Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report
Abstract
1. Introduction
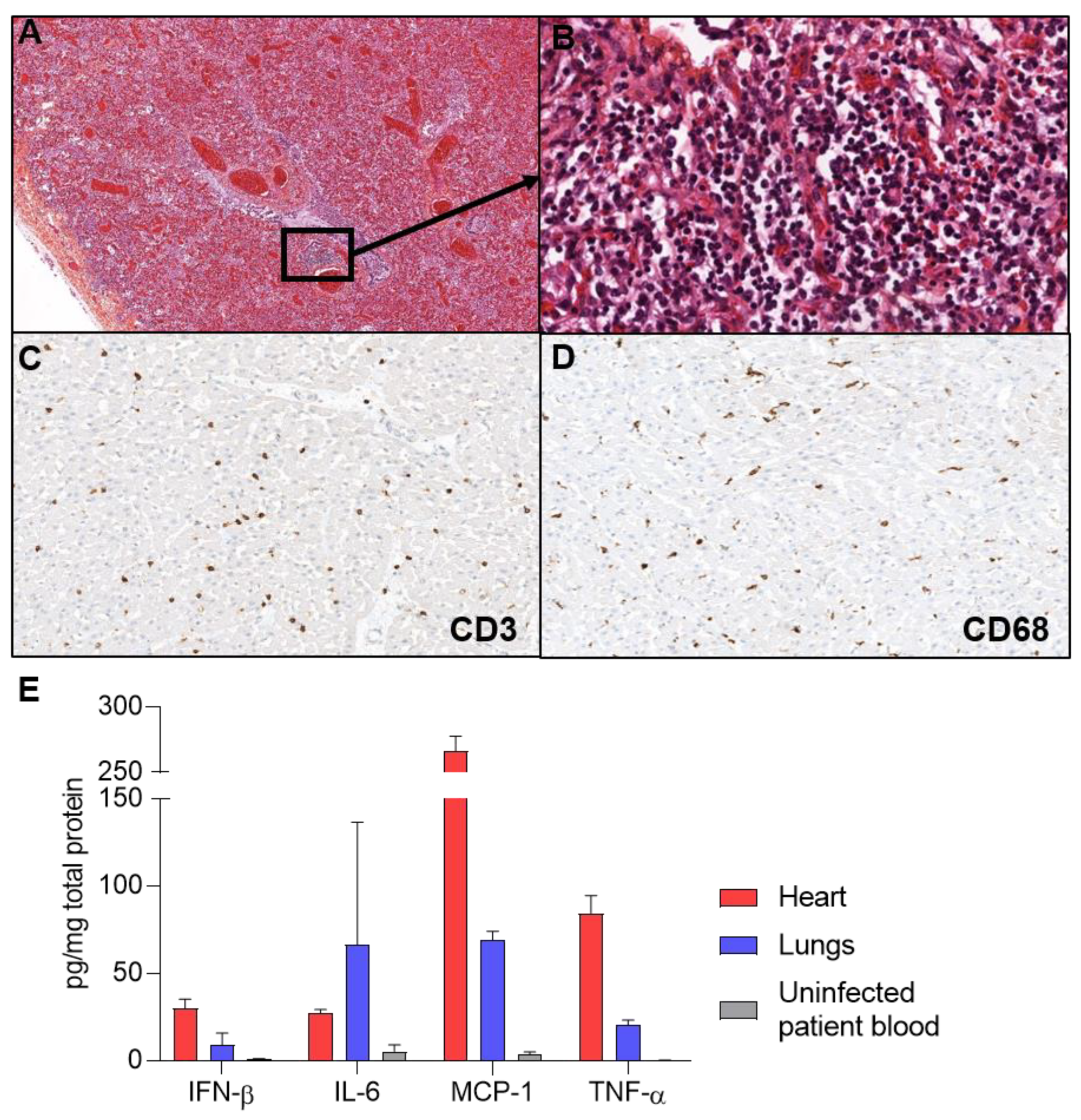
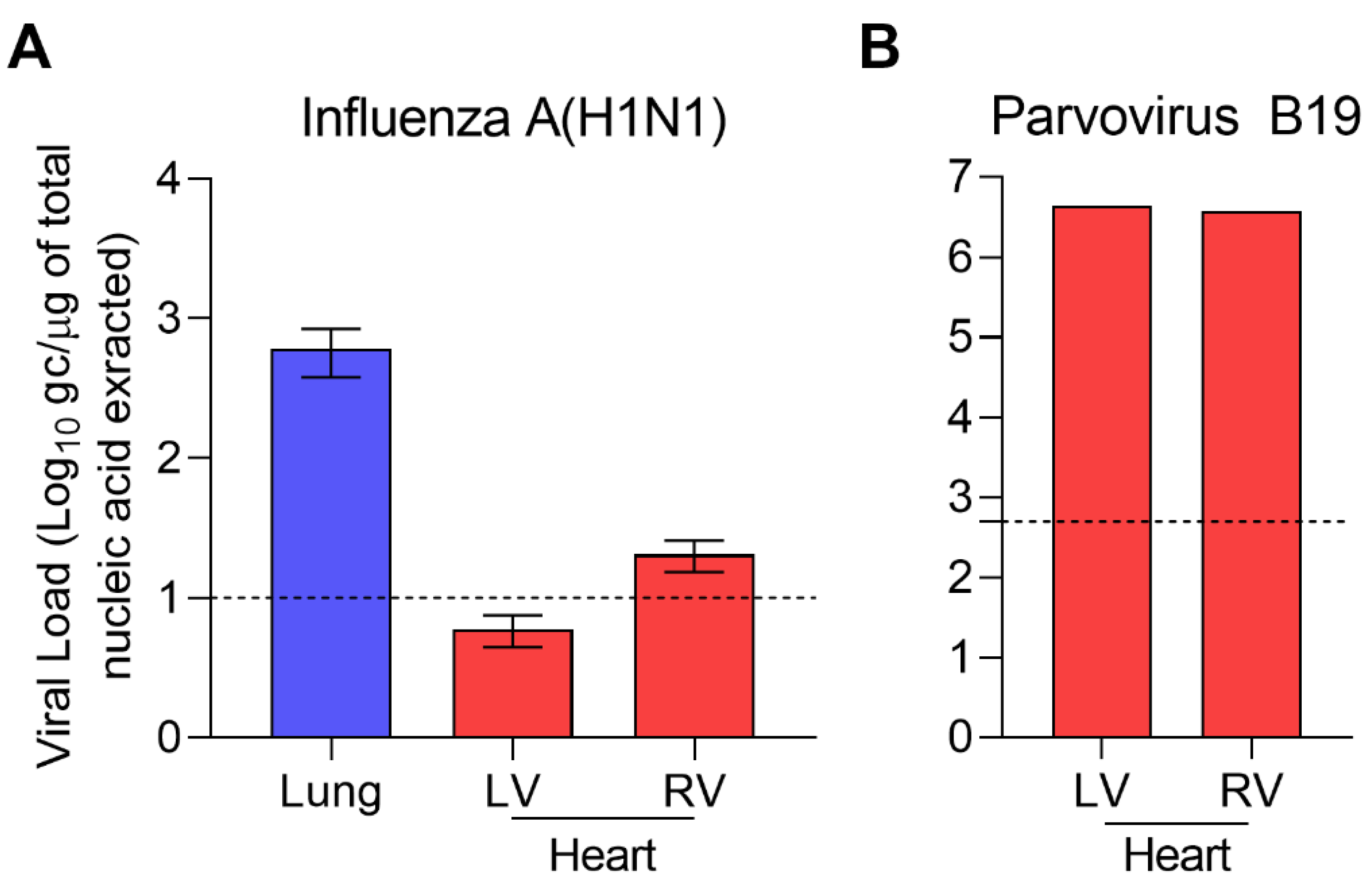
2. Case Presentation
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andréoletti, L.; Lévêque, N.; Boulagnon, C.; Brasselet, C.; Fornes, P. Viral Causes of Human Myocarditis. Arch. Cardiovasc. Dis. 2009, 102, 559–568. [Google Scholar] [CrossRef] [PubMed]
- Stewart, G.C.; Lopez-Molina, J.; Gottumukkala, R.V.; Rosner, G.F.; Rosner, G.F.; Anello, M.S.; Hecht, J.L.; Winters, G.L.; Padera, R.F.; Baughman, K.L.; et al. Myocardial Parvovirus B19 Persistence: Lack of Association with Clinicopathologic Phenotype in Adults with Heart Failure. Circ. Heart Fail. 2011, 4, 71–78. [Google Scholar] [CrossRef]
- Bock, C.-T.; Klingel, K.; Kandolf, R. Human Parvovirus B19-Associated Myocarditis. N. Engl. J. Med. 2010, 362, 1248–1249. [Google Scholar] [CrossRef] [PubMed]
- Bock, C.-T.; Düchting, A.; Utta, F.; Brunner, E.; Sy, B.T.; Klingel, K.; Lang, F.; Gawaz, M.; Felix, S.B.; Kandolf, R. Molecular Phenotypes of Human Parvovirus B19 in Patients with Myocarditis. World J. Cardiol. 2014, 6, 183–195. [Google Scholar] [CrossRef]
- Juhasz, Z.; Tiszlavicz, L.; Kele, B.; Terhes, G.; Deak, J.; Rudas, L.; Kereszty, E. Sudden Cardiac Death from Parvovirus B19 Myocarditis in a Young Man with Brugada Syndrome. J. Forensic Leg. Med. 2014, 25, 8–13. [Google Scholar] [CrossRef] [PubMed]
- Mamas, M.A.; Fraser, D.; Neyses, L. Cardiovascular Manifestations Associated with Influenza Virus Infection. Int. J. Cardiol. 2008, 130, 304–309. [Google Scholar] [CrossRef]
- Kuiken, T.; Taubenberger, J.K. Pathology of Human Influenza Revisited. Vaccine 2008, 26, D59–D66. [Google Scholar] [CrossRef] [PubMed]
- Shieh, W.-J.; Blau, D.M.; Denison, A.M.; DeLeon-Carnes, M.; Adem, P.; Bhatnagar, J.; Sumner, J.; Liu, L.; Patel, M.; Batten, B.; et al. 2009 Pandemic Influenza A (H1N1). Am. J. Pathol. 2010, 177, 166–175. [Google Scholar] [CrossRef]
- Gill, J.R.; Sheng, Z.-M.; Ely, S.F.; Guinee, D.G.; Beasley, M.B.; Suh, J.; Deshpande, C.; Mollura, D.J.; Morens, D.M.; Bray, M.; et al. Pulmonary Pathologic Findings of Fatal 2009 Pandemic Influenza A/H1N1 Viral Infections. Arch. Pathol. Lab. Med. 2010, 134, 235–243. [Google Scholar] [CrossRef]
- Caforio, A.L.P.; Pankuweit, S.; Arbustini, E.; Basso, C.; Gimeno-Blanes, J.; Felix, S.B.; Fu, M.; Helio, T.; Heymans, S.; Jahns, R.; et al. Current State of Knowledge on Aetiology, Diagnosis, Management, and Therapy of Myocarditis: A Position Statement of the European Society of Cardiology Working Group on Myocardial and Pericardial Diseases. Eur. Heart J. 2013, 34, 2636–2648. [Google Scholar] [CrossRef]
- Fernández-Rodríguez, A.; Burton, J.L.; Andreoletti, L.; Alberola, J.; Fornes, P.; Merino, I.; Martínez, M.J.; Castillo, P.; Sampaio-Maia, B.; Caldas, I.M.; et al. Post-Mortem Microbiology in Sudden Death: Sampling Protocols Proposed in Different Clinical Settings. Clin. Microbiol. Infect. 2019, 25, 570–579. [Google Scholar] [CrossRef]
- Mukhopadhyay, S.; Philip, A.T.; Stoppacher, R. Pathologic Findings in Novel Influenza A (H1N1) Virus (“Swine Flu”) Infection: Contrasting Clinical Manifestations and Lung Pathology in Two Fatal Cases. Am. J. Clin. Pathol. 2010, 133, 380–387. [Google Scholar] [CrossRef][Green Version]
- Harms, P.W.; Schmidt, L.A.; Smith, L.B.; Newton, D.W.; Pletneva, M.A.; Walters, L.L.; Tomlins, S.A.; Fisher-Hubbard, A.; Napolitano, L.M.; Park, P.K.; et al. Autopsy Findings in Eight Patients with Fatal H1N1 Influenza. Am. J. Clin. Pathol 2010, 134, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Soto-Abraham, M.V.; Soriano-Rosas, J.; Díaz-Quiñónez, A.; Silva-Pereyra, J.; Vazquez-Hernandez, P.; Torres-López, O.; Roldán, A.; Cruz-Gordillo, A.; Alonso-Viveros, P.; Navarro-Reynoso, F. Pathological Changes Associated with the 2009 H1N1 Virus. N. Engl. J. Med. 2009, 361, 2001–2003. [Google Scholar] [CrossRef]
- Ukimura, A.; Izumi, T.; Matsumori, A. Clinical Research Committee on Myoc a National Survey on Myocarditis Associated with the 2009 Influenza A (H1N1) Pandemic in Japan. Circ. J. 2010, 74, 2193–2199. [Google Scholar] [CrossRef] [PubMed]
- Boulagnon, C.; Leveque, N.; Renois, F.; Andreoletti, L.; Fornes, P. Influenza A/H1N1 (2009) Infection as a Cause of Unexpected Out-of-Hospital Death in the Young. J. Forensic Sci. 2012, 57, 1650–1655. [Google Scholar] [CrossRef]
- Hamoudi, A.; Vais, D.; Taqi, V. H1N1 Influenza Causing Fulminant Myocarditis Requiring Extracorporeal Membrane Oxygenation. Cureus 2019, 11, e4665. [Google Scholar] [CrossRef]
- N’Guyen, Y.; Lesaffre, F.; Metz, D.; Tassan, S.; Saade, Y.; Boulagnon, C.; Fornes, P.; Renois, F.; Andreoletti, L. Enterovirus but Not Parvovirus B19 Is Associated with Idiopathic Dilated Cardiomyopathy and Endomyocardial CD3, CD68, or HLA-DR Expression: Virus & Inflammatory Marker Detection in DCM. J. Med. Virol. 2017, 89, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Kühl, U.; Lassner, D.; Wallaschek, N.; Gross, U.M.; Krueger, G.R.F.; Seeberg, B.; Kaufer, B.B.; Escher, F.; Poller, W.; Schultheiss, H.-P. Chromosomally Integrated Human Herpesvirus 6 in Heart Failure: Prevalence and Treatment: Prevalence and Reactivation of CiHHV-6. Eur. J. Heart Fail. 2015, 17, 9–19. [Google Scholar] [CrossRef]
- Verdonschot, J.; Hazebroek, M.; Merken, J.; Debing, Y.; Dennert, R.; Brunner-La Rocca, H.-P.; Heymans, S. Relevance of Cardiac Parvovirus B19 in Myocarditis and Dilated Cardiomyopathy: Review of the Literature: Parvovirus B19 in Myocarditis and DCM. Eur. J. Heart Fail. 2016, 18, 1430–1441. [Google Scholar] [CrossRef]
- Kodama, M. Influenza Myocarditis. Circ. J. 2010, 74, 2060–2061. [Google Scholar] [CrossRef]
- Lalueza, A.; Folgueira, D.; Muñoz-Gallego, I.; Trujillo, H.; Laureiro, J.; Hernández-Jiménez, P.; Moral-Jiménez, N.; Castillo, C.; Ayuso, B.; Díaz-Pedroche, C.; et al. Influence of Viral Load in the Outcome of Hospitalized Patients with Influenza Virus Infection. Eur. J. Clin. Microbiol. Infect. Dis 2019, 38, 667–673. [Google Scholar] [CrossRef]
- Li, C.-C.; Wang, L.; Eng, H.-L.; You, H.-L.; Chang, L.-S.; Tang, K.-S.; Lin, Y.-J.; Kuo, H.-C.; Lee, I.-K.; Liu, J.-W.; et al. Correlation of Pandemic (H1N1) 2009 Viral Load with Disease Severity and Prolonged Viral Shedding in Children. Emerg. Infect. Dis. 2010, 16, 1265–1272. [Google Scholar] [CrossRef] [PubMed]
- Pronier, C.; Gacouin, A.; Lagathu, G.; Le Tulzo, Y.; Tadié, J.-M.; Thibault, V. Respiratory Influenza Viral Load as a Marker of Poor Prognosis in Patients with Severe Symptoms. J. Clin. Virol. 2021, 136, 104761. [Google Scholar] [CrossRef]
- De Jong, M.D.; Simmons, C.P.; Thanh, T.T.; Hien, V.M.; Smith, G.J.D.; Chau, T.N.B.; Hoang, D.M.; Van Vinh Chau, N.; Khanh, T.H.; Dong, V.C.; et al. Fatal Outcome of Human Influenza A (H5N1) Is Associated with High Viral Load and Hypercytokinemia. Nat. Med. 2006, 12, 1203–1207. [Google Scholar] [CrossRef]
- Thuy, H.T.D. Co-Infection of Parvovirus B19, CMV and BK Virus after Renal Transplantation. Int. J. Clin. Med. IJCM 2018, 9, 820–825. [Google Scholar] [CrossRef][Green Version]
- Kühl, U.; Pauschinger, M.; Noutsias, M.; Seeberg, B.; Bock, T.; Lassner, D.; Poller, W.; Kandolf, R.; Schultheiss, H.-P. High Prevalence of Viral Genomes and Multiple Viral Infections in the Myocardium of Adults With “Idiopathic” Left Ventricular Dysfunction. Circulation 2005, 111, 887–893. [Google Scholar] [CrossRef] [PubMed]
- Karrasch, M.; Felber, J.; Keller, P.M.; Kletta, C.; Egerer, R.; Bohnert, J.; Hermann, B.; Pfister, W.; Theis, B.; Petersen, I.; et al. Primary Epstein-Barr Virus Infection and Probable Parvovirus B19 Reactivation Resulting in Fulminant Hepatitis and Fulfilling Five of Eight Criteria for Hemophagocytic Lymphohistiocytosis. Int. J. Infect. Dis. 2014, 28, 143–146. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Shehi, E.; Ghazanfar, H.; Fortuzi, K.; Gonzalez, E.; Zeana, C. A Rare Case of Parvovirus B19 Infection Manifesting as Chronic Aplastic Anemia and Neutropenia in a Human Immunodeficiency Virus-Infected Patient. Cureus 2020, 12, e12174. [Google Scholar] [CrossRef]
- Krell, S.; Adams, I.; Arnold, U.; Kalinski, T.; Aumann, V.; König, W.; König, B. Influenza B Pneumonia with Staphylococcus Aureus Superinfection Associated with Parvovirus B19 and Concomitant Agranulocytosis. Infection 2003, 31, 353–358. [Google Scholar] [CrossRef]
- Escobar-Sevilla, J.; Bustos Merlo, A.; Garcia Martínez, C.; Mediavilla Garcia, J.D. Severe Refractory Anaemia and Fever of Unknow Origin: Human Parvovirus B19 Reactivation. Eur. J. Case Rep. Intern. Med. 2020, 7, 001596. [Google Scholar] [CrossRef] [PubMed]
- Short, K.R.; Veeris, R.; Leijten, L.M.; van den Brand, J.M.; Jong, V.L.; Stittelaar, K.; Osterhaus, A.D.M.E.; Andeweg, A.; van Riel, D. Proinflammatory Cytokine Responses in Extra-Respiratory Tissues During Severe Influenza. J. Infect. Dis 2017, 216, 829–833. [Google Scholar] [CrossRef] [PubMed]
- Gautam, S.; Cohen, A.J.; Stahl, Y.; Valda Toro, P.; Young, G.M.; Datta, R.; Yan, X.; Ristic, N.T.; Bermejo, S.D.; Sharma, L.; et al. Severe Respiratory Viral Infection Induces Procalcitonin in the Absence of Bacterial Pneumonia. Thorax 2020, 75, 974. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Callon, D.; Berri, F.; Lebreil, A.-L.; Fornès, P.; Andreoletti, L. Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report. Pathogens 2021, 10, 958. https://doi.org/10.3390/pathogens10080958
Callon D, Berri F, Lebreil A-L, Fornès P, Andreoletti L. Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report. Pathogens. 2021; 10(8):958. https://doi.org/10.3390/pathogens10080958
Chicago/Turabian StyleCallon, Domitille, Fatma Berri, Anne-Laure Lebreil, Paul Fornès, and Laurent Andreoletti. 2021. "Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report" Pathogens 10, no. 8: 958. https://doi.org/10.3390/pathogens10080958
APA StyleCallon, D., Berri, F., Lebreil, A.-L., Fornès, P., & Andreoletti, L. (2021). Coinfection of Parvovirus B19 with Influenza A/H1N1 Causes Fulminant Myocarditis and Pneumonia. An Autopsy Case Report. Pathogens, 10(8), 958. https://doi.org/10.3390/pathogens10080958





