Abstract
Multivalency is a strategy commonly used by medicinal carbohydrate chemists to increase the affinity of carbohydrate-based small molecules for their protein targets. Although this approach has been very successful in enhancing binding to isolated carbohydrate-binding proteins, anticipating the multivalent presentations that will improve biological activity in cellular assays remains challenging. In this work we investigate linear molecular scaffolds for the synthesis of a low valency presentation of a divalent galactoside 1, previously identified by us as an inhibitor of the adhesion of opportunistic fungal pathogen Candida albicans to buccal epithelial cells (BECs). Adhesion inhibition assays revealed that multivalent glycoconjugate 3 is more effective at blocking C. albicans adherence to BECs upon initial exposure to epithelial cells. Interestingly, 3 did not seem to have any effect when it was pre-incubated with yeast cells, in contrast to the original lead compound 1, which caused a 25% reduction of adhesion. In competition assays, where yeast cells and BECs were co-incubated, multivalent glycoconjugate 3 inhibited up to 49% C. albicans adherence in a dose-dependent manner. The combined effect of compound 1 towards both yeast cells and BECs allowed it to achieve over 60% inhibition of the adhesion of C. albicans to BECs in competition assays.
1. Introduction
Protein-carbohydrate recognition is the first step in the initiation of many human diseases. In many cases, for microorganisms to infect their hosts, microbe proteins initially adhere to carbohydrate epitopes displayed on the host cell surface [1]. Small-molecule inhibitors of the adhesion process have been successfully developed and studied for many years [2]. The use of glycoconjugates as anti-adhesion ligands is desirable since they can mimic the host cell surface glycans and block pathogen attachment, thus preventing infection. However, carbohydrates interact with their protein receptors (lectins) with low affinity (generally millimolar to micromolar dissociation constants) [3]. Consequently, the development of strategies for increasing the lectin–ligand binding affinities to levels required for therapeutic use has received much attention in glycoscience research [4]. In nature, carbohydrate epitopes are expressed multiple times on the cell surface: many lectins have more than one binding site to counteract the low-affinity problem, leading to stronger interactions. This phenomenon has become known as the “multivalent effect” or “cluster glycoside effect”, which was first reported by Lee and co-workers in 1995 [5]. Numerous multivalent glycoconjugates with various valencies and spatial arrangement of carbohydrate ligands have been developed to enhance carbohydrate–lectin interactions. The multivalent glycoconjugates can have well-defined molecular structures and display a specific number of carbohydrate ligands when they are built around scaffolds such as calixarenes [6], dendrimers [7], cyclodextrins [8], cyclopeptides [9] and fullerenes [7]; they can also have higher valencies such as those provided by polymers [10], nanoparticles [11] and quantum dots [12]. There are many reports of multivalent glycoconjugates with increased affinity for isolated lectins, in comparison to their monovalent counterparts [13]. Most of these studies involve lectins whose structures have been well characterized by X-ray crystallography, and the strength of lectin–ligand binding interactions is measured using techniques such as haemagglutination inhibition assays, enzyme-linked lectin assays (ELLA) and isothermal titration calorimetry [14,15]. The multivalent effect in ligand–protein interactions has also been investigated with ligands other than carbohydrates, to potentiate different types of biological effects [16,17]. Despite the many achievements in this field, the design of high-affinity multivalent presentations is still a challenging task, as it strongly depends on multiple factors: these include structural parameters of the glycoconjugates such as linker length, density of the ligands in the glycoconstruct and heterogeneity of the carbohydrate epitopes presented [18]. Moreover, the impact of multivalency on biological activity is particularly difficult to anticipate in cellular assays, given the structural complexity encountered by the ligands at the cell surface [19,20].
C. albicans is an opportunistic pathogenic yeast and the most prevalent cause of hospital-acquired fungal infections worldwide. It is therefore hugely important that new treatments are developed for these infections, which are now becoming resistant to conventional antifungal medicines [21]. The molecular mechanisms of the adherence processes of C. albicans to host cells and abiotic surfaces are complex, albeit essential for infection and biofilm formation [22]. Hence, targeting adhesion in C. albicans offers an interesting approach towards antifungal therapies as alternatives to conventional drugs [23]. Early reports indicate that C. albicans adhesins recognise and bind to many cell surface glycans and carbohydrates, including mono- and disaccharides (e.g., l-fucose, α-d-methyl mannoside and N-acetyl-d-glucosamine) [24,25]. Glycosphingolipids have also been shown to act as adhesion receptors for yeasts. C. albicans bound specifically to lactosylceramide (Galβ(1-4)Glcβ(1-1)Cer), with the terminal galactose essential for binding [26], and to asialo-GM1 (gangliotetraosylceramide: βGal(1-3)βGalNAc(1-4)βGal(1-4)βGlc(1-1)Cer), with the minimal carbohydrate sequence required for binding being βGalNAc(1-4)βGal [27]. Previous research in our group hence screened a small library of synthetic glycoconjugates with various sugars and valencies, and tested their ability to inhibit the adhesion of C. albicans to human BEC. This study showed that the divalent galactoside 1 (Figure 1) was a successful inhibitor of the adhesion of C. albicans to BECs [28]. Ongoing research in the group is aiming to identify the adhesin on the cell surface of the C. albicans with which this galactosylated compound 1 is interacting. In this work, we developed a low valency-multivalent derivative of the original lead compound 1 to assess the effect of multivalent displays in the inhibition of the adhesion of C. albicans to BECs. Recently, we reported the multivalent presentation of lead compound 1 on RAFT cyclopeptide- and polylysine-based scaffolds [29]. Tetra-, hexa- and hexadecavalent displays of compound 1 were thus evaluated as inhibitors of C. albicans adhesion, revealing a better performance for the lower valency glycoconjugates. In our investigations towards an optimal multivalent architecture to enhance the activity of lead compound 1, in the current study we opted for linear peptoid scaffold 2 [30] to prepare glycoconjugate 3 (Figure 1). Since the structure of the target lectin in C. albicans is not known, a tetravalent display of compound 1 in a flexible scaffold such as 2 could provide a very useful comparison with the results observed when more rigid and cyclic scaffolds were used. Peptoid scaffolds have been successfully investigated by Taillefumier and co-workers in the design of defined valency glycoconjugates [31,32]. Thus, lead compound 1 was modified with a linker to facilitate connection with this scaffold using Copper(I)-catalyzed Azide–Alkyne Cycloaddition (CuAAC) methodology. The tetravalent display of compound 1 as presented in glycoconjugate 3 may significantly affect how it interacts with carbohydrate-binding proteins in C. albicans and BECs surface, which, in turn, should allow for identification of the most suitable structural features to inhibit pathogen–cell interaction.
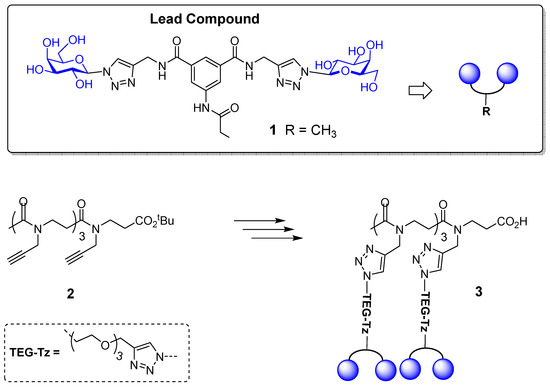
Figure 1.
Shows the structure of lead compound 1, peptoid scaffold 2 and multivalent glycoconjugate 3.
2. Results and Discussion
2.1. Chemical Synthesis
In order to install lead compound 1 onto the alkyne-featuring scaffold 2 via the CuAAC reaction, it had to be functionalized with an azido group in a manner that would not compromise its interaction with target proteins in the C. albicans cell wall. Our previous work showed that functionalization of 5-aminobenzene position in compound 1 with a fluorescent label still allowed for the recognition of the digalactoside motifs and localization of the compound at the cell wall in C. albicans [28]. Thus, 5-amino-isophthalic acid was protected using tert-butoxycabonyl (Boc) anhydride, followed by reaction with propargylamine and TBTU to give compound 4 (Scheme 1). CuAAC chemistry was used to conjugate the per-acetylated-1-β-azido galactose [33] moieties to dialkyne 4 to give compound 5, which was then treated with TFA to remove the N-Boc-protecting group yielding compound 6 [28]. Reaction with bromoacetyl bromide to give compound 7 and subsequent bromide displacement by treatment with sodium azide gave key azido-functionalized compound 8 [29]. Attempts of direct reaction of this compound with alkynated scaffolds using CuAAC reaction conditions failed to yield any of the desired product, likely due to steric effects. Oligo(ethyleneglycol)s are commonly used as linkers between the carbohydrate moieties and the scaffold in multivalent glycoconjugates, because of their flexibility, water-solubility, the availability of various lengths and the presence of functionalizable hydroxy groups [34]. Therefore, it was decided to use a triethylene glycol (TEG) derivative as a linker to join the divalent galactoside 8 to the alkyne scaffolds 2.
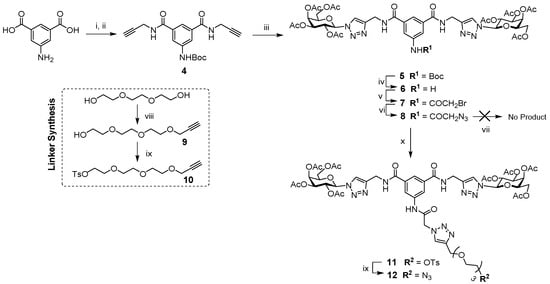
Scheme 1.
Synthesis of azido-TEG divalent galactoside 12 (inset shows the synthesis of TEG linker): Reagents and conditions: (i) Di-tert-butyl dicarbonate, NaOH, 1,4-dioxane, 0 °C to rt, 3 h, 86%; (ii) DMTMM, propargylamine, THF, 48 h, 95%; (iii) 2,3,4,6-tetra-O-acetyl-1-β-azido-galactoside, CuSO4·5H2O/Na Asc, CH3COCH3/H2O, rt, 16 h, 71%; (iv) TFA, DCM, 2 h, rt, 99%; (v) bromoacetyl bromide, NEt3, anhydrous DCM, 16 h, 83%; (vi) NaN3, anhydrous DMF, N2, 80 °C, 16 h, quant%; (vii) CuAAC methodology, propargylated scaffolds, no product isolated; (viii) propargyl bromide, NaH, anhydrous THF, N2, 16 h, 75%; (ix) TsCl, KOH, DCM, 0 °C, 2 h, 87%; (x) 10, CuSO4·5H2O/Na Asc, CH3CN/H2O, MW (microwave), 100 °C, 30 min, 74%; (xi) NaN3, CH3CN, DMF, 80 °C, 24 h, 95%.
Using well-documented procedures, TEG was reacted with propargyl bromide to give the mono-propargylated linker 9 [35]. To ensure mono-propargylation, the reaction was carried out in excess of TEG. Compound 9 was then tosylated, yielding compound 10 [36]. This was then reacted with the azido di-galactoside 8 using microwave (MW) assisted CuAAC methodology to give compound 11. The tosyl group was then replaced by an azide upon treatment with sodium azide to give compound 12. This key synthetic intermediate presents the acetylated divalent galactoside with a TEG linker functionalized with a terminal azide, available for CuAAC conjugation to various propargylated scaffolds.
β-peptoid scaffolds designed by Faure, Taillefumier and coworkers have been used to create linear and cyclic displays of various recognition motifs, including carbohydrates [30,31,32]. Linear scaffold 2 [30] was reacted with compound 12 using, once again, microwave-assisted CuAAC conditions (Scheme 2); acetylated glycocluster 13 was thus obtained and subjected to mild base hydrolysis that resulted in the removal of the tert-butyl ester and acetyl protecting groups, yielding glycoconjugate 3, which presents a tetravalent display of the lead divalent galactoside 1. In addition, CuAAC reaction of linear scaffold 2 with per-acetylated-1-β-azido galactose [33] gave tetragalacoside 14, which was deprotected under mild basic conditions to give 15 (Scheme 2). This compound, lacking structural features of lead compound 1 other than terminal β-triazolyl-galactosides, would serve as a control in subsequent biological evaluation of anti-adhesion activity against C. albicans.
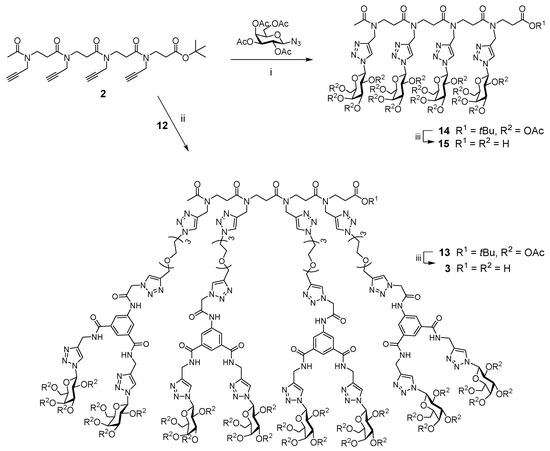
Scheme 2.
Synthesis of glycoconjugate 3. Reagents and conditions: (i) CuSO4·5H2O/Na Asc, CH3CN/H2O, MW, 100 °C, 30 min, 58–68%; (ii) MeOH, NEt3, H2O, 45 °C, 6 h, 82–92%.
2.2. Biological Evaluation
In our previous work [28,29] we established that compound 1 and derivatives were not toxic to C. albicans yeast cells at the concentrations used in the current study. Glycoconjugates 3, featuring multivalent displays of the original lead compound 1, along with tetra-β-triazolyl-galactoside 15, were then evaluated for their anti-adhesive properties against C. albicans using several assays.
2.2.1. Exclusion Assay
The initial adherence assay was performed by treating the C. albicans with compounds 3 and 15. After an incubation period of 90 min, the treated yeast cells were exposed to exfoliated BECs. The average number of yeast cells attached to each BEC was calculated (Figure 2a), and the percentage increase or decrease of the number of C. albicans cells adhering to the BECs was compared to a control (untreated yeast) and the lead compound 1 (Figure 2b). In this assay, compound 15 only slightly reduced the adhesion of the yeast to the BECs, whereas compound 3 did not have any appreciable effect. Compound 1 showed moderate inhibition of adhesion at this concentration (up to 25% reduction at 10 mg/mL). On the other hand, when the BECs were treated with the above compounds, allowing the incubation period and followed by exposure to C. albicans cells, the average number of yeasts attached per BEC was reduced (Figure 2c). The tetra-galactoside 15 caused similar reduction of yeast adhesion as in the previous assay, while multivalent glycoconjugate 3 inhibited C. albicans adhesion by 35%, similarly to the inhibition produced by lead compound 1 (Figure 2d). These results suggest that the multivalent presentation in compound 3 disfavours significantly the interactions with structural components of the cell wall in C. albicans, which had been previously proposed for compound 1 [28]. However, this effect was not observed upon preliminary exposure to BECs, since both compounds 1 and 3 caused a similar decrease in yeast adhesion.

Figure 2.
Exclusion assays of glycoconjugates 3 and 15: (a) Shows the average number of yeast attached per BEC (buccal epithelial cells), after the yeast cells were pre-treated with glycoconjugates; (b) Shows the percentage change in the adherence of yeast to the BEC versus the control and the lead compound 1 after the yeast were pre-treated; (c) Shows the average number of yeast attached per BEC, after the BEC were pre-treated with compounds; (d) Shows the percentage change in the adherence of yeast to the BEC versus the control and the lead compound 1 after the BEC were pre-treated. All assays were performed at a glycoconjugate concentration of 10 mg/mL.
2.2.2. Competition Assay
The glycoconjugates were then evaluated in a competition assay, in which their anti-adhesion abilities were tested in the presence of both C. albicans and BECs. Co-incubation with the compounds resulted in a reduction in yeast adhesion in all cases (Figure 3a), with compound 3 causing up to a 49% decrease. Interestingly, lead compound 1, which showed activity in both exclusion assays discussed above (i.e., when pre-incubated with C. albicans and BECs, independently), was able to inhibit up to 64% the adhesion of C. albicans to BECs in the competition assay.
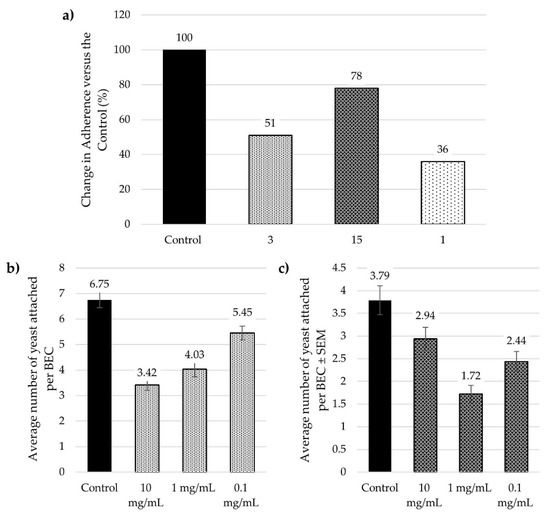
Figure 3.
Competition assays of glycoconjugates 1, 3 and 15: (a) Shows the change in adherence compared to the control and the lead compound 1 at 10 mg/mL; (b) Shows the average number of yeast attached to BEC in the presence of compound 3 (10, 1, 0.1 mg/mL); (c) Shows the average number of yeast attached to BEC in the presence of compound 15 (10, 1, 0.1 mg/mL).
The compounds were then tested at lower concentrations. Glycoconjugate 3 exhibited a typical dose–response pattern, with ca. 50% inhibition of adhesion at the highest concentration (Figure 3b). Compound 15 (Figure 3c) did not show a linear relationship between concentration and activity, where the best inhibition of adhesion was observed at 1 mg/mL.
Overall, this study shows that the tetravalent presentation of lead compound 1 generated in glycoconjugate 3 significantly impaired its ability to interact with C. albicans cells, although it maintained anti-adhesion activity when pre-incubated with BECs. While no significant reduction of adhesion was observed when C. albicans cells were pre-treated with glycoconjugate 3, this compound was able to inhibit adhesion of the yeast when BECs were pre-treated or in competition assays. This clearly indicates that the multivalent compounds in this study do not interact effectively with C. albicans cell wall components; the observed reduction in adhesion appears to be due to preferential binding of the compounds to BECs over the yeast cells. Tetra-galactoside 15, which does not display the structural features of lead compound 1 nor a TEG linker, generally showed the lowest inhibition of adhesion, although at lower concentration it showed better activity; however, this compound did not follow a dose–response pattern. The original lead compound 1 was the most effective inhibitor of yeast adhesion. Interestingly, the exclusion assays show that compound 1 produces a decrease in adhesion not only when pre-exposed to C. albicans cells, as our previous work has shown, but also there is a significant contribution to the activity of compound 1 arising from interaction with BECs. These results highlight that the optimization of activity of derivatives of compound 1 should consider also interactions with BECs as a potential approach to broad-spectrum anti-adhesive glycoconjugates.
3. Materials and Methods
3.1. Chemistry
3.1.1. General Methods
All reagents for synthesis were bought commercially and used without further purification. Tetrahydrofuran (THF) was freshly distilled over sodium wire and benzophenone. Dichloromethane (DCM) was freshly distilled over CaH2 prior to use. Reactions were monitored with thin layer chromatography (TLC) on Merck Silica Gel F254 plates. Detection was effected by UV (λ = 254 nm) or charring in a mixture of 5% sulfuric acid-ethanol. NMR spectra were recorded using Bruker Ascend 500 spectrometer at 293K. All chemical shifts were referenced relative to the relevant deuterated solvent residual peaks. Assignments of the NMR spectra were deduced using 1H NMR and 13C NMR, along with 2D experiments (COSY, HSQC and HMBC). Chemical shifts are reported in ppm. Flash chromatography was performed with Merck Silica Gel 60. Microwave reactions were carried out using a CEM Discover Microwave Synthesizer. Optical rotations were obtained from an AA-100 polarimeter, and
values are given in 10−1 cm2·g−1. High-performance liquid chromatography analysis (HPLC, Waters Alliance 2695) was performed in final compounds and indicated purity of 95% based on integrations without the use of an internal standard. High-resolution mass spectrometry (HRMS) was performed on an Agilent-LC 1200 Series coupled to a 6210 Agilent Time-Of-Flight (TOF) mass spectrometer equipped with an electrospray source in both positive and negative (ESI+/−) modes. Infrared spectra were obtained as a film on NaCl plates or as KBr disks in the region 4000–400 cm−1 on a Perkin Elmer Spectrum 100 FT-IR spectrophotometer. Spectroscopic data for all compounds are provided in the Supplementary Materials.
3.1.2. Synthetic Procedures
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethylamide)-N”-(2-bromoacetamido)-5-aminobenzene-1,3-dicarboxamide (7)
6 [21] (1.128 g, 1.13 mmol) was dissolved in dry DCM (20 mL). NEt3 (0.19 mL, 1.35 mmol) was added to this solution. Bromoacetyl bromide (0.12 mL, 1.35 mmol) was dissolved in dry DCM (5 mL) in a separate round-bottom flask. The first solution was added to the second dropwise via a cannula and the resulting reaction mixture was allowed to stir for 16 h. The reaction mixture was washed with water (20 mL), HCl (1 N, 20 mL), sat. NaHCO3 solution (20 mL), followed by brine (20 mL). The organic phase was dried (MgSO4) and the solvent was removed in vacuo to obtain the pure product 7 without further purification as a brown, sticky solid (1.056 g, 83%). Rf = 0.65 (DCM, 5% MeOH). −4.0 (c 1.0, DCM). 1H NMR (500 MHz, CDCl3) δ 9.10 (s, 1H, NHCOCH2Br), 8.09–7.90 (m, 6H, triaz-H, CONHCH2-triaz and Ar-H), 7.75 (s, 1H, Ar-H), 5.93 (d, J = 9.2 Hz, 2H, H-1), 5.60 (t, J = 9.7 Hz, 2H, H-2), 5.54 (d, J = 2.9 Hz, 2H, H-4), 5.32–5.26 (m, 2H, H-3), 4.67 (ddd, J = 39.3, 15.1, 5.4 Hz, 4H, CH2-triaz), 4.31 (t, J = 6.4 Hz, 2H, H-5), 4.22–4.11 (m, 4H, H-6 and H-6′), 3.98 (s, 2H, CH2-Br), 2.21 (s, 6H, OAc), 2.00 (app d, J = 2.7 Hz, 12H, OAc × 2), 1.82 (s, 6H, OAc). 13C NMR (125 MHz, CDCl3) δ 170.4 (CO of OAc), 170.1 (CO of OAc), 169.8 (CO of OAc), 169.4 (CO of OAc), 166.5 (CONHCH2-triaz), 165.0 (COCH2Br), 145.6 (C-triaz), 138.3 (Ar-C), 135.0 (Ar-C), 121.6 (CH-triaz), 121.4 (Ar-CH), 121.2 (Ar-CH), 86.2 (C-1), 74.0 (C-5), 70.8 (C-3), 68.1 (C-2), 66.8 (C-4), 61.2 (C-6), 35.5 (CH2-triaz), 29.6 (NHCOCH2Br), 20.7 (CH3 of OAc), 20.6 (CH3 of OAc), 20.5 (CH3 of OAc), 20.3 (CH3 of OAc). IR (film on NaCl): 3345, 3087, 2975, 1752, 1651, 1536, 1446, 1371, 1227, 1063, 924 732 cm−1. HRMS (ESI+): m/z calculated for C44H52BrN12O21 + H+ [M+H+]: 1122.2539, found 1122.2545.
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethyl amide)-N”-(2-azidoacetamido)-5-aminobenzene-1,3-dicarboxamide (8)
7 (231 mg, 0.206 mmol) and NaN3 (30 mg, 0.412 mmol) were dissolved in anhydrous DMF (10 mL) and heated to 80 °C. The reaction mixture was allowed to stir for 16 h. The solvent was removed in vacuo, and the resulting residue was re-dissolved in DCM (20 mL) and was washed with brine (20 mL × 3). The organic phase was dried over MgSO4 and the solvent was removed in vacuo to obtain the pure product 8 without further purification as a yellow solid (1.056 g, 83%). Rf = 0.41 (DCM:MeOH 9:1).
-5.6 (c 0.9, DCM). 1H NMR (500 MHz, CDCl3) δ 9.10 (s, 1H, NHCOCH2N3), 8.18 (s, 2H, NHCH2CCH), 8.02 (s, 2H, Ar-H), 7.97 (s, 2H, CH-triaz), 7.82 (s, 1H, Ar-H), 5.95 (d, J = 9.2 Hz, 2H, H-1), 5.61 (t, J = 9.7 Hz, 2H, H-2), 5.56 (d, J = 3.1 Hz, 2H, H-4), 5.32 (dd, J = 10.1, 3.5 Hz, 2H, H-3), 4.67 (ddd, J = 20.4, 15.4, 5.5 Hz, 4H, CH2-triaz), 4.34 (t, J = 6.6 Hz, 2H, H-5), 4.23–4.13 (m, 4H, H-6 and H-6′), 4.06 (s, 2H, CH2-N3), 2.21 (s, 6H, OAc), 2.01 (s, 12H, OAc × 2), 1.82 (s, 6H, OAc). 13C NMR (125 MHz, CDCl3) δ 170.4 (CO of OAc), 170.1 (CO of OAc), 169.9 (CO of OAc), 169.3 (CO of OAc), 166.5 (CONHCH2CCH), 166.3 (COCH2N3), 145.5 (C-triaz), 138.0 (Ar-C), 134.9 (Ar-C), 121.6 (Ar-CH and CH-triaz), 121.4 (Ar-CH), 86.1 (C-1), 73.9 (C-5), 70.8 (C-3), 68.1 (C-2), 66.9 (C-4), 61.2 (C-6), 52.5 (CH2N3), 35.4 (CH2-triaz), 20.7 (CH3 of OAc), 20.6 (CH3 of OAc), 20.5 (CH3 of OAc), 20.2 (CH3 of OAc). IR (film on NaCl): 3342, 2942, 2110, 1747, 1655, 1528, 1427, 1368, 1211, 1046, 923, 733 cm−1. HRMS (ESI+): m/z calculated for C44H52N12O21 + Na+ [M+Na+]: 1107.3268, found 1107.3303.
Synthesis of 2-[2-(2-Propargyloxyethoxy)ethoxy]ethanol (9)
Procedure adapted from Weil et al. [30]. Triethylene glycol (1 mL, 7.48 mmol, 3 equiv) was diluted with dry THF (10 mL) under N2. The solution was cooled to 0 °C and NaH (60% oil dispersion) (0.1 g, 2.49 mmol) was added portion-wise. The reaction was allowed to warm up to rt and was stirred for 20 min. Propargyl bromide (0.27 mL, 2.49 mmol) was added dropwise. The reaction mixture was allowed to stir overnight. Column chromatography (100% EtOAc) eluted the pure product 9 as a clear oil (0.292 g, 75%). (Rf = 0.42: EtOAc) 1H NMR (500 MHz, CDCl3) δ 4.09–4.08 (m, 2H, CH2CCH), 3.63–3.53 (m, 10H, CH2 × 5), 3.50–3.46 (m, 2H, CH2-OH), 3.11 (s, 1H, OH), 2.39 (t, J = 2.8 Hz, 1H, CH2CCH). The NMR data is in agreement with the data reported in the literature [30].
Synthesis of 2-(2-(2-Propargyloxyethoxy)ethoxy)ethyl-4-methylbenzenesulfonate (10)
This procedure was adapted from Ramström et al. [31]. 9 (0.288 g, 1.53 mmol) was dissolved in DCM (5 mL). TsCl (0.321 g, 1.68 mmol, 1.1 equiv) was added and the mixture was cooled to 0 °C on ice. KOH (0.343 g, 6.12 mmol, 4 equiv) was added slowly after grinding. The mixture was vigorously stirred for 2 h. The mixture was poured onto ice-water and extracted with DCM (3 × 20 mL). The combined organic layers were dried over MgSO4, filtered and concentrated in vacuo to give the pure product 10 as a clear oil (0.457 g, 87%). 1H NMR (500 MHz, CDCl3) δ 7.79 (d, J = 8.3 Hz, 2H, Ar-H), 7.33 (d, J = 8.2 Hz, 2H, Ar-H), 4.18 (d, J = 2.4 Hz, 2H, CH2CCH), 4.16–4.13 (m, 2H, CH2OTs), 3.69–3.65 (m, 4H, CH2 × 2), 3.65–3.61 (m, 2H, CH2), 3.58 (s, 4H, CH2 × 2), 2.43 (s, 3H, CH3-Ar), 2.42 (t, J = 2.4 Hz, 1H, CH2CCH). The NMR data are in agreement with the data reported in the literature [31].
Synthesis of N, N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethyl amide)-N”-(2-4-((2-(2-(2-(4-methylbenzenesulfonate)ethoxy)ethoxy)ethoxy) methyl)-1H-1,2,3-triazol-1-yl)acetamido)-5-aminobenzene-1,3-dicarboxamide (11)
Copper sulphate pentahydrate (20 mg) and sodium ascorbate (40 mg) were added to a solution 8 (0.516 g, 0.476 mmol) and 10 (0.163 g, 0.476 mmol) in CH3CN/H2O (4 mL/2 mL). The reaction was allowed to stir in the MW at 100 °C until deemed complete by TLC analysis (15 min). The solvent was removed in vacuo. The residue was dissolved in DCM (30 mL), washed with brine (20 mL × 3), and dried (MgSO4). The mixture was filtered and the solvent was removed in vacuo to yield the crude product, which was purified by silica gel column chromatography (DCM:MeOH 98:2–95:5) to give the pure product 11 as a yellow solid (0.307 g, 74%). Rf = 0.45 (DCM:MeOH 9:1).
-5 (c 1, DCM). 1H NMR (500 MHz, CDCl3) δ 9.82 (s, 1H, NHCH2N3), 8.16 (s, 2H, NHCH2CCH), 7.97 (s, 2H, CH-triaz), 7.84 (appd, J = 2.8 Hz, 3H, Ar-H × 2 and CH-triaz), 7.76 (s, 1H, Ar-H), 7.69 (d, J = 8.3 Hz, 2H, Ar-H of OTs), 7.26 (d, J = 8.1 Hz, 2H, Ar-H of OTs), 5.89 (d, J = 9.2 Hz, 2H, H-1), 5.60 (t, J = 9.7 Hz, 2H, H-2) 5.50 (d, J = 2.9 Hz, 2H, H-4), 5.26 (dd, J = 10.3, 3.3 Hz, 2H, H-3), 5.21 (s, 2H, CH2), 4.67–4.55 (m, 6H, CH2-triaz and CH2CN3), 4.28 (t, J = 6.6 Hz, 2H, H-5), 4.11 (qd, J = 11.5, 6.6 Hz, 2H, H-6 and H-6′), 4.06–4.04 (m, 2H, CH2), 3.65–3.59 (m, 2H, CH2), 3.58–3.54 (m, 4H, CH2 × 2), 3.48 (s, 2H, CH2), 2.37 (s, 3H, CH3 of OTs), 2.14 (s, 6H, CH3 of OAc), 1.96 (s, 6H, CH3 of OAc), 1.95 (s, 6H, CH3 of OAc), 1.75 (s, 6H, CH3 of OAc). 13C NMR (125 MHz, CDCl3) δ 170.4 (CO of OAc), 170.1 (CO of OAc), 169.9 (CO of OAc), 169.3 (CO of OAc), 166.6 (CONHCH2CCH), 164.5 (COCH2N3), 145.4 (C-triaz), 144.9 (Ar-C of OTs), 144.8 (CHCN3), 138.1 (Ar-C), 134.8 (Ar-C), 132.7 (Ar-C of OTs), 129.9 (Ar-CH of OTs), 127.9 (Ar-CH of OTs), 125.3 (CHCN3), 121.9 (CH-triaz), 121.6 (Ar-CH), 121.3 (Ar-CH), 86.0 (C-1), 73.8 (C-5), 70.9 (C-3), 70.5 (CH2), 70.4 (CH2), 70.3 (CH2), 69.7 (CH2), 69.4 (CH2), 68.6 (CH2), 68.0 (C-2), 66.9 (C-4), 64.3 (NHCOCH2N3), 61.1 (C-6), 52.8 (CH2), 35.4 (CH2-triaz), 21.6 (CH3 of OAc), 20.6 (CH3 of OAc), 20.5 (CH3 of OAc), 20.2 (CH3 of OAc). IR (film on NaCl): 3344, 3091, 2939, 1754, 1657, 1599, 1535, 1448, 1370, 1223, 1176, 1095, 1054, 924 cm−1. HRMS (ESI+): m/z calculated for C60H74N12O27S + Na+ [M+Na+]: 1449.4405, found 1449.4332.
Synthesis of N,N’-di-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl-1,2,3-triazol-4-ylmethylamide)-N”-(2-4-((2-(2-(2-azidoethoxy)ethoxy)ethoxy)methyl)-1H-1,2,3-triazol-1-yl)acetamido)-5-aminobenzene-1,3-dicarboxamide (12)
Compound 11 (183 mg, 0.128 mmol) and NaN3 (17 mg, 0.256 mmol) were dissolved in anhydrous DMF (10 mL) and heated to 80 °C. The reaction mixture was allowed to stir for 16 h. The solvent was removed in vacuo, and the resulting residue was dissolved in DCM (20 mL) and was washed with brine (20 mL × 3). The organic phase was dried (MgSO4) and the solvent was removed in vacuo to obtain the pure product 12 without further purification as a yellow solid (167 g, 100%). Rf = 0.42 (DCM:MeOH 9:1).
-3 (c 1, DCM). 1H NMR (500 MHz, CDCl3) δ 9.79 (s, 1H, NHCH2N3), 8.13 (s, 2H, NHCH2-triaz), 7.94 (s, 2H, CH-triaz), 7.82 (s, 1H, CH-triaz), 7.77 (s, 2H, Ar-H), 7.70 (s, 1H, Ar-H), 5.85 (d, J = 9.2 Hz, 2H, H-1), 5.57 (t, J = 9.8 Hz, 2H, H-2), 5.47 (d, J = 2.7 Hz, 2H, H-4), 5.25–5.15 (m, 4H, H-3 and CH2), 4.66–4.48 (m, 6H, CH2-triaz × 3), 4.24 (t, J = 6.3 Hz, 2H, H-5), 4.13–4.04 (m, 4H, H-6 and H-6′), 3.67–3.47 (m, 8H, CH2 × 4), 3.27–3.24 (m, 2H, CH2), 2.12 (s, 6H, CH3 of OAc), 1.94 (s, 6H, CH3 of OAc), 1.93 (s, 6H, CH3 of OAc), 1.73 (s, 6H, CH3 of OAc). 13C NMR (125 MHz, CDCl3) δ 170.5 (CO of OAc), 170.2 (CO of OAc), 170.0 (CO of OAc), 169.4 (CO of OAc), 166.7 (CONHCH2-triaz), 164.5 (COCH2N3), 145.6 (C-triaz), 145.0 (CHCN3), 134.9 (Ar-C), 125.4 (CHCN3), 121.9 (CH-triaz), 121.7 (Ar-CH), 121.3 (Ar-CH), 86.2 (C-1), 74.0 (C-5), 71.0 (C-3), 70.6 (CH2 × 2), 70.0 (CH2), 69.9 (CH2), 68.1 (C-2), 67.0 (C-4), 64.5 (NHCOCH2N3), 61.2 (C-6), 50.7 (CH2), 35.5 (CH2-triaz), 20.7 (CH3 of OAc × 2), 20.6 (CH3 of OAc), 20.4 (CH3 of OAc). IR (film on NaCl): 3335, 3088, 2924, 2109, 1754, 1658, 1600, 1534, 1447, 1370, 1222, 1054, 924 cm−1. HRMS (ESI+): m/z calculated for C53H67N15O24 + Na+ [M+Na+]: 1320.4381, found 1320.4375.
Synthesis of tert-butyl-(4,8,12,16-tetra-aza)(5,9,13,17-tetra-oxo)(4,8,12,16-tetra-N-propargyl)octadeconoate (2)
The method outlined by Faure, Taillefumier and coworkers was adapted to synthesise the tetrameric propargylated scaffold [25]. The secondary amine was then acetylated using the following procedure:
Tetrameric propargylated scaffold (0.490 g, 0.96 mmol) was dissolved in DCM (20 mL). Acetic anhydride (0.91 mL, 9.6 mmol) was added to the solution. The reaction mixture was allowed to stir for 6 h at rt and was concentrated under reduced pressure. The crude mixture was dissolved in ethyl acetate (20 mL) and was washed with sat NaHCO3 (20 mL × 2) and brine (20 mL × 2). The organic layer was dried (MgSO4), filtered and then concentrated in vacuo. The crude product was purified by silica gel column chromatography (DCM:MeOH 9:1) to give the pure product as a pale-yellow oil (515 mg, 97%). 1H NMR (500 MHz, CDCl3) δ 4.24–4.06 (m, 8H), 3.83–3.70 (m, 3H), 3.72–3.60 (m, 5H), 2.91–2.68 (m, 6H), 2.55 (m, 2H), 2.37–2.23 (m, 2H), 2.24–2.10 (m, 4H), 1.43 (s, 9H, C(CH3)3). 13C NMR (125 MHz, CDCl3) δ 171.5, 171.2, 170.9, 170.1, 81.7, 81.6, 81.4, 80.9, 78.9, 78.9, 78.6, 72.9, 72.8, 72.6, 72.5, 72.5, 72.1, 71.9, 71.7, 44.4, 44.2, 43.9, 43.7, 43.6, 43.5, 43.0, 42.8, 39.8, 39.1, 39.0, 38.9, 38.6, 38.4, 34.8, 34.5, 34.4, 34.3, 34.1, 32.2, 31.8, 29.7, 28.1, 21.8, 21.5. HRMS (ESI+): m/z calculated for C30H40N4O6 + H+ [M+H+]:553.3026, found 553.3015. 1H NMR and 13C NMR spectroscopic data corresponded to that found in the literature [25].
Synthesis of Acetylated Tetravalent β-Peptoid Glycocluster (13)
Copper sulphate pentahydrate (40 mg) and sodium ascorbate (80 mg) were added to a solution of 10 (30 mg, 0.0231 mmol) and 14 (13 mg, 0.0231 mmol) in CH3CN/H2O (4 mL/ 2 mL). The reaction was allowed to stir in the MW at 100 °C for 10 min × 2. The solvent was removed in vacuo. The residue was dissolved in DCM (30 mL), washed with brine (20 mL × 3), and dried (MgSO4). The mixture was filtered and the solvent was removed in vacuo to yield the crude product, which was purified by silica gel column chromatography (DCM:MeOH 98:2–95:5) to give the pure product 13 as a yellow sticky solid (77 mg, 58%). Rf = 0.48 (DCM:MeOH 9:1).
-7 (c 1, DCM). 1H NMR (500 MHz, CDCl3) δ 10.08 (s, 4H, NHCOCH2N3), 8.56–7.59 (m, 36H, NHCH2CCH × 8, CH2CCH × 16 and Ar-H × 12), 5.91 (s, 8H, H-1), 5.63 (t, J = 10.2 Hz, 8H, H-2), 5.53 (s, 8H, H-4), 5.28 (d, J = 10.2 Hz, 16H, H-3 and CH2 × 4), 4.80–4.39 (m, 6H, CH2-triaz, CH2′s), 4.38–4.05 (m, H, H-5 and H-6, CH2′s), 3.86–3.31 (m, 13H, CH2′s), 3.11–2.31 (m, 4H, CH2′s), 2.18 (s, 7H, OAc and NAc), 1.99 (s, 15H, OAc), 1.79 (s, 6H, OAc), 1.43 (s, 3H, tert-butyl). 13C NMR (125 MHz, CDCl3) δ 170.59, 170.35, 170.09, 122.1, 121.6, 120.7, 86.3 (C-1), 74.1 (C-5), 71.1 (C-3), 71.0 (CH2), 70.7 (CH2), 70.6 (CH2), 70.5 (CH2), 69.8 (CH2), 69.3 (CH2), 68.2 (C-2), 67.1 (C-4), 64.7 (CH2), 61.3 (C-6), 52.9 (CH2), 50.3 (CH2), 44.2 (CH2), 43.5 (CH2), 43.2 (CH2), 42.8 (CH2), 40.0 (CH2), 39.8 (CH2), 38.7 (CH2), 35.5 (CH2-triaz), 34.3 (CH2), 33.8 (CH2), 32.1 (CH2), 29.89, 28.3 (C(CH3)), 21.7 (CH3 of NHAc), 20.84 (CH3 of OAc), 20.72 (CH3 of OAc), 20.45 (CH3 of OAc), 1.21. IR (film on NaCl): 3392, 2927, 1753, 1647, 1536, 1448, 1370, 1223, 1092, 1060, 923, 732 cm−1. MALDI-TOF-MS [M+H]+: m/z calculated for C242H309N64O102 +H+: 5744.104, found 5744.346.
Synthesis of Tetravalent β-Peptoid Glycocluster (3)
Compound 13 (70 mg, 0.0122 mmol) was dissolved in methanol/H2O (4 mL, 2 mL). NEt3 (0.1 mL) was added, and the reaction mixture was allowed to stir at 45 °C for 6 h. The solution was cooled, Amberlite H+ was added and the mixture was allowed to stir for 30 min. The solution was filtered, and the solvent was removed in vacuo. Excess NEt3 was removed using the Schlenk line. The product was freeze-dried overnight to yield the pure product 3 as a white fluffy solid (44 mg, 82%). 1H NMR (500 MHz, D2O) δ 8.62–7.72 (m, 28H, Ar-H and triaz-H), 5.69 (s, 10H, H-1 and CH2s), 5.47 (s, 6H, CH2s), 4.74–4.44 (m, 24H, CH2-triaz and CH2s), 4.22 (s, 14H, H-2 and CH2s), 4.11 (d, J = 20.3 Hz, 14H, H-4 and CH2s), 3.99 (s, 8H, H-5), 3.88 (d, J = 9.8 Hz, 14H, H-3 and CH2s), 3.84–3.36 (m, 60H, H-6, H-6′ and CH2s), 2.99–2.31 (m, 24H, CH2s), 1.99–1.86 (m, 3H CH3). 13C NMR (125 MHz, DMSO) δ 171.1, 170.9, 166.1, 166.0, 165.1, 162.8, 145.5, 144.3, 139.1, 135.6, 126.2, 124.0, 122.3, 121.7, 88.5, 78.9, 74.2, 70.2, 70.1, 70.0, 69.8, 69.5, 69.4, 69.1, 68.9, 63.9, 60.9, 60.7, 52.6, 49.9, 49.8, 45.9, 40.5, 40.4, 40.2, 40.0, 39.9, 39.7, 39.5, 36.3, 35.4, 31.3, 28.2, 9.1. IR (ATR): 3301, 2925, 1650, 1540, 1443, 1388, 1253, 1092, 1055, 891 cm−1. MALDI-TOF-MS [M+H]+: m/z calculated for C242H309N64O102 +Na+: 4422.7465, found 4422.807.
Synthesis of 2,6,10,14-tetraoxo-3,7,11,15-tetrakis((1-(2,3,4,6-tetra-O-acetyl-β-d-galactopyranosyl -1H-1,2,3-triazol-4-yl)methyl)-3,7,11,15-tetraazaoctadecan-18-oic acid (14)
Copper sulphate pentahydrate (40 mg) and sodium ascorbate (80 mg) were added to a solution of per-acetylated-1-β-azido galactose [28] (567 mg, 1.520 mmol) and 2 [25] (200 mg, 0.362 mmol) in CH3CN/H2O (4 mL/2 mL). The reaction was stirred in the MW at 100 °C for 20 min (10 min × 2). The solvent was removed in vacuo. The residue was dissolved in DCM (30 mL), washed with brine (20 mL × 3), and dried (MgSO4). The mixture was filtered and the solvent was removed in vacuo to yield the crude product, which was purified by silica gel column chromatography (DCM:MeOH 98:2–95:5) to give the pure product 14 as a yellow sticky solid (503 mg, 68%). Rf = 0.47 (DCM:MeOH 9:1).
-4.71 (c 0.85, DCM). 1H NMR (500 MHz, CDCl3) δ 7.98–7.77 (m, 4H, triaz-H), 5.98–5.74 (m, 4H, H-1), 5.59–5.42 (m, 8H, H-2 and H-4), 5.37–5.18 (m, 4H, H-3), 4.81–4.49 (m, 8H, CH2 × 4), 4.35–4.08 (m, 12H, H-5, H-6 and H-6′), 3.83–3.53 (m, 8H, CH2 × 4), 3.02–2.68 (m, 6H, CH2 × 3), 2.65–2.47 (m, 2H, CH2), 2.27–2.21 (m, 12H, OAc), 2.20–2.15 (m, 3H, NAc), 2.06–1.98 (m, 24H, OAc), 1.92–1.80 (m, 12H, OAc), 1.47–1.40 (m, 9H, C(CH3)3). 13C NMR (125 MHz, CDCl3) δ 171.3, 170.3, 170.1, 169.8, 168.8, 144.8, 144.6, 122.3, 122.2, 86.3, 77.3, 77.0, 76.8, 74.0, 70.8, 68.0, 66.8, 61.1, 45.2, 44.2, 40.8, 34.6, 31.9, 31.8, 28.1, 22.0, 21.5, 20.7, 20.6, 20.5, 20.2. IR (ATR): 2938, 1746, 1638, 1423, 1368, 1211, 1157, 1091, 1044, 952, 922, 841, 733 cm−1. HRMS (ESI+): m/z calculated for C86H116N16O42 + Na+ [M+Na+]: 2067.7331, found 2067.7223.
Synthesis of 2,6,10,14-tetraoxo-3,7,11,15-tetrakis((1-(β-d-galactopyranosyl-1H-1,2,3-triazol-4-yl)methyl)-3,7,11,15-tetraazaoctadecan-18-oic acid (15)
Compound 14 (452 mg, 0.221 mmol) was dissolved in methanol/H2O (4 mL, 2 mL). NEt3 (0.1 mL) was added, and the reaction mixture was allowed to stir at 45 °C for 6 h. The solution was cooled, Amberlite H+ was added and the mixture was allowed to stir for 30 min. The solution was filtered, and the solvent was removed in vacuo. Excess NEt3 was removed using the Schlenk line. The product was freeze-dried overnight to yield the pure product 15 as a white fluffy solid (267 mg, 92%).
+14.0 (c 0.5, H2O). 1H NMR (500 MHz, D2O) δ 8.23–8.16 (m, 2H), 8.08 (s, 2H), 5.64–5.54 (m, 4H, H-1), 4.65–4.42 (m, 8H, CH2-triaz and CH2), 4.19–4.06 (m, 4H, H-2), 4.01 (appdd, J = 5.2, 3.2 Hz, 4H, H-4), 3.97–3.86 (m, 4H, H-5), 3.85–3.76 (m, 4H, H-3), 3.75–3.61 (m, 12H, H-6, H-6′ and CH2 × 2), 3.58–3.51 (m, 4H), 2.80–2.46 (m, 8H, CH2 × 4), 2.13–2.04 (m, 1H), 1.16 (s, 3H, CH3). 13C NMR (126 MHz, DMSO) δ 173.1, 170.5, 170.3, 144.6, 122.5, 122.3, 88.6, 88.5, 78.9, 75.6, 74.2, 69.9, 68.9, 60.9, 58.6, 45.7, 44.7, 43.8, 43.0, 42.9, 39.9, 22.1, 21.7, 9.0. IR (film on NaCl): 3299, 2916, 1719, 1620, 1451, 1421, 1365, 1224, 1087, 1053, 885 cm−1. HRMS (ESI+): m/z calculated for C50H76N16O26 + Na+ [M+Na+]: 1339.5014, found 1339.5032.
3.2. Biological Evaluation
Fungal Strain: C. albicans (clinical isolate from a corneal infection) was maintained on sabouraud dextrose agar and cultures were grow to the stationary phase (1–2 × 108 cells/mL) overnight in YEPD broth (1% (w/v) yeast extract, 2% (w/v) bacteriological peptone, 2% (w/v) glucose) at 30 °C and 200 rpm. Stationary phase yeast cells were harvested, washed with PBS and resuspended at a density of 1 × 108 cells/mL in PBS.
Buccal epithelial cells: Buccal epithelial cells (BECs) were harvested from healthy volunteers by gently scraping the inside of the cheek with a sterile tongue depressor. Cells were washed in PBS and resuspended at a density of 5 × 105 cells/mL.
Adherence assays: Yeast cells were mixed with BECs in a ratio of 50:1 in a final volume of 2 mL and incubated at 30 °C and 200 rpm for 90 min. The BEC/yeast cell mixture was harvested by passing through a polycarbonate membrane containing 30 µm pores which trapped the BECs but allowed unattached yeast cells to pass through. This was washed ×2 with 10 mL PBS and cells remaining on the membrane were collected and placed on glass slides which were left to air-dry overnight. The cells were heat fixed and stained using 0.5% (w/v) crystal violet, rinsed using cold water to remove any surplus stain and left to air-dry for 30 min. The number of C. albicans cells adhering to a sample of 200 BECs per treatment was assessed microscopically. In the exclusion assay, the yeast cells or BECs were incubated for 90 min in the presence of each compound at the given concentration. After this time, the cells were harvested and washed twice with PBS before being resuspended in 1 mL PBS before being mixed with BECs or yeast cells (as described). In the competition assay format yeast cells, BECs and compound (at the given concentration) were co-incubated for 90 min prior to harvesting.
Statistics: All experiments were performed on three independent occasions. In each assay, the number of yeast cells adhering to 200 randomly chosen BECs was determined. Results are mean ± SEM.
4. Conclusions
In summary, we report the synthesis of a multivalent display of divalent galactoside 1, a potent inhibitor of the adhesion of C. albicans to BECSs. Glycoconjugate 3 was built upon a linear peptoid scaffold, to access distinct spatial presentations of the recognition motifs provided by lead compound 1. This compound, together with control compound 15, were successfully synthesised using a series of sequential CuAAC reactions. The ability of these compounds to inhibit adhesion of C. albicans to BECs was then evaluated. Although multivalent presentations of carbohydrate epitopes are commonly used to increase their biological activity, we did not observe this effect for the compounds studied herein. In fact, the exclusion assays indicated that the multivalent glycoconjugates did not bind effectively to C. albicans cell wall, in contrast to their parent compound 1. Since the fungal target for lead compound 1 is not known, the results highlight the importance of detailed structural knowledge of the target proteins when considering the design of multivalent presentation. Interestingly, the inhibition of C. albicans adhesion observed for the compounds in this study is then likely due to interactions with BECs. Further studies are currently underway in our laboratory to establish if these are specific interactions and to optimize structural parameters, as this could extend the scope of activity of these compounds to other pathogens in the oral cavity.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10050572/s1; it includes spectroscopic data for the synthetic compounds described herein.
Author Contributions
Conceptualization, K.K. and T.V.-T.; methodology, H.M. (Harlei Martin) and H.M. (Hannah Masterson); formal analysis, H.M. (Harlei Martin); investigation, H.M. (Harlei Martin); resources, K.K. and T.V.-T.; data curation, all authors; writing—original draft preparation, H.M. (Harlei Martin); writing—review and editing, H.M. (Harlei Martin), K.K. and T.V.-T.; supervision, K.K. and T.V.-T. All authors have read and agreed to the published version of the manuscript.
Funding
We would like to acknowledge Maynooth University for the provision of the John and Pat Hume Scholarship to H. Martin.
Institutional Review Board Statement
All experiments were performed in accordance with the Guidelines stipulated in Directive 2004/23/EC of the European Parliament and of the Council (31 March 2004). Experiments were conducted according to the guidelines by the Ethics Committee at Maynooth University.
Informed Consent Statement
Informed consent was not necessary for this study.
Data Availability Statement
The data presented in this study are available within the article and in the supplementary material.
Acknowledgments
We would like to acknowledge Maynooth University for the provision of the John and Pat Hume Scholarship to H. Martin.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Varki, A. Biological roles of glycans. Glycobiology 2012, 27, 3–49. [Google Scholar] [CrossRef]
- Cozens, D.; Read, R.C. Anti-adhesion methods as novel therapeutics for bacterial infections. Expert Rev. Anti-Infect. Ther. 2012, 10, 1457–1468. [Google Scholar] [CrossRef]
- Linman, M.J.; Taylor, J.D.; Yu, H.; Chen, X.; Cheng, Q. Surface Plasmon Resonance Study of Protein−Carbohydrate Interactions Using Biotinylated Sialosides. Anal. Chem. 2008, 80, 4007–4013. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Dimick, S.M.; Powell, S.C.; McMahon, S.A.; Moothoo, D.N.; Naismith, J.H.; Toone, E.J. On the Meaning of Affinity: Cluster Glycoside Effects and Concanavalin, A.J. Am. Chem. Soc. 1999, 121, 10286–10296. [Google Scholar] [CrossRef]
- Lee, Y.C.; Lee, R.T. Carbohydrate-Protein Interactions: Basis of Glycobiology. Acc. Chem. Res. 1995, 28, 321–327. [Google Scholar] [CrossRef]
- Boukerb, A.M.; Rousset, A.; Galanos, N.; Méar, J.-B.; Thépaut, M.; Grandjean, T.; Gillon, E.; Cecioni, S.; Abderrahmen, C.; Faure, K.; et al. Antiadhesive Properties of Glycoclusters against Pseudomonas aeruginosa Lung Infection. J. Med. Chem. 2014, 57, 10275–10289. [Google Scholar] [CrossRef] [PubMed]
- Chabre, Y.M.; Roy, R. Multivalent glycoconjugate syntheses and applications using aromatic scaffolds. Chem. Soc. Rev. 2013, 42, 4657–4708. [Google Scholar] [CrossRef]
- Martínez, Á.; Ortiz Mellet, C.; García Fernández, J.M. Cyclodextrin-based multivalent glycodisplays: Covalent and supramolecular conjugates to assess carbohydrate–protein interactions. Chem. Soc. Rev. 2013, 42, 4746–4773. [Google Scholar] [CrossRef]
- Galan, M.C.; Dumy, P.; Renaudet, O. Multivalent glyco(cyclo)peptides. Chem. Soc. Rev. 2013, 42, 4599–4612. [Google Scholar] [CrossRef]
- Miura, Y. Design and synthesis of well-defined glycopolymers for the control of biological functionalities. Polym. J. 2012, 44, 679–689. [Google Scholar] [CrossRef]
- Yilmaz, G.; Becer, C.R. Glyconanoparticles and their interactions with lectins. Polym. Chem. 2015, 6, 5503–5514. [Google Scholar] [CrossRef]
- Hill, S.; Galan, M.C. Fluorescent carbon dots from mono- and polysaccharides: Synthesis, properties and applications. Beilstein J. Org. Chem. 2017, 13, 675–693. [Google Scholar] [CrossRef] [PubMed]
- Bernardi, A.; Jimenez-Barbero, J.; Casnati, A.; De Castro, C.; Darbre, T.; Fieschi, F.; Finne, J.; Funken, H.; Jaeger, K.-E.; Lahmann, M.; et al. Multivalent glycoconjugates as anti-pathogenic agents. Chem. Soc. Rev. 2013, 42, 4709–4727. [Google Scholar] [CrossRef]
- Pieters, R.J. Maximising multivalency effects in protein–carbohydrate interactions. Org. Biomol. Chem. 2009, 7, 2013–2025. [Google Scholar] [CrossRef]
- Pifferi, C.; Goyard, D.; Gillon, E.; Imberty, A.; Renaudet, O. Synthesis of Mannosylated Glycodendrimers and Evaluation against BC2L-A Lectin from Burkholderia cenocepacia. ChemPlusChem 2017, 82, 390–398. [Google Scholar] [CrossRef] [PubMed]
- Arsiwala, A.; Castro, A.; Frey, S.; Stathos, M.; Kane, R.S. Designing Multivalent Ligands to Control Biological Interactions: From Vaccines and Cellular Effectors to Targeted Drug Delivery. Chem. Asian J. 2019, 14, 244–255. [Google Scholar] [CrossRef]
- Brissonnet, Y.; Araoz, R.; Sousa, R.; Percevault, L.; Brument, S.; Deniaud, D.; Servant, D.; Le Questel, J.Y.; Lebreton, J.; Gouin, S.G. Di- and heptavalent nicotinic analogues to interfere with α7 nicotinic acetylcholine receptors. Bioorg. Med. Chem. 2019, 27, 700–707. [Google Scholar] [CrossRef]
- González-Cuesta, M.; Ortiz-Mellet, C.; García Fernández, J.M. Carbohydrate supramolecular chemistry: Beyond the multivalent effect. Chem. Commun. 2020, 56, 5207–5222. [Google Scholar] [CrossRef] [PubMed]
- Chalopin, T.; Brissonnet, Y.; Sivignon, A.; Deniaud, D.; Cremet, L.; Barnich, N.; Bouckaert, J.; Gouin, S. Inhibition profiles of mono- and polyvalent FimH antag onists against 10 different Escherichia coli strains. Org. Biomol. Chem. 2015, 13, 11369–11375. [Google Scholar] [CrossRef]
- Lehot, V.; Brissonnet, Y.; Dussouy, C.; Brument, S.; Cabanettes, A.; Gillon, E.; Deniaud, D.; Varrot, A.; Pape, P.L. Multivalent fucosides with nanomolar affinity for the Aspergillus fumigatus lectin FleA prevent spore adhesion to Pneumocytes. Chem. Eur. J. 2018, 24, 19243–19249. [Google Scholar] [CrossRef]
- Revie, N.M.; Iyer, K.R.; Robbins, R.; Cowen, L.E. Antifungal drug resistance: Evolution, mechanisms and impact. Curr. Opin. Microbiol. 2018, 45, 70–76. [Google Scholar] [CrossRef]
- Ciurea, C.N.; Kosovski, I.B.; Mare, A.D.; Toma, F.; Pintea-Simon, I.A.; Man, A. Candida and Candidiasis-Opportunism Versus Pathogenicity: A Review of the Virulence Traits. Microorganisms 2020, 8, 857. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Govern, M.M.; Abbey, L.; Gilroy, A.; Mullins, S.; Howell, S.; Kavanagh, K.; Velasco-Torrijos, T. Inhibition of adherence of the yeast Candida albicans to buccal epithelial cells by synthetic aromatic glycoconjugates. Eur. J. Med. Chem. 2018, 160, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Critchley, I.A.; Douglas, L.J. Role of glycosides as epithelial cell receptors for Candida albicans. J. Gen. Microbiol. 1987, 133, 637–643. [Google Scholar] [CrossRef]
- Tosh, F.D.; Douglas, L.J. Characterization of a fucoside-binding adhesin of Candida albicans. Infect. Immun. 1992, 60, 4734–4739. [Google Scholar] [CrossRef]
- Jimenez-Lucho, V.; Ginsburg, V.; Krivan, H.C. Cryptococcus neoformans, Candida albicans, and other fungi bind specifically to the glycosphingolipid lactosylceramide (Galβl-4Glcβ3-lCer), a possible adhesion receptor for yeasts. Infect. Immun. 1990, 58, 2085–2090. [Google Scholar] [CrossRef]
- Yu, L.; Lee, K.K.; Hodges, R.S.; Paranchych, W.; Irvin, R.T. Adherence of Pseudomonas aeruginosa and Candida albicans to Glycosphingolipid (Asialo-GM1) Receptors is Achieved by a Conserved Receptor-Binding Domain Present on Their Adhesins. Infect. Immun. 1994, 62, 5213–5219. [Google Scholar] [CrossRef] [PubMed]
- Martin, H.; Kavanagh, K.; Velasco-Torrijos, T. Targeting adhesion in fungal pathogen Candida albicans. Future Med. Chem. 2021, 13, 313–334. [Google Scholar] [CrossRef]
- Martin, H.; Goyard, D.; Margalit, A.; Doherty, K.; Renaudet, O.; Kavanagh, K.; Velasco-Torrijos, T. Multivalent Presentations of Glycomimetic Inhibitor of the Adhesion of Fungal Pathogen Candida albicans to Human Buccal Epithelial Cells. Bioconjug. Chem. 2021. ahead of print. [Google Scholar] [CrossRef]
- Roy, O.; Faure, S.; Thery, V.; Didierjean, C.; Taillefumier, C. Cyclic β-Peptoids. Org. Lett. 2008, 10, 921–924. [Google Scholar] [CrossRef]
- Cecioni, S.; Faure, S.; Darbost, U.; Bonnamour, I.; Parrot-Lopez, H.; Roy, O.; Taillefumier, C.; Wimmerová, M.; Praly, J.P.; Imberty, A.; et al. Selectivity among Two Lectins: Probing the Effect of Topology, Multivalency and Flexibility of “Clicked” Multivalent Glycoclusters. Chem. Eur. J. 2011, 17, 2146–2159. [Google Scholar] [CrossRef]
- Szekely, T.; Roy, O.; Dériaud, E.; Job, A.; Lo-Man, R.; Leclerc, C.; Taillefumier, C. Design, Synthesis, and Immunological Evaluation of a Multicomponent Construct Based on a Glycotripeptoid Core Comprising B and T Cell Epitopes and a Toll-like Receptor 7 Agonist That Elicits Potent Immune Responses. J. Med. Chem. 2018, 61, 9568–9582. [Google Scholar] [CrossRef] [PubMed]
- Tropper, F.D.; Andersson, F.O.; Braun, S.; Roy, R. Phase Transfer Catalysis as a General and Stereoselective Entry into Glycosyl Azides from Glycosyl Halides. Synthesis 1992, 1992, 618–620. [Google Scholar] [CrossRef]
- Lu, G.; Lam, S.; Burgess, K. An iterative route to “decorated” ethylene glycol-based linkers. Chem. Comm. 2006, 15, 1652–1654. [Google Scholar] [CrossRef] [PubMed]
- Ng, D.Y.W.; Fahrer, J.; Wu, Y.; Eisele, K.; Kuan, S.L.; Barth, H.; Weil, T. Efficient Delivery of p53 and Cytochrome C by Supramolecular Assembly of a Dendritic Multi-Domain Delivery System. Adv. Healthc. Mater. 2013, 2, 1620–1629. [Google Scholar] [CrossRef] [PubMed]
- Norberg, O.; Deng, L.; Yan, M.; Ramström, O. Photo-Click Immobilization of Carbohydrates on Polymeric Surfaces—A Quick Method to Functionalize Surfaces for Biomolecular Recognition Studies. Bioconjug. Chem. 2009, 20, 2364–2370. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).