An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection
Abstract
1. Introduction
2. Results
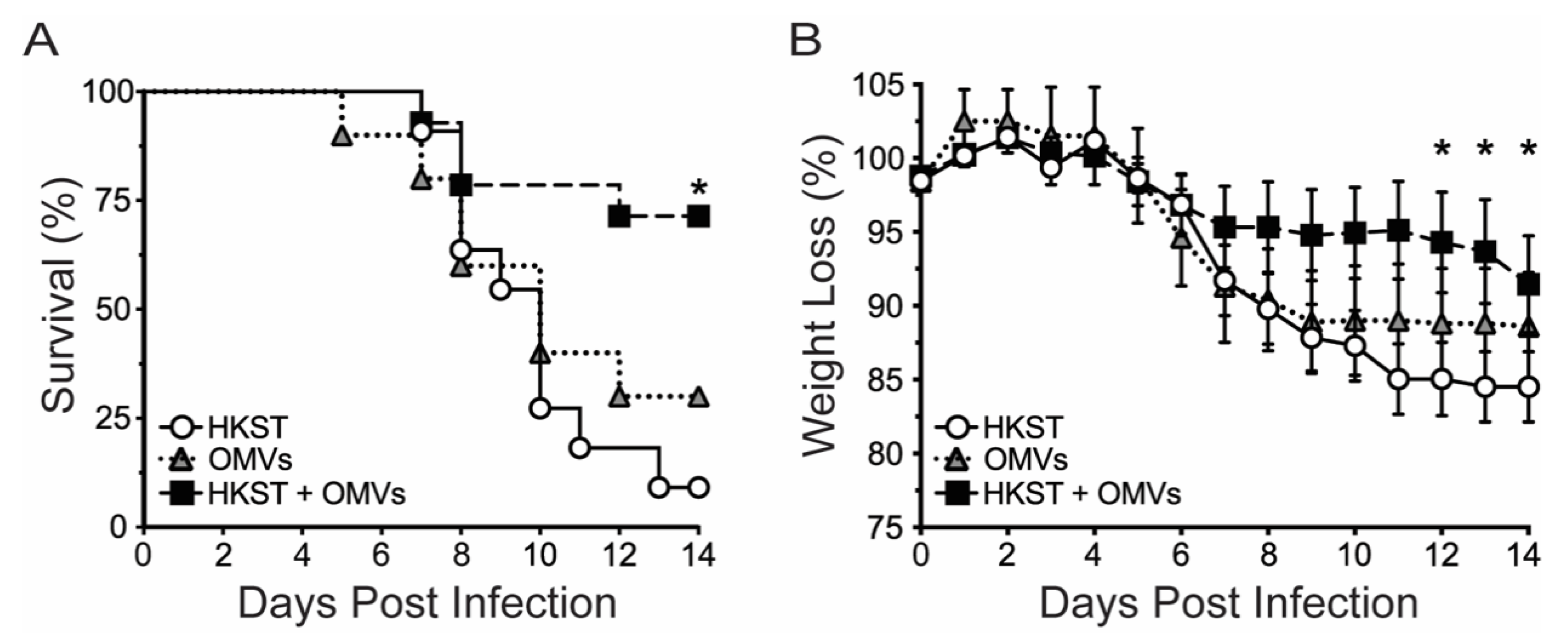
2.1. OMV-Adjuvanted Inactivated S. Typhimurium Vaccine Protects Against Lethal, Oral Challenge with S. Typhimurium
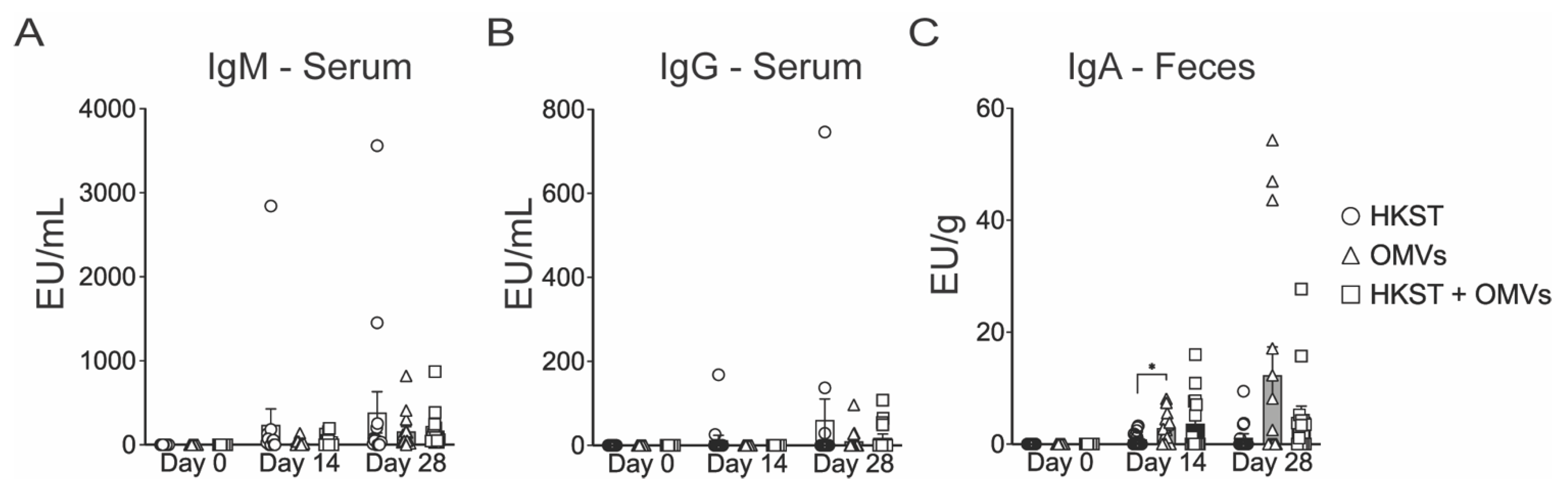
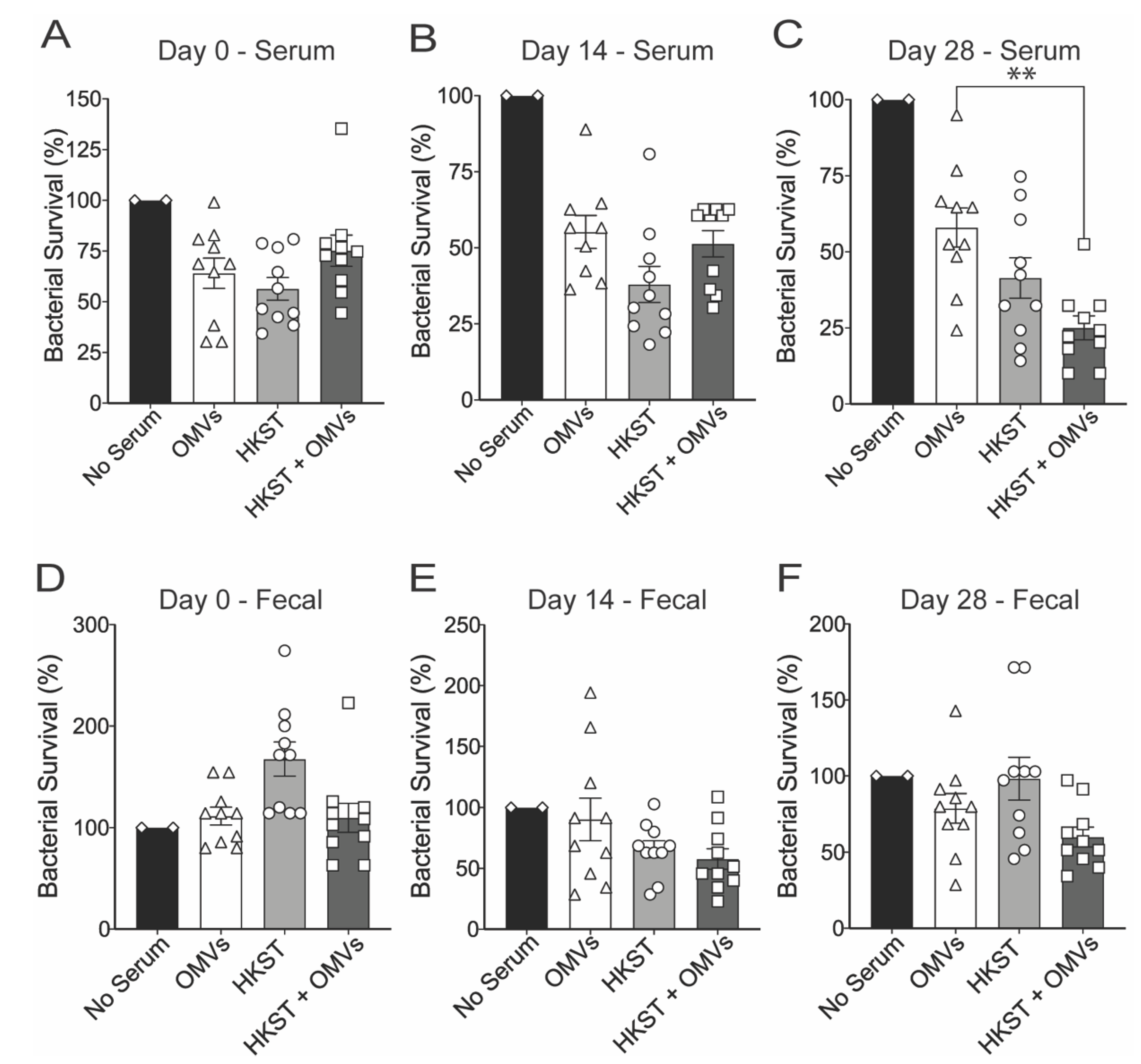
2.2. Oral Immunization with OMV-Adjuvanted Inactivated S. Typhimurium Vaccine Induces Neutralizing Systemic and Mucosal Antibodies
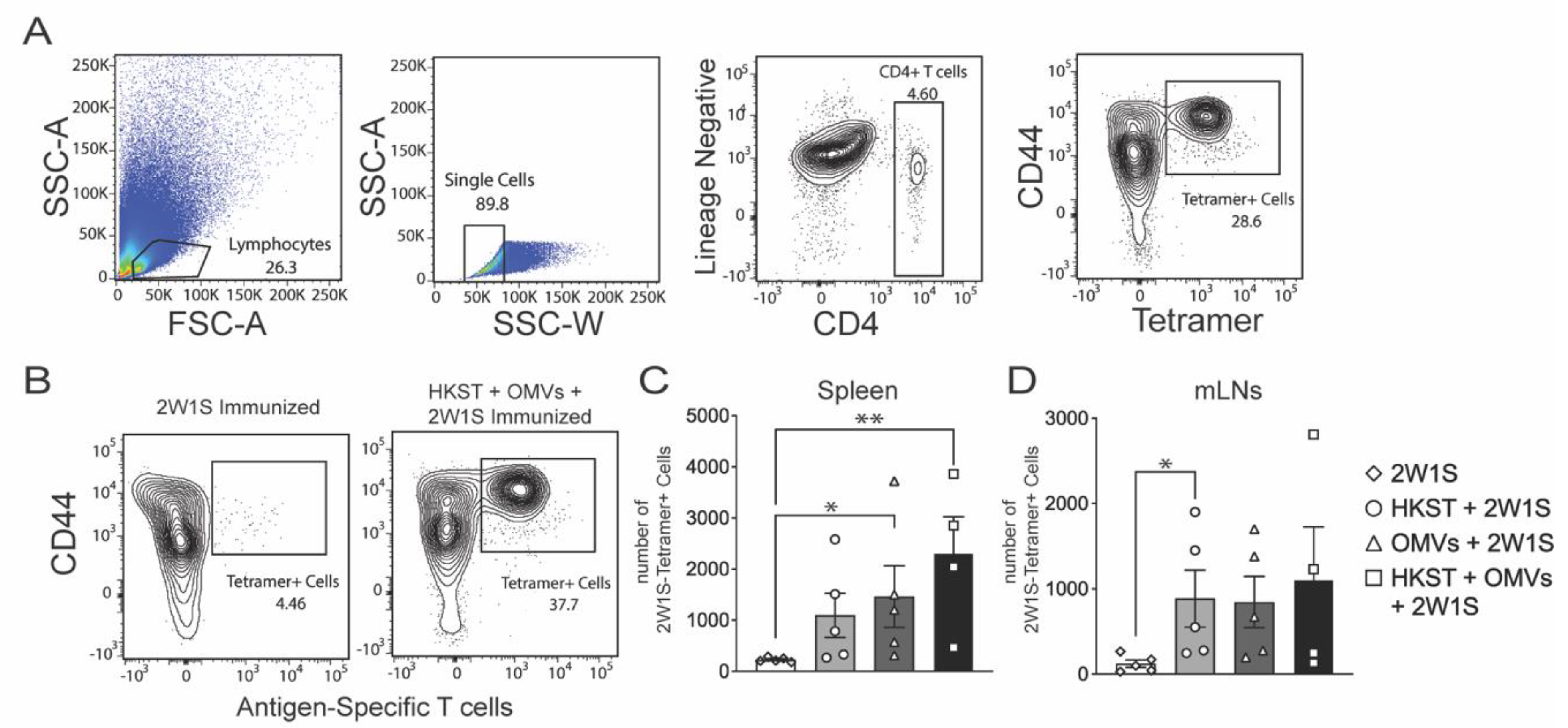
2.3. The Salmonella-Specific CD4 T-Cell Responses Increase in Spleen and MLNs after Oral Immunization
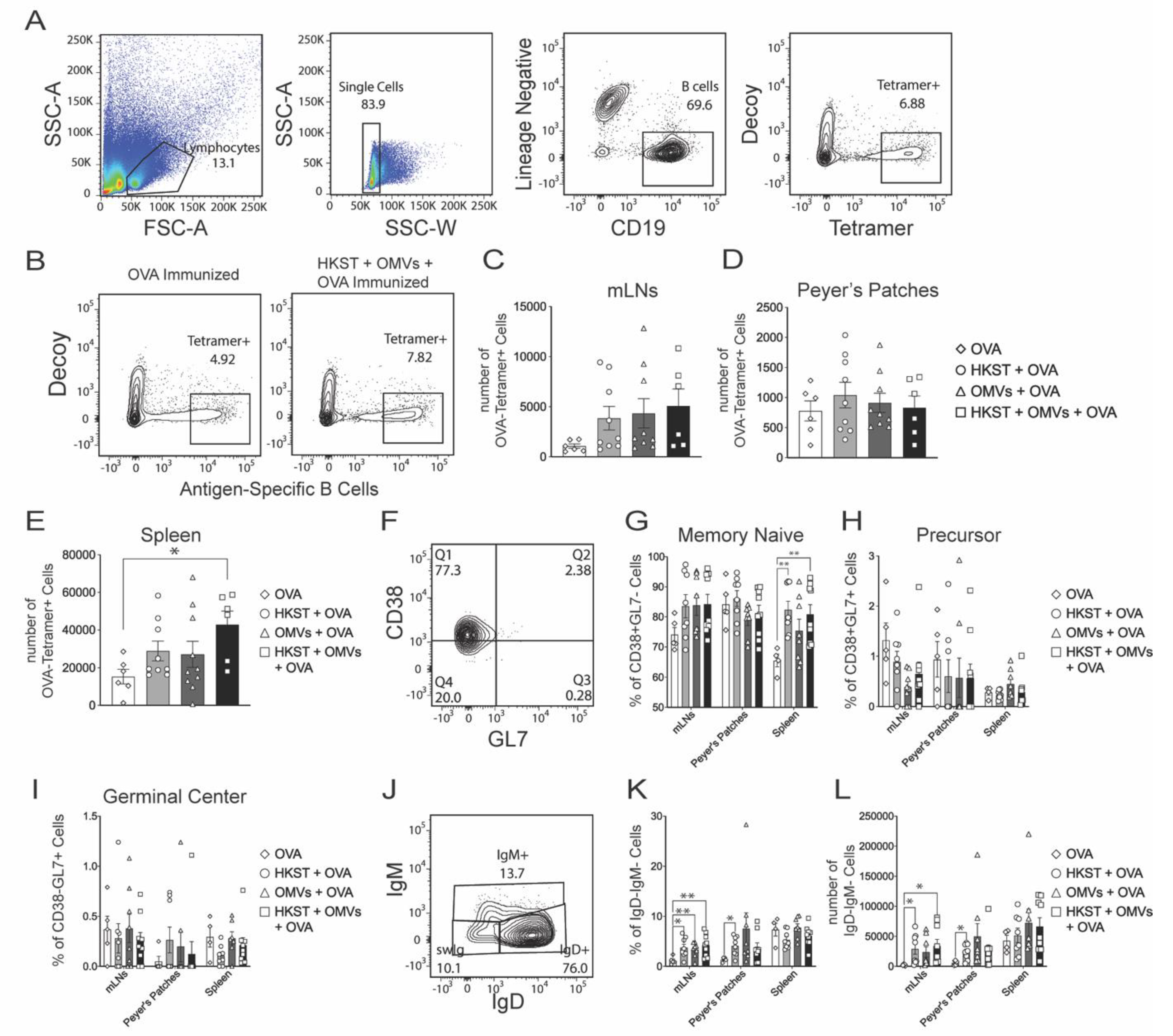
2.4. Antigen-Specific B Cells Increase and Isotype Switch Following Oral Immunization with the Combination of Heat-Killed Salmonella and B. pseudomallei OMVs
3. Discussion
4. Materials and Methods
4.1. Ethics Statement
4.2. Bacterial Strains
4.3. Mice and Immunizations
4.4. Burkholderia pseudomallei Outer Membrane Vesicle Purification
4.5. Antibody ELISAs
4.6. Opsonophagocytosis Assay for Macrophage Survival
4.7. Single-Cell Suspension Generation, MHC-II Tetramer Staining, Magnetic Assisted Cell Sorting, Flow Cytometry
4.8. Single-Cell Suspension Generation, B-Cell Tetramer Staining, Magnetic Assisted Cell Sorting, Flow Cytometry
4.9. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Greenwood, B. The contribution of vaccination to global health: Past, present and future. Philos. Trans. R. Soc. B Biol. Sci. 2014, 369, 20130433. [Google Scholar] [CrossRef] [PubMed]
- Coffman, R.L.; Sher, A.; Seder, R.A. Vaccine Adjuvants: Putting Innate Immunity to Work. Immunity 2010, 33, 492–503. [Google Scholar] [CrossRef] [PubMed]
- Savelkoul, H.F.J.; Ferro, V.A.; Strioga, M.M.; Schijns, V.E.J.C. Choice and Design of Adjuvants for Parenteral and Mucosal Vaccines. Vaccines 2015, 3, 148–171. [Google Scholar] [CrossRef] [PubMed]
- Kammona, O.; Bourganis, V.; Karamanidou, T.; Kiparissides, C. Recent developments in nanocarrier-aided mucosal vaccination. Nanomedicine 2017, 12, 1057–1074. [Google Scholar] [CrossRef]
- Neutra, M.R.; Kozlowski, P.A. Mucosal vaccines: The promise and the challenge. Nat. Rev. Immunol. 2006, 6, 148–158. [Google Scholar] [CrossRef]
- Davitt, C.J.; Lavelle, E.C. Delivery strategies to enhance oral vaccination against enteric infections. Adv. Drug Deliv. Rev. 2015, 91, 52–69. [Google Scholar] [CrossRef]
- Kuehn, M.J. Bacterial outer membrane vesicles and the host-pathogen interaction. Genes Dev. 2005, 19, 2645–2655. [Google Scholar] [CrossRef]
- Jan, A.T. Outer Membrane Vesicles (OMVs) of Gram-negative Bacteria: A Perspective Update. Front. Microbiol. 2017, 8, 1053. [Google Scholar] [CrossRef]
- Holst, J.; Martin, D.; Arnold, R.; Huergo, C.C.; Oster, P.; O’Hallahan, J.; Rosenqvist, E. Properties and clinical performance of vaccines containing outer membrane vesicles from Neisseria meningitidis. Vaccine 2009, 27, B3–B12. [Google Scholar] [CrossRef]
- Baker, S.M.; Davitt, C.J.H.; Motyka, N.; Kikendall, N.L.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Cross Protection against Inhalational Glanders in Mice and Non-Human Primates. Vaccines 2017, 5, 49. [Google Scholar] [CrossRef]
- Nieves, W.; Asakrah, S.; Qazi, O.; Brown, K.A.; Kurtz, J.; AuCoin, D.P.; McLachlan, J.B.; Roy, C.J.; Morici, L.A. A naturally derived outer-membrane vesicle vaccine protects against lethal pulmonary Burkholderia pseudomallei infection. Vaccine 2011, 29, 8381–8389. [Google Scholar] [CrossRef]
- Nieves, W.; Petersen, H.; Judy, B.M.; Blumentritt, C.A.; Russell-Lodrigue, K.; Roy, C.J.; Torres, A.G.; Morici, L.A. A Burkholderia pseudomallei Outer Membrane Vesicle Vaccine Provides Protection against Lethal Sepsis. Clin. Vaccine Immunol. 2014, 21, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Petersen, H.; Nieves, W.; Russell-Lodrigue, K.; Roy, C.J.; Morici, L.A. Evaluation of a Burkholderia Pseudomallei Outer Membrane Vesicle Vaccine in Nonhuman Primates. Procedia Vaccinol. 2014, 8, 38–42. [Google Scholar] [CrossRef]
- Prior, J.; Davitt, C.; Kurtz, J.; Gellings, P.; McLachlan, J.; Morici, L. Bacterial-Derived Outer Membrane Vesicles Are Potent Adjuvants That Drive Humoral and Cellular Immune Responses. Pharmaceutics 2021, 13, 131. [Google Scholar] [CrossRef]
- Schild, S.; Nelson, E.J.; Camilli, A. Immunization with Vibrio cholerae Outer Membrane Vesicles Induces Protective Immunity in Mice. Infect. Immun. 2008, 76, 4554–4563. [Google Scholar] [CrossRef] [PubMed]
- Egal-Mor, O.; Boyle, E.C.; Grassl, G.A. Same species, different diseases: How and why typhoidal and non-typhoidal Salmonella enterica serovars differ. Front. Microbiol. 2014, 5, 391. [Google Scholar] [CrossRef]
- Shakya, M.; Colin-Jones, R.; Theiss-Nyland, K.; Voysey, M.; Pant, D.; Smith, N.; Liu, X.; Tonks, S.; Mazur, O.; Farooq, Y.G.; et al. Phase 3 Efficacy Analysis of a Typhoid Conjugate Vaccine Trial in Nepal. N. Engl. J. Med. 2019, 381, 2209–2218. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, W.; Wang, S. Effect of vaccine administration modality on immunogenicity and efficacy. Expert Rev. Vaccines 2015, 14, 1509–1523. [Google Scholar] [CrossRef]
- McSorley, S.J.; Cookson, B.T.; Jenkins, M.K. Characterization of CD4+T Cell Responses During Natural Infection withSalmonella typhimurium. J. Immunol. 2000, 164, 986–993. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Petersen, H.E.; Frederick, D.R.; Morici, L.A.; McLachlan, J.B. Vaccination with a Single CD4 T Cell Peptide Epitope from a Salmonella Type III-Secreted Effector Protein Provides Protection against Lethal Infection. Infect. Immun. 2014, 82, 2424–2433. [Google Scholar] [CrossRef]
- Kurtz, J.R.; Nieves, W.; Bauer, D.L.; Israel, K.E.; Adcox, H.E.; Gunn, J.S.; Morici, L.A.; McLachlan, J.B. Salmonella Persistence and Host Immunity Are Dictated by the Anatomical Microenvironment. Infect. Immun. 2020, 88. [Google Scholar] [CrossRef]
- Gayet, R.; Bioley, G.; Rochereau, N.; Paul, S.; Corthésy, B. Vaccination against Salmonella Infection: The Mucosal Way. Microbiol. Mol. Biol. Rev. 2017, 81, 7–17. [Google Scholar] [CrossRef]
- Harrell, J.E.; Hahn, M.M.; D’Souza, S.J.; Vasicek, E.M.; Sandala, J.L.; Gunn, J.S.; McLachlan, J.B. Salmonella Biofilm Formation, Chronic Infection, and Immunity Within the Intestine and Hepatobiliary Tract. Front. Cell. Infect. Microbiol. 2021, 10, 624622. [Google Scholar] [CrossRef]
- Kurosaki, T.; Kometani, K.; Ise, W. Memory B cells. Nat. Rev. Immunol. 2015, 15, 149–159. [Google Scholar] [CrossRef]
- Ravindran, R.; McSorley, S.J. Tracking the dynamics of T-cell activation in response to Salmonella infection. Immunology 2005, 114, 450–458. [Google Scholar] [CrossRef]
- Chu, H.H.; Moon, J.J.; Takada, K.; Pepper, M.; Molitor, J.A.; Schacker, T.W.; Hogquist, K.A.; Jameson, S.C.; Jenkins, M.K. Positive selection optimizes the number and function of MHCII-restricted CD4+ T cell clones in the naive polyclonal repertoire. Proc. Natl. Acad. Sci. USA 2009, 106, 11241–11245. [Google Scholar] [CrossRef]
- Schmiel, S.E.; Yang, J.A.; Jenkins, M.K.; Mueller, D.L. Cutting Edge: Adenosine A2a Receptor Signals Inhibit Germinal Center T Follicular Helper Cell Differentiation during the Primary Response to Vaccination. J. Immunol. 2016, 198, 623–628. [Google Scholar] [CrossRef] [PubMed]
- Nanton, M.R.; Way, S.S.; Shlomchik, M.J.; McSorley, S.J. Cutting Edge: B Cells Are Essential for Protective Immunity againstSalmonellaIndependent of Antibody Secretion. J. Immunol. 2012, 189, 5503–5507. [Google Scholar] [CrossRef]
- Reboldi, A.; Cyster, J.G. Peyer’s patches: Organizing B-cell responses at the intestinal frontier. Immunol. Rev. 2016, 271, 230–245. [Google Scholar] [CrossRef] [PubMed]
- Victora, G.D.; Schwickert, T.A.; Fooksman, D.R.; Kamphorst, A.O.; Meyer-Hermann, M.; Dustin, M.L.; Nussenzweig, M.C. Germinal Center Dynamics Revealed by Multiphoton Microscopy with a Photoactivatable Fluorescent Reporter. Cell 2010, 143, 592–605. [Google Scholar] [CrossRef]
- Stanaway, J.D.; Parisi, A.; Sarkar, K.; Blacker, B.F.; Reiner, R.C.; Hay, S.I.; Nixon, M.R.; Dolecek, C.; James, S.L.; Mokdad, A.H.; et al. The global burden of non-typhoidal salmonella invasive disease: A systematic analysis for the Global Burden of Disease Study 2017. Lancet Infect. Dis. 2019, 19, 1312–1324. [Google Scholar] [CrossRef]
- Crump, J.A.; Sjölund-Karlsson, M.; Gordon, M.A.; Parry, C.M. Epidemiology, Clinical Presentation, Laboratory Diagnosis, Antimicrobial Resistance, and Antimicrobial Management of Invasive Salmonella Infections. Clin. Microbiol. Rev. 2015, 28, 901–937. [Google Scholar] [CrossRef] [PubMed]
- Ao, T.T.; Feasey, N.A.; Gordon, M.A.; Keddy, K.H.; Angulo, F.J.; Crump, J.A. Global Burden of Invasive NontyphoidalSalmonellaDisease, 20101. Emerg. Infect. Dis. 2015, 21, 941–949. [Google Scholar] [CrossRef]
- Feasey, N.A.; Dougan, G.; Kingsley, R.A.; Heyderman, R.S.; Gordon, M.A. Invasive non-typhoidal salmonella disease: An emerging and neglected tropical disease in Africa. Lancet 2012, 379, 2489–2499. [Google Scholar] [CrossRef]
- Shakya, A.K.; Chowdhury, M.; Tao, W.; Gill, H.S. Mucosal vaccine delivery: Current state and a pediatric perspective. J. Control. Release 2016, 240, 394–413. [Google Scholar] [CrossRef]
- Ramirez, J.E.V.; Sharpe, L.A.; Peppas, N.A. Current state and challenges in developing oral vaccines. Adv. Drug Deliv. Rev. 2017, 114, 116–131. [Google Scholar] [CrossRef]
- Baker, S.M.; Settles, E.W.; Davitt, C.; Gellings, P.; Kikendall, N.; Hoffmann, J.; Wang, Y.; Bitoun, J.; Lodrigue, K.-R.; Sahl, J.W.; et al. Burkholderia pseudomallei OMVs derived from infection mimicking conditions elicit similar protection to a live-attenuated vaccine. NPJ Vaccines 2021, 6, 18. [Google Scholar] [CrossRef]
- Levine, M.M.; Ferreccio, C.; Abrego, P.; Martin, O.S.; Ortiz, E.; Cryz, S. Duration of efficacy of Ty21a, attenuated Salmonella typhi live oral vaccine. Vaccine 1999, 17, S22–S27. [Google Scholar] [CrossRef]
- Ferreira, R.; Valdez, Y.; Coombes, B.K.; Sad, S.; Gouw, J.W.; Brown, E.M.; Li, Y.; Grassl, G.A.; Antunes, L.C.M.; Gill, N.; et al. A Highly Effective Component Vaccine against Nontyphoidal Salmonella enterica Infections. MBio 2015, 6, e01421-15. [Google Scholar] [CrossRef]
- Tennant, S.M.; Wang, J.-Y.; Galen, J.E.; Simon, R.; Pasetti, M.F.; Gat, O.; Levine, M.M. Engineering and Preclinical Evaluation of Attenuated Nontyphoidal Salmonella Strains Serving as Live Oral Vaccines and as Reagent Strains. Infect. Immun. 2011, 79, 4175–4185. [Google Scholar] [CrossRef]
- Liu, Q.; Yi, J.; Liang, K.; Liu, T.; Roland, K.L.; Jiang, Y.; Kong, Q. Outer membrane vesicles derived from Salmonella Typhimurium mutants with truncated LPS induce cross-protective immune responses against infection of Salmonella enterica serovars in the mouse model. Int. J. Med. Microbiol. 2016, 306, 697–706. [Google Scholar] [CrossRef] [PubMed]
- Taylor, P.W. Bactericidal and Bacteriolytic Activity of Serum against Gram-Negative Bacteria. Microbiol. Rev. 1983, 47, 46–83. [Google Scholar] [CrossRef]
- Casadevall, A. Antibody-mediated protection against intracellular pathogens. Trends Microbiol. 1998, 6, 102–107. [Google Scholar] [CrossRef]
- Ault, A.; Tennant, S.M.; Gorres, J.P.; Eckhaus, M.; Sandler, N.G.; Roque, A.; Livio, S.; Bao, S.; Foulds, K.E.; Kao, S.-F.; et al. Safety and tolerability of a live oral Salmonella typhimurium vaccine candidate in SIV-infected nonhuman primates. Vaccine 2013, 31, 5879–5888. [Google Scholar] [CrossRef]
- MacLennan, C.A.; Gondwe, E.N.; Msefula, C.L.; Kingsley, R.A.; Thomson, N.R.; White, S.; Goodall, M.; Pickard, D.J.; Graham, S.M.; Dougan, G.; et al. The neglected role of antibody in protection against bacteremia caused by nontyphoidal strains of Salmonella in African children. J. Clin. Investig. 2008, 118, 1553–1562. [Google Scholar] [CrossRef] [PubMed]
- Michetti, P.; Porta, N.; Mahan, M.J.; Slauch, J.M.; Mekalanos, J.J.; Blum, A.; Kraehenbuhl, J.-P.; Neutra, M.R. Monoclonal immunoglobulin a prevents adherence and invasion of polarized epithelial cell monolayers by Salmonella typhimurium. Gastroenterology 1994, 107, 915–923. [Google Scholar] [CrossRef]
- Carlin, N.I.; Svenson, S.B.; Lindberg, A.A. Role of monoclonal O-antigen antibody epitope specificity and isotype in protection against experimental mouse typhoid. Microb. Pathog. 1987, 2, 171–183. [Google Scholar] [CrossRef]
- Frank, M.M.; Joiner, K.; Hammer, C. The Function of Antibody and Complement in the Lysis of Bacteria. Clin. Infect. Dis. 1987, 9, S537–S545. [Google Scholar] [CrossRef]
- Robbins, J.D. Reexamination of the Protective Role of the Capsular Polysaccharide (Vi antigen) of Salmonella typhi. J. Infect. Dis. 1984, 150, 436–449. [Google Scholar] [CrossRef]
- Di Niro, R.; Lee, S.-J.; Heiden, J.A.V.; Elsner, R.A.; Trivedi, N.; Bannock, J.M.; Gupta, N.T.; Kleinstein, S.H.; Vigneault, F.; Gilbert, T.J.; et al. Salmonella Infection Drives Promiscuous B Cell Activation Followed by Extrafollicular Affinity Maturation. Immunity 2015, 43, 120–131. [Google Scholar] [CrossRef]
- Propst, K.L.; Mima, T.; Choi, K.-H.; Dow, S.W.; Schweizer, H.P. A Burkholderia pseudomallei ΔpurM Mutant Is Avirulent in Immunocompetent and Immunodeficient Animals: Candidate Strain for Exclusion from Select-Agent Lists. Infect. Immun. 2010, 78, 3136–3143. [Google Scholar] [CrossRef]
- Moon, J.J.; Chu, H.H.; Hataye, J.; Pagán, A.J.; Pepper, M.; McLachlan, J.B.; Zell, T.; Jenkins, M.K. Tracking epitope-specific T cells. Nat. Protoc. 2009, 4, 565–581. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.J.; Martinez, R.J.; Titcombe, P.J.; Barsness, L.O.; Thomas, S.R.; Zhang, N.; Katzman, S.D.; Jenkins, M.K.; Mueller, D.L. Deletion and anergy of polyclonal B cells specific for ubiquitous membrane-bound self-antigen. J. Exp. Med. 2012, 209, 2065–2077. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Harrell, J.E.; Kurtz, J.R.; Bauer, D.L.; Prior, J.T.; Gellings, P.S.; Morici, L.A.; McLachlan, J.B. An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection. Pathogens 2021, 10, 616. https://doi.org/10.3390/pathogens10050616
Harrell JE, Kurtz JR, Bauer DL, Prior JT, Gellings PS, Morici LA, McLachlan JB. An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection. Pathogens. 2021; 10(5):616. https://doi.org/10.3390/pathogens10050616
Chicago/Turabian StyleHarrell, Jaikin E., Jonathan R. Kurtz, David L. Bauer, J. Timothy Prior, Patrick S. Gellings, Lisa A. Morici, and James B. McLachlan. 2021. "An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection" Pathogens 10, no. 5: 616. https://doi.org/10.3390/pathogens10050616
APA StyleHarrell, J. E., Kurtz, J. R., Bauer, D. L., Prior, J. T., Gellings, P. S., Morici, L. A., & McLachlan, J. B. (2021). An Outer Membrane Vesicle-Adjuvanted Oral Vaccine Protects Against Lethal, Oral Salmonella Infection. Pathogens, 10(5), 616. https://doi.org/10.3390/pathogens10050616






