Abstract
Uropathogenic Escherichia coli is the most common cause of urinary tract infections, resulting in about 150 million reported annual cases. With multidrug resistance on the rise and the need for global and region surveillance, this investigation looks at the UPEC isolates collected for a 3-year period, with a view of ascertaining their antimicrobial susceptibility patterns and associated virulence determinants. The identification of bacteria isolates, antimicrobial susceptibility, and extended-spectrum beta-lactamases (ESBLs) production was determined with a Vitek 2 Compact Automated System (BioMerieux, Marcy L’Etoile, France). ESBLs were confirmed by the combined disc test (CDT) and basic biochemical test. The isolates were distributed into A (11%), B1 (6%), B2 (62.4%), and D (20.6%). Resistance to the penicillin group was high, between 88% and 100%. Additionally, resistance was high to cephalosporins (100%) in 2017 and 2018. The isolates were all sensitive to tigecycline, while resistance against imipenem and meropenem was low, at 4–12% in 2017 and 2018 and 0% in 2019. The results also showed that ESBL isolates were seen in 2017 and 2018. They were confirmed positive to CTX/CLA (88.5%) and CAZ/CLA (85%). By 2019, the number of resistant isolates reduced, showing only 4% ESBL isolates. Two virulence genes, fimH (46%) and papE/F (15%), were detected among the isolates by PCR. In conclusion, this study found that phylogroups B2 and D carried the most virulence genes as well as MDR and ESBL characteristics, suggesting the UPEC strains to be extraintestinal pathogens responsible for UTIs.
Keywords:
uropathogenic; Escherichia coli; MDR; phylogroup; multidrug resistant; ESBL; virulence genes 1. Introduction
Urinary tract infections (UTIs) place a huge annual financial burden on healthcare systems globally. An estimated 150 million cases are reported globally on an annual basis [1]. It is estimated that there are about seven million hospital visits, of which one million are admitted to emergency units/departments in the USA annually [2,3], impacting patients’ quality of life [4]. Generally, UTIs can be categorized as either community-associated (CAUTIs) or hospital-acquired (HAUTIs) infections [5]. In Saudi Arabia, the incidences are high and are said to be the most prevalent cause of infections [6], predominantly so among women [7]. Uropathogenic Escherichia coli (UPEC) are highly versatile pathogens capable of both commensal existence in the human GIT as well as becoming opportunistic pathogens in the urinary tract [8]. Of UTI visits to hospitals, 14.6% were to emergency departments, reported in Riyadh, Saudi Arabia during a quarter of one year by adults and elderly patients [9]. This percentage was higher than that of an earlier report in the same region [10]. Additionally, higher frequencies of UTIs have been consistently reported among women patients in Saudi Arabia compared to male patients, the reasons for which are attributed to anatomical and physiological differences [9]. A report by the General Authority for Statistics, Saudi Arabia [11] placed the population of women at about 8.5 million in January 2016. Of these, there were an estimated 880,000 women in that year who visited primary care due to UTIs, with half of that number (440,000) being to emergency departments. Though frequencies of UTIs in women are indicated to decrease with age while increasing in adult males [12], there has generally been a financial burden in the management of UTIs in Saudi Arabia, similar to that found in global reports [9]. Treatment has become more costly with the rise in antimicrobial resistance by bacterial isolates and clinicians being faced with limited options, particularly with ESBL-producing pathogens.
A high prevalence of ESBL-MDR E. coli strains has been reported [13,14,15], with a recent study confirming E. coli as the most common cause [16] of UTIs. The prevalence of ESBL carriage by MDR UTI clinical isolates has been reported [16,17], and this suggests additional therapeutic implications as they are resistant to a wide range of antibiotics [16].
While there are several bacterial pathogens that cause UTIs [9,16], those originating from Escherichia coli form the majority. Sometimes, the symbiotic relationship is compromised, and the violation of the gastrointestinal barriers could lead to a wide range of infections. On the other hand, there are pathogenic strains that are classified as either enteric or extraintestinal E. coli (ExPEC) [18]. Uropathogenic E. coli (UPEC) is one of two pathotypes of ExPEC, a strain that exists in the gut and can disseminate to other parts of the human anatomy, initiating disease [18]. Generally, urinary tract infections are caused by uropathogenic E. coli [19] with strains that are marked by diversity in mobility and metabolism, and bacterial behaviors that predict pathogenicity rather than the carriage of any specified sets of genes [19]. Virulence factors play critical roles throughout the process of invasion, colonization, and bacterial multiplication, and thereby continue to gain attention from researchers globally [11,12,13,20,21,22], particularly in this era of difficult-to-treat bacterial infections. However, differences in virulence phenotypes and host susceptibility determine the associated risk of UTIs [19]. Additionally, antimicrobial resistance is linked to reduced virulence [23], while phylogenetic diversity among E. coli isolates is further highlighted [24]. However, reports on virulence determinants are scarce in the region of the present study.
As with other bacterial pathogens, antimicrobial resistance by uropathogens is reportedly on a global increase [21,25]. Researchers reveal that there are limited options available for treatment [26,27,28,29], while recommending steps to help curtail antimicrobial resistance and the spread of resistant genes associated with them. Global and regional antimicrobial surveillance programs are one such step. Such programs have been instituted in regions of Saudi Arabia [9,25,30], where they update regional surveillance as recommended to curtail the rise in antimicrobial resistance. The literature is, however, silent on the region of the present investigation regarding virulence factors associated with bacterial infections and resistance. To bridge this gap in knowledge, the present report looks at the antimicrobial phenotypes of Escherichia coli isolates associated with urinary tract UPEC infections in Al-Ahsa, a town in the southeast of Saudi Arabia. This study also examines the antimicrobial susceptibility pattern of E. coli clinical isolates collected from urine samples between 2017 and 2019. Additionally, virulence genes associated with the isolates were ascertained to find any possible correlation between antimicrobial resistances. Therefore, the objective of this investigation was to examine the phylogenetic groups of UPEC isolates, their antimicrobial susceptibility pattern, and ESBL carriage among the isolates. In addition, associated virulence factors were also examined. The investigation provides an insight into a three-year antimicrobial resistance pattern.
2. Materials and Methods
2.1. Ethical Consideration
E. coli clinical isolates were those of routine hospital diagnoses, which served as part of patient care. They were obtained from the microbial bank of the medical microbiology laboratory in the College of Medicine of King Faisal University. Patients were not involved in the study.
2.2. Bacterial Isolates
A retrospective study was carried out on uropathogenic E. coli isolates collected between 2017 and 2019. Confirmed E. coli isolates had been inoculated into MicrobankTM ready-to-use tubes (Pro-lab Diagnostic, Round Rock, TX, USA) incorporated with treated beads and cryopreservative fluid by the manufacturers. The methods of preservation and bacterial retrieval were performed according to the guidelines provided by the manufacturers (https://www.pro-lab-direct.com/v/vspfiles/microbank/microbank-wwp-portfolio.pdf, accessed on 19 June 2021). A total of 170 bacteria strains were used for the antimicrobial susceptibility assay. The isolates were given codes beginning with letters followed by a number. Bacteria IDs were confirmed with a Vitek Compact 2 (BioMerieux, Marcy L’Etoile, France) using GN ID cards. Additionally, basic biochemical tests that included citrate and lactose fermenting in addition to urease and indole tests were carried out. However, 152 isolates were used for ESBLs assay, while 48 of them were used for molecular analysis.
2.3. Antimicrobial Susceptibility Assay, Detection and Confirmation of ESBLs
Vitek 2 AST cards were used to test the susceptibility of the isolates against the following antibiotics: amoxicillin, amoxicillin/clavulanic acid, piperacillin/tazobactam, imipenem (Imp), meropenem (Mer), ciprofloxacin (Cp), cephalotin (Kf), cefuroxime (Cxm), ceftriaxone (Cax), cefotaxime (Cft), cefazolin (Cfz), ceftazidime (Caz), cefepime (Pime), aztreonam (AZT), Augmentin (Aug), ampicillin (Amp), gentamicin (Gm), tigecycline (Tig), nitrofurantoin (Fd), and trimethoprim/sulfamethoxazole (Sxt). The production of extended-spectrum beta-lactamases (ESBLs) was detected by a Vitek 2 Automated System and confirmed by the combined disc test (CDT) as recommended by the CLSI [31], using cefotaxime (CTX) or ceftazidime (CAZ) combined with clavulanic acid (CLA) as inhibitors. Tests were carried out on Muller–Hinton agar incubated aerobically at 35 °C for 24 h. They were seeded with strains of UPEC isolates, discs of 30 µg cefotaxime (CTX), 30 µg ceftazidime (CAZ), combined with 10 µg clavulanic acid (CLA). The results were interpreted as recommended by the CLSI [32] based on resistance to a single test against cefotaxime and ceftazidime individually and separately in combination with clavulanate.
The following primers were used for the amplification and detection of the carriage of ESBL (tem, ctx, and shv) genes by PCR, with the resultant products being stained with ethidium bromide and visualized by agarose gel electrophoresis: TEM-F-AGATCAGTTGGGTGCACGAG, TEM-R CAGTGCTGCAATGATACCG; CTX-F-ATGTGCAGYACCAGTAARGTKATGGC, CTX-R-TGGGTRAARTARGTSACCAGAAYCAGCGG; and SHV-F-GGGTTATTCTTATTTGTCGC, SHV-R-TTAGCGTTGCCAGTGCTC.
2.4. Hemolysin Production and Cell Surface Hydrophobicity Test
The isolates were initially screened for hemolysin production as described earlier [33] by culturing them overnight on 5% blood agar at 37 °C to detect hemolysis by the isolates. Additionally, the salt aggregation test (SAT) was used to determine cell surface hydrophobicity according to methods described earlier [33]. A bacterial suspension of 10 μL was prepared in a phosphate buffer and mixed with an ammonium sulphate solution with a molarity that ranged from 0.3125 to 0.5 m on a glass slide. SAT values were interpreted as described earlier [34].
2.5. DNA Extraction and Determination of the Phylogenetic Grouping of the Isolates
A Qiagen DNA extraction kit was used to extract the genomic DNA of the isolates according to the guidelines of the manufacturer. Briefly, a final volume of 50 μL, made up of 25 μL of the master mixture, 2 μL of each of the primers, and 100 ng of the DNA template, was used for PCR amplification. The cycling program was performed in AB Applied Biosystems thermal cycler (Foster City, California 94404, USA) and consisted of 30 cycles of 94 °C for 1 min, 62 °C for 45 s, and 72 °C for 1 min, The resultant product was stained with 0.5 µg/mL of ethidium bromide and resolved by gel electrophoresis on 1% agarose gel.
The isolates were grouped phylogenetically by the PCR method described earlier [35], using chuA and yjaA DNA markers in combination with TspE4.C2, an anonymous DNA fragment. The presence or absence of these three DNA fragments was used to group the isolates into the following: group A (chuA−, TspE4.C2−), group B1 (chuA+, yjaA+), group B2 (chuA+, yjaA−), and group D (yjaA). The obtained groupings were categorized according to the profiles previously described [35], with the primers shown in Table 1.

Table 1.
Oligonucleotide sequences of primers and the molecular weight (bp) used for the research.
2.6. Determination of Associated Virulence Factors of Strains’ UPEC Isolates
The assay of urovirulence genes in the isolates that were detected by multiplex PCR using the extracted DNA product of each isolate was amplified with specific virulence primers in Table 1. The manufacturer’s guidelines were used to constitute a multiplex reaction mix, “providing a final concentration of 3 mM MgCl2 (3 × 0.85 mL), 5× Q-Solution (1 × 2.0 mL), RNase-Free Water (2 × 1.7 mL)”. To the multiplex reaction, a mix of 1 µL of extracted DNA template was added to obtain 50 µL of the final reaction. Cycling conditions were also as recommended by the manufacturers: an initial heat activation of HotStar Tag DNA polymerase at 95 °C for 15 min; 35 cycles of 30 s denaturation at 94 °C and annealing for 90 s at 63 °C followed by extension at 72 °C for 90 s, with 10 min of the final extension at 72 °C (www.qiagen.com/HB-0453, accessed on 19 June 2021). The amplified PCR product was stained with 0.5 µg/mL ethidium bromide and resolved by gel electrophoresis on 2% agarose gel. The experiment was repeated twice.
2.7. Statistical Analysis
GraphPad Prism version 9.2.0 was used to analyze the data pertaining to the susceptibility of the antimicrobials and presented as percentages. A Z-test for the difference of two proportions was used for pairwise comparison between resistance versus resistance and intermediate versus intermediate percentages using Stata MP version 13 with significance taken at p-value < 0.05. In addition, a Chi-test for homogeneity of proportion was utilized to test significant differences across the various phylogenetic groups. A p-value of less than 0.01 was considered statistically significant.
3. Results
3.1. Demography and Source of Samples
The UPEC isolates were from urine specimens that had been obtained from patients in wards, the out-patient department (OPD), the emergency room (ER), and the intensive care unit (ICU). Samples were more often from female patients than males, and this was consistently the case for the 3 years of observation. The age group ranged from less than 1 year to 90 years old; however, the age range of 31 to 40 years old was more prevalent compared to the other age groups (Figure 1).
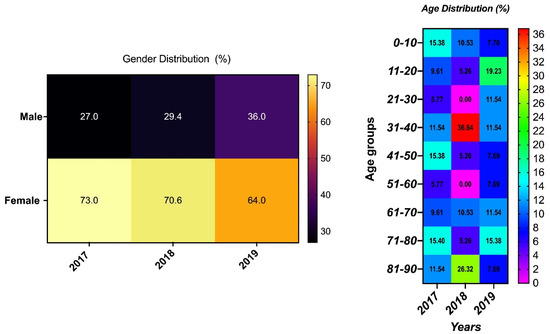
Figure 1.
Demographic distribution of isolates’ sample sources with the year of isolation.
3.2. Hemolysis, Cell Surface Hydrophobicity, and Phylogenetic Groups of the UPEC Isolates
Alpha (α) hemolysis on blood agar was detected in 48 (28.2%) of the initial 170 isolates while 64 (37.65%) of the isolates with visible bacterial clumping with an SAT of less than 1.25 were seen to be cell-surface hydrophobic [33,34]. Further investigation on this aspect was not carried out as they were basic initial microbial analysis.
Isolates were grouped into phylogroups A, BI, B2, and D (Table 2) by PCR gel electrophoresis results. Of the 170 UPEC strains, the majority (62.4%) were classified into the B2 phylogroup, next to which were those in the D phylogroup, which constituted 20.6% of the isolates. The least common were the A and B1 phylogroups which formed 11% and 6% of the UPEC isolates, respectively (Table 2).

Table 2.
Phylogenetic groups of the isolates and the distribution of the virulence genes.
3.3. Antimicrobial Susceptibility of the Isolates
The isolates were resistant to the penicillin group of antibiotics for the 3 years observed, showing high resistance to ampicillin (Aml) and amoxicillin (Am). Additionally, resistance to cephalotin (Kf), ceftazidime (Caz), ceftriaxone (Cax), and cefepime (Pime) was high (100%) in 2017 and remained at 100% for Kf with a slight decrease in resistance (94%) to Caz, PIME, and Cax (96%) in 2018. Resistance to the other cephalosporins (Cfz, Cft, and Cxm) was between 94% and 100% in the first 2 years observed, with a significant (p = 0.00) decrease to between 27% and 15% in the third year. Resistance to the carbapenems was low for all 3 years observed (4–12% in 2017 and 2018 to 0% in 2019). However, there was a high susceptibility of the isolates to tigecycline (100%) for the 3 years observed, but lower susceptibility was seen for tetracycline (20%, 35%, and 35%) for the 3-year duration. No specific pattern in the percentage of resistance to amikacin was seen for the duration observed (Table 3).

Table 3.
Percentage (%) of antimicrobial susceptibility of clinical strains of E. coli urine from urine samples for a period of 3 years.
Among the fluoroquinolones (ciprofloxacin and levofloxacin), resistance remained between 70% and 90% among the isolates. For this group of antibiotics, there was no specific pattern of susceptibility during the 3 years observed. However, resistance by the isolates to trimethoprim/sulfamethoxazole (Sxt) increased nonsignificantly (p = 0.69, 0.09), from 73% in 2017, to 75% in 2018, and significantly (p = 0.04) to 85% in 2019 (Table 3).
3.4. Antimicrobial Pattern of ESBLs UPEC Strains
An overall look at the antimicrobial susceptibility of the isolates showed that those collected in 2019 with serial numbers (S/N) 1–26 were more sensitive to tested antibiotics compared to those with S/N 27–48 collected in 2018 and 2017 (Figure 2). All the 2017 and 2018 isolates are ESBLs, the majority of which were in the B2 and D phylogroups, while only AC 37 was an ESBL 2019 isolate (Figure 2).
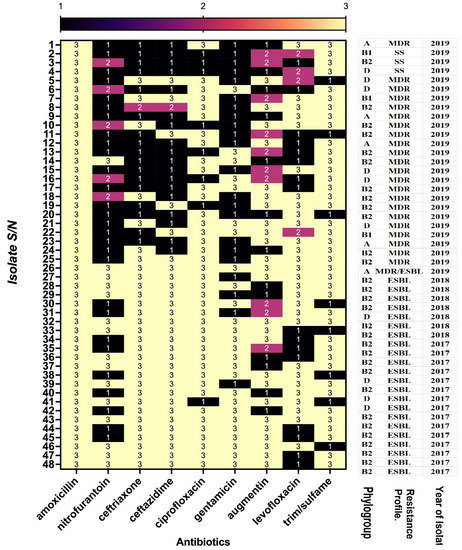
Figure 2.
Comparison of antimicrobial resistance, isolate phylogenetic group, and the year of isolation: multidrug resistant (MDR); susceptible strain (SS); and extended-spectrum beta-lactamases (ESBL). Resistant (3); intermediate (2); sensitive (1). The figure shows the 48 UPEC isolates and their susceptibility to antimicrobials commonly used for treatment.
UPEC–ESBL isolates in the first year of the investigation (2017) were confirmed positive to CTX/CLA (88.5%) and CAZ/CLA (85%); these percentages were reduced in 2018 (40% and 15%, respectively) and subsequently to 4% in 2019 (Table 4). Three investigated ESBL genes were detected among the isolates with varying proportions, more so with the 2017 isolates. CTX was detected in the majority of the isolates, irrespective of the year of sampling (Table 4). The results also showed some multiple carriage of antimicrobial-resistant genes among the isolates, while none of the investigated genes was detected in 15.4% and 2% of the samples in 2017 and 2018, respectively (Table 4).

Table 4.
ESBLs and associated resistant determinant genes.
3.5. Susceptibility to Antimicrobials According to Phylogenetic Group
All the phylogroups were resistant to the penicillins while exhibiting no specific pattern of susceptibility against the other tested antibiotics. There were, however, some variations among the groups as seen in the isolates in phylogroups A and D being significantly (p = 0.001) less resistant to Augmentin (Table 5) when compared to the other phylogroups. Additionally, no significant (p = 0.55) difference in the percentage numbers of UPEC isolates in phylogroups (A, B1, B2 and D) that were resistant to ceftriaxone were noted. Isolates in group A and D were significantly (p = 0.001) less resistant to the cephalosporin antibiotic ceftazidime. For ciprofloxacin, significant differences (p < 0.001) were seen in percentage resistance between phylogroup A and all others. However, comparing phylogroup B1 and D did not show any significant (p < 0.291) difference. For the second fluoroquinolone antibiotic (levofloxacin), differences seen in the phylogroups were not significant (p = 0.37). There were nonsignificant (0.15) differences in the susceptibility of all groups of the UPEC isolates to nitrofurantoin. Furthermore, while all of the B1 isolates were highly sensitive to nitrofurantoin, they were highly resistant to trimethoprim/sulfamethoxazole. Generally, susceptibility to tobramycin was low, with 94% (A), 75% (B1), 93% (B2), and 83% (D) resistance recorded among the phylogroups.

Table 5.
Antimicrobial susceptibility distribution among the phylogenetic groups.
3.6. Associated Virulence Genes
Six virulence genes (sfa, fimH, papE/F, ironN, papA, and hlyA) were investigated by PCR. Only two genes, fimH and papE/F, were detected. A total of 46% of UPEC strains presented the fimH gene, and 15% of the isolates presented the papE/F gene.
4. Discussion
This study describes the antimicrobial pattern and associated virulence genes in strains of Escherichia coli strains from urinary tract infections (UPECs) as seen over a period of three years in the Al-Ahsa region of Saudi Arabia. Samples were more often from females than males, and the highest amount came from the 30–40 year-old age group, findings that are consistent with those of a previous report [40]. Additionally, the isolates here were classified into A, B1, B2, and D phylogroups, with significantly more isolates in B2 (62.4%) and D (20.6%), suggesting the UPEC strains could be extraintestinal pathogens responsible for the UTIs [18]. The least common phylogroups were A and B1, which are considered as commensals. These findings are similar to those of other researchers [41,42,43].
The isolates in this investigation were MDR, being resistant to three or more groups of antimicrobials. For the penicillin group (Aml, Am), a high percentage of resistance (88–100%) was observed for the period of study. This is consistent with previous reports from other regions in the Kingdom [44,45], while in a recent report, resistance to ampicillin was low [16]. Nonetheless, the penicillin group of antibiotics might not be suitable in the management of UPEC urinary tract infections attributed to strains of E. coli in this locality. The 2019 clinical UPEC isolates were more susceptible to the tested drugs than the isolates from the preceding (2017, 2018) years were. This is consistent with reports on findings by researchers in the study region [46,47,48]. They reported that the implementation of antimicrobial stewardship by some hospitals in the Kingdom could also be a probable contributory factor [49]. In addition to this, there is the implemented regulation by the Saudi Arabia Ministry of Health (MOH) for dispensing nonprescribed antibiotics across the counter by community pharmacies [50]; this could also be taken into consideration. However, this trend is worth monitoring in antimicrobial surveillance when taking into consideration that the excessive use of broad-spectrum antibiotics has led to MDR–UPEC strains [51,52]. Generally, there is a high incidence of resistance to antibiotics in the treatment of UTI isolates in the region of this study [9,16,44], while it is thought that MDR seen in UTIs is on the rise [53]. Thus, the results here further highlight challenges posed by difficult-to-treat bacterial infections. The pattern of resistance to nitrofurantoin (Fd), seen for the three years observed, can, however, be considered high when compared with those of other reports [9,52,54,55,56]. However, the 8% recorded resistance to Fd in 2019 is similar to that of reported strains in hospitalized patients in England [57] and Bosnia-Herzegovina [58]. Thus, Fd could still serve as a first-line treatment in uncomplicated cases of UTIs caused by UPEC, as suggested earlier [52]. Other antibiotics recommended by the European Association of Urology in managing uncomplicated UTIs are fosfomycin and trimethoprim/sulfamethoxazole (SXT) [51], both of which were not suitable for the management of the UPEC isolates in this investigation. Similarly, for low susceptibility to SXT, a frontline antibiotic had been reported [59,60], with the suggested reasons for such resistance being the inappropriate wide use of these antibiotics [59]. Thus, it might not be suitable for managing infections resulting from MDR–UPEC isolates. Contrary to the present findings for fosfomycin are reports from Germany, Spain, and Belgium which found an antibiotic suitable for managing UPEC infections [55,61]. This, therefore, suggests variations that might be the result of regional differences, which should be taken into consideration in the management UPEC infections [51].
The UPEC isolates here were sensitive to tigecycline and exhibited low resistance to amikacin, imipenem, and meropenem. A recent report in Saudi Arabia listed amikacin and meropenem as the best antimicrobials in the management of UTIs [16]. These drugs are still the gold standard in the management of MDR–UPEC infections in this region of study. However, research findings vary, with reports stating that MDR–UPEC isolates are sensitive to amikacin on the one hand and resistant to carbapenem on the other [41]. Carbapenems are commonly recommended for the management of ESBL–UPEC infections according to Kot [52].
The highest percentage number of ESBL–UPEC strains was found among the 2017 isolates, findings that are similar to those of others in Saudi Arabia [44,62,63] as well as other regions of the world [41]. The ESBL–UPEC isolates in this research were predominant in the B2 phylogroup, with blaCTX as the main amplified gene, and in some cases, multiple ESBL genes were detected, findings that agree with those of other researchers [41,64]. Here, the predominance of the blaCTX gene, as well as the encountered coexistence with other ESBL genes, could give bacteria selective advantage against antibiotic pressure [41]. The prevalence of the blaCTX gene has also been reported in different regions of the world [40,65,66,67].
There is also the carriage of virulence genes either chromosomally or extra-chromosomally by UPEC isolates, all of which are established during the course of an infection. Generally, the B2 and D phylogroups are stipulated to be the most virulent strains associated with UTIs [68], both of which were in the majority in this study. Only two of the six investigated virulence genes were detected in this study, of which the adhesion gene fimH was significantly more common than papE/F. This might be considered, overall, as a low carriage of virulence genes when compared to findings by other researchers [68]. The reasons for the low carriage of virulence genes can be attributed to several reasons. First is the number of investigated genes compared to the vast number of those associated with strains of UPEC. Bearing in mind the genomic diversity of E. coli, the mobile genetic elements collectively determine pathotype and ecotype trait [69]. Numerous virulence elements result from different genes that are detectable by PCR [65,70,71]. Another reason that should be taken into consideration is the genetically diverse subset of extraintestinal pathogenic E. coil which cause UTIs and are reported to have no core sets of virulence factors [8]. There is also a functional redundancy which can lead to the expression of mixed bacterial populations. In addition, hemolysis as seen in the preliminary study is generally associated with cell lysis and considered a cytotoxic necrotizing factor [33]. In addition, the observed hydrophobicity in some of the strains here has been described as virulence mechanism [33].
However, due to possible gene mutations, PCR amplification could lead to not detecting virulence genes, while negative PCR results could therefore not be considered as being the absence of a targeted gene, a view suggested earlier [72]. Again, UPEC isolates are postulated to parade a high degree in the diverse possession of specific virulence genes on their pathogenicity island [18,73]. The course of infection, as well as the duration of infection, could also be a leading factor in the process of virulence, but this was not investigated here. There is a need for further investigation.
5. Conclusions
We have reported the phylogenetic groupings of UPEC isolates collected in the Al-Ahsa region in the southeast of Saudi Arabia for a three-year period. The majority of the isolates were MDR, with those collected before 2019 being less susceptible to recommended first-line antibiotics. In addition, the four phylogroups, A, B1, B2, and D, showed nonspecific patterns in their drug-resistant profiles. Interestingly, the 2019 antimicrobial susceptibility showed a better sensitivity pattern with the isolates of that year. A minimal number of virulence genes was detected among the UPEC isolates irrespective of the year of isolation. We therefore suggest further investigation, particularly on recurrent infections with UPEC.
Author Contributions
Conceptualization, L.I.B.-E.; methodology, L.I.B.-E., N.K., E.E. and G.B.A.; software, L.I.B.-E. and G.B.A.; validation, L.I.B.-E.; formal analysis, L.I.B.-E.; investigation, L.I.B.-E., N.K., E.E. and G.B.A.; resources, L.I.B.-E.; data curation, L.I.B.-E., E.E. and G.B.A.; writing—original draft preparation, L.I.B.-E., N.K., E.E. and G.B.A.; writing—review and editing, L.I.B.-E., N.K., E.E. and G.B.A.; visualization, L.I.B.-E.; supervision, L.I.B.-E.; project administration, L.I.B.-E.; funding acquisition, L.I.B.-E. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Deanship of Scientific Research, Vice Presidency for Graduate Studies and Scientific Research, King Faisal University, under annual research grant no. GRANT239.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data are present in the paper, but should the need arise, the corresponding author can also be contacted.
Acknowledgments
The researchers thank the Deanship for Scientific Research, King Faisal University, for funding the research.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the study design; in the collection, analyses, or interpretation of the data; in the writing of the manuscript; or in the decision to publish the results.
References
- Stamm, W.E.; Norrby, S.R. Urinary tract infections: Disease panorama and challenges. J. Infect. Dis. 2001, 183 (Suppl. 1), S1–S4. [Google Scholar] [CrossRef]
- Taur, Y.; Smith, M.A. Adherence to the Infectious Diseases Society of America guidelines in the treatment of uncomplicated urinary tract infection. Clin. Infect. Dis. 2007, 44, 769–774. [Google Scholar] [CrossRef]
- Schappert, S.M.; Burt, C.W. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency de-partments: United States, 2001–2002. Vital. Health Stat. 2006, 13, 1–66. [Google Scholar]
- Kostakioti, M.; Hultgren, S.J.; Hadjifrangiskou, M. Molecular blueprint of uropathogenic Escherichia coli virulence provides clues toward the development of anti-virulence therapeutics. Virulence 2012, 3, 592–594. [Google Scholar] [CrossRef] [PubMed]
- Tandogdu, Z.; Wagenlehner, F.M. Global epidemiology of urinary tract infections. Curr. Opin. Infect. Dis. 2016, 29, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Alanazi, M.Q.; Al-Jeraisy, M.I.; Salam, M. Prevalence and predictors of antibiotic prescription errors in an emergency de-partment, Central Saudi Arabia. Drug Healthc. Patient Saf. 2015, 7, 103–111. [Google Scholar] [CrossRef]
- Car, J. Urinary tract infections in women: Diagnosis and management in primary care. BMJ 2006, 332, 94–97. [Google Scholar] [CrossRef]
- Spurbeck, R.R.; Mobley, H.L.T. Chapter 9—Uropathogenic Escherichia coli. In Donnenberg, Escherichia coli, 2nd ed.; Michael, S., Ed.; Elsevier Inc., Academic Press: Amsterdam, The Netherlands, 2013; pp. 275–304. ISBN 9780123970480. [Google Scholar] [CrossRef]
- Alanazi, M.Q. An evaluation of community-acquired urinary tract infection and appropriateness of treatment in an emergency department in Saudi Arabia. Ther. Clin. Risk Manag. 2018, 14, 2363–2373. [Google Scholar] [CrossRef]
- Akbar, D.H. Urinary tract infection. Diabetics and non-diabetic patients. Saudi Med. J. 2001, 22, 326–329. [Google Scholar]
- General Authority for Statistics. Statistical Yearbook of 2016. In Kingdom of Saudi Arabia: General Authority for Statistics; 2016. Available online: https://www.stats.gov.sa/en/169 (accessed on 3 May 2022).
- Garout, W.A.; Kurdi, H.S.; Shilli, A.H.; Kari, J.A. Urinary tract infection in children younger than 5 years. Etiology and asso-ciated urological anomalies. Saudi Med. J. 2015, 36, 497–501. [Google Scholar] [CrossRef]
- Yadav, K.K.; Adhikari, N.; Khadka, R.; Pant, A.D.; Shah, B. Multidrug resistant Enterobacteriaceae and extended spectrum β-lactamase producing Escherichia coli: A cross-sectional study in National Kidney Center, Nepal. Antimicrob Resist. Infect Control. 2015, 4, 42. [Google Scholar] [CrossRef] [PubMed]
- Gharavi, M.J.; Zarei, J.; Roshani-Asl, P.; Yazdanyar, Z.; Sharif, M.; Rashidi, N. Comprehensive study of antimicrobial suscep-tibility pattern and extended spectrum beta-lactamase (ESBL) prevalence in bacteria isolated from urine samples. Sci. Rep. 2021, 11, 578. [Google Scholar] [CrossRef] [PubMed]
- Norafika, N.A.; Arbianti, N.; Prihatiningsih, S.; Indriani, D.W. A retrospective cross-sectional study of urinary tract infections and prevalence of antibiotic resistant pathogens in patients with diabetes mellitus from a public hospital in Surabaya, Indonesia. Germs 2020, 10, 157–166. [Google Scholar] [CrossRef] [PubMed]
- Alasmary, M.Y. Antimicrobial Resistance Patterns and ESBL of Uropathogens Isolated from Adult Females in Najran Region of Saudi Arabia. Clin. Pract. 2021, 11, 650–658. [Google Scholar] [CrossRef]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence of Multidrug Resistance and Extended-Spectrum β-Lactamase Carriage of Clinical Uropathogenic Escherichia coli Isolates in Riyadh, Saudi Arabia. Int. J. Microbiol. 2018, 2018, 3026851. [Google Scholar] [CrossRef]
- Bien, J.; Sokolova, O.; Bozko, P. Role of Uropathogenic Escherichia coli Virulence Factors in Development of Urinary Tract Infection and Kidney Damage. Int. J. Nephrol 2012, 2012, 681473. [Google Scholar] [CrossRef]
- Schreiber, H.L.; Conover, M.S.; Chou, W.C.; Hibbing, M.E.; Manson, A.L.; Dodson, K.W.; Hannan, T.J.; Roberts, P.L.; Stapleton, A.E.; Hooton, T.M.; et al. Bacterial virulence phenotypes of Escherichia coli and host susceptibility determine risk for urinary tract infections. Sci. Transl. Med. 2017, 9, eaaf1283. [Google Scholar] [CrossRef]
- Asadi, S.; Kargar, M.; Solhjoo, K.; Najafi, A.; Ghorbani-Dalini, S. The Association of Virulence Determinants of Uropathogenic Escherichia coli With Antibiotic Resistance. Jundishapur J. Microbiol. 2014, 7, e9936. [Google Scholar] [CrossRef]
- Shah, C.; Baral, R.; Bartaula, B.; Shrestha, L.B. Virulence factors of uropathogenic Escherichia coli (UPEC) and correlation with antimicrobial resistance. BMC Microbiol. 2019, 19, 204. [Google Scholar] [CrossRef]
- Alghoribi, M.F.; Gibreel, T.M.; Farnham, G.; Al-Johani, S.M.; Balkhy, H.H.; Upton, M. Antibiotic-resistant ST38, ST131 and ST405 strains are the leading uropathogenic Escherichia coli clones in Riyadh, Saudi Arabia. J. Antimicrob. Chemother 2015, 70, 2757–2762. [Google Scholar] [CrossRef]
- Johnson, J.R.; Kuskowski, M.A.; Owens, K.; Gajewski, A.; Winokur, P.L. Phylogenetic origin and virulence genotype in relation to resistance to fluoroquinolones and/or extended-spectrum cephalosporins and cephamycins among Escherichia coli isolates from animals and humans. J. Infect. Dis. 2003, 188, 759–768. [Google Scholar] [CrossRef]
- Alqasim, A.; Abu Jaffal, A.; Alyousef, A.A. Prevalence and molecular characteristics of sequence type 131 clone among clinical uropathogenic Escherichia coli isolates in Riyadh, Saudi Arabia. Saudi J. Biol. Sci. 2020, 27, 296–302. [Google Scholar] [CrossRef] [PubMed]
- Bazaid, A.S.; Saeed, A.; Alrashidi, A.; Alshaghdali, K.; A Hammam, S.; Alreshidi, T.; Alshammary, M.; Alarfaj, A.; Thallab, R.; Aldarhami, A. Antimicrobial Surveillance for Bacterial Uropathogens in Hail, Saudi Arabia: A Five-Year Multi-center Retrospective Study. Infect. Drug Resist. 2021, 14, 1455–1465. [Google Scholar] [CrossRef] [PubMed]
- Al Yousef, S.A.; Younis, S.; Farrag, E.; Moussa, H.S.h.; Bayoumi, F.S.; Ali, A.M. Clinical and Laboratory Profile of Urinary Tract Infections Associated with Extended Spectrum β-Lactamase Producing Escherichia coli and Klebsiella pneumoniae. Ann. Clin. Lab Sci. 2016, 46, 393–400. [Google Scholar] [PubMed]
- Al-Tawfiq, J.A.; Anani, A.A. Antimicrobial susceptibility pattern of bacterial pathogens causing urinary tract infections in a Saudi Arabian hospital. Chemotherapy 2009, 55, 127–131. [Google Scholar] [CrossRef] [PubMed]
- Al-Harthi, A.A.; Al-Fifi, S.H. Antibiotic resistance pattern and empirical therapy for urinary tract infections in children. Saudi Med. J. 2008, 29, 854–858. [Google Scholar]
- Kader, A.A.; Kumar, A. Prevalence and antimicrobial susceptibility of extended-spectrum beta-lactamase-producing Esche-richia coli and Klebsiella pneumoniae in a general hospital. Ann. Saudi. Med. 2005, 25, 239–242. [Google Scholar] [CrossRef]
- Eltahawy, A.T.; Khalaf, R.M.F. Urinary tract infection at a University Hospital in Saudi Arabia: Incidence, microbiology, and antimicrobial susceptibility. Ann. Saudi Med. 1988, 8, 261–266. [Google Scholar] [CrossRef][Green Version]
- CLSI. Performance Standards for Antimicrobial Susceptibility Testing, 30th ed.; CLSI supplement M100; Clinical and La-bor-atory Standards Institute: Pittsburgh, PA, USA, 2020; Available online: https://www.nih.org.pk/wp-content/uploads/2021/02/CLSI2020.pdf (accessed on 1 August 2021).
- Clinical Laboratory Standards Institute. Methods for Dilution Antimicrobial Susceptibility Testing for Bacteria That Grows Aerobically, 9th ed.; (M07-A9); Clinical Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
- Ranjan, K.P.; Ranjan, N.; Chakraborty, A.; Arora, D.R. An approach to uropathogenic Escherichia coli in urinary tract infec-tions. J. Lab Physicians 2010, 2, 70–73. [Google Scholar] [CrossRef]
- Siegfried, L.; Kmetová, M.; Puzová, H.; Molokácová, M.; Filka, J. Virulence-associated factors in Escherichia coli strains isolated from children with urinary tract infections. J. Med. Microbiol. 1994, 41, 127–132. [Google Scholar] [CrossRef]
- Clermont, O.; Bonacorsi, S.; Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 2000, 66, 4555–4558. [Google Scholar] [CrossRef] [PubMed]
- Le Bouguenec, C.; Archambaud, M.; Labigne, A. Rapid and specific detection of the pap, afa, and sfa adhesin-encoding operons in uropathogenic Escherichia coli strains by polymerase chain reaction. J. Clin. Microbiol. 1992, 30, 1189–1193. [Google Scholar] [CrossRef] [PubMed]
- Yamamoto, S.; Terai, A.; Yuri, K.; Kurazono, H.; Takeda, Y.; Yoshida, O. Detection of urovirulence factors in Escherichia coli by multiplex polymerase chain reaction. FEMS Immunol. Med. Microbiol. 1995, 12, 85–90. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Stell, A.L. Extended virulence genotypes of Escherichia coli strains from patients with urosepsis in relation to phylogeny and host compromise. J. Infect. Dis. 2000, 181, 261–272. [Google Scholar] [CrossRef] [PubMed]
- Johnson, J.R.; Russo, T.A.; Tarr, P.I.; Carlino, U.; Bilge, S.S.; Vary, J.C.; Stell, A.L. Molecular epidemiological and phylogenetic associations of two novel putative virulence genes, iha and iroN (E. coli), among Escherichia coli isolates from patients with urosepsis. Infect. Immun. 2000, 68, 3040–3047. [Google Scholar] [CrossRef]
- Dadi, B.R.; Abebe, T.; Zhang, L.; Mihret, A.; Abebe, W.; Amogne, W. Distribution of virulence genes and phylogenetics of uropathogenic Escherichia coli among urinary tract infection patients in Addis Ababa, Ethiopia. BMC Infect. Dis. 2020, 20, 108. [Google Scholar] [CrossRef] [PubMed]
- Mazumder, R.; Abdullah, A.; Ahmed, D.; Hussain, A. High Prevalence of blaCTX-M-15 Gene among Extended-Spectrum β-Lactamase-Producing Escherichia coli Isolates Causing Extraintestinal Infections in Bangladesh. Antibiotics 2020, 9, 796. [Google Scholar] [CrossRef]
- Lee, J.H.; Subhadra, B.; Son, Y.J.; Kim, D.H.; Park, H.S.; Kim, J.M.; Koo, S.H.; Oh, M.H.; Kim, H.J.; Choi, C.H. Phylogenetic group distributions, virulence factors and antimicrobial resistance properties of uropathogenic Escherichia coli strains isolated from patients with urinary tract infections in South Korea. Lett. Appl. Microbiol. 2016, 62, 84–90. [Google Scholar] [CrossRef]
- Iranpour, D.; Hassanpour, M.; Ansari, H.; Tajbakhsh, S.; Khamisipour, G.; Najafi, A. Phylogenetic groups of Escherichia coli strains from patients with urinary tract infection in Iran based on the new Clermont phylotyping method. BioMed Res. Int. 2015, 2015, 846219. [Google Scholar] [CrossRef]
- Al-Zahrani, J.; Al Dossari, K.; Gabr, A.H.; Ahmed, A.-F.; Al Shahrani, S.A.; Al-Ghamdi, S. Antimicrobial resistance patterns of Uropathogens isolated from adult women with acute uncomplicated cystitis. BMC Microbiol. 2019, 19, 237. [Google Scholar] [CrossRef]
- Al Wutayd, O.; Al Nafeesah, A.; Adam, I.; Babikir, I. The antibiotic susceptibility patterns of uropathogens isolated in Qassim, Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 946–952. [Google Scholar] [CrossRef] [PubMed]
- Haseeb, A.; Faidah, H.S.; Al-Gethamy, M.; Iqbal, M.S.; Barnawi, A.M.; Elahe, S.S.; Bukhari, D.N.; Noor Al-Sulaimani, T.M.; Fadaaq, M.; Alghamdi, S.; et al. Evaluation of a Multidisciplinary Antimicrobial Stewardship Program in a Saudi Critical Care Unit: A Quasi-Experimental Study. Front. Pharmacol. 2020, 11, 570238. [Google Scholar] [CrossRef] [PubMed]
- Al-Omari, A.; Al Mutair, A.; Alhumaid, S.; Salih, S.; Alanazi, A.; Albarsan, H.; Abourayan, M.; Al Subaie, M. The impact of antimicrobial stewardship program implementation at four tertiary private hospitals: Results of a five-years pre-post analysis. Antimicrob Resist. Infect. Control 2020, 9, 95. [Google Scholar] [CrossRef] [PubMed]
- Zowawi, H.M. Antimicrobial resistance in Saudi Arabia. An urgent call for an immediate action. Saudi Med. J. 2016, 37, 935–940. [Google Scholar] [CrossRef]
- Alghamdi, S.; Berrou, I.; Aslanpour, Z.; Mutlaq, A.; Haseeb, A.; Albanghali, M.; Hammad, M.A.; Shebl, N. Antimicrobial Stewardship Programmes in Saudi Hospitals: Evidence from a National Survey. Antibiotics 2021, 10, 193. [Google Scholar] [CrossRef]
- AlOtieschan, S.; Alsalim, A.; Albabtain, S.; Almujahid, M.; Obeid, D.; Alhabradi, F.; Alenazi, T. Public Perception Toward Ministry of Health Regulations for Antibiotic Dispensing and Its Impact on Pharmacy and Family Physician Visits. Cureus 2021, 13, e14981. [Google Scholar] [CrossRef]
- Bartoletti, R.; Cai, T.; Wagenlehner, F.M.; Naber, K.; Bjerklund, J.T.E. Treatment of urinary tract infections and antibiotic stewardship. Eur. Urol. Suppl. 2016, 15, 81–87. [Google Scholar] [CrossRef]
- Kot, B. Antibiotic Resistance Among Uropathogenic Escherichia coli. Pol. J. Microbiol 2019, 68, 403–415. [Google Scholar] [CrossRef]
- Adamus-Białek, W.; Baraniak, A.; Wawszczak, M.; Głuszek, S.; Gad, B.; Wróbel, K.; Bator, P.; Majchrzak, M.; Parniewski, P. The genetic background of antibiotic resistance among clinical uropathogenic Escherichia coli strains. Mol. Biol. Rep. 2018, 45, 1055–1065. [Google Scholar] [CrossRef]
- Delpech, G.; Allende, N.G.; Lissarrague, S.; Sparo, M. Antimicrobial resistance of uropathogenic Escherichia coli from elderly patients at a general hospital, Argentina. Open Infect. Dis. J. 2018, 10, 79–87. [Google Scholar] [CrossRef]
- Kresken, M.; Körber-Irrgang, B.; Biedenbach, D.J.; Batista, N.; Besard, V.; Cantón, R.; García-Castillo, M.; Kalka-Moll., W.; Pascual, A.; Schwarz, R.; et al. Comparative in vitro activity of oral antimicrobial agents against Enterobacteriaceae from patients with community acquired urinary tract infections in three European countries. Clin. Microbiol. Infect. 2016, 22, 63.e1–63.e5. [Google Scholar] [CrossRef] [PubMed]
- Cunha, M.A.; Assunção, G.L.M.; Medeiros, I.M.; Freitas, M.R. Antibiotic resistance patterns of urinary tract infections in a north-eastern Brazilian capital. Rev. Inst. Med. Trop São Paulo 2016, 58, 2. [Google Scholar] [CrossRef] [PubMed]
- Abernethy, J.; Guy, R.; Sheridan, E.A.; Hopkins, S.; Kiernan, M.; Wilcox, M.H.; Johnson, A.P.; Hope, R.E. coli bacteraemia sentinel surveillance group. Epidemiology of Escherichia coli bacteraemia in England: Results of an enhanced sentinel surveil-lance programme. J. Hosp. Infect. 2017, 95, 365–375. [Google Scholar] [CrossRef]
- Abduzaimovic, A.; Aljicevic, M.; Rebic, V.; Vranic, S.M.; Abduzaimovic, K.; Sestic, S. Antibiotic Resistance in Urinary Isolates of Escherichia coli. Mater Sociomed 2016, 28, 416–419. [Google Scholar] [CrossRef] [PubMed]
- Yamaji, R.; Rubin, J.; Thys, E.; Friedman, C.R.; Riley, L.W. Persistent Pandemic Lineages of Uropathogenic Escherichia coli in a College Community from 1999 to 2017. J. Clin. Microbiol. 2018, 56, e01834–e17. [Google Scholar] [CrossRef] [PubMed]
- Prasada, S.; Bhat, A.; Bhat, S.; Shenoy Mulki, S.; Tulasidas, S. Changing antibiotic susceptibility pattern in uropathogenic Escherichia coli over a period of 5 years in a tertiary care center. Infect. Drug Resist. 2019, 12, 1439–1443. [Google Scholar] [CrossRef]
- Neuner, E.A.; Sekeres, J.; Hall, G.S.; van Duin, D. Experience with fosfomycin for treatment of urinary tract infections due to multidrug-resistant organisms. Antimicrob. Agents Chemother 2012, 56, 5744–5748. [Google Scholar] [CrossRef]
- Balkhi, B.; Mansy, W.; Alghadeer, S.; Alnuaim, A.; AlShehri, A.; Somily, A. Antimicrobial susceptibility of microorganisms causing Urinary Tract Infections in Saudi Arabia. J. Infect. Dev. Ctries. 2018, 12, 220–227. [Google Scholar] [CrossRef]
- Alyamani, E.J.; Khiyami, A.M.; Booq, R.Y.; Majrashi, M.A.; Bahwerth, F.S.; Rechkina, E. The occurrence of ESBL-producing Escherichia coli carrying aminoglycoside resistance genes in urinary tract infections in Saudi Arabia. Ann. Clin. Microbiol. An-timicrob. 2017, 16, 1–13. [Google Scholar] [CrossRef]
- Hawkey, P.M. Prevalence and clonality of extended-spectrum beta-lactamases in Asia. Clin. Microbiol. Infect. 2008, 14 (Suppl. 1), 159–165. [Google Scholar] [CrossRef]
- Usein, C.R.; Damian, M.; Tatu-Chitoiu, D.; Capusa, C.; Fagaras, R.; Tudorache, D.; Nica, M.; Le Bouguénec, C. Prevalence of virulence genes in Escherichia coli strains isolated from Romanian adult urinary tract infection cases. J. Cell Mol. Med. 2001, 5, 303–310. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, S.; Han, L.; Guo, X.; Chen, M.; Ni, Y.; Zhang, Y.; Cui, Z.; He, P. Drug resistance and virulence of uropa-tho-genic Escherichia coli from Shanghai, China. J. Antibiot (Tokyo) 2014, 67, 799–805. [Google Scholar] [CrossRef] [PubMed]
- Munkhdelger, Y.; Gunregjav, N.; Dorjpurev, A.; Juniichiro, N.; Sarantuya, J. Detection of virulence genes, phylogenetic group and antibiotic resistance of uropathogenic Escherichia coli in Mongolia. J. Infect. Dev. Ctries 2017, 11, 51–57. [Google Scholar] [CrossRef]
- Khairy, R.M.; Mohamed, E.S.; Ghany, H.M.A.; Abdelrahim, S.S. Phylogenic classification and virulence genes profiles of uropathogenic E. coli and diarrheagenic E. coli strains isolated from community acquired infections. PLoS ONE 2019, 14, e0222441. [Google Scholar] [CrossRef]
- Tan, C.W.; Chlebicki, M.P. Urinary tract infections in adults. Singapore Med. J. 2016, 57, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Momtaz, H.; Karimian, A.; Madani, M.; Safarpoor Dehkordi, F.; Ranjbar, R.; Sarshar, M.; Souod, N. Uropathogenic Escherichia coli in Iran: Serogroup distributions, virulence factors and antimicrobial resistance properties. Ann. Clin. Microbiol. Antimicrob 2013, 12, 8. [Google Scholar] [CrossRef] [PubMed]
- Tabasi, M.; Karam, M.R.; Habibi, M.; Mostafavi, E.; Bouzari, S. Genotypic Characterization of Virulence Factors in Escherichia coli Isolated from Patients with Acute Cystitis, Pyelonephritis and Asymptomatic Bacteriuria. J. Clin. Diagn Res. 2016, 10, DC01–DC07. [Google Scholar] [CrossRef]
- Tarchouna, M.; Ferjani, A.; Ben-Selma, W.; Boukadida, J. Distribution of uropathogenic virulence genes in Escherichia coli isolated from patients with urinary tract infection. Int. J. Infect. Dis. 2013, 17, e450–e453. [Google Scholar] [CrossRef]
- Wiles, T.J.; Kulesus, R.R.; Mulvey, M.A. Origins and virulence mechanisms of uropathogenic Escherichia coli. Exp. Mol. Pathol. 2008, 85, 11–19. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).