 1 (1/0) | 0 (0/0) | 0.03 | 0.00 |
| Lingen East 3 | Emsland | 2222 | 249 | 8 (7/1) | 1 (0/1) | 7 (7/0) | 0.36 | 0.33 |
| Lingen West 2 | Emsland | 3136 | 367 | 7 (3/4) | 7 (3/4) | 0 (0/0) | 0.13 | 0.51 |
| Lake “Die Rolle” 3 | Nienburg | 1007 | 98 | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0.00 | 0.00 |
| Rauher Busch 2 | Nienburg | 4004 | 446 | 1 (1/0) | 0 (0/0) | 1 (1/0) | 0.03 | 0.00 |
| Barsinghausen/Mooshuette 2 | Hannover | 1856 | 283 | 1 (0/1) | 1 (0/1) | 0 (0/0) | 0.00 | 0.19 |
| Celle 3 | Celle | 3742 | 654 | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0.00 | 0.00 |
| Garden near Flettmar 3 | Gifhorn | 177 | 30 | 0 (0/0) | 0 (0/0) | 0 (0/0) | 0.00 | 0.00 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Topp, A.-K.; Springer, A.; Dobler, G.; Bestehorn-Willmann, M.; Monazahian, M.; Strube, C. New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks. Pathogens 2022, 11, 126. https://doi.org/10.3390/pathogens11020126
Topp A-K, Springer A, Dobler G, Bestehorn-Willmann M, Monazahian M, Strube C. New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks. Pathogens. 2022; 11(2):126. https://doi.org/10.3390/pathogens11020126
Chicago/Turabian StyleTopp, Anna-Katharina, Andrea Springer, Gerhard Dobler, Malena Bestehorn-Willmann, Masyar Monazahian, and Christina Strube. 2022. "New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks" Pathogens 11, no. 2: 126. https://doi.org/10.3390/pathogens11020126
APA StyleTopp, A.-K., Springer, A., Dobler, G., Bestehorn-Willmann, M., Monazahian, M., & Strube, C. (2022). New and Confirmed Foci of Tick-Borne Encephalitis Virus (TBEV) in Northern Germany Determined by TBEV Detection in Ticks. Pathogens, 11(2), 126. https://doi.org/10.3390/pathogens11020126