Avian Macrophage Responses to Virulent and Avirulent Clostridium perfringens
Abstract
:1. Introduction
2. Results
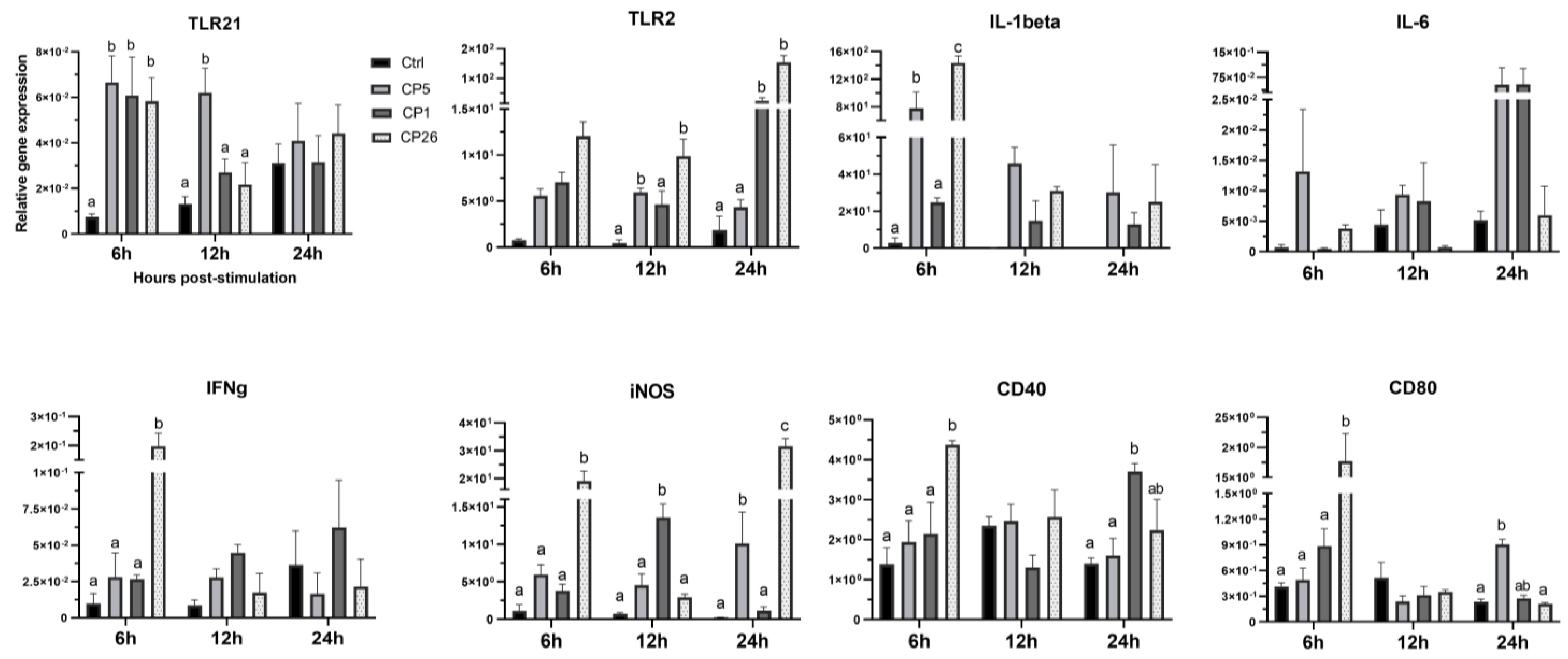
2.1. Macrophage Responses to Clostridium Perfringens Whole Cells
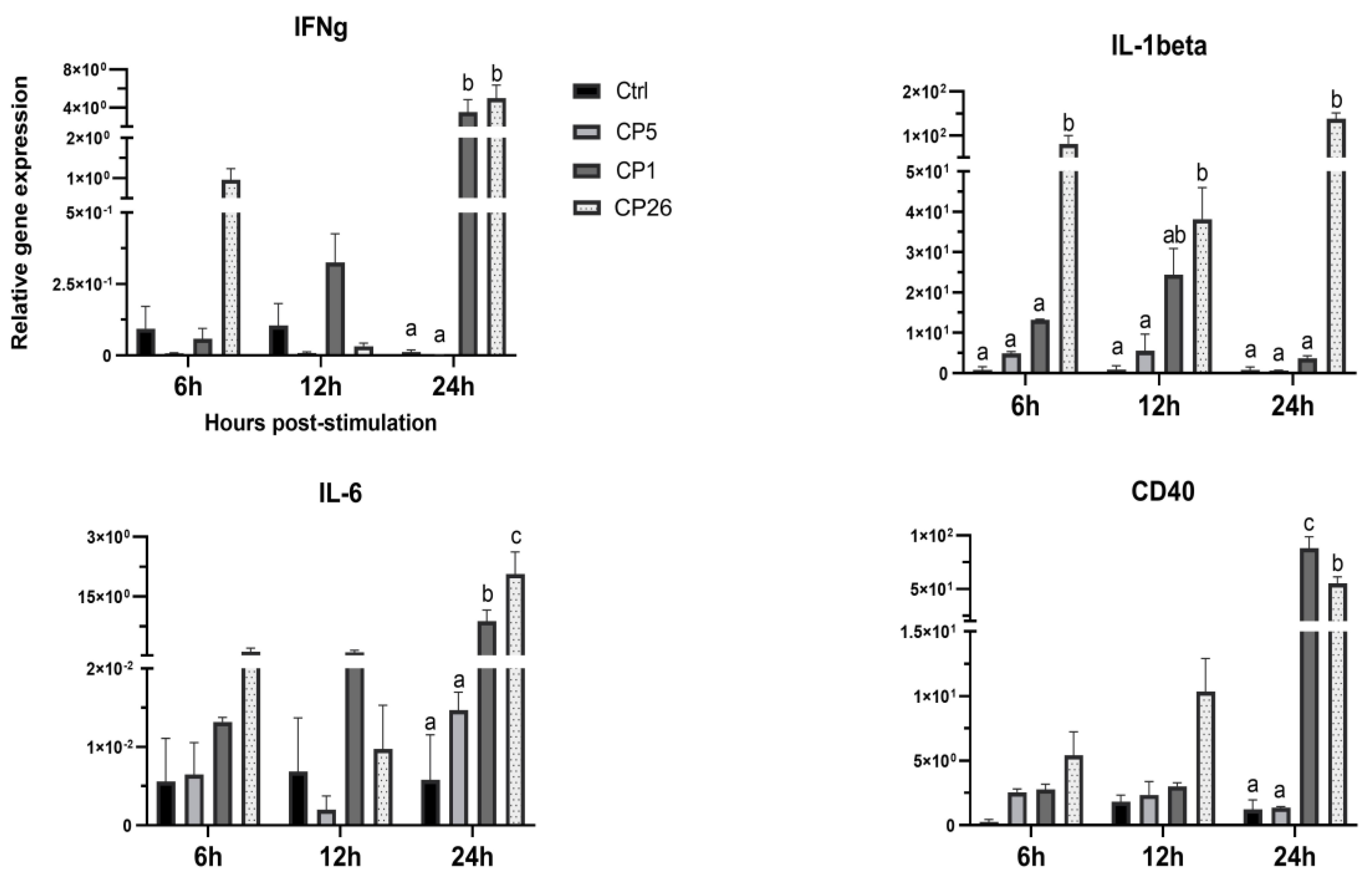
2.2. Macrophage Responses to Clostridium Perfringens Secretory Products
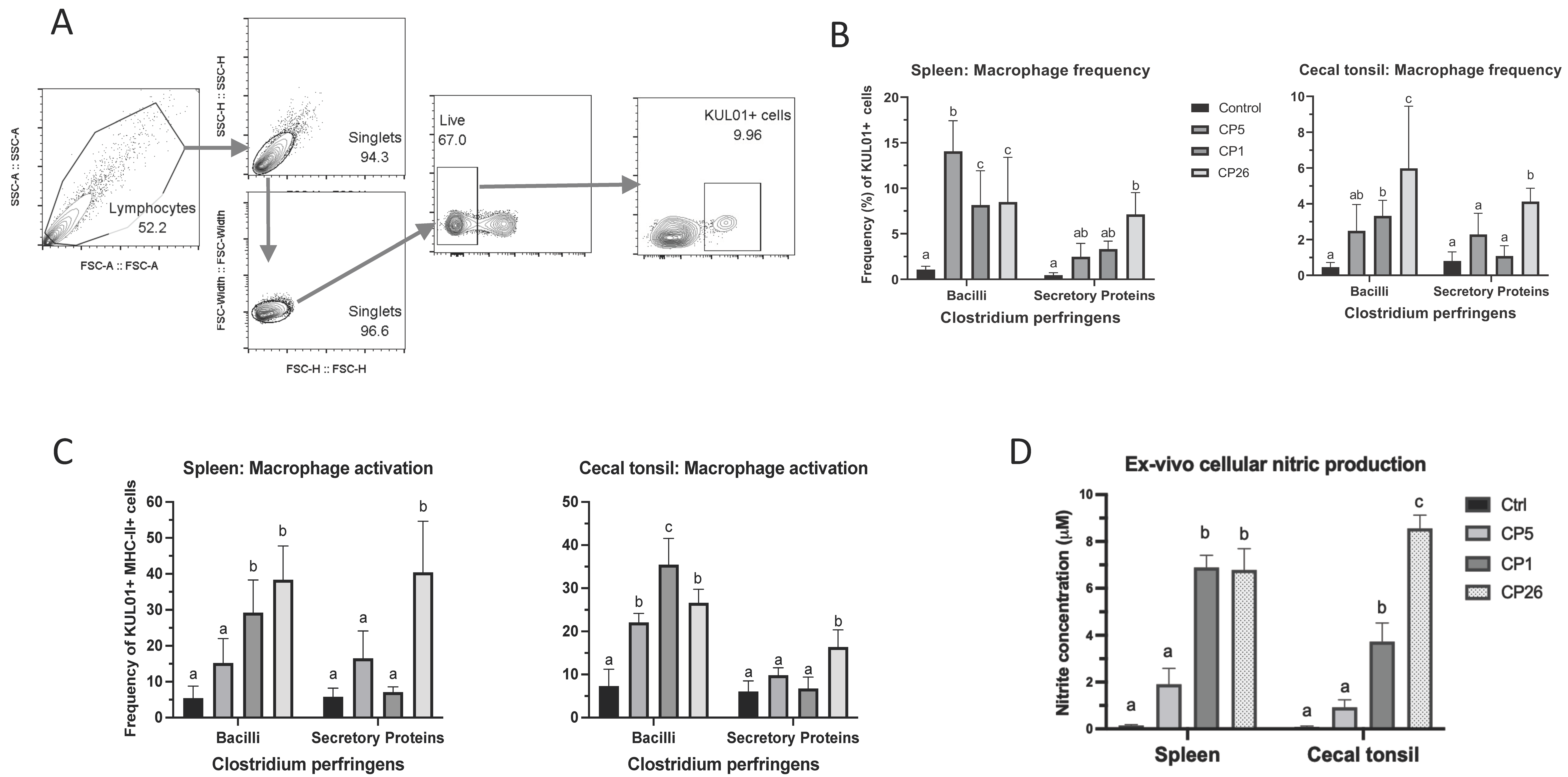
2.3. Macrophage Expression of MHC-II and Production of Nitric Oxide
2.4. Ex Vivo Cellular Responses and Nitric Oxide Production
3. Discussion
4. Materials and Methods
4.1. Cells
4.2. Bacteria
4.3. Secretory Proteins Preparation
4.4. Animals
4.5. In Vitro Cellular Stimulation
4.6. Ex Vivo Cellular Stimulation
4.7. Immune Gene Expression
4.8. Flow Cytometry
4.9. Nitric Oxide (NO) Measurement
4.10. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mosser, D.M.; Edwards, J.P. Exploring the full spectrum of macrophage activation. Nat. Rev. Immunol. 2008, 8, 958–969. [Google Scholar] [CrossRef]
- Froidevaux, C.; Roger, T.; Martin, C.; Glauser, M.P.; Calandra, T. Macrophage migration inhibitory factor and innate immune responses to bacterial infections. Crit. Care Med. 2001, 29, S13–S15. [Google Scholar] [CrossRef]
- Mege, J.L.; Mehraj, V.; Capo, C. Macrophage polarization and bacterial infections. Curr. Opin. Infect. Dis. 2011, 24, 230–234. [Google Scholar] [CrossRef]
- Mellata, M.; Dho-Moulin, M.; Dozois, C.M.; Curtiss, R., 3rd; Lehoux, B.; Fairbrother, J.M. Role of avian pathogenic Escherichia coli virulence factors in bacterial interaction with chicken heterophils and macrophages. Infect. Immun. 2003, 71, 494–503. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.K.; Melville, S.B. Effects of Clostridium perfringens alpha-toxin (PLC) and perfringolysin O (PFO) on cytotoxicity to macrophages, on escape from the phagosomes of macrophages, and on persistence of C. perfringens in host tissues. Infect. Immun. 2004, 72, 5204–5215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paredes-Sabja, D.; Sarker, M.R. Interactions between Clostridium perfringens spores and Raw 264.7 macrophages. Anaerobe 2012, 18, 148–156. [Google Scholar] [CrossRef] [PubMed]
- Wade, B. The True Cost of Necrotic Enteritis; World Poultry: Cambridge, UK, 2015. [Google Scholar]
- Prescott, J.F.; Parreira, V.R.; Mehdizadeh Gohari, I.; Lepp, D.; Gong, J. The pathogenesis of necrotic enteritis in chickens: What we know and what we need to know: A review. Avian Pathol. 2016, 45, 288–294. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.R.; Parreira, V.R.; Sharif, S.; Prescott, J.F. Clostridium perfringens antigens recognized by broiler chickens immune to necrotic enteritis. Clin. Vaccine Immunol. 2006, 13, 1358–1362. [Google Scholar] [CrossRef] [Green Version]
- Kulkarni, R.R.; Parreira, V.R.; Sharif, S.; Prescott, J.F. Immunization of broiler chickens against Clostridium perfringens-induced necrotic enteritis. Clin. Vaccine Immunol. 2007, 14, 1070–1077. [Google Scholar] [CrossRef] [Green Version]
- Coursodon, C.F.; Glock, R.D.; Moore, K.L.; Cooper, K.K.; Songer, J.G. TpeL-producing strains of Clostridium perfringens type A are highly virulent for broiler chicks. Anaerobe 2012, 18, 117–121. [Google Scholar] [CrossRef]
- Gu, C.; Lillehoj, H.S.; Sun, Z.; Lee, Y.; Zhao, H.; Xianyu, Z.; Yan, X.; Wang, Y.; Lin, S.; Liu, L.; et al. Characterization of Virulent netB (+)/tpeL (+) Clostridium perfringens Strains from Necrotic Enteritis-Affected Broiler Chicken Farms. Avian Dis. 2019, 63, 461–467. [Google Scholar] [CrossRef]
- Lepp, D.; Roxas, B.; Parreira, V.R.; Marri, P.R.; Rosey, E.L.; Gong, J.; Songer, J.G.; Vedantam, G.; Prescott, J.F. Identification of novel pathogenicity loci in Clostridium perfringens strains that cause avian necrotic enteritis. PLoS ONE 2010, 5, e10795. [Google Scholar] [CrossRef]
- de Geus, E.D.; Vervelde, L. Regulation of macrophage and dendritic cell function by pathogens and through immunomodulation in the avian mucosa. Dev. Comp. Immunol. 2013, 41, 341–351. [Google Scholar] [CrossRef]
- Fasina, Y.O.; Lillehoj, H.S. Characterization of intestinal immune response to Clostridium perfringens infection in broiler chickens. Poult. Sci. 2019, 98, 188–198. [Google Scholar] [CrossRef]
- Guo, S.; Li, C.; Liu, D.; Guo, Y. Inflammatory responses to a Clostridium perfringens type A strain and alpha-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015, 44, 81–91. [Google Scholar] [CrossRef]
- Park, S.S.; Lillehoj, H.S.; Allen, P.C.; Park, D.W.; FitzCoy, S.; Bautista, D.A.; Lillehoje, E.P. Immunopathology and cytokine responses in broiler chickens coinfected with Eimeria maxima and Clostridium perfringens with the use of an animal model of necrotic enteritis. Avian Dis. 2008, 52, 14–22. [Google Scholar] [CrossRef]
- Wang, B.; Hussain, A.; Zhou, Y.; Zeng, Z.; Wang, Q.; Zou, P.; Gong, L.; Zhao, P.; Li, W. Saccharomyces boulardii attenuates inflammatory response induced by Clostridium perfringens via TLR4/TLR15-MyD8 pathway in HD11 avian macrophages. Poult. Sci. 2020, 99, 5356–5365. [Google Scholar] [CrossRef]
- Zaytsoff, S.J.M.; Lyons, S.M.; Garner, A.M.; Uwiera, R.R.E.; Zandberg, W.F.; Abbott, D.W.; Inglis, G.D. Host responses to Clostridium perfringens challenge in a chicken model of chronic stress. Gut Pathog. 2020, 12, 24. [Google Scholar] [CrossRef]
- Alber, A.; Morris, K.M.; Bryson, K.J.; Sutton, K.M.; Monson, M.S.; Chintoan-Uta, C.; Borowska, D.; Lamont, S.J.; Schouler, C.; Kaiser, P.; et al. Avian Pathogenic Escherichia coli (APEC) Strain-Dependent Immunomodulation of Respiratory Granulocytes and Mononuclear Phagocytes in CSF1R-Reporter Transgenic Chickens. Front. Immunol. 2019, 10, 3055. [Google Scholar] [CrossRef]
- Peng, L.; Matthijs, M.G.R.; Haagsman, H.P.; Veldhuizen, E.J.A. Avian pathogenic Escherichia coli-induced activation of chicken macrophage HD11cells. Dev. Comp. Immunol. 2018, 87, 75–83. [Google Scholar] [CrossRef]
- Lu, Y.; Sarson, A.J.; Gong, J.; Zhou, H.; Zhu, W.; Kang, Z.; Yu, H.; Sharif, S.; Han, Y. Expression profiles of genes in Toll-like receptor-mediated signaling of broilers infected with Clostridium perfringens. Clin. Vaccine Immunol. 2009, 16, 1639–1647. [Google Scholar] [CrossRef] [Green Version]
- Gao, Q.; Qi, L.; Wu, T.; Wang, J. Clostridium butyricum activates TLR2-mediated MyD88-independent signaling pathway in HT-29 cells. Mol. Cell Biochem. 2012, 361, 31–37. [Google Scholar] [CrossRef]
- Jin, X.; Zhang, X.; Li, J.; Yu, W.; Chen, F. Activation of chicken macrophages during in vitro stimulation and expression of immune genes. Am. J. Vet. Res. 2018, 79, 1306–1312. [Google Scholar] [CrossRef]
- do Vale, A.; Cabanes, D.; Sousa, S. Bacterial Toxins as Pathogen Weapons Against Phagocytes. Front. Microbiol. 2016, 7, 42. [Google Scholar] [CrossRef]
- Jessen, D.L.; Osei-Owusu, P.; Toosky, M.; Roughead, W.; Bradley, D.S.; Nilles, M.L. Type III secretion needle proteins induce cell signaling and cytokine secretion via Toll-like receptors. Infect. Immun. 2014, 82, 2300–2309. [Google Scholar] [CrossRef] [Green Version]
- Takehara, M.; Seike, S.; Sonobe, Y.; Bandou, H.; Yokoyama, S.; Takagishi, T.; Miyamoto, K.; Kobayashi, K.; Nagahama, M. Clostridium perfringens alpha-toxin impairs granulocyte colony-stimulating factor receptor-mediated granulocyte production while triggering septic shock. Commun. Biol. 2019, 2, 45. [Google Scholar] [CrossRef] [Green Version]
- Lai, Y.H.; Tsai, B.Y.; Hsu, C.Y.; Chen, Y.H.; Chou, P.H.; Chen, Y.L.; Liu, H.C.; Ko, W.C.; Tsai, P.J.; Hung, Y.P. The Role of Toll-Like Receptor-2 in Clostridioides difficile Infection: Evidence from a Mouse Model and Clinical Patients. Front. Immunol. 2021, 12, 691039. [Google Scholar] [CrossRef]
- Simpson, M.; Frisbee, A.; Kumar, P.; Schwan, C.; Aktories, K.; Petri, W.A. Clostridioides difficile binary toxin (CDT) is recognized by the TLR2/6 heterodimer to induce an NF-kappaB response. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Gaudino, S.J.; Kumar, P. Cross-Talk between Antigen Presenting Cells and T Cells Impacts Intestinal Homeostasis, Bacterial Infections, and Tumorigenesis. Front. Immunol. 2019, 10, 360. [Google Scholar] [CrossRef] [Green Version]
- Rajput, I.R.; Hussain, A.; Li, Y.L.; Zhang, X.; Xu, X.; Long, M.Y.; You, D.Y.; Li, W.F. Saccharomyces boulardii and Bacillus subtilis B10 modulate TLRs mediated signaling to induce immunity by chicken BMDCs. J. Cell. Biochem. 2014, 115, 189–198. [Google Scholar] [CrossRef]
- Mortada, M.; Cosby, D.E.; Akerele, G.; Ramadan, N.; Oxford, J.; Shanmugasundaram, R.; Ng, T.T.; Selvaraj, R.K. Characterizing the immune response of chickens to Campylobacter jejuni (Strain A74C). PLoS ONE 2021, 16, e0247080. [Google Scholar] [CrossRef]
- Collier, C.T.; Hofacre, C.L.; Payne, A.M.; Anderson, D.B.; Kaiser, P.; Mackie, R.I.; Gaskins, H.R. Coccidia-induced mucogenesis promotes the onset of necrotic enteritis by supporting Clostridium perfringens growth. Vet. Immunol. Immunopathol. 2008, 122, 104–115. [Google Scholar] [CrossRef]
- Sarson, A.J.; Wang, Y.; Kang, Z.; Dowd, S.E.; Lu, Y.; Yu, H.; Han, Y.; Zhou, H.; Gong, J. Gene expression profiling within the spleen of Clostridium perfringens-challenged broilers fed antibiotic-medicated and non-medicated diets. BMC Genom. 2009, 10, 260. [Google Scholar] [CrossRef] [Green Version]
- Crippen, T.L.; Sheffield, C.L.; He, H.; Lowry, V.K.; Kogut, M.H. Differential nitric oxide production by chicken immune cells. Dev. Comp. Immunol. 2003, 27, 603–610. [Google Scholar] [CrossRef]
- Zhang, B.; Lv, Z.; Li, H.; Guo, S.; Liu, D.; Guo, Y. Dietary l-arginine inhibits intestinal Clostridium perfringens colonisation and attenuates intestinal mucosal injury in broiler chickens. Brit. J. Nutr. 2017, 118, 321–332. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Brien, D.K.; Therit, B.H.; Woodman, M.E.; Melville, S.B. The role of neutrophils and monocytic cells in controlling the initiation of Clostridium perfringens gas gangrene. FEMS Immunol. Med. Microbiol. 2007, 50, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ou, X.; Mao, S.; Cao, J.; Cheng, A.; Wang, M.; Zhu, D.; Chen, S.; Jia, R.; Liu, M.; Sun, K.; et al. Comparative analysis of virus-host interactions caused by a virulent and an attenuated duck hepatitis A virus genotype 1. PLoS ONE 2017, 12, e0178993. [Google Scholar] [CrossRef]
- Skjesol, A.; Skjaeveland, I.; Elnaes, M.; Timmerhaus, G.; Fredriksen, B.N.; Jorgensen, S.M.; Krasnov, A.; Jorgensen, J.B. IPNV with high and low virulence: Host immune responses and viral mutations during infection. Virol. J. 2011, 8, 396. [Google Scholar] [CrossRef] [Green Version]
- Qureshi, M.A.; Miller, L.; Lillehoj, H.S.; Ficken, M.D. Establishment and characterization of a chicken mononuclear cell line. Vet. Immunol. Immunopathol. 1990, 26, 237–250. [Google Scholar] [CrossRef]
- Thompson, D.R.; Parreira, V.R.; Kulkarni, R.R.; Prescott, J.F. Live attenuated vaccine-based control of necrotic enteritis of broiler chickens. Vet. Microbiol. 2006, 113, 25–34. [Google Scholar] [CrossRef]
- Nowell, V.J.; Poppe, C.; Parreira, V.R.; Jiang, Y.F.; Reid-Smith, R.; Prescott, J.F. Clostridium perfringens in chicken. Anaerobe 2010, 16, 314–315. [Google Scholar] [CrossRef] [PubMed]
- Fernandes da Costa, S.P.; Mot, D.; Bokori-Brown, M.; Savva, C.G.; Basak, A.K.; Van Immerseel, F.; Titball, R.W. Protection against avian necrotic enteritis after immunisation with NetB genetic or formaldehyde toxoids. Vaccine 2013, 31, 4003–4008. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laursen, A.M.S.; Kulkarni, R.R.; Taha-Abdelaziz, K.; Plattner, B.L.; Read, L.R.; Sharif, S. Characterizaton of T cells in Marek’s disease virus (Gallid herpesvirus 2) infection of chickens. Virology 2018, 522, 56–64. [Google Scholar] [CrossRef] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]


| Target Gene | Primer Sequence (5′-3′) | Annealing Temp | GeneBank Accession Number |
|---|---|---|---|
| TLR2 | F-5′-ATCCTGCTGGAGCCCATTCAGAG3′ R-5′-TTGCTCTTCATCAGGAGGCCACT-3′ | 60 | NM_204278.1 |
| TLR21 | F-5′-CCTGCGCAAGTGTCCGCTCA-3′ R-5′-GCCCCAGGTCCAGGAAGCAG-3′ | 60 | NM_001030558.1 |
| IL-1β | F-5′-GTGAGGCTCAACATTGCGCTGTA-3′ R-5′-TGTCCAGGCGGTAGAAGATGAAG-3′ | 64 | AJ009800 |
| IL-6 | F-5′- CGTGTGCGAGAACAGCATGGAGA-3′ R-5′-TCAGGCATTTCTCCTCGTCGAAGC-3′ | 60 | NM_204628.1 |
| IFNγ | F-5′-ACACTGACAAGTCAAAGCCGCACA-3′ R-5′-AGTCGTTCATCGGGAGCTTGGC-3′ | 60 | X99774 |
| iNOS | F-5′-GGCAGCAGCGTCTCTATGACTTG-3′ R-5′-GACTTTAGGCTGCCCAGGTTG-3′ | 64 | NM 204961 |
| CD80 | F-5′-CTGTTCCTTCACATCCTGAGAG-3′ R-5′-CTTCAACACCATCTATTTGCCAG-3′ | 58 | NM_001079739 |
| CD40 | F-5′-CCTGGTGATGCTGTGAATTG-3′ R-5′-CTTCTGTGTCGTTGCATTCAG-3′ | 58 | NM_204665 |
| β-actin | F-5′-CAACACAGTGCTGTCTGGTGGTA-3′ R-5′-ATCGTACTCCTGCTTGCTGATCC-3′ | 58 | X00182 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kulkarni, R.R.; Gaghan, C.; Mohammed, J. Avian Macrophage Responses to Virulent and Avirulent Clostridium perfringens. Pathogens 2022, 11, 100. https://doi.org/10.3390/pathogens11010100
Kulkarni RR, Gaghan C, Mohammed J. Avian Macrophage Responses to Virulent and Avirulent Clostridium perfringens. Pathogens. 2022; 11(1):100. https://doi.org/10.3390/pathogens11010100
Chicago/Turabian StyleKulkarni, Raveendra R., Carissa Gaghan, and Javid Mohammed. 2022. "Avian Macrophage Responses to Virulent and Avirulent Clostridium perfringens" Pathogens 11, no. 1: 100. https://doi.org/10.3390/pathogens11010100
APA StyleKulkarni, R. R., Gaghan, C., & Mohammed, J. (2022). Avian Macrophage Responses to Virulent and Avirulent Clostridium perfringens. Pathogens, 11(1), 100. https://doi.org/10.3390/pathogens11010100







