Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus
Abstract
1. Introduction
2. Materials and Methods
2.1. Human Subjects
2.2. Control Subjects
2.3. CF Patients
2.4. PMN and Serum Isolation
2.5. Sputum Collection and Processing
2.6. In vitro “CF Sputum Model”
2.7. Bacteria
2.8. Cell Viability
2.9. High Throughput Bacterial Killing Assay
2.10. Attachment and Phagocytosis
2.11. NADPH Oxidase Activity Measurements
2.12. NET Release
2.13. DNAse Activity Measurement
2.14. Apoptosis
2.15. Statistical Analysis
3. Results
3.1. In vitro Model of CF Airway-Like Conditions
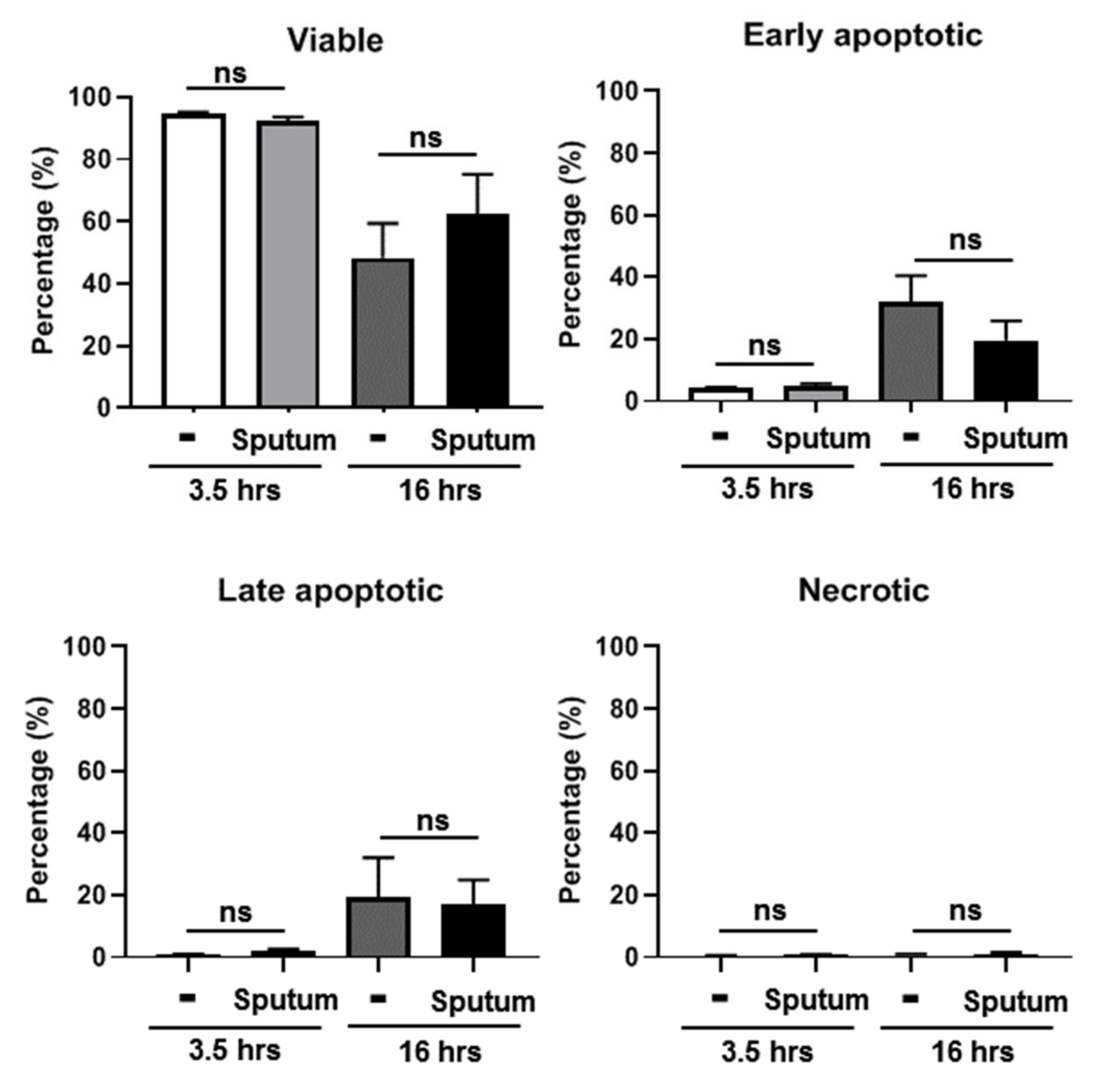
3.2. CF Sputum Exposure Does Not Impair PMN Viability
3.3. CF Sputum Does Not Induce Apoptosis in Human PMNs
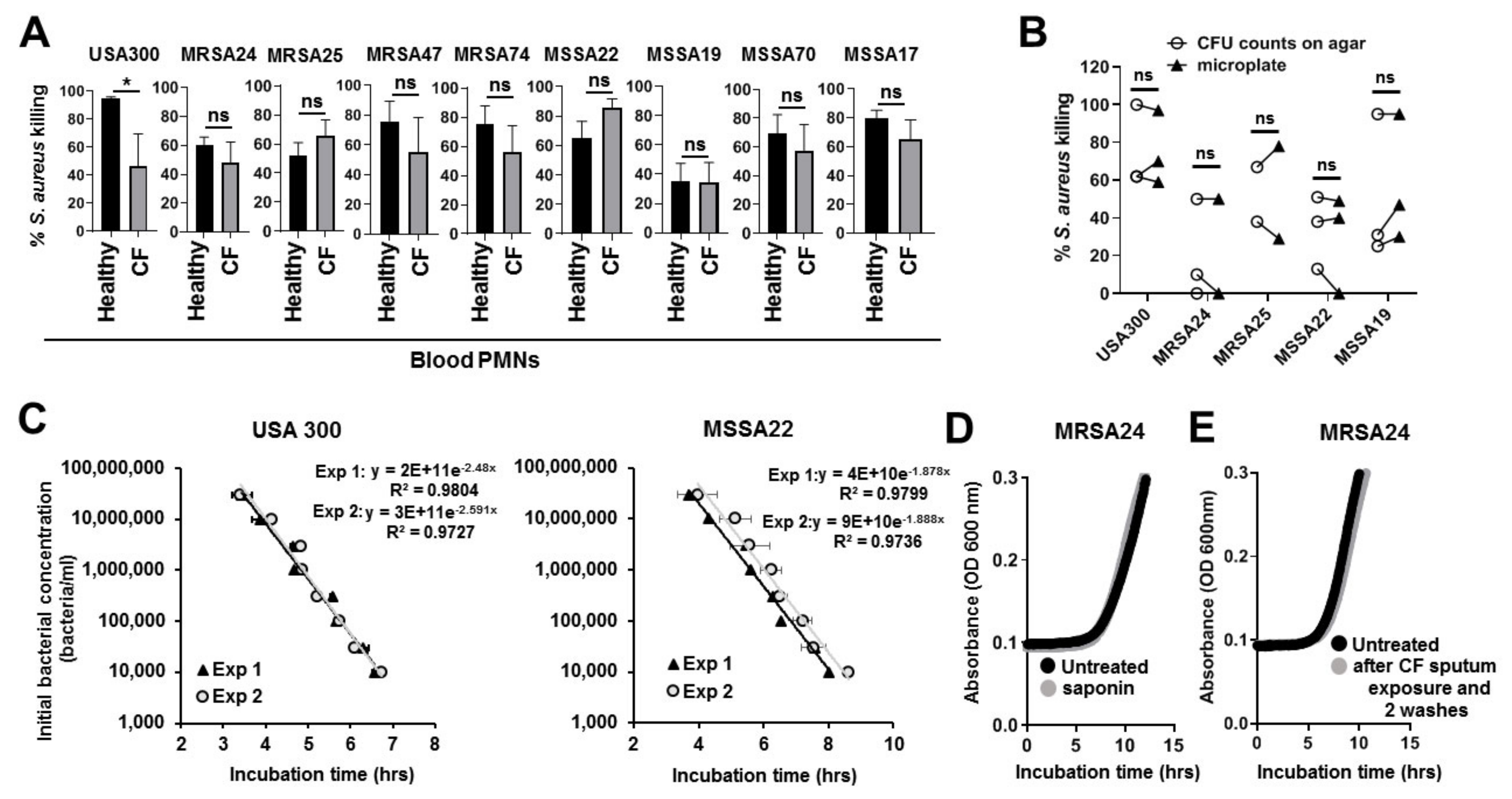
3.4. CF Isolates of S. aureus Are Killed by Human PMNs
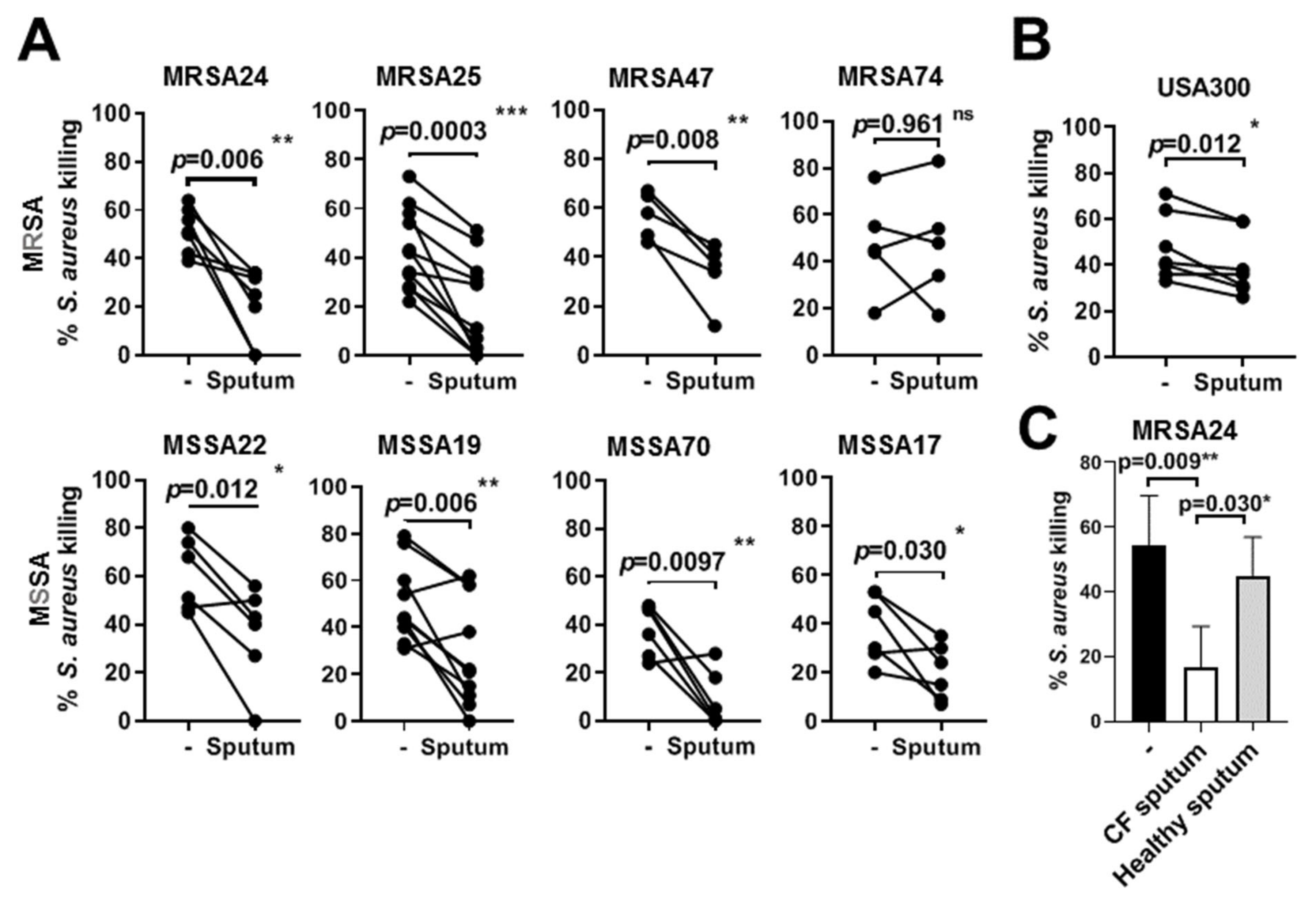
3.5. CF Sputum Compromises the Killing of S. aureus Clinical Isolates by PMNs
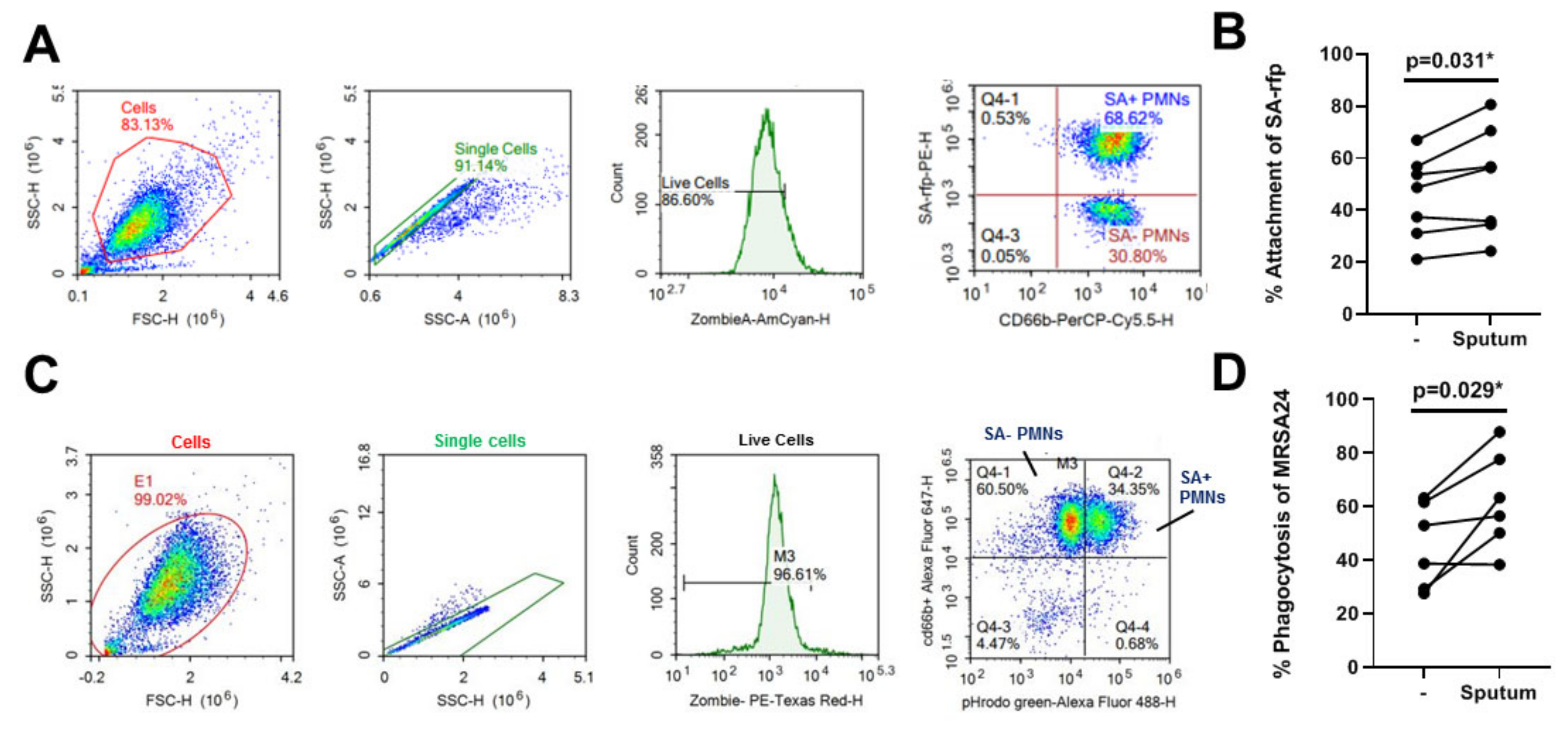
3.6. CF Sputum Does Not Inhibit PMN Attachment and Phagocytosis of S. aureus
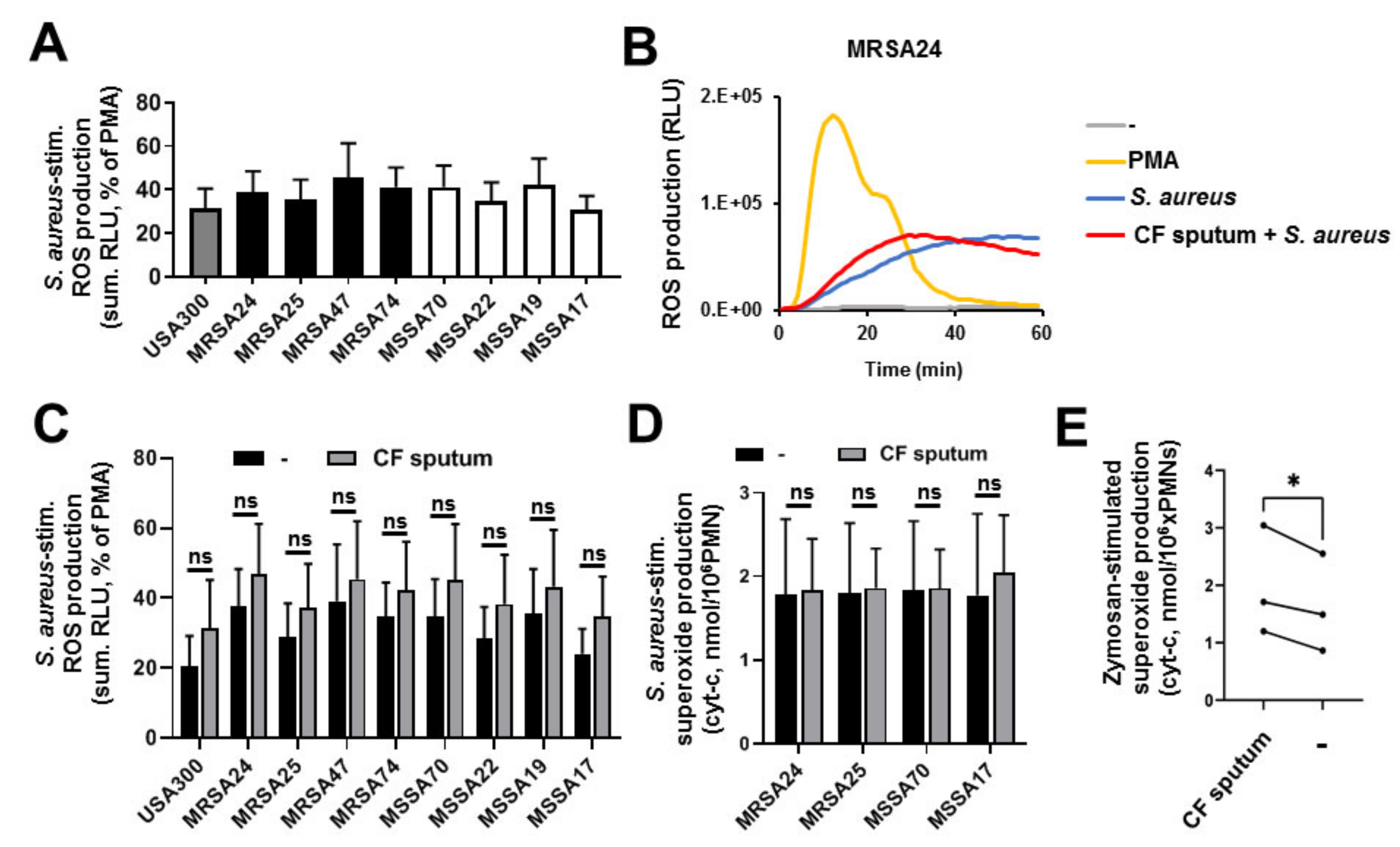
3.7. CF Sputum Does Not Inhibit PMN Superoxide Production in Response to S. aureus
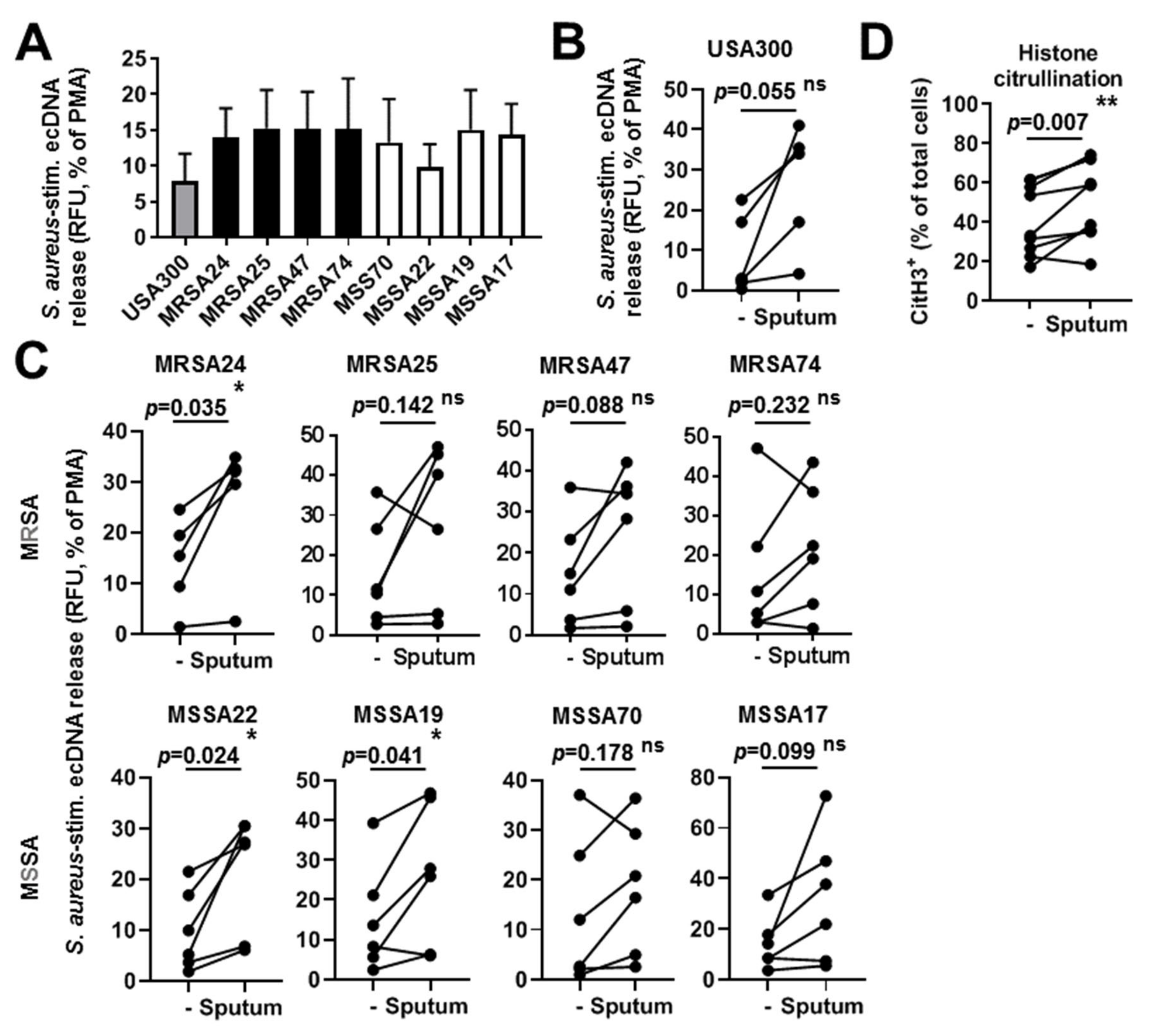
3.8. CF Sputum Does Not Inhibit NET Release of PMNs in Response to S. aureus
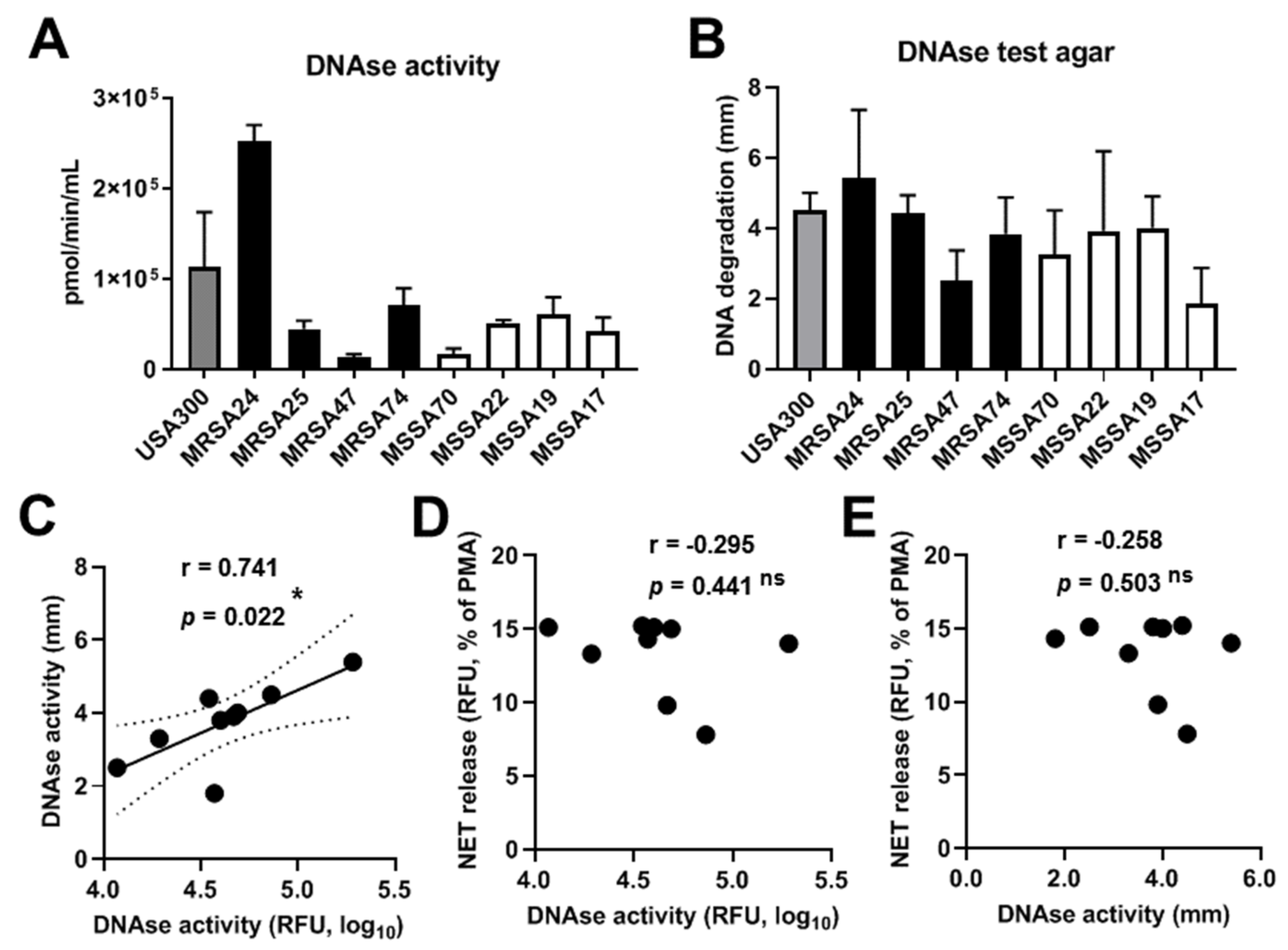
3.9. S. aureus CF Clinical Isolates Possess DNAse I Activity
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PMN | polymorphonuclear neutrophil granulocyte |
| CF | cystic fibrosis |
| NET | neutrophil extracellular traps |
| MOI | multiplicity of infection |
| MRSA | methicillin-resistant S. aureus |
| MSSA | methicillin-sensitive S. aureus |
References
- Ahlgren, H.G.; Benedetti, A.; Landry, J.S.; Bernier, J.; Matouk, E.; Radzioch, D.; Lands, L.C.; Rousseau, S.; Nguyen, D. Clinical outcomes associated with Staphylococcus aureus and Pseudomonas aeruginosa airway infections in adult cystic fibrosis patients. BMC Pulm. Med. 2015, 15, 67. [Google Scholar] [CrossRef] [PubMed]
- Cystic Fibrosis Foundation Patient Registry. 2017 Annual Data Report; Cystic Fibrosis Foundation Patient Registry: Bethesda, MD, USA, 2018. [Google Scholar]
- Akil, N.; Muhlebach, M.S. Biology and management of methicillin resistant Staphylococcus aureusin cystic fibrosis. Pediatr. Pulmonol. 2018, 53, S64–S74. [Google Scholar] [CrossRef] [PubMed]
- Lo, D.K.; Muhlebach, M.S.; Smyth, A.R. Interventions for the eradication of meticillin-resistant Staphylococcus aureus (MRSA) in people with cystic fibrosis. Cochrane Database Syst. Rev. 2018, 7, CD009650. [Google Scholar] [CrossRef] [PubMed]
- Muhlebach, M.S.; Zorn, B.T.; Esther, C.R.; Hatch, J.E.; Murray, C.P.; Turkovic, L.; Ranganathan, S.C.; Boucher, R.C.; Stick, S.M.; Wolfgang, M.C. Initial acquisition and succession of the cystic fibrosis lung microbiome is associated with disease progression in infants and preschool children. PLoS Pathog. 2018, 14, e1006798. [Google Scholar] [CrossRef] [PubMed]
- Dasenbrook, E.C.; Checkley, W.; Merlo, C.A.; Konstan, M.W.; Lechtzin, N.; Boyle, M.P. Association between respiratory tract methicillin-resistant Staphylococcus aureus and survival in cystic fibrosis. JAMA J. Am. Med. Assoc. 2010, 303, 2386–2392. [Google Scholar] [CrossRef] [PubMed]
- Ren, C.L.; Morgan, W.J.; Konstan, M.W.; Schechter, M.S.; Wagener, J.S.; Fisher, K.A.; Regelmann, W.E.; for The Investigators and Coordinators of the Epidemiologic Study of Cystic Fibrosis. Presence of methicillin resistantStaphylococcus aureus in respiratory cultures from cystic fibrosis patients is associated with lower lung function. Pediatr. Pulmonol. 2007, 42, 513–518. [Google Scholar] [CrossRef] [PubMed]
- Takei, H.; Araki, A.; Watanabe, H.; Ichinose, A.; Sendo, F. Rapid killing of human neutrophils by the potent activator phorbol 12-myristate 13-acetate (PMA) accompanied by changes different from typical apoptosis or necrosis. J. Leukoc. Biol. 1996, 59, 229–240. [Google Scholar] [CrossRef]
- Brinkmann, V.; Reichard, U.; Goosmann, C.; Fauler, B.; Uhlemann, Y.; Weiss, D.S.; Weinrauch, Y.; Zychlinsky, A. Neutrophil Extracellular Traps Kill Bacteria. Science 2004, 303, 1532–1535. [Google Scholar] [CrossRef]
- De Jong, N.W.M.; Van Kessel, K.P.M.; Van Strijp, J.A.G. Immune Evasion by Staphylococcus aureus. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Nasser, A.; Moradi, M.; Jazireian, P.; Safari, H.; Alizadeh-Sani, M.; Pourmand, M.R.; Azimi, T. Staphylococcus aureus versus neutrophil: Scrutiny of ancient combat. Microb. Pathog. 2019, 131, 259–269. [Google Scholar] [CrossRef]
- Liu, Q.; Mazhar, M.; Miller, L.S. Immune and Inflammatory Reponses to Staphylococcus aureus Skin Infections. Curr. Dermatol. Rep. 2018, 7, 338–349. [Google Scholar] [CrossRef]
- Harrison, C.J. Innate immunity as a key element in host defense against methicillin resistant Staphylococcus aureus. Minerva Pediatr. 2009, 61, 503–514. [Google Scholar]
- Rawat, A.; Bhattad, S.; Singh, S. Chronic Granulomatous Disease. Indian J. Pediatr. 2016, 83, 345–353. [Google Scholar] [CrossRef]
- Kahl, B.C.; Becker, K.; Löffler, B. Clinical significance and pathogenesis of staphylococcal small colony variants in persistent infections. Clin. Microbiol. Rev. 2016, 29, 401–427. [Google Scholar] [CrossRef]
- Tirouvanziam, R.; Gernez, Y.; Conrad, C.K.; Moss, R.B.; Schrijver, I.; Dunn, C.E.; Davies, Z.A.; Herzenberg, L.A. Profound functional and signaling changes in viable inflammatory neutrophils homing to cystic fibrosis airways. Proc. Natl. Acad. Sci. USA 2008, 105, 4335–4339. [Google Scholar] [CrossRef]
- Highlander, S.K.; Hultén, K.G.; Qin, X.; Jiang, H.; Yerrapragada, S.; O Mason, E.; Shang, Y.; Williams, T.M.; Fortunov, R.M.; Liu, Y.; et al. Subtle genetic changes enhance virulence of methicillin resistant and sensitive Staphylococcus aureus. BMC Microbiol. 2007, 7, 99. [Google Scholar] [CrossRef]
- Monecke, S.; Coombs, G.; Shore, A.C.; Coleman, D.C.; Akpaka, P.E.; Borg, M.; Chow, H.; Ip, M.; Jatzwauk, L.; Jonas, D.; et al. A field guide to pandemic, epidemic and sporadic clones of methicillin-resistant staphylococcus aureus. PLoS ONE 2011, 6, e17936. [Google Scholar] [CrossRef]
- Ibberson, C.B.; Parlet, C.P.; Kwiecinski, J.; Crosby, H.A.; Meyerholz, D.; Horswill, A.R. Hyaluronan modulation impacts staphylococcus aureus biofilm infection. Infect. Immun. 2016, 84, 1917–1929. [Google Scholar] [CrossRef]
- Nair, D.; Memmi, G.; Hernandez, D.; Bard, J.; Beaume, M.; Gill, S.; Francois, P.; Cheung, A.L. Whole-genome sequencing of staphylococcus aureus strain rn4220, a key laboratory strain used in virulence research, identifies mutations that affect not only virulence factors but also the fitness of the strain. J. Bacteriol. 2011, 193, 2332–2335. [Google Scholar] [CrossRef] [PubMed]
- Löfblom, J.; Kronqvist, N.; Uhlen, M.; Stahl, S.; Wernerus, H. Optimization of electroporation-mediated transformation: Staphylococcus carnosus as model organism. J. Appl. Microbiol. 2007, 102, 736–747. [Google Scholar] [CrossRef] [PubMed]
- Bernardy, E.E.; Petit, R.A.; Raghuram, V.; Alexander, A.M.; Read, T.D.; Goldberg, J.B. Genotypic and phenotypic diversity of staphylococcus aureus isolates from cystic fibrosis patient lung infections and their interactions with pseudomonas aeruginosa. mBio 2020, 11, e00735-20. [Google Scholar] [CrossRef] [PubMed]
- Yoo, D.-G.; Winn, M.; Pang, L.; Moskowitz, S.M.; Malech, H.L.; Leto, T.L.; Rada, B. Release of Cystic Fibrosis Airway Inflammatory Markers fromPseudomonas aeruginosa–Stimulated Human Neutrophils Involves NADPH Oxidase-Dependent Extracellular DNA Trap Formation. J. Immunol. 2014, 192, 4728–4738. [Google Scholar] [CrossRef] [PubMed]
- Rada, B.K.; Geiszt, M.; Káldi, K.; Timár, C.; Ligeti, E. Dual role of phagocytic NADPH oxidase in bacterial killing. Blood 2004, 104, 2947–2953. [Google Scholar] [CrossRef] [PubMed]
- Pang, L.; Hayes, C.P.; Buac, K.; Yoo, D.-G.; Rada, B. Pseudogout-Associated Inflammatory Calcium Pyrophosphate Dihydrate Microcrystals Induce Formation of Neutrophil Extracellular Traps. J. Immunol. 2013, 190, 6488–6500. [Google Scholar] [CrossRef]
- Rada, B.; Jendrysik, M.A.; Pang, L.; Hayes, C.P.; Yoo, D.-G.; Park, J.J.; Moskowitz, S.M.; Malech, H.L.; Leto, T.L. Pyocyanin-Enhanced Neutrophil Extracellular Trap Formation Requires the NADPH Oxidase. PLoS ONE 2013, 8, e54205. [Google Scholar] [CrossRef]
- Yoo, D.-G.; Floyd, M.; Winn, M.; Moskowitz, S.M.; Rada, B. NET formation induced by Pseudomonas aeruginosa cystic fibrosis isolates measured as release of myeloperoxidase–DNA and neutrophil elastase–DNA complexes. Immunol. Lett. 2014, 160, 186–194. [Google Scholar] [CrossRef]
- Femling, J.K.; Cherny, V.V.; Morgan, D.; Rada, B.; Davis, A.P.; Czirják, G.; Enyedi, P.; England, S.K.; Moreland, J.G.; Ligeti, E.; et al. The antibacterial activity of human neutrophils and eosinophils requires proton channels but not bk channels. J. Gen. Physiol. 2006, 127, 659–672. [Google Scholar] [CrossRef]
- Sil, P.; Yoo, D.-G.; Floyd, M.; Gingerich, A.; Rada, B. High throughput measurement of extracellular dna release and quantitative net formation in human neutrophils in vitro. J. Vis. Exp. 2016, e52779. [Google Scholar] [CrossRef]
- Bernardy, E.E.; Petit, R.A.; Moller, A.G.; Blumenthal, J.A.; McAdam, A.J.; Priebe, G.P.; Chande, A.T.; Rishishwar, L.; Jordan, I.K.; Read, T.D.; et al. Whole-Genome Sequences of Staphylococcus aureus Isolates from Cystic Fibrosis Lung Infections. Microbiol. Resour. Announc. 2019, 8, e01564-18. [Google Scholar] [CrossRef]
- Buvelot, H.; Posfay-Barbe, K.M.; Linder, P.; Schrenzel, J.; Krause, K.-H. Staphylococcus aureus, phagocyte NADPH oxidase and chronic granulomatous disease. FEMS Microbiol. Rev. 2016, 41, 139–157. [Google Scholar] [CrossRef]
- Martínez-Alemán, S.R.; Campos-García, L.; Palma-Nicolas, J.P.; Hernández-Bello, R.; González, G.M.; Sánchez-González, A. Understanding the entanglement: Neutrophil extracellular traps (nets) in cystic fibrosis. Front. Cell. Infect. Microbiol. 2017, 7, 104. [Google Scholar] [CrossRef]
- Herzog, S.; Dach, F.; De Buhr, N.; Niemann, S.; Schlagowski, J.; Chaves-Moreno, D.; Neumann, C.; Goretzko, J.; Schwierzeck, V.; Mellmann, A.; et al. High Nuclease Activity of Long Persisting Staphylococcus aureus Isolates Within the Airways of Cystic Fibrosis Patients Protects Against NET-Mediated Killing. Front. Immunol. 2019, 10, 2552. [Google Scholar] [CrossRef]
- Cystic Fibrosis Foundation Patient Registry. 2015 Annual Data Report; Cystic Fibrosis Foundation Patient Registry: Bethesda, MD, USA, 2016. [Google Scholar]
- Pattison, S.H.; Gibson, D.S.; Johnston, E.; Peacock, S.; Rivera, K.; Tunney, M.; Pappin, D.J.; Elborn, J.S. Proteomic profile of cystic fibrosis sputum cells in adults chronically infected with Pseudomonas aeruginosa. Eur. Respir. J. 2017, 50, 1601569. [Google Scholar] [CrossRef]
- Jia, S.H.; Li, Y.; Parodo, J.; Kapus, A.; Fan, L.; Rotstein, O.D.; Marshall, J.C. Pre–B cell colony–enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J. Clin. Investig. 2004, 113, 1318–1327. [Google Scholar] [CrossRef]
- Scannell, M.; Flanagan, M.B.; Destefani, A.; Wynne, K.; Cagney, G.; Godson, C.; Maderna, P. Annexin-1 and Peptide Derivatives Are Released by Apoptotic Cells and Stimulate Phagocytosis of Apoptotic Neutrophils by Macrophages. J. Immunol. 2007, 178, 4595–4605. [Google Scholar] [CrossRef]
- Gueders, M.M.; Balbin, M.; Rocks, N.; Foidart, J.-M.; Gosset, P.; Louis, R.; Shapiro, S.; Lopez-Otin, C.; Noël, A.; Cataldo, D.D. Matrix Metalloproteinase-8 Deficiency Promotes Granulocytic Allergen-Induced Airway Inflammation. J. Immunol. 2005, 175, 2589–2597. [Google Scholar] [CrossRef]
- Sloane, A.J.; Lindner, R.A.; Prasad, S.S.; Sebastian, L.T.; Pedersen, S.K.; Robinson, M.; Bye, P.T.; Nielson, D.W.; Harry, J.L. Proteomic analysis of sputum from adults and children with cystic fibrosis and from control subjects. Am. J. Respir. Crit. Care Med. 2005, 172, 1416–1426. [Google Scholar] [CrossRef]
- Castellani, S.; Di Gioia, S.; Di Toma, L.; Conese, M. Human Cellular Models for the Investigation of Lung Inflammation and mucus production in cystic fibrosis. Anal. Cell. Pathol. 2018, 2018, 3839803. [Google Scholar] [CrossRef]
- Painter, R.G.; Valentine, V.G.; Lanson, N.A.; Leidal, K.; Zhang, Q.; Lombard, G.; Thompson, C.; Viswanathan, A.; Nauseef, W.M.; Wang, G.; et al. CFTR expression in human neutrophils and the phagolysosomal chlorination defect in cystic fibrosis. Biochemistry 2006, 45, 10260–10269. [Google Scholar] [CrossRef]
- Dickerhof, N.; Isles, V.; Pattemore, P.; Hampton, M.B.; Kettle, A.J. Exposure of Pseudomonas aeruginosa to bactericidal hypochlorous acid during neutrophil phagocytosis is compromised in cystic fibrosis. J. Biol. Chem. 2019, 294, 13502–13514. [Google Scholar] [CrossRef]
- Painter, R.G.; Bonvillain, R.W.; Valentine, V.G.; Lombard, G.A.; LaPlace, S.G.; Nauseef, W.M.; Wang, G. The role of chloride anion and CFTR in killing of Pseudomonas aeruginosa by normal and CF neutrophils. J. Leukoc. Biol. 2008, 83, 1345–1353. [Google Scholar] [CrossRef] [PubMed]
- Hampton, M.B.; Kettle, A.J.; Winterbourn, C.C. Involvement of superoxide and myeloperoxidase in oxygen-dependent killing of Staphylococcus aureus by neutrophils. Infect. Immun. 1996, 64, 3512–3517. [Google Scholar] [CrossRef] [PubMed]
- Morris, M.R.; Doull, I.J.M.; Dewitt, S.; Hallett, M.B. Reduced iC3b-mediated phagocytotic capacity of pulmonary neutrophils in cystic fibrosis. Clin. Exp. Immunol. 2005, 142, 68–75. [Google Scholar] [CrossRef] [PubMed]
- Forrest, O.A.; Ingersoll, S.A.; Preininger, M.K.; Laval, J.; Limoli, D.H.; Brown, M.R.; Lee, F.E.; Bedi, B.; Sadikot, R.T.; Goldberg, J.B.; et al. Frontline Science: Pathological conditioning of human neutrophils recruited to the airway milieu in cystic fibrosis. J. Leukoc. Biol. 2018, 104, 665–675. [Google Scholar] [CrossRef]
- Houston, N.; Stewart, N.; Smith, D.S.; Bell, S.; Champion, A.C.; Reid, D. Sputum neutrophils in cystic fibrosis patients display a reduced respiratory burst. J. Cyst. Fibros. 2013, 12, 352–362. [Google Scholar] [CrossRef]
- Mittal, M.; Siddiqui, M.R.; Tran, K.; Reddy, S.P.; Malik, A.B. Reactive Oxygen Species in Inflammation and Tissue Injury. Antioxid. Redox Signal. 2014, 20, 1126–1167. [Google Scholar] [CrossRef]
- Guerra, F.E.; Addison, C.B.; De Jong, N.W.M.; Azzolino, J.; Pallister, K.B.; Van Strijp, J. Staphylococcus aureus SaeR/S-regulated factors reduce human neutrophil reactive oxygen species production. J. Leukoc. Biol. 2016, 100, 1005–1010. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Allen, R.C.; Paulais, M.; Nguyen, A.T.; Bessou, G.; Lenoir, G.; Descamps-Latscha, B. Disturbed Myeloperoxidase-Dependent Activity of Neutrophils in Cystic Fibrosis Homozygotes and Heterozygotes, and Its Correction by Amiloride. J. Immunol. 1996, 157, 2728–2735. [Google Scholar]
- Ibberson, C.B.; Whiteley, M. TheStaphylococcus aureustranscriptome during cystic fibrosis lung infection. mBio 2019, 10, e01990-15. [Google Scholar] [CrossRef]
- Lopatkin, A.J.; Stokes, J.M.; Zheng, E.J.; Yang, J.H.; Takahashi, M.K.; You, L.; Collins, J.J. Bacterial metabolic state more accurately predicts antibiotic lethality than growth rate. Nat. Microbiol. 2019, 4, 2109–2117. [Google Scholar] [CrossRef]
- Beiter, K.; Wartha, F.; Albiger, B.; Normark, S.; Zychlinsky, A.; Henriques-Normark, B. an endonuclease allows streptococcus pneumoniae to escape from neutrophil extracellular traps. Curr. Biol. 2006, 16, 401–407. [Google Scholar] [CrossRef]
- Walker, M.J.; Hollands, A.; Sanderson-Smith, M.L.; Cole, J.N.; Kirk, J.K.; Henningham, A.; McArthur, J.D.; Dinkla, K.; Aziz, R.; Kansal, R.G.; et al. DNase Sda1 provides selection pressure for a switch to invasive group A streptococcal infection. Nat. Med. 2007, 13, 981–985. [Google Scholar] [CrossRef]
- Buchanan, J.T.; Simpson, A.J.; Aziz, R.; Liu, G.Y.; Kristian, S.A.; Kotb, M.; Feramisco, J.; Nizet, V. DNase expression allows the pathogen group a streptococcus to escape killing in neutrophil extracellular traps. Curr. Biol. 2006, 16, 396–400. [Google Scholar] [CrossRef]
- Pressler, T. Review of recombinant human deoxyribonuclease (rhDNase) in the management of patients with cystic fibrosis. Biol. Targets Ther. 2008, 2, 611–617. [Google Scholar] [CrossRef]
- Jang, S. Multidrug efflux pumps in Staphylococcus aureus and their clinical implications. J. Microbiol. 2016, 54, 1–8. [Google Scholar] [CrossRef]
- Kaatz, G.W.; McAleese, F.; Seo, S.M.; Petersen, P.; Ruzin, A.; Dunman, P.M.; Murphy, E.; Projan, S.J.; Bradford, P.A. Multidrug resistance in staphylococcus aureus due to overexpression of a novel multidrug and toxin extrusion (mate) transport protein. Antimicrob. Agents Chemother. 2005, 49, 1865–1871. [Google Scholar] [CrossRef]
- Marcos, V.; Zhou-Suckow, Z.; Yildirim, A.; Önder Yildirim, A.; Bohla, A.; Hector, A.; Vitkov, L.; Krautgartner, W.D.; Stoiber, W.; Griese, M.; et al. Free DNA in cystic fibrosis airway fluids correlates with airflow obstruction. Mediat. Inflamm. 2015, 2015, 408935. [Google Scholar] [CrossRef]
- Pincus, S.H.; Klebanoff, S.J. Quantitative Leukocyte Iodination. N. Engl. J. Med. 1971, 284, 744–750. [Google Scholar] [CrossRef]
- Klebanoff, S.J.; Kettle, A.J.; Rosen, H.; Winterbourn, C.C.; Nauseef, W.M. Myeloperoxidase: A front-line defender against phagocytosed microorganisms. J. Leukoc. Biol. 2013, 93, 185–198. [Google Scholar] [CrossRef]
- Chapman, A.L.P.; Hampton, M.B.; Senthilmohan, R.; Winterbourn, C.C.; Kettle, A.J. Chlorination of bacterial and neutrophil proteins during phagocytosis and killing of staphylococcus aureus. J. Biol. Chem. 2002, 277, 9757–9762. [Google Scholar] [CrossRef]
- Metzler, K.D.; Goosmann, C.; Lubojemska, A.; Zychlinsky, A.; Papayannopoulos, V. A Myeloperoxidase-containing complex regulates neutrophil elastase release and actin dynamics during NETosis. Cell Rep. 2014, 8, 883–896. [Google Scholar] [CrossRef]
- Branzk, N.; Lubojemska, A.; Hardison, S.E.; Wang, Q.; Gutierrez, M.G.; Brown, G.D.; Papayannopoulos, V. Neutrophils sense microbe size and selectively release neutrophil extracellular traps in response to large pathogens. Nat. Immunol. 2014, 15, 1017–1025. [Google Scholar] [CrossRef] [PubMed]
- Voyich, J.M.; Braughton, K.R.; Sturdevant, D.E.; Whitney, A.R.; Saïd-Salim, B.; Porcella, S.F.; Long, R.D.; Dorward, D.W.; Gardner, D.J.; Kreiswirth, B.N.; et al. Insights into Mechanisms Used byStaphylococcus aureusto Avoid Destruction by Human Neutrophils. J. Immunol. 2005, 175, 3907–3919. [Google Scholar] [CrossRef] [PubMed]
- Rogers, R.; Tompsett, R. The survival of staphylococci within human leucocytes. Bull. N. Y. Acad. Med. 1952, 95, 209–230. [Google Scholar]

| Identifier in This Work | CF Sample Name | Methicillin Sensitivity | Strains or Isolates | CF Patient ID | References |
|---|---|---|---|---|---|
| MRSA24 | CFBRSa24 | resistant | CFBR-219 | Bernardy et al. [22] | |
| MRSA25 | CFBRSa25 | resistant | CFBR-134 | ||
| MRSA47 | CFBRSa47 | resistant | Cystic | CFBR-105 | |
| MRSA74 | CFBRSa74 | resistant | Fibrosis | CFBR-201 | |
| MSSA17 | CFBR_EB_Sa117 | sensitive | clinical | CFBR-280 | |
| MSSA19 | CFBR_EB_Sa119 | sensitive | isolates | CFBR-309 | |
| MSSA22 | CFBR_EB_Sa122 | sensitive | CFBR-322 | ||
| MSSA70 | CFBRSa70 | sensitive | CFBR-171 | ||
| USA 300 | - | resistant | Reference strain | - | S. aureus subsp. aureus (ATCC BAA1717TM) |
| SA-rfp | - | - | JE2 background | - | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fantone, K.; Tucker, S.L.; Miller, A.; Yadav, R.; Bernardy, E.E.; Fricker, R.; Stecenko, A.A.; Goldberg, J.B.; Rada, B. Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus. Pathogens 2021, 10, 703. https://doi.org/10.3390/pathogens10060703
Fantone K, Tucker SL, Miller A, Yadav R, Bernardy EE, Fricker R, Stecenko AA, Goldberg JB, Rada B. Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus. Pathogens. 2021; 10(6):703. https://doi.org/10.3390/pathogens10060703
Chicago/Turabian StyleFantone, Kayla, Samantha L. Tucker, Arthur Miller, Ruchi Yadav, Eryn E. Bernardy, Rachel Fricker, Arlene A. Stecenko, Joanna B. Goldberg, and Balázs Rada. 2021. "Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus" Pathogens 10, no. 6: 703. https://doi.org/10.3390/pathogens10060703
APA StyleFantone, K., Tucker, S. L., Miller, A., Yadav, R., Bernardy, E. E., Fricker, R., Stecenko, A. A., Goldberg, J. B., & Rada, B. (2021). Cystic Fibrosis Sputum Impairs the Ability of Neutrophils to Kill Staphylococcus aureus. Pathogens, 10(6), 703. https://doi.org/10.3390/pathogens10060703






