Fire Ant Venom Alkaloids: Possible Control Measure for Soilborne and Foliar Plant Pathogens
Abstract
1. Introduction
2. Results
2.1. Test of Venom Alkaloids and Alarm Pheromone against Plant Fungal and Oomycete Pathogens
2.2. Test of Venom Alkaloids and Alarm Pheromone against Plant Bacterial Pathogens
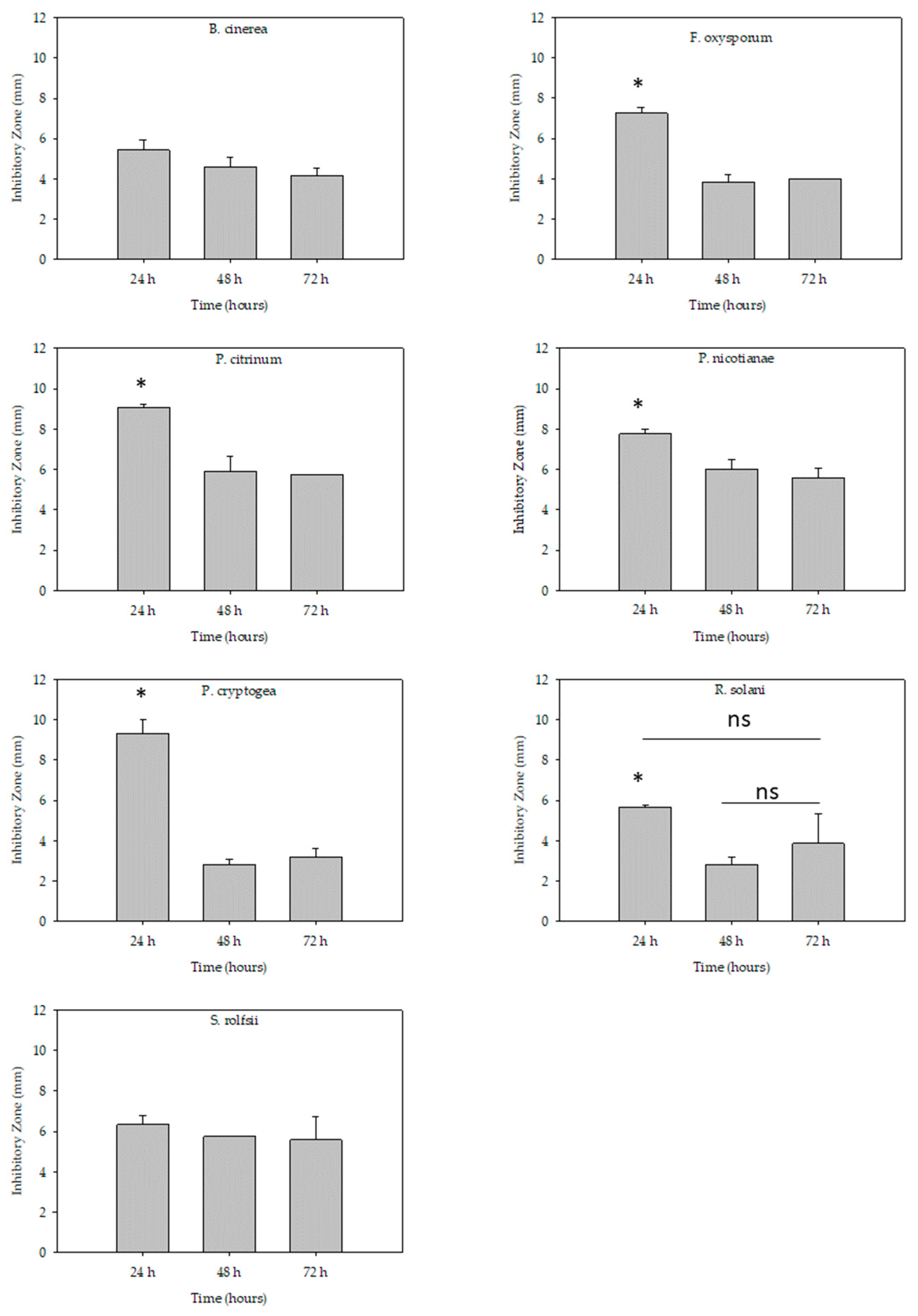
2.3. Test of Stability Effect of Venom Alkaloids against Plant Fungal and Oomycete Pathogens
2.4. Greenhouse Bioassay against Soilborne Plant Pathogens
2.4.1. Rhizoctonia solani
2.4.2. Fusarium oxysporum
2.4.3. Phytophthora nicotianae
2.4.4. Botrytis cinerea
3. Discussion
4. Materials and Methods
4.1. Fire Ant Collection, Venom Alkaloids Extraction
4.2. Fungal, Oomycetes, and Bacterial Culture Preparation
4.3. Test of Venom Alkaloids and Alarm Pheromone against Plant Fungal and Oomycete Pathogens
4.4. Test of Stability Effect of Venom Alkaloids against Fungal Pathogens and Oomycete Pathogens
4.5. Test of Venom Alkaloids and Alarm Pheromone against Bacterial Pathogens
4.6. Greenhouse Bioassays
4.6.1. Soilborne Pathogens
4.6.2. Foliar Pathogen
4.7. Evaluation of Crop Health
4.7.1. Soilborne Pathogens
4.7.2. Foliar Pathogen
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Andrivon, D.; de Vallavieille-Pope, C. Race diversity and complexity in selected populations of fungal biotrophic pathogens of cereals. Phytopathology 1995, 85, 897–905. [Google Scholar] [CrossRef]
- Chupp, C.; Sherf, A.F. Vegetable Diseases and Their Control, 2nd ed.; Ronald Press: New York, NY, USA, 1960. [Google Scholar]
- Dawadi, S.; Baysal-Gurel, F.; Addesso, K.M.; Oliver, J.B.; Simmons, T. Impact of Cover Crop Usage on Soilborne Diseases in Field Nursery Production. Agronomy 2019, 9, 753. [Google Scholar] [CrossRef]
- Benson, D.M.; Cartwright, D.K. Ornamental diseases incited by Rhizoctonia spp. In Rhizoctonia Species: Taxonomy, Molecular Biology, Ecology, Pathology and Disease Control; Sneh, B., Jabaji-Hare, S., Neate, S.M., Dijst, G., Eds.; Springer: Dordrecht, The Netherlands, 1996; pp. 303–314. [Google Scholar]
- Erwin, D.C.; Ribeiro, O.K. Phytophthora Diseases Worldwide; American Phytopathological Society: St. Paul, MN, USA, 1996. [Google Scholar]
- IPM. Pest Management Strategic Plan for Container and Field-Produced Nursery Crops. 2009. Available online: http://www.ipmcenters.org/pmsp/pdf/GA-KY-NC-SCTNnurserycropsPMSP.pdf (accessed on 7 December 2019).
- Mihajlović, M.; Rekanović, E.; Hrustić, J.; Tanović, B. Methods for management of soilborne plant pathogens. Pestic. Fitomedicina 2017, 32, 9–24. [Google Scholar] [CrossRef]
- Cline, E.T.; Farr, D.F.; Rossman, A.Y. A synopsis of Phytophthora with accurate scientific names, host range, and geographic distribution. Plant Health Prog. 2008, 9, 32. [Google Scholar] [CrossRef]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef]
- Bika, R.; Baysal-Gurel, F.; Jennings, C. Botrytis cinerea management in ornamental production: A continuous battle. Can. J Plant Pathol. 2020, 1–21. [Google Scholar] [CrossRef]
- Hildebrand, P.D. Dispersal of plant pathogens. In Encyclopedia of Pest Management; Pimental, D., Ed.; Marcel Dekker: Ithaca, NY, USA, 2002; pp. 193–194. [Google Scholar]
- Ellis, S.D.; Boehm, M.J.; Coplin, D. Bacterial diseases of plants. Agric. Nat. Resour. 2008, 401–406. [Google Scholar]
- Parry, D.W. Plant Pathology in Agriculture; Cambridge University Press: Cambridge, UK, 1990. [Google Scholar]
- Baysal-Gurel, F.; Gardener, B.M.; Miller, S.A. Soilborne Disease Management in Organic Vegetable Production. 2012. Available online: https://eorganic.org/node/7581 (accessed on 6 January 2020).
- Panth, M.; Hassler, S.C.; Baysal-Gurel, F. Methods for Management of Soilborne Diseases in Crop Production. Agriculture 2020, 10, 16. [Google Scholar] [CrossRef]
- Triky-Dotan, S.; Westerdahl, B.; Martin, F.N.; Subbarao, K.; Koike, S.T.; Ajwa, H.A. Fumigant dosages below maximum label rate control some soilborne pathogens. Calif. Agric. 2016, 70, 130–136. [Google Scholar] [CrossRef]
- Baysal-Gurel, F.; Liyanapathiranage, P.; Mullican, J. Biofumigation: Opportunities and challenges for control of soilborne diseases in nursery production. Plant Health Prog. 2018, 19, 332–337. [Google Scholar] [CrossRef]
- Huber, D.M.; Haneklaus, S. Managing nutrition to control plant disease. Landbauforsch. Volkenrode 2007, 57, 313–322. [Google Scholar]
- Shafique, H.A.; Sultana, V.; Ehteshamul-Haque, S.; Athar, M. Management of soil-borne diseases of organic vegetables. J. Plant Prot. Res. 2016, 56, 221–230. [Google Scholar] [CrossRef]
- Vallance, J.; Déniel, F.; Le Floch, G.; Guérin-Dubrana, L.; Blancard, D.; Rey, P. Pathogenic and beneficial microorganisms in soilless cultures. Sustain. Agric. 2011, 2, 711–726. [Google Scholar]
- Fokkema, N.J. Opportunities and problems of control of foliar pathogens with micro-organisms. Pestic. Sci. 1993, 37, 411–416. [Google Scholar] [CrossRef]
- Köhl, J.; Fokkema, N.J. Strategies for biological control of necrotrophic fungal foliar pathogens. In Plant Microbe Interactions and Biological Control; Boland, G., Bolis, L., Eds.; Marcel Dekker: New York, NY, USA, 1998; pp. 49–88. [Google Scholar]
- Elad, Y. Biocontrol of foliar pathogens: Mechanisms and application. Commun. Agric. Appl. Biol. Sci. 2003, 68, 17–24. [Google Scholar]
- Elad, Y.; Freeman, S. Biological control of fungal plant pathogens. In Agricultural Applications; Kempken, F., Ed.; Springer: Berlin/Heidelberg, Germany, 2002; pp. 93–109. [Google Scholar]
- Chellemi, D.O.; Gamliel, A.; Katan, J.; Subbarao, K.V. Development and deployment of system-based approaches for the management of soilborne plant pathogens. Phytopathology 2016, 106, 216–225. [Google Scholar] [CrossRef]
- Li, S.; Jin, X.; Chen, J. Effects of piperidine and piperideine alkaloids from the venom of red imported fire ants, Solenopsis invicta Buren, on Pythium ultimum Trow growth in vitro and the application of piperideine alkaloids to control cucumber damping-off in the greenhouse. Pest Manag. Sci. 2012, 68, 1546–1552. [Google Scholar] [CrossRef]
- Jespers, A.B.K.; De Waard, M.A. Natural products in plant protection. Eur. J. Plant Pathol. 1993, 99, 109–117. [Google Scholar] [CrossRef]
- Alves, D.S.; Ascari, J. Use of Natural Chemical Products for Pest. In Natural Enemies of Insect Pests in Neotropical Agroecosystems: Biological Control and Functional Biodiversity; Souza, B., Vázquez, L.L., Rosangela, C., Eds.; Springer: Gewerbestrasse, Switzerland, 2020; p. 479. [Google Scholar]
- Ross, K.G.; Meer, R.K.V.; Fletcher, D.J.; Vargo, E.L. Biochemical phenotypic and genetic studies of two introduced fire ants and their hybrid (Hymenoptera: Formicidae). Evology 1987, 41, 280–293. [Google Scholar] [CrossRef]
- Chen, J.; Cantrell, C.L.; Shang, H.W.; Rojas, M.G. Piperideine alkaloids from the poison gland of the red imported fire ant (Hymenoptera: Formicidae). J. Agric. Food Chem. 2009, 57, 3128–3133. [Google Scholar] [CrossRef]
- Chen, J.; Shang, H.; Jin, X. Interspecific variation of Δ1, 6-piperideines in imported fire ants. Toxicon 2010, 55, 1181–1187. [Google Scholar] [CrossRef] [PubMed]
- Zamith-Miranda, D.; Fox, E.G.; Monteiro, A.P.; Gama, D.; Poublan, L.E.; de Araujo, A.F.; Araujo, M.F.; Atella, G.C.; Machado, E.A.; Diaz, B.L. The allergic response mediated by fire ant venom proteins. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Blum, M.S.; Walker, J.R.; Callahan, P.S.; Novak, A.F. Chemical, insecticidal, and antibiotic properties of fire ant venom. Science 1958, 128, 306–307. [Google Scholar] [CrossRef] [PubMed]
- Jouvenaz, D.P.; Blum, M.S.; MacConnell, J.G. Antibacterial Activity of Venom Alkaloids from the Imported Fire Ant, Solenopsis invicta Buren1. Antimicrob. Agents Chemother. 1972, 2, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Storey, G.K.; Vander Meer, R.K.; Boucias, D.G.; McCoy, C.W. Effect of fire ant (Solenopsis invicta) venom alkaloids on the in vitro germination and development of selected entomogenous fungi. J. Invertebr. Pathol. 1991, 58, 88–95. [Google Scholar] [CrossRef]
- Sharma, K.R.; Fadamiro, H.Y. Fire ant alarm pheromone and venom alkaloids act in concert to attract parasitic phorid flies, Pseudacteon spp. J. Insect Physiol. 2013, 59, 1119–1124. [Google Scholar] [CrossRef] [PubMed]
- Vander Meer, R.K.; Preston, C.A.; Choi, M.Y. Isolation of a pyrazine alarm pheromone component from the fire ant, Solenopsis invicta. J. Chem. Ecol. 2010, 36, 163–170. [Google Scholar] [CrossRef]
- Sullivan, D.C.; Flowers, H.; Rockhold, R.; Herath, H.B.; Nanayakkara, N.D. Antibacterial activity of synthetic fire ant venom: The solenopsins and isosolenopsins. Am. J. Med. Sci. 2009, 338, 287–291. [Google Scholar] [CrossRef]
- Honeycutt, E.W.; Benson, D.M. Formulation of binucleate Rhizoctonia spp. and biocontrol of Rhizoctonia solani on impatiens. Plant Dis. 2001, 85, 1241–1248. [Google Scholar] [CrossRef]
- Elad, Y.; Pertot, I.; Prado, A.M.C.; Stewart, A. Plant hosts of Botrytis spp. In Botrytis–The Fungus, the Pathogen and its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar]
- Mammella, M.A.; Martin, F.N.; Cacciola, S.O.; Coffey, M.D.; Faedda, R.; Schena, L. Analyses of the population structure in a global collection of Phytophthora nicotianae isolates inferred from mitochondrial and nuclear DNA sequences. Phytopathology 2013, 103, 610–622. [Google Scholar] [CrossRef]
- Panabieres, F.; Ali, G.S.; Allagui, M.B.; Dalio, R.J.; Gudmestad, N.C.; Kuhn, M.L.; Guha Roy, S.; Schena, L.; Zampounis, A. Phytophthora nicotianae diseases worldwide: New knowledge of a long-recognised pathogen. Phytopathol. Mediterr. 2016, 20–40. [Google Scholar] [CrossRef]
- Najberek, K.; Pusz, W.; Solarz, W.; Olejniczak, P. The seeds of success: Release from fungal attack on seeds may influence the invasiveness of alien Impatiens. Plant Ecol. 2018, 219, 1197–1207. [Google Scholar] [CrossRef]
| Pathogen | Treatment | Inhibitory Zone (mm) ± SEM | Within Treatment Statistics | |||
|---|---|---|---|---|---|---|
| 2.4 µg/µL * | 3.0 µg/µL | 3.6 µg/µL | 4.8 µg/µL | |||
| B. cinerea | Venom | 0.7 ± 0.3 a ** (C) | 2.4 ± 0.2 a (A) | 1.4 ± 0.7 a (B) | 2.3 ± 0.4 a (A) | χ2(3,8) = 10.61, p = 0.014 |
| Alarm | 0 b | 0 b | 0 b | 0 b | ||
| Control *** | 0 b | |||||
| Between treatment statistics | χ2(2,6) = 9.85, p = 0.007 | χ2 (2,6) = 240.29, p < 0.001 | χ2(2,6) = 7.92, p = 0.019 | χ2 (2,6) = 82.53, p < 0.001 | ||
| F. oxysporum | Venom | 0.6 ± 0.2 a (B) | 1.9 ± 0.2 a (A) | 2.0 ± 0.3 a (A) | 1.9 ± 0.2 a (A) | χ2(3,8) = 26.77, p < 0.001 |
| Alarm | 0 b | 0 b | 0 b | 0 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 14, p = 0.001 | χ2 (2,6) = 151.14, p < 0.001 | χ2 (2,6) = 96, p < 0.001 | χ2 (2,6) = 264.5, p < 0.001 | ||
| P. citrinum | Venom | 3.8 ± 0.7 a (B) | 5.0 ± 0.0 a (A) | 5.0 ± 0.0 a (A) | 4.7 ± 0.3 a (AB) | χ2(3,8) = 6.85, p = 0.077 |
| Alarm | 0 b | 0 b | 0 b | 0 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 54, p < 0.001 | χ2 (2,6) = 50, p < 0.001 | χ2 (2,6) = 50, p < 0.001 | χ2 (2,6) = 482.46, p < 0.001 | ||
| P. cryptogea | Venom | 0.6 ± 0.3 a (B) | 0.8 ± 0.1 a (AB) | 1.2 ± 0.1 a (A) | 1.1 ± 0.2 a (AB) | χ2(3,8) = 6.32, p = 0.097 |
| Alarm | 0 b | 0 b | 0 b | 0 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 7.54, p = 0.023 | χ2 (2,6) = 200, p < 0.001 | χ2 (2,6) = 392, p < 0.001 | χ2 (2,6) = 84.50, p < 0.001 | ||
| P. nicotianae | Venom | 2.8 ± 0.5 a | 2.7 ± 0.2 a | 2.3 ± 0.5 a | 3.2 ± 0.2 a | χ2(3,8) = 2.26, p = 0.521 |
| Alarm | 0.4 ± 0.4 b | 0.3 ± 0.1 b | 0.5 ± 0.1 b | 0.5 ± 0.1 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 29.66, p < 0.001 | χ2 (2,6) = 288, p < 0.001 | χ2 (2,6) = 32.6, p < 0.001 | χ2 (2,6) = 250.4, p < 0.001 | ||
| R. solani | Venom | 1.3 ± 0.3 a (B) | 2.4 ± 0.3 a (A) | 2.5 ± 0.2 a (A) | 2.5 ± 0.1 a (A) | χ2(3,8) = 12.46, p = 0.006 |
| Alarm | 0 b | 0 b | 0 b | 0 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 37.50, p < 0.001 | χ2 (2,6) = 105.12, p < 0.001 | χ2 (2,6) = 600, p < 0.001 | χ2 (2,6) = 85.71, p < 0.001 | ||
| S. rolfsii | Venom | 3.8 ± 0.7 a (B) | 5.3 ± 0.3 a (A) | 3.5 ± 0.4 a (B) | 4.7 ± 0.5 a (AB) | χ2(3,8) = 8.64, p = 0.035 |
| Alarm | 1.3 ± 0.5 b | 0.7 ± 0.2 b | 0.9 ± 0.4 b | 0.7 ± 0.1 b | ||
| Control | 0 b | |||||
| Between treatment statistics | χ2 (2,6) = 31.82, p < 0.001 | χ2 (2,6) = 317.22, p < 0.001 | χ2 (2,6) = 61.87, p < 0.001 | χ2 (2,6) = 144.84, p < 0.001 | ||
| Treatment | Descriptions (Rates of Alkaloid *) | Damping-Off (%) | Root Rot Severity (%) | Total Plant Weight (g) | Root Weight (g) | Pathogen Recovery (%) | |
|---|---|---|---|---|---|---|---|
| 1st Trial *** | 2nd Trial | ||||||
| Venom | 30 µg/mL + pathogen | 6 ± 3.1 b ** | 5 ± 3.8 c | 14.1 ± 1.3 a | 11.7 ± 1.8 a | 3.1 ± 0.5 ab | 19 ± 3.5 c |
| 3 µg/mL + pathogen | 4 ± 2.7 b | 1 ± 0.6 cd | 12.0 ± 1.2 ab | 11.0 ± 1.8 a | 2.6 ± 0.4 a | 19 ± 2.8 c | |
| 0.3 µg/mL + pathogen | 4 ± 2.7 b | 26 ± 5.1 b | 10.3 ± 1.6 ab | 7.2 ± 0.7 b | 1.8 ± 0.3 cd | 34 ± 8.1 c | |
| 0.15 µg/mL + pathogen | 12 ± 6.7 b | 31 ± 5.0 b | 10.1 ± 1.5 ab | 8.0 ± 0.7 b | 2.2 ± 0.4 bc | 54 ± 9.8 b | |
| Control | + pathogen | 40 ± 7.4 a | 64 ± 2.2 a | 7.7 ± 1.3 b | 5.0 ± 0.8 b | 0.8 ± 0.1 d | 99 ± 1.0 a |
| Water only | 10 ± 10.0 b | 2 ± 1.3 cd | 12.1 ± 2.5 ab | 11.1 ± 2.9 a | 3.7 ± 0.6 a | 17 ± 6.5 c | |
| Acetone only | 0 b | 0 cd | 13.5 ± 1.9 a | 10.0 ± 1.1 a | 3.1 ± 0.5 a b | 11 ± 4.6 c | |
| Treatment | Descriptions (Rates of Alkaloid *) | Damping-Off (%) | Root Rot Severity (%) | Total Plant Weight (g) | Root Weight (g) | Pathogen Recovery (%) | ||
|---|---|---|---|---|---|---|---|---|
| 1st Trial *** | 2nd Trial | 1st Trial | 2nd Trial | |||||
| Venom | 30 µg/mL + pathogen | 14 ± 4.3 ab ** | 0 c | 3 ± 1.5 bc | 12.0 ± 1.3 a | 12.7 ± 0.8 a | 3.1 ± 0.4 a | 18 ± 4.9 c |
| 3 µg/mL + pathogen | 10 ± 4.5 b | 8 ± 5.0 c | 0 c | 12.4 ± 1.8 a | 13.6 ± 1.4 a | 2.9 ± 0.5 a | 21 ± 4.1 c | |
| 0.3 µg/mL + pathogen | 10 ± 3.3 b | 40 ± 2.5 b | 5 ± 2.3 b | 7.3 ± 0.8 b | 11.7 ± 0.3 ab | 1.8 ± 0.3 b | 40 ± 8.4 b | |
| 0.15 µg/mL + pathogen | 4 ± 2.7 bc | 43 ± 8.5 b | 14 ± 5.0 b | 7.9 ± 1.1 b | 11.5 ± 1.7 b | 2.0 ± 0.4 b | 52 ± 10.8 b | |
| Control | + pathogen | 20 ± 5.2 a | 65 ± 13.9 a | 50 ± 7.9 a | 7.8 ± 0.9 b | 8.4 ± 0.9 b | 1.1 ± 0.1 b | 92 ± 2.9 a |
| Water only | 0 c | 3 ± 2.5 c | 6 ± 4.8 b | 12.3 ± 3.6 a | 12.8 ± 2.2 a | 3.3 ± 0.4 a | 16 ± 5.8 c | |
| Acetone only | 0 c | 7.5 ± 7.5 c | 2.5 ± 2.5 b c | 12.8 ± 0.5 a | 13.0 ± 1.5 a | 2.9 ± 0.3 a | 14 ± 4.3 c | |
| Treatment | Descriptions (Rates of Alkaloid *) | Damping-Off (%) | Root Rot Severity (%) | Total Plant Weight (g) | Root Weight (g) | Pathogen Recovery (%) | |
|---|---|---|---|---|---|---|---|
| 1st Trial *** | 2nd Trial | ||||||
| Venom | 30 µg/mL + pathogen | 10 ± 4.5 cd ** | 0 d | 12.3 ± 0.9 ab | 12.2 ± 0.8 a | 3.3 ± 0.3 a | 18 ± 3.9 c |
| 3 µg/mL + pathogen | 6 ± 3.1 c | 3 ± 1.7 d | 11.7 ± 1.3 ab | 11.2 ± 1.3 a | 3.2 ± 0.4 a | 17 ± 5.0 c | |
| 0.3 µg/mL + pathogen | 14 ± 5.2 bc | 25 ± 3.2 b | 10.6 ± 1.2 abc | 9.5 ± 0.5 bc | 2.0 ± 0.3 b | 27 ± 7.8 bc | |
| 0.15 µg/mL + pathogen | 18 ± 3.6 b | 15 ± 6.1 c | 9.1 ± 1.2 bc | 6.6 ± 0.6 cc | 1.6 ± 0.2 b | 36 ± 9.6 b | |
| Control | + pathogen | 34 ± 6.0 a | 61 ± 2.9 a | 8.2 ± 1.3 c | 6.5 ± 0.6 c | 1.6 ± 0.2 b | 96 ± 3.1 a |
| Water only | 0 d | 2 ± 1.3 d | 13.2 ± 1.1 a | 11.8 ± 0.6 a | 3.8 ± 0.3 a | 17 ± 6.2 c | |
| Acetone only | 0 d | 1 ± 1.3 d | 12.4 ± 1.4 a | 11.3 ± 0.5 a | 3.3 ± 0.4 a | 17 ± 6.0 c | |
| Treatment | Descriptions (Rates of Alkaloid *) | Botrytis Blight Severity (%) | Disease Incidence (%) | Phytotoxicity (%) | Total Plant Weight (g) |
|---|---|---|---|---|---|
| Venom | 30 µg/mL + pathogen | 4 ± 1.9 c ** | 4 ± 1.1 c | 67 ± 3.2 a | 14.5 ± 1.8 a |
| 3 µg/mL + pathogen | 8 ± 2.6 bc | 4 ± 0.8 c | 25 ± 7.3 b | 15.2 ± 1.4 ab | |
| 0.3 µg/mL + pathogen | 18 ± 7.2 b | 10 ± 2.3 b | 10 ± 1.6 c | 12.3 ± 1.6 b | |
| 0.15 µg/mL + pathogen | 19 ± 6.0 b | 13 ± 2.5 b | 1 ± 0.7 d | 13.5 ± 2.5 b | |
| Control | + pathogen | 63 ± 8.3 a | 52 ± 5.2 a | 0 d | 9.2 ± 0.8 c |
| Water only | 0 c | 1 ± 0.6 c | 0 d | 18.1 ± 1.6 a | |
| Acetone only | 0 c | 1 ± 0.4 c | 0 d | 14.9 ± 1.4 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dawadi, S.; Baysal-Gurel, F.; Addesso, K.M.; Liyanapathiranage, P.; Simmons, T. Fire Ant Venom Alkaloids: Possible Control Measure for Soilborne and Foliar Plant Pathogens. Pathogens 2021, 10, 659. https://doi.org/10.3390/pathogens10060659
Dawadi S, Baysal-Gurel F, Addesso KM, Liyanapathiranage P, Simmons T. Fire Ant Venom Alkaloids: Possible Control Measure for Soilborne and Foliar Plant Pathogens. Pathogens. 2021; 10(6):659. https://doi.org/10.3390/pathogens10060659
Chicago/Turabian StyleDawadi, Sujan, Fulya Baysal-Gurel, Karla M. Addesso, Prabha Liyanapathiranage, and Terri Simmons. 2021. "Fire Ant Venom Alkaloids: Possible Control Measure for Soilborne and Foliar Plant Pathogens" Pathogens 10, no. 6: 659. https://doi.org/10.3390/pathogens10060659
APA StyleDawadi, S., Baysal-Gurel, F., Addesso, K. M., Liyanapathiranage, P., & Simmons, T. (2021). Fire Ant Venom Alkaloids: Possible Control Measure for Soilborne and Foliar Plant Pathogens. Pathogens, 10(6), 659. https://doi.org/10.3390/pathogens10060659






