Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Isolation of Fusarium Fungi from Plant Material
2.2. Identification of Fusarium Fungi
2.3. Preparation of Spore Suspensions
2.4. Description of Greenhouse Experiment
2.5. Analysis of FHB Parameters of Inoculated Spring Wheat
2.6. Statistical Analysis
3. Results
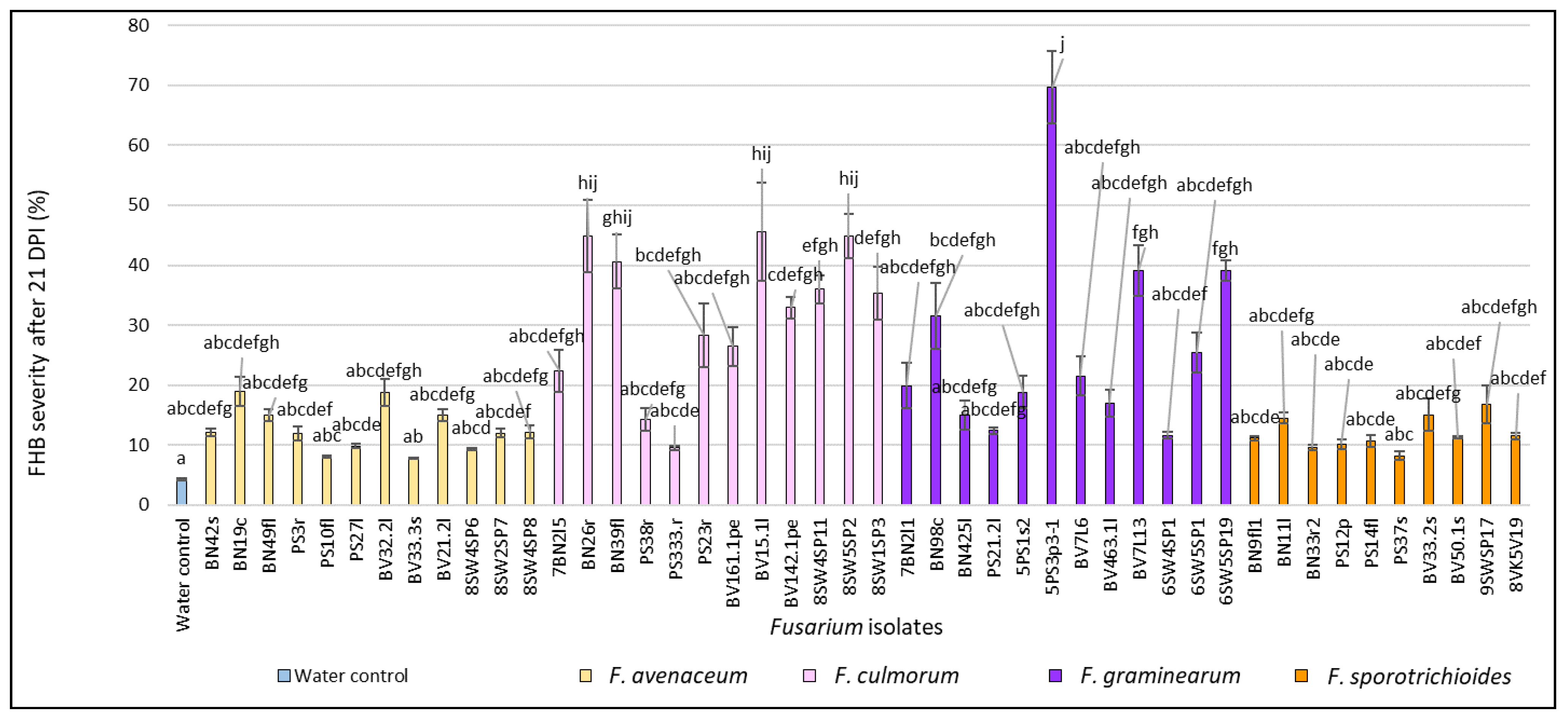
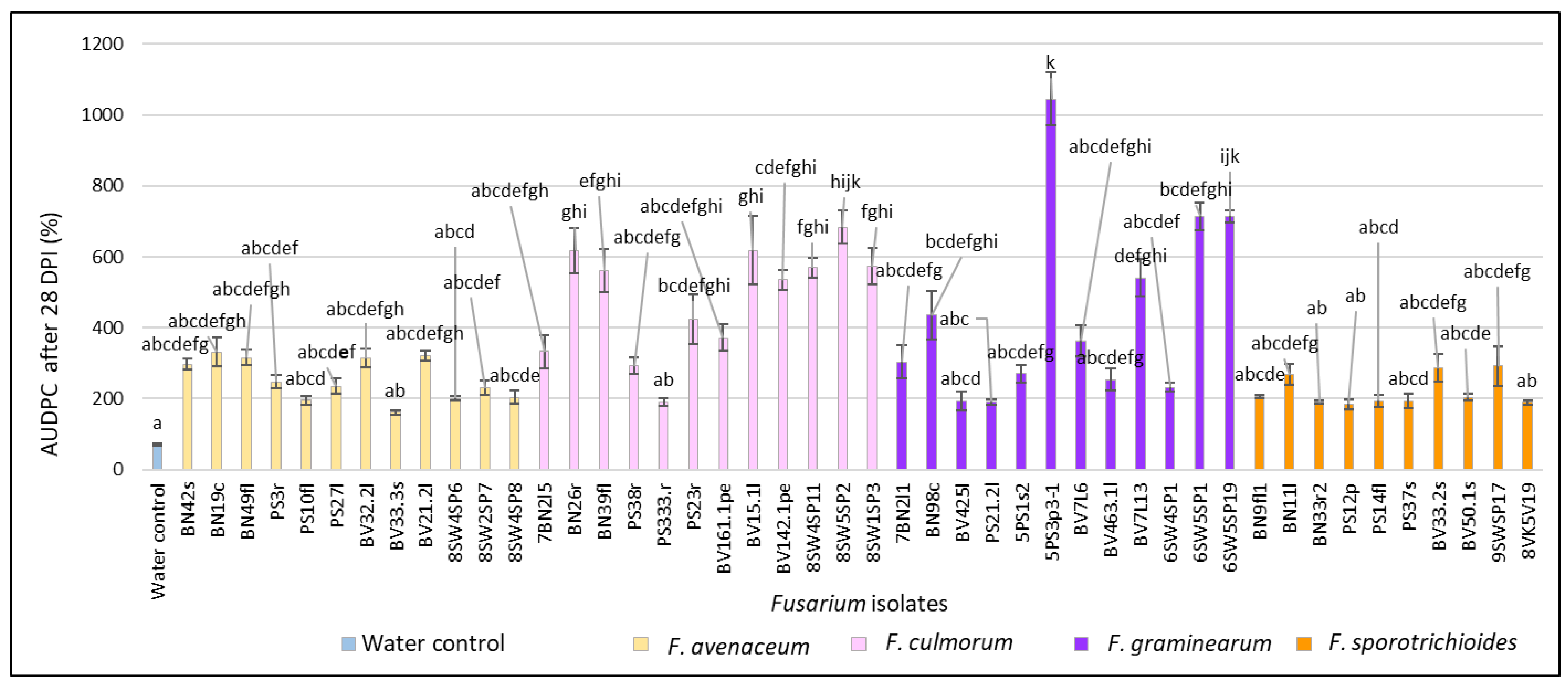
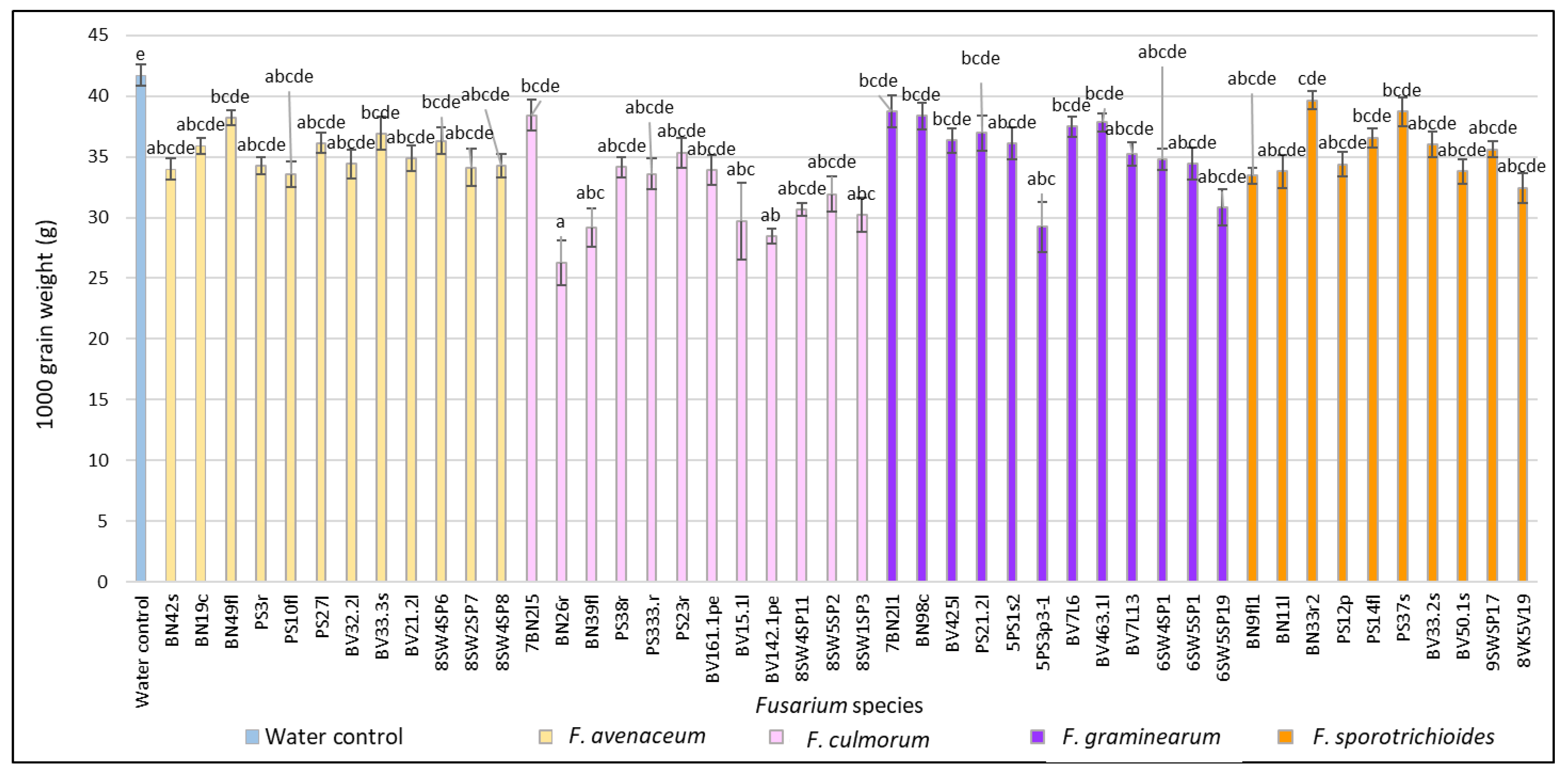
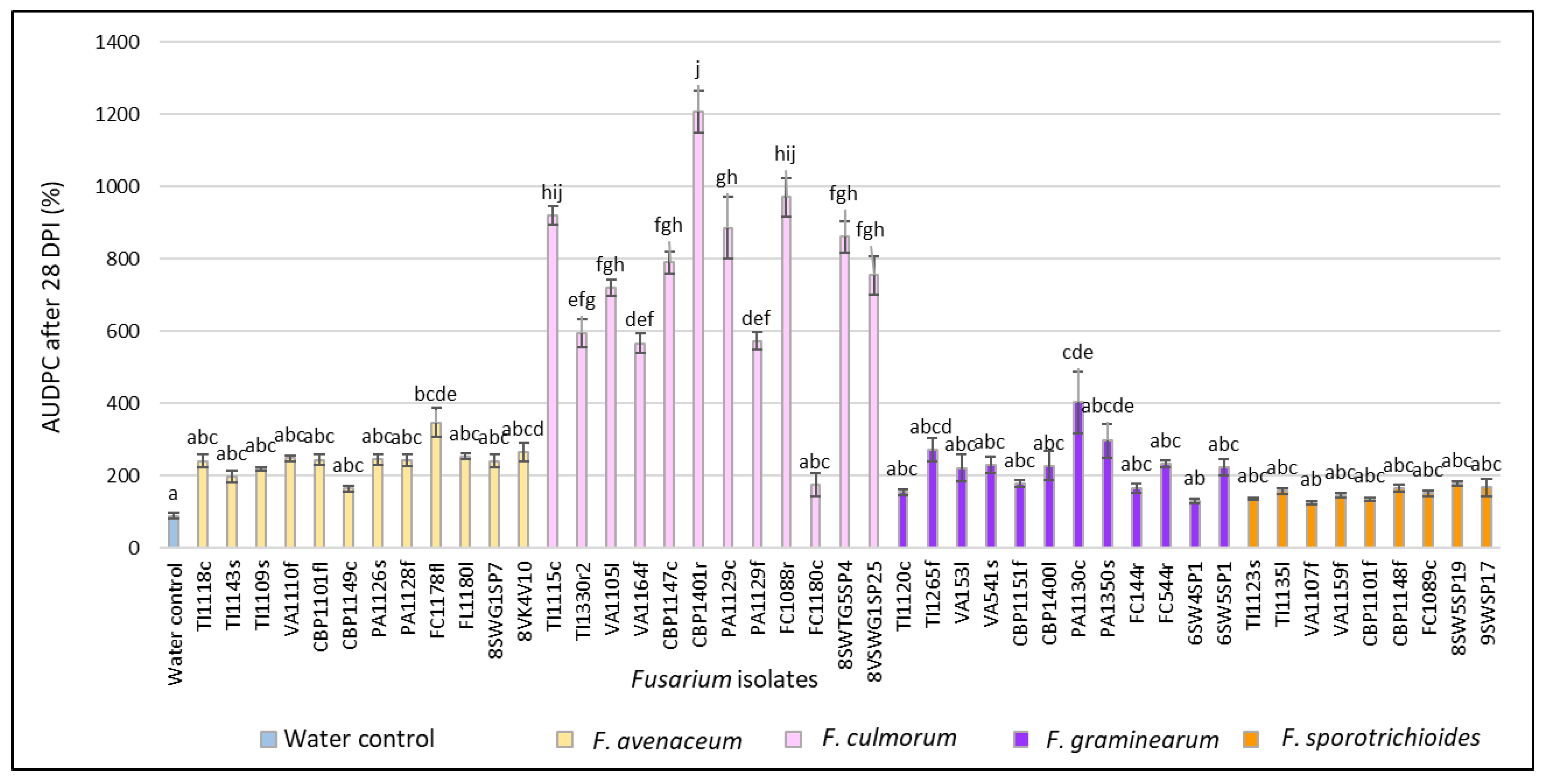
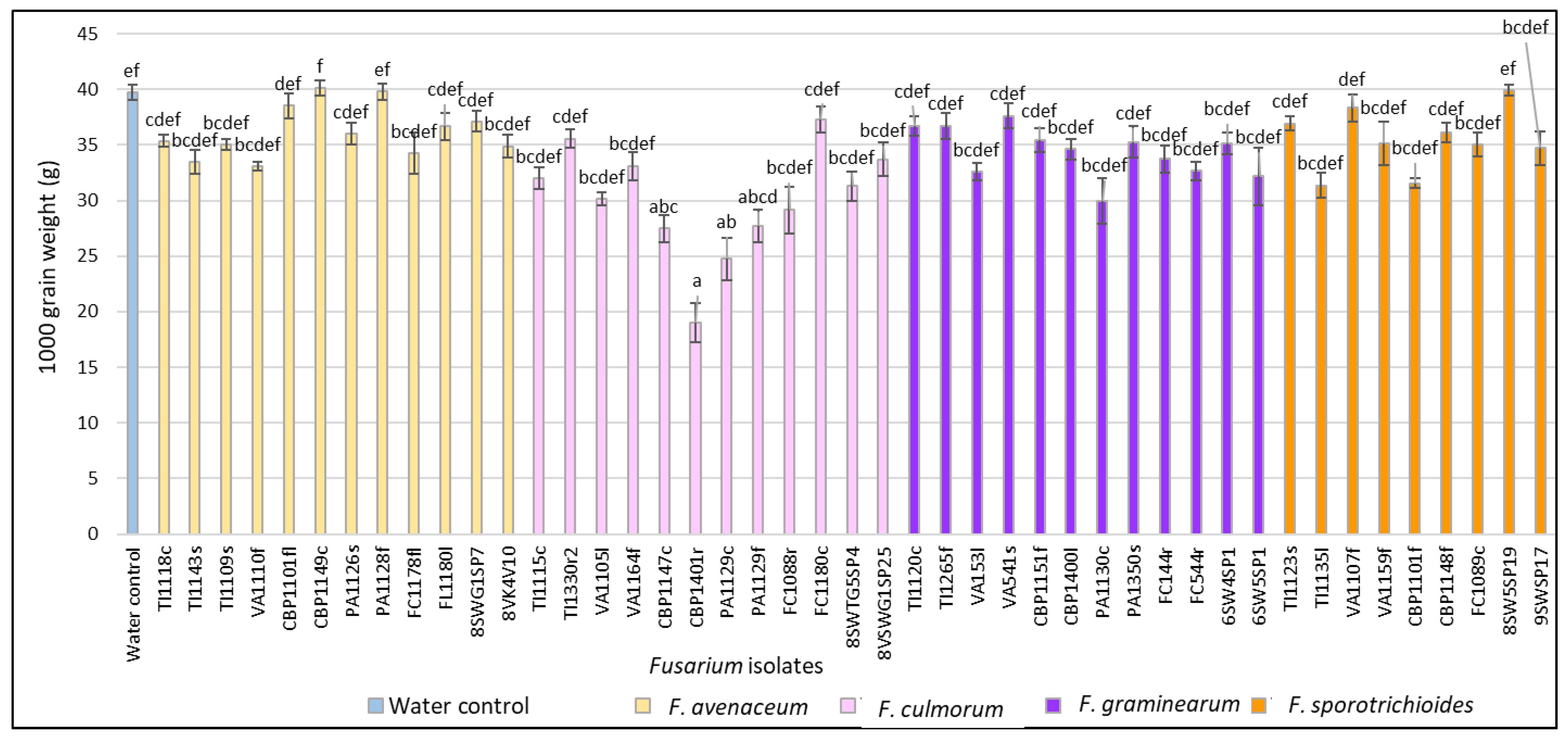
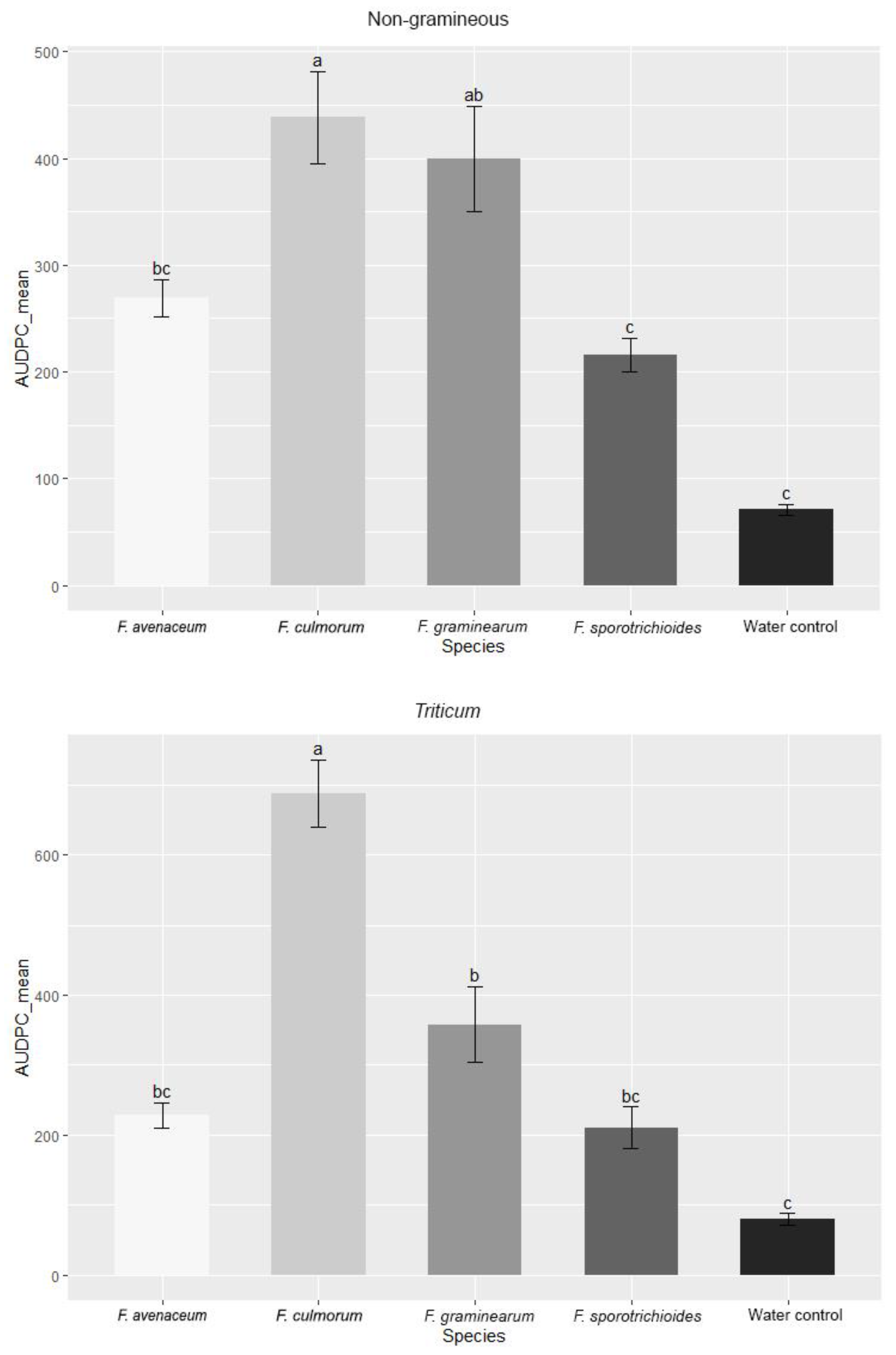
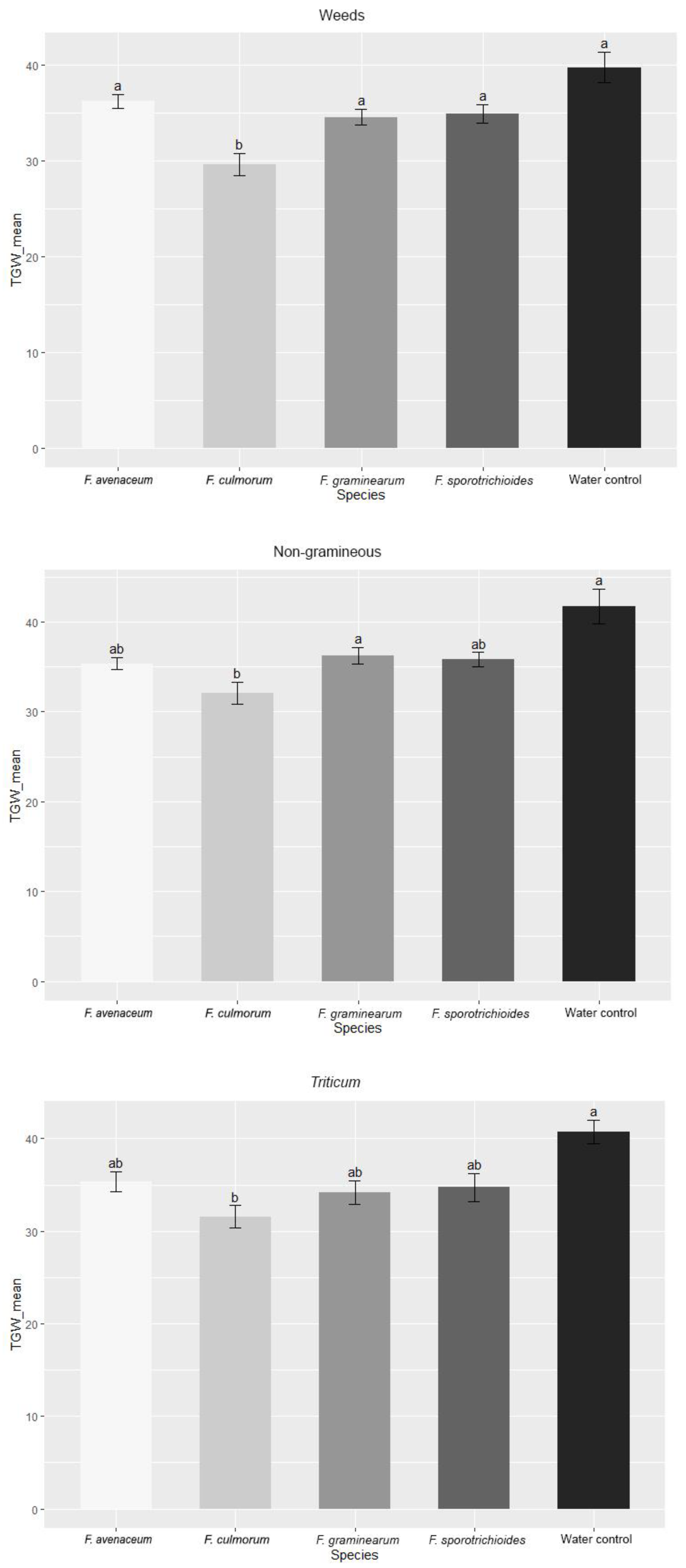
3.1. Evaluation of FHB Severity, AUDPC and TGW under Wheat Inoculation with Fusarium Fungi Isolated from the Non-Gramineous Plants
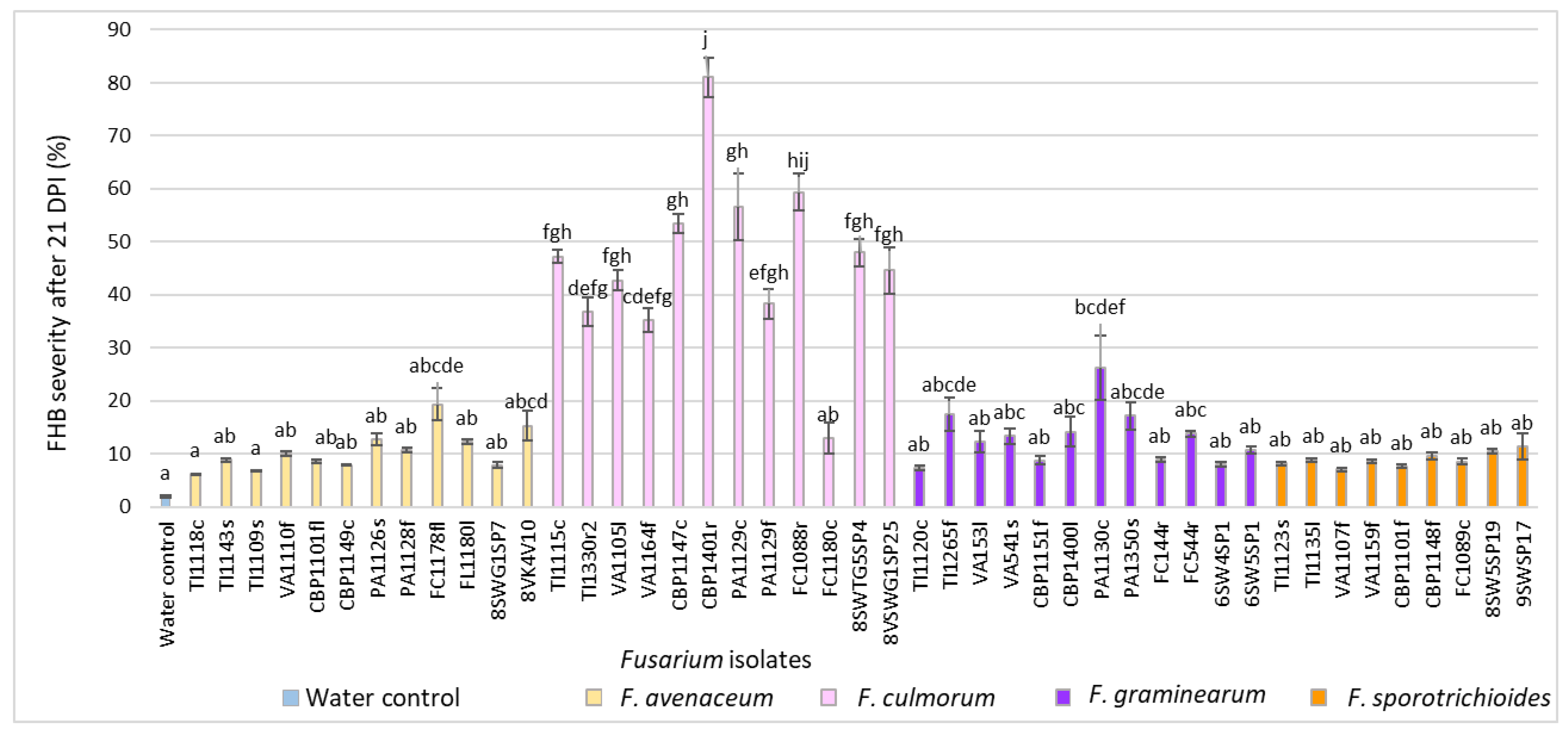
3.2. Evaluation of FHB Severity, AUDPC and TGW under Wheat Inoculation with Fusarium Fungi Isolated from Weeds
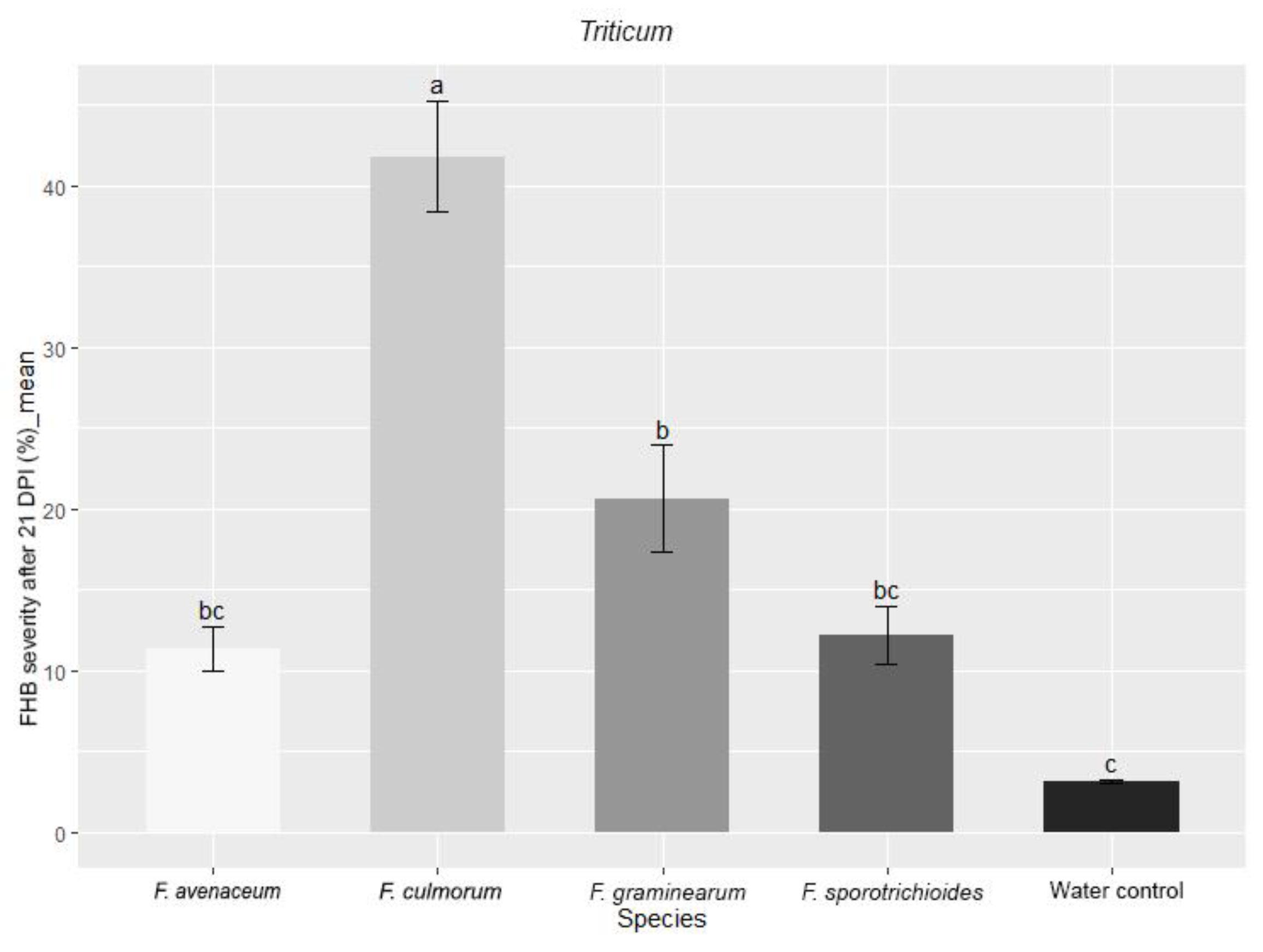
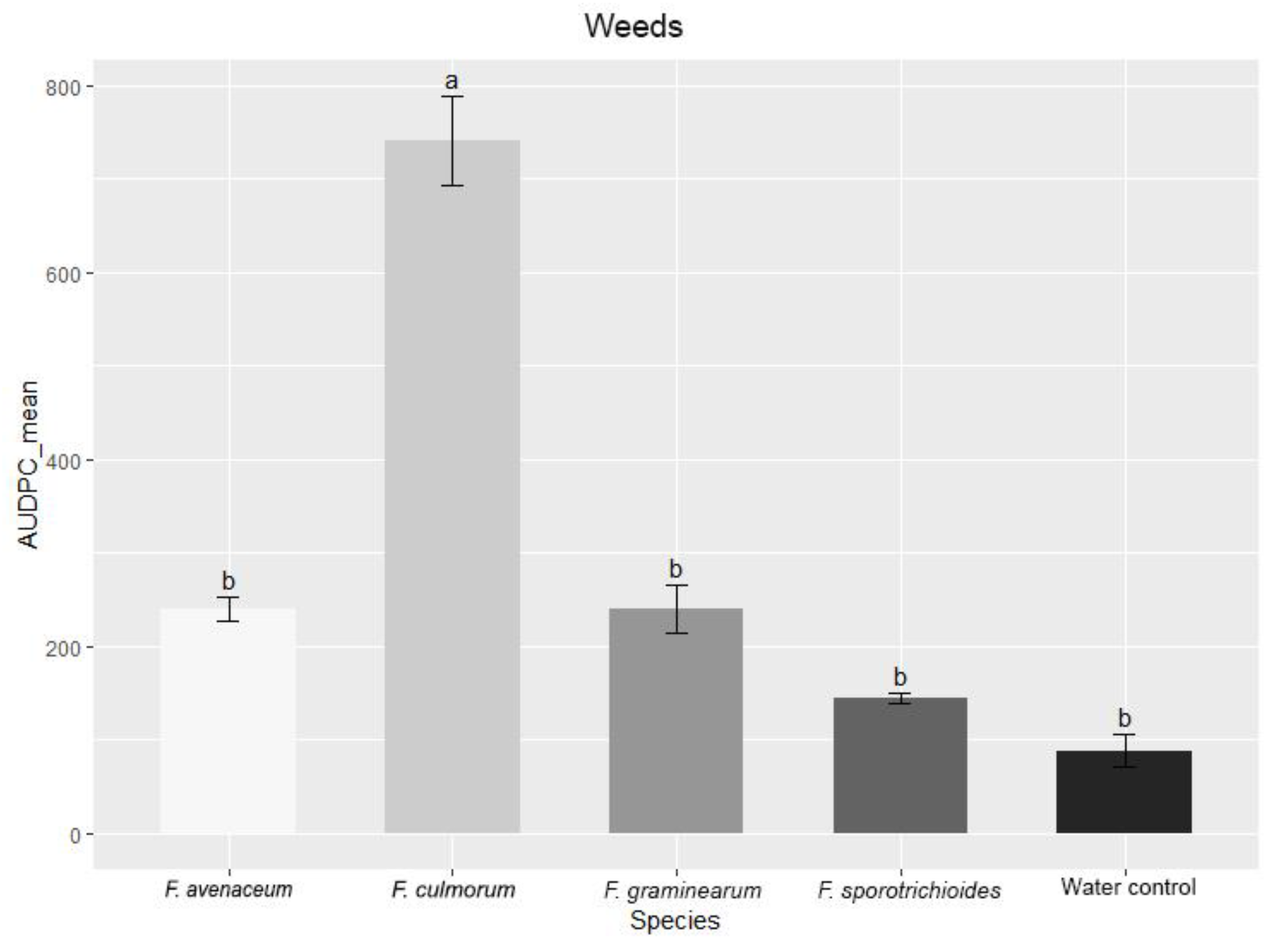
3.3. Comparison of the Pathogenicity of Fusarium Fungi Isolated from Different Hostplants
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yli-Mattila, T.; Ward, T.J.; O’Donnell, K.; Proctor, R.H.; Burkin, A.A.; Kononenko, G.P.; Gavrilova, O.P.; Aoki, T.; McCormick, S.P.; Gagkaeva, T.Y. Fusarium sibiricum sp. nov, a novel type A trichothecene-producing Fusarium from northern Asia closely related to F. sporotrichioides and F. langsethiae. Int. J. Food Microbiol. 2011, 147, 58–68. [Google Scholar] [CrossRef] [PubMed]
- O’Donnell, K.; Ward, T.J.; Robert, V.A.R.G.; Crous, P.W.; Geiser, D.M.; Kang, S. DNA sequence-based identification of Fusarium: Current status and future directions. Phytoparasitica 2015, 43, 583–595. [Google Scholar] [CrossRef]
- Covarelli, L.; Beccari, G.; Prodi, A.; Generotti, S.; Etruschi, F.; Juan, C.; Ferrer, E.; Mañes, J. Fusarium species, chemotype characterisation and trichothecene contamination of durum and soft wheat in an area of central Italy. J. Sci. Food Agric. 2014, 95, 540–551. [Google Scholar] [CrossRef] [PubMed]
- Supronienė, S.; Sakalauskas, S.; Mankevičienė, A.; Barčauskaitė, K.; Jonavičienė, A. Distribution of B type trichothecene producing Fusarium species in wheat grain and relation to mycotoxins DON and NIV concentrations. Zemdirbyste-Agriculture 2016, 103, 281–288. [Google Scholar] [CrossRef][Green Version]
- Schöneberg, T.; Musa, T.; Forrer, H.-R.; Mascher, F.; Bucheli, T.D.; Bertossa, M.; Keller, B.; Vogelgsang, S. Infection conditions of Fusarium graminearum in barley are variety specific and different from those in wheat. Eur. J. Plant Pathol. 2018, 151, 975–989. [Google Scholar] [CrossRef]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The global burden of pathogens and pests on major food crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Matny, O.N. Fusarium Head Blight and Crown Rot on Wheat & Barley: Losses and Health Risks. Adv. Plants Agric. Res. 2015, 2, 39. [Google Scholar] [CrossRef]
- Lofgren, L.A.; LeBlanc, N.R.; Certano, A.K.; Nachtigall, J.; LaBine, K.M.; Riddle, J.; Broz, K.; Dong, Y.; Bethan, B.; Kafer, C.W.; et al. Fusarium graminearum: Pathogen or endophyte of North American grasses? New Phytol. 2017, 217, 1203–1212. [Google Scholar] [CrossRef]
- Fulcher, M.R.; Garcia, J.P.; Damann, K.C.M.; Bergstrom, G.C. Variable interactions between non-cereal grasses and Fusarium graminearum. Can. J. Plant Pathol. 2019, 41, 450–456. [Google Scholar] [CrossRef]
- Martínez, M.; Arata, A.F.; Fernández, M.D.; Stenglein, S.A.; Dinolfo, M.I. Fusarium species richness in mono- and dicotyledonous weeds and their ability to infect barley and wheat. Mycol. Prog. 2021, 20, 1203–1216. [Google Scholar] [CrossRef]
- Sakalauskas, S.; Stumbriene, K.; Suproniene, S.; Svegzda, P. Changes in Fusarium Link Species Composition From Lithuanian Wheat Grain in Years 2005-2007 to 2011-2013. Proc. Latv. Univ. Agric. 2014, 32, 45–50. [Google Scholar] [CrossRef]
- Brennan, J.M.; Egan, D.; Cooke, B.M.; Doohan, F.M. Effect of temperature on head blight of wheat caused by Fusarium culmorum and F. graminearum. Plant Pathol. 2005, 54, 156–160. [Google Scholar] [CrossRef]
- Alisaac, E.; Rathgeb, A.; Karlovsky, P.; Mahlein, A.-K. Fusarium Head Blight: Effect of Infection Timing on Spread of Fusarium graminearum and Spatial Distribution of Deoxynivalenol within Wheat Spikes. Microorganisms 2020, 9, 79. [Google Scholar] [CrossRef] [PubMed]
- Stein, J.; Kirby, C.; Osborne, L. Environmental factors influencing scab of barley in the northern great plains. In Proceedings of the 2005 National Fusarium Head Blight Forum, Brookings, SD, USA, 11–13 December 2005; p. 151. [Google Scholar]
- Llorens, A.; Mateo, R.; Hinojo, M.; Valle-Algarra, F.; Jiménez, M. Influence of environmental factors on the biosynthesis of type B trichothecenes by isolates of Fusarium spp. from Spanish crops. Int. J. Food Microbiol. 2004, 94, 43–54. [Google Scholar] [CrossRef] [PubMed]
- Nita, M.; Tilley, K.; De Wolf, E.; Kuldau, G. Effects of moisture during and after anthesis on the development of Fusarium head blight of wheat and mycotoxin production. In Proceedings of the National Fusarium Head Blight Forum, Milwaukee, WI, USA, 11–13 December 2005; pp. 11–13. [Google Scholar]
- Shah, L.; Ali, A.; Yahya, M.; Zhu, Y.; Wang, S.; Si, H.; Rahman, H.; Ma, C. Integrated control of fusarium head blight and deoxynivalenol mycotoxin in wheat. Plant Pathol. 2017, 67, 532–548. [Google Scholar] [CrossRef]
- Mourelos, C.; Malbrán, I.; Balatti, P.; Ghiringhelli, P.; Lori, G. Gramineous and non-gramineous weed species as alternative hosts of Fusarium graminearum, causal agent of Fusarium head blight of wheat, in Argentina. Crop. Prot. 2014, 65, 100–104. [Google Scholar] [CrossRef]
- Zakaria, L.; Ning, C.H. Endophytic Fusarium spp. from Roots of Lawn Grass (Axonopus compressus). Trop. Life Sci. Res. 2013, 24, 85–90. [Google Scholar]
- Aoki, T.; O’Donnell, K.; Geiser, D.M. Systematics of key phytopathogenic Fusarium species: Current status and future challenges. J. Gen. Plant Pathol. 2014, 80, 189–201. [Google Scholar] [CrossRef]
- Atanasova-Penichon, V.; Pons, S.; Pinson-Gadais, L.; Picot, A.; Marchegay, G.; Bonnin-Verdal, M.-N.; Ducos, C.; Barreau, C.; Roucolle, J.; Sehabiague, P.; et al. Chlorogenic Acid and Maize Ear Rot Resistance: A Dynamic Study Investigating Fusarium graminearum Development, Deoxynivalenol Production, and Phenolic Acid Accumulation. Mol. Plant-Microbe Interactions® 2012, 25, 1605–1616. [Google Scholar] [CrossRef]
- Geraldo, M.R.F.; Tessmann, D.J.; Kemmelmeier, C. Production of mycotoxins by Fusarium graminearum isolated from small cereals (wheat, triticale and barley) affected with scab disease in Southern Brazil. Braz. J. Microbiol. 2006, 37, 58–63. [Google Scholar] [CrossRef]
- Blandino, M.; Haidukowski, M.; Pascale, M.; Plizzari, L.; Scudellari, D.; Reyneri, A. Integrated strategies for the control of Fusarium head blight and deoxynivalenol contamination in winter wheat. Field Crop. Res. 2012, 133, 139–149. [Google Scholar] [CrossRef]
- Jestoi, M.N.; Paavanen-Huhtala, S.; Parikka, P.; Yli-Mattila, T. In vitro and in vivo mycotoxin production of Fusarium species isolated from Finnish grains. Arch. Phytopathol. Plant Prot. 2008, 41, 545–558. [Google Scholar] [CrossRef]
- Kokkonen, M.; Ojala, L.; Parikka, P.; Jestoi, M. Mycotoxin production of selected Fusarium species at different culture conditions. Int. J. Food Microbiol. 2010, 143, 17–25. [Google Scholar] [CrossRef] [PubMed]
- Marin, S.; Ramos, A.J.; Cano-Sancho, G.; Sanchis, V. Mycotoxins: Occurrence, toxicology, and exposure assessment. Food Chem. Toxicol. 2013, 60, 218–237. [Google Scholar] [CrossRef] [PubMed]
- Kaushik, G. Effect of Processing on Mycotoxin Content in Grains. Crit. Rev. Food Sci. Nutr. 2013, 55, 1672–1683. [Google Scholar] [CrossRef] [PubMed]
- Habler, K.; Geissinger, C.; Hofer, K.; Schüler, J.; Moghari, S.; Hess, M.; Gastl, M.; Rychlik, M. Fate of Fusarium Toxins during Brewing. J. Agric. Food Chem. 2016, 65, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Pereyra, S.A.; Dill-Macky, R. Colonization of the Residues of Diverse Plant Species by Gibberella zeae and Their Contribution to Fusarium Head Blight Inoculum. Plant Dis. 2008, 92, 800–807. [Google Scholar] [CrossRef] [PubMed]
- Landschoot, S.; Audenaert, K.; Waegeman, W.; Pycke, B.; Bekaert, B.; De Baets, B.; Haesaert, G. Connection between primary Fusarium inoculum on gramineous weeds, crop residues and soil samples and the final population on wheat ears in Flanders, Belgium. Crop. Prot. 2011, 30, 1297–1305. [Google Scholar] [CrossRef]
- Petit, S.; Boursault, A.; Guilloux, M.; Munier-Jolain, N.; Reboud, X. Weeds in agricultural landscapes. A review. Agron. Sustain. Dev. 2010, 31, 309–317. [Google Scholar] [CrossRef]
- Postic, J.; Cosic, J.; Vrandecic, K.; Jurkovic, D.; Saleh, A.A.; Leslie, J.F. Diversity of Fusarium Species Isolated from Weeds and Plant Debris in Croatia. J. Phytopathol. 2011, 160, 76–81. [Google Scholar] [CrossRef]
- Ilic, J.; Cosic, J.; Jurkovic, D.; Vrandecic, K. Pathogenicity of Fusarium spp. isolated from weeds and plant debris in eastern Croatia to wheat and maize. Poljopriverda 2012, 18, 7–11. Available online: https://hrcak.srce.hr/94622 (accessed on 22 August 2022).
- Burlakoti, R.R.; Ali, S.; Secor, G.A.; Neate, S.M.; McMullen, M.P.; Adhikari, T.B. Genetic Relationships Among Populations of Gibberella zeae from Barley, Wheat, Potato, and Sugar Beet in the Upper Midwest of the United States. Phytopathology® 2008, 98, 969–976. [Google Scholar] [CrossRef] [PubMed]
- Christ, D.S.; Gödecke, R.; von Tiedemann, A.; Varrelmann, M. Pathogenicity, Symptom Development, and Mycotoxin Formation in Wheat by Fusarium Species Frequently Isolated from Sugar Beet. Phytopathology® 2011, 101, 1338–1345. [Google Scholar] [CrossRef] [PubMed]
- Dong, F.; Xu, J.; Zhang, X.; Wang, S.; Xing, Y.; Mokoena, M.P.; Olaniran, A.O.; Shi, J. Gramineous weeds near paddy fields are alternative hosts for the Fusarium graminearum species complex that causes fusarium head blight in rice. Plant Pathol. 2020, 69, 433–441. [Google Scholar] [CrossRef]
- Svitlica, B.; Cosic, J.; Simic, B.; Jurkovic, D.; Vrandecic, K.; Purar, B.; Teklic, T. Pathogenicity of Fusarium species to maize ears. Cereal Res. Commun. 2008, 36, 543–544. Available online: http://fiver.ifvcns.rs/handle/123456789/637 (accessed on 23 August 2022).
- Rasiukeviciute, N.; Suproniene, S.; Kelpsiene, J.; Svegzda, P.; Kadziene, G.; Sneideris, D.; Ivanauskas, A.; Treikale, O. Susceptibility of non-cereal crops to Fusarium graminearum complex and their role within cereal crop rotation as a source of inoculum for Fusarium head blight. Span. J. Agric. Res. 2019, 16, e1012. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Kelpsiene, J.; Pranaitiene, S.; et al. Asymptomatic weeds are frequently colonised by pathogenic species of Fusarium in cereal-based crop rotations. Weed Res. 2019, 59, 312–323. [Google Scholar] [CrossRef]
- Suproniene, S.; Kadziene, G.; Irzykowski, W.; Sneideris, D.; Ivanauskas, A.; Sakalauskas, S.; Serbiak, P.; Svegzda, P.; Auskalniene, O.; Jedryczka, M. Weed species within cereal crop rotations can serve as alternative hosts for Fusarium graminearum causing Fusarium head blight of wheat. Fungal Ecol. 2019, 37, 30–37. [Google Scholar] [CrossRef]
- Sneideris, D.; Ivanauskas, A.; Prakas, P.; Butkauskas, D.; Treikale, O.; Kadziene, G.; Rasiukeviciute, N.; Kelpsiene, J.; Suproniene, S. Population Structure of Fusarium graminearum Isolated from Different Sources in One Area over the Course of Three Years. Phytopathology® 2020, 110, 1312–1318. [Google Scholar] [CrossRef]
- Nirenberg, H. Untersuchungen Über Die Morphologische und Biologische Differenzierung in der Fusarium-Sektion Liseola; Kommissionsverlag Paul Parey: Berlin, Germany. [CrossRef]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An Illustrated Manual for Identification; Pennsylvania State University Press: London, UK, 1983; 193p, ISBN 0-271-00349-9. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. The Fusarium Laboratory Manual; Blackwell Professional: Ames, IA, USA, 2006; 388p. [Google Scholar]
- Demeke, T.; Clear, R.M.; Patrick, S.K.; Gaba, D. Species-specific PCR-based assays for the detection of Fusarium species and a comparison with the whole seed agar plate method and trichothecene analysis. Int. J. Food Microbiol. 2005, 103, 271–284. [Google Scholar] [CrossRef]
- Purahong, W.; Nipoti, P.; Pisi, A.; Lemmens, M.; Prodi, A. Aggressiveness of different Fusarium graminearum chemotypes within a population from Northern-Central Italy. Mycoscience 2014, 55, 63–69. [Google Scholar] [CrossRef]
- Engle, J.S.; Lipps, P.E.; Mills, D. Fusarium head blight severity scale for winter wheat. In Bulletin AC-48-03, Extension Factsheet; Ohio State University: Columbus, OH, USA, 2003. [Google Scholar]
- Matelionienė, N.; Supronienė, S.; Shamshitov, A.; Zavtrikovienė, E.; Janavičienė, S.; Kadžienė, G. Weeds in Cereal Crop Rotations May Host Fusarium Species That Cause Fusarium Head Blight and Grain Weight Losses in Wheat. Agronomy 2022, 12, 2741. [Google Scholar] [CrossRef]
- Jenkinson, P.; Parry, D. Isolation of Fusarium species from common broad-leaved weeds and their pathogenicity to winter wheat. Mycol. Res. 1994, 98, 776–780. [Google Scholar] [CrossRef]
- Janaviciene, S.; Suproniene, S.; Kadziene, G.; Pavlenko, R.; Berzina, Z.; Bartkevics, V. Toxigenicity of F. graminearum Residing on Host Plants Alternative to Wheat as Influenced by Environmental Conditions. Toxins 2022, 14, 541. [Google Scholar] [CrossRef] [PubMed]
- Gerling, M.; Pätzig, M.; Hempel, L.; Büttner, C.; Müller, M.E.H. Arable Weeds at the Edges of Kettle Holes as Overwintering Habitat for Phytopathogenic Fungi. Agronomy 2022, 12, 823. [Google Scholar] [CrossRef]
- Linde, C.C.; Smith, L.M.; Peakall, R. Weeds, as ancillary hosts, pose disproportionate risk for virulent pathogen transfer to crops. BMC Evol. Biol. 2016, 16, 1–12. [Google Scholar] [CrossRef]

| Experiment | Isolates per Fusarium Species | Isolate Number per Hostplant |
|---|---|---|
| I experiment | F. avenaceum—12 | T. aestivum—11 |
| F. culmorum—12 | B. napus—12 | |
| F. graminearum—12 | P. sativum—12 | |
| F. sporotrichioides—10 | B. vulgaris—11 | |
| Total in I | 46 isolates | 46 isolates |
| II experiment | F. avenaceum—12 | T. aestivum—8 |
| F. culmorum—12 | V. arvensis—8 | |
| F. graminearum—12 | C. bursa pastoris—8 | |
| F. sporotrichioides—9 | P. annua—6 | |
| F. convolvulus—7 | ||
| T. inodorum—8 | ||
| Total in II | 45 isolates | 45 isolates |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zavtrikovienė, E.; Gorash, A.; Kadžienė, G.; Matelionienė, N.; Supronienė, S. Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions. Pathogens 2022, 11, 1467. https://doi.org/10.3390/pathogens11121467
Zavtrikovienė E, Gorash A, Kadžienė G, Matelionienė N, Supronienė S. Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions. Pathogens. 2022; 11(12):1467. https://doi.org/10.3390/pathogens11121467
Chicago/Turabian StyleZavtrikovienė, Evelina, Andrii Gorash, Gražina Kadžienė, Neringa Matelionienė, and Skaidrė Supronienė. 2022. "Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions" Pathogens 11, no. 12: 1467. https://doi.org/10.3390/pathogens11121467
APA StyleZavtrikovienė, E., Gorash, A., Kadžienė, G., Matelionienė, N., & Supronienė, S. (2022). Pathogenicity of Asymptomatically Residing Fusarium Species in Non-Gramineous Plants and Weeds to Spring Wheat under Greenhouse Conditions. Pathogens, 11(12), 1467. https://doi.org/10.3390/pathogens11121467








