Genome-Based Analysis of Klebsiella spp. Isolates from Animals and Food Products in Germany, 2013–2017
Abstract
1. Introduction
2. Results
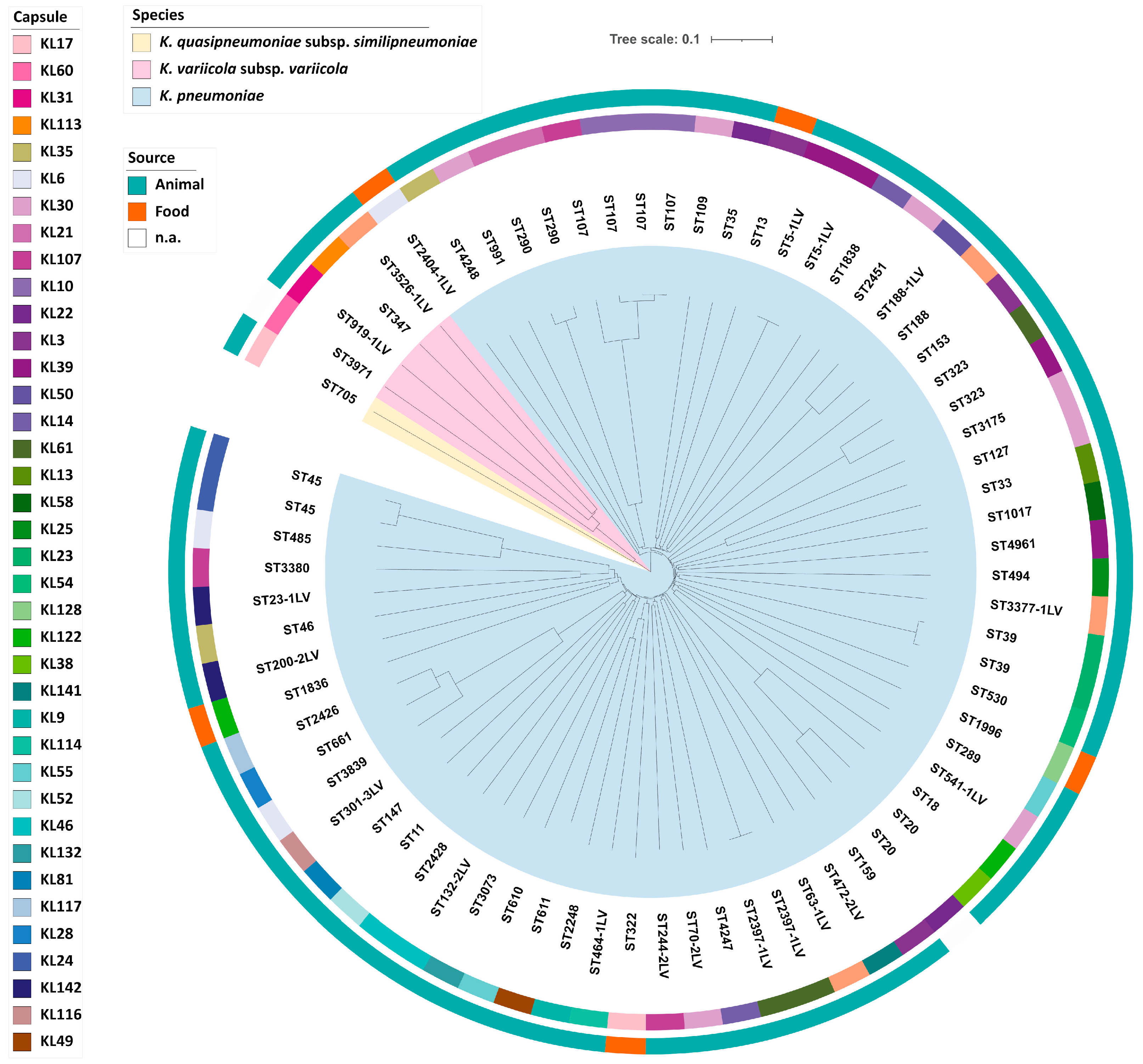
2.1. Klebsiella spp. Population Analysis
2.2. Klebsiella spp. Antibiotic Resistance and Resistance Genes
2.3. Molecular Typing
2.4. Presence of Virulence Factors
3. Discussion
4. Materials and Methods
4.1. Bacterial Isolates
4.2. Species Identification and Antibiotic Susceptibility Testing (AST)
4.3. Whole Genome Sequencing (WGS) and De Novo Assembly
4.4. WGS-Based Typing and Virulence and Resistance Gene Prediction
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Podschun, R.; Ullmann, U. Klebsiella spp. as nosocomial pathogens: Epidemiology, taxonomy, typing methods, and pathogenicity factors. Clin. Microbiol. Rev. 1998, 11, 589–603. [Google Scholar] [CrossRef]
- Davis, G.S.; Waits, K.; Nordstrom, L.; Weaver, B.; Aziz, M.; Gauld, L.; Grande, H.; Bigler, R.; Horwinski, J.; Porter, S.; et al. Intermingled Klebsiella pneumoniae Populations between Retail Meats and Human Urinary Tract Infections. Clin. Infect. Dis. 2015, 61, 892–899. [Google Scholar] [CrossRef]
- Rodriguez-Medina, N.; Barrios-Camacho, H.; Duran-Bedolla, J.; Garza-Ramos, U. Klebsiella variicola: An emerging pathogen in humans. Emerg. Microbes Infect. 2019, 8, 973–988. [Google Scholar] [CrossRef] [PubMed]
- Wyres, K.L.; Lam, M.M.C.; Holt, K.E. Population genomics of Klebsiella pneumoniae. Nat. Rev. Microbiol. 2020, 18, 344–359. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, C.; Passet, V.; Rakotondrasoa, A.; Brisse, S. Identification of Klebsiella pneumoniae, Klebsiella quasipneumoniae, Klebsiella variicola and Related Phylogroups by MALDI-TOF Mass Spectrometry. Front. Microbiol. 2018, 9, 3000. [Google Scholar] [CrossRef] [PubMed]
- Paczosa, M.K.; Mecsas, J. Klebsiella pneumoniae: Going on the Offense with a Strong Defense. Microbiol. Mol. Biol. Rev. 2016, 80, 629–661. [Google Scholar] [CrossRef] [PubMed]
- Martin, R.M.; Bachman, M.A. Colonization, Infection, and the Accessory Genome of Klebsiella pneumoniae. Front. Cell. Infect. Microbiol. 2018, 8, 4. [Google Scholar] [CrossRef] [PubMed]
- Tian, L.; Tan, R.; Chen, Y.; Sun, J.; Liu, J.; Qu, H.; Wang, X. Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: Factors related to the carbapenem resistance and patient mortality. Antimicrob. Resist. Infect. Control 2016, 5, 48. [Google Scholar] [CrossRef]
- Tanner, W.D.; VanDerslice, J.A.; Goel, R.K.; Leecaster, M.K.; Fisher, M.A.; Olstadt, J.; Gurley, C.M.; Morris, A.G.; Seely, K.A.; Chapman, L.; et al. Multi-state study of Enterobacteriaceae harboring extended-spectrum beta-lactamase and carbapenemase genes in US drinking water. Sci. Rep. 2019, 9, 3938. [Google Scholar] [CrossRef]
- Effah, C.Y.; Sun, T.W.; Liu, S.H.; Wu, Y.J. Klebsiella pneumoniae: An increasing threat to public health. Ann. Clin. Microbiol. Antimicrob. 2020, 19, 1–9. [Google Scholar] [CrossRef]
- Dinkelacker, A.G.; Vogt, S.; Oberhettinger, P.; Mauder, N.; Rau, J.; Kostrzewa, M.; Rossen, J.W.A.; Autenrieth, I.B.; Peter, S.; Liese, J. Typing and Species Identification of Clinical Klebsiella Isolates by Fourier Transform Infrared Spectroscopy and Matrix-Assisted Laser Desorption Ionization-Time of Flight Mass Spectrometry. J. Clin. Microbiol. 2018, 56, e00843-18. [Google Scholar] [CrossRef]
- Wang, J.H.; Liu, Y.C.; Lee, S.S.J.; Yen, M.Y.; Chen, Y.S.; Wang, J.H.; Wann, S.R.; Lin, H.H. Primary liver abscess due to Klebsiella pneumoniae in Taiwan. Clin. Infect. Dis. 1998, 26, 1434–1438. [Google Scholar] [CrossRef] [PubMed]
- Bialek-Davenet, S.; Criscuolo, A.; Ailloud, F.; Passet, V.; Jones, L.; Delannoy-Vieillard, A.S.; Garin, B.; Le Hello, S.; Arlet, G.; Nicolas-Chanoine, M.H.; et al. Genomic definition of hypervirulent and multidrug-resistant Klebsiella pneumoniae clonal groups. Emerg. Infect. Dis. 2014, 20, 1812–1820. [Google Scholar] [CrossRef]
- Tang, M.; Kong, X.; Hao, J.; Liu, J. Epidemiological Characteristics and Formation Mechanisms of Multidrug-Resistant Hypervirulent Klebsiella pneumoniae. Front. Microbiol. 2020, 11, 581543. [Google Scholar] [CrossRef] [PubMed]
- Russo, T.A.; Marr, C.M. Hypervirulent Klebsiella pneumoniae. Clin. Microbiol. Rev. 2019, 32. [Google Scholar] [CrossRef]
- Shon, A.S.; Bajwa, R.P.S.; Russo, T.A. Hypervirulent (hypermucoviscous) Klebsiella pneumoniae A new and dangerous breed. Virulence 2013, 4, 107–118. [Google Scholar] [CrossRef] [PubMed]
- Heiden, S.E.; Hubner, N.O.; Bohnert, J.A.; Heidecke, C.D.; Kramer, A.; Balau, V.; Gierer, W.; Schaefer, S.; Eckmanns, T.; Gatermann, S.; et al. A Klebsiella pneumoniae ST307 outbreak clone from Germany demonstrates features of extensive drug resistance, hypermucoviscosity, and enhanced iron acquisition. Genome Med. 2020, 12, 113. [Google Scholar] [CrossRef] [PubMed]
- Roberts, D.E.; McClain, H.M.; Hansen, D.S.; Currin, P.; Howerth, E.W. An outbreak of Klebsiella pneumoniae infection in dogs with severe enteritis and septicemia. J. Vet. Diagn. Investig. 2000, 12, 168–173. [Google Scholar] [CrossRef]
- Manges, A.R. Genomic Epidemiology: Revealing Hidden Reservoirs for Klebsiella pneumoniae. Clin. Infect. Dis. 2015, 61, 900–902. [Google Scholar] [CrossRef]
- Liu, L.; Feng, Y.; Hu, Y.Y.; Kang, M.; Xie, Y.; Zong, Z.Y. Klebsiella grimontii, a New Species Acquired Carbapenem Resistance. Front. Microbiol. 2018, 9, 2170. [Google Scholar] [CrossRef]
- Passet, V.; Brisse, S. Description of Klebsiella grimontii sp nov. Int. J. Syst. Evol. Microbiol. 2018, 68, 377–381. [Google Scholar] [CrossRef]
- Pinto, N.A.; D’Souza, R.; Hwang, I.S.; Choi, J.; In, Y.H.; Park, H.S.; Ryu, C.M.; Yong, D.; Lee, K. Whole genome and transcriptome analysis reveal MALDI-TOF MS and SDS-PAGE have limited performance for the detection of the key outer membrane protein in carbapenem-resistant Klebsiella pneumoniae isolates. Oncotarget 2017, 8, 84818–84826. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Zhou, H.; Qin, L.; Pang, Z.; Qin, T.; Ren, H.; Pan, Z.; Zhou, J. Frequency, Antimicrobial Resistance and Genetic Diversity of Klebsiella pneumoniae in Food Samples. PLoS ONE 2016, 11, e0153561. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, G.; Ye, Q.; Wu, Q.; Zhang, J.; Huang, Y. Phenotypic and Genotypic Characterization of Klebsiella pneumoniae Isolated From Retail Foods in China. Front. Microbiol. 2018, 9, 289. [Google Scholar] [CrossRef]
- Davis, G.S.; Price, L.B. Recent Research Examining Links Among Klebsiella pneumoniae from Food, Food Animals, and Human Extraintestinal Infections. Curr. Environ. Health Rep. 2016, 3, 128–135. [Google Scholar] [CrossRef] [PubMed]
- Anzai, E.K.; de Souza, J.C.; Peruchi, A.R.; Fonseca, J.M.; Gumpl, E.K.; Pignatari, A.C.C.; Hirano, Z.M.B.; Silveira, A.C.D. First case report of non-human primates (Alouatta clamitans) with the hypervirulent Klebsiella pneumoniae serotype K1 strain ST 23: A possible emerging wildlife pathogen. J. Med. Primatol. 2017, 46, 337–342. [Google Scholar] [CrossRef]
- Bowring, B.G.; Fahy, V.A.; Morris, A.; Collins, A.M. An unusual culprit: Klebsiella pneumoniae causing septicaemia outbreaks in neonatal pigs? Vet. Microbiol. 2017, 203, 267–270. [Google Scholar] [CrossRef]
- Marques, C.; Betas, A.; Aboim, C.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.T.; Pomba, C. Evidence of Sharing of Klebsiella pneumoniae Strains between Healthy Companion Animals and Cohabiting Humans. J. Clin. Microbiol. 2019, 57. [Google Scholar] [CrossRef]
- Marques, C.; Menezes, J.; Belas, A.; Aboim, C.; Cavaco-Silva, P.; Trigueiro, G.; Gama, L.T.; Pomba, C. Klebsiella pneumoniae causing urinary tract infections in companion animals and humans: Population structure, antimicrobial resistance and virulence genes. J. Antimicrob. Chemother. 2019, 74, 594–602. [Google Scholar] [CrossRef]
- Navon-Venezia, S.; Kondratyeva, K.; Carattoli, A. Klebsiella pneumoniae: A major worldwide source and shuttle for antibiotic resistance. FEMS Microbiol. Rev. 2017, 41, 252–275. [Google Scholar] [CrossRef]
- Zhang, R.M.; Li, J.Y.; Wang, Y.; Shen, J.Z.; Shen, Z.Q.; Wang, S.L. Presence of NDM in non-E. coli Enterobacteriaceae in the poultry production environment. J. Antimicrob. Chemother. 2019, 74, 2209–2213. [Google Scholar] [CrossRef]
- Ovejero, C.M.; Escudero, J.A.; Thomas-Lopez, D.; Hoefer, A.; Moyano, G.; Montero, N.; Martin-Espada, C.; Gonzalez-Zorn, B. Highly Tigecycline-Resistant Klebsiella pneumoniae Sequence Type 11 (ST11) and ST147 Isolates from Companion Animals. Antimicrob. Agents Chemother. 2017, 61, e02640-16. [Google Scholar] [CrossRef]
- Wang, X.; Li, H.; Zhao, C.; Chen, H.; Liu, J.; Wang, Z.; Wang, Q.; Zhang, Y.; He, W.; Zhang, F.; et al. Novel NDM-9 metallo-beta-lactamase identified from a ST107 Klebsiella pneumoniae strain isolated in China. Int. J. Antimicrob. Agents 2014, 44, 90–91. [Google Scholar] [CrossRef]
- Patil, S.; Chen, X.; Wen, F. Exploring the phenotype and genotype of multi-drug resistant Klebsiella pneumoniae harbouring blaCTX-M group extended-spectrum beta-lactamases recovered from paediatric clinical cases in Shenzhen, China. Ann. Clin. Microbiol. Antimicrob. 2019, 18, 32. [Google Scholar] [CrossRef] [PubMed]
- Ku, Y.H.; Chuang, Y.C.; Chen, C.C.; Lee, M.F.; Yang, Y.C.; Tang, H.J.; Yu, W.L. Klebsiella pneumoniae Isolates from Meningitis: Epidemiology, Virulence and Antibiotic Resistance. Sci. Rep. 2017, 7, 6634. [Google Scholar] [CrossRef]
- Aarestrup, F.M.; McDermott, P.F.; Wegener, H.C. Transmission of Antibiotic Resistance from Food Animals to Humans. In Campylobacter, 3rd ed.; ASM Press: Washington, DC, USA, 2008; pp. 645–665. [Google Scholar]
- Rahman, M.M.; Husna, A.; Elshabrawy, H.A.; Alam, J.; Runa, N.Y.; Badruzzaman, A.T.M.; Banu, N.A.; Al Mamun, M.; Paul, B.; Das, S.; et al. Isolation and molecular characterization of multidrug-resistant Escherichia coli from chicken meat. Sci. Rep. 2020, 10, 21999. [Google Scholar] [CrossRef]
- Vidovic, N.; Vidovic, S. Antimicrobial Resistance and Food Animals: Influence of Livestock Environment on the Emergence and Dissemination of Antimicrobial Resistance. Antibiotics 2020, 9, 52. [Google Scholar] [CrossRef] [PubMed]
- Irrgang, A.; Roschanski, N.; Tenhagen, B.A.; Grobbel, M.; Skladnikiewicz-Ziemer, T.; Thomas, K.; Roesler, U.; Kasbohrer, A. Prevalence of mcr-1 in E. coli from Livestock and Food in Germany, 2010–2015. PLoS ONE 2016, 11, e0159863. [Google Scholar] [CrossRef] [PubMed]
- Zhai, Y.; Zhang, Z.; Wang, Z.; Chen, Y.; Wang, Q.; Lv, Y.; Yang, J.; Zhao, T.; Guo, Y.; Gao, Z. Relative Strengths and Regulation of Different Promoter-Associated Sequences for Various blaSHV Genes and Their Relationships to β-Lactam Resistance. J. Mol. Microbiol. Biotechnol. 2017, 27, 91–101. [Google Scholar] [CrossRef] [PubMed]
- Haeili, M.; Javani, A.; Moradi, J.; Jafari, Z.; Feizabadi, M.M.; Babaei, E. MgrB Alterations Mediate Colistin Resistance in Klebsiella pneumoniae Isolates from Iran. Front. Microbiol. 2017, 8, 2470. [Google Scholar] [CrossRef]
- Prashar, A.; Bhatia, S.; Gigliozzi, D.; Martin, T.; Duncan, C.; Guyard, C.; Terebiznik, M.R. Filamentous morphology of bacteria delays the timing of phagosome morphogenesis in macrophages. J. Cell Biol. 2013, 203, 1081–1097. [Google Scholar] [CrossRef]
- Lam, M.M.C.; Wyres, K.L.; Judd, L.M.; Wick, R.R.; Jenney, A.; Brisse, S.; Holt, K.E. Tracking key virulence loci encoding aerobactin and salmochelin siderophore synthesis in Klebsiella pneumoniae. Genome Med. 2018, 10. [Google Scholar] [CrossRef] [PubMed]
- Rau, J.; Eisenberg, T.; Männig, A.; Wind, C.; Lasch, P.; Sting, R. MALDI-UP—An internet platform for the exchange of MALDI-TOF mass spectra. Asp. Food Control Anim. Health (ejournal) 2016, 2016, 1–17. [Google Scholar]
- Neumann, B.; Rackwitz, W.; Hunfeld, K.P.; Fuchs, S.; Werner, G.; Pfeifer, Y. Genome sequences of two clinical Escherichia coli isolates harboring the novel colistin-resistance gene variants mcr-1.26 and mcr-1.27. Gut Pathog. 2020, 12, 40. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Bankevich, A.; Nurk, S.; Antipov, D.; Gurevich, A.A.; Dvorkin, M.; Kulikov, A.S.; Lesin, V.M.; Nikolenko, S.I.; Pham, S.; Prjibelski, A.D.; et al. SPAdes: A new genome assembly algorithm and its applications to single-cell sequencing. J. Comput. Biol. 2012, 19, 455–477. [Google Scholar] [CrossRef] [PubMed]
- Wick, R.R.; Judd, L.M.; Gorrie, C.L.; Holt, K.E. Unicycler: Resolving bacterial genome assemblies from short and long sequencing reads. PLoS Comput. Biol. 2017, 13, e1005595. [Google Scholar] [CrossRef]
- Letunic, I.; Bork, P. Interactive Tree Of Life (iTOL) v4: Recent updates and new developments. Nucleic Acids Res. 2019, 47, W256–W259. [Google Scholar] [CrossRef] [PubMed]


| Beta-Lactam Resistance | Isolates |
|---|---|
| n = 94 | |
| Beta-lactamase genes | |
| blaSHV-1 | 9 (9.6%) |
| blaSHV-11 | 27 (28.7%) |
| blaSHV-33 | 1 (1.1%) |
| blaSHV-37 | 2 (2.1%) |
| blaSHV-61 | 1 (1.1%) |
| blaSHV-108 | 2 (2.1%) |
| blaSHV-119 | 1 (1.1%) |
| blaSHV-157 | 1 (1.1%) |
| blaSHV-168 | 1 (1.1%) |
| blaSHV-187 | 1 (1.1%) |
| blaSHV-194 | 1 (1.1%) |
| blaSHV-199 | 2 (2.1%) |
| blaSHV-211 | 1 (1.1%) |
| blaSHV-215 | 1 (1.1%) |
| blaSHV-234 | 1 (1.1%) |
| blaSHV-244 | 1 (1.1%) |
| blaLAP-2 | 2 (1.1%) |
| blaLEN-16 | 1 (1.1%) |
| blaLEN-19 | 1 (1.1%) |
| blaLEN-24 | 1 (1.1%) |
| blaLEN-32 | 1 (1.1%) |
| blaOKP-B-6 | 1 (1.1%) |
| blaOXA-1 | 2 (2.1%) |
| blaOXY1-1 | 3 (3.2%) |
| blaOXY1-4 | 3 (3.2%) |
| blaOXY1-5 | 2 (2.1%) |
| blaOXY2-1 | 2 (2.1%) |
| blaOXY5-1 | 1 (1.1%) |
| blaOXY6-2 | 4 (4.3%) |
| blaOXY6-3 | 1 (1.1%) |
| blaOXY6-4 | 6 (6.4%) |
| blaTEM-1 | 3 (3.2%) |
| Extended-spectrum beta-lactamase genes | |
| blaSHV-27 * | 9 (9.6%) |
| blaSHV-41 * | 2 (2.1%) |
| blaCTX-M-1 | 1 (1.1%) |
| blaCTX-M-14 | 2 (2.1%) |
| blaCTX-M-15 | 2 (2.1%) |
| Sequence Types | Novel Sequence Types | ||
|---|---|---|---|
| ST | n = 72 | ST | n = 72 |
| ST11 | 1 (1.4%) | ST5-1LV | 2 (2.8%) |
| ST13 | 1 (1.4%) | ST23-1LV | 1 (1.4%) |
| ST18 | 1 (1.4%) | ST63-1LV | 1 (1.4%) |
| ST20 | 2 (2.8%) | ST70-2LV | 1 (1.4%) |
| ST33 | 1 (1.4%) | ST132-2LV | 1 (1.4%) |
| ST35 | 1 (1.4%) | ST188-1LV | 1 (1.4%) |
| ST39 | 2 (2.8%) | ST200-2LV | 1 (1.4%) |
| ST45 | 2 (2.8%) | ST244-2LV | 1 (1.4%) |
| ST46 | 1 (1.4%) | ST301-3LV | 1 (1.4%) |
| ST107 | 4 (5.6%) | ST464-1LV | 1 (1.4%) |
| ST109 | 1 (1.4%) | ST472-2LV | 1 (1.4%) |
| ST127 | 1 (1.4%) | ST541-1LV | 1 (1.4%) |
| ST147 | 1 (1.4%) | ST919-1LV | 1 (1.4%) |
| ST153 | 1 (1.4%) | ST2397-1LV | 2 (2.8%) |
| ST159 | 1 (1.4%) | ST2404-1LV | 1 (1.4%) |
| ST188 | 1 (1.4%) | ST3377-1LV | 1 (1.4%) |
| ST289 | 1 (1.4%) | ST3381-1LV | 1 (1.4%) |
| ST290 | 2 (2.8%) | ST3526-1LV | 1 (1.4%) |
| ST322 | 1 (1.4%) | ||
| ST323 | 2 (2.8%) | ||
| ST347 | 1 (1.4%) | ||
| ST485 | 1 (1.4%) | ||
| ST494 | 1 (1.4%) | ||
| ST530 | 1 (1.4%) | ||
| ST610 | 1 (1.4%) | ||
| ST611 | 1 (1.4%) | ||
| ST661 | 1 (1.4%) | ||
| ST705 | 1 (1.4%) | ||
| ST991 | 1 (1.4%) | ||
| ST1017 | 1 (1.4%) | ||
| ST1836 | 1 (1.4%) | ||
| ST1838 | 1 (1.4%) | ||
| ST1996 | 1 (1.4%) | ||
| ST2248 | 1 (1.4%) | ||
| ST2426 | 1 (1.4%) | ||
| ST2428 | 1 (1.4%) | ||
| ST2451 | 1 (1.4%) | ||
| ST3073 | 1 (1.4%) | ||
| ST3175 | 1 (1.4%) | ||
| ST3380 | 1 (1.4%) | ||
| ST3839 | 1 (1.4%) | ||
| ST3971 | 1 (1.4%) | ||
| ST4247 | 1 (1.4%) | ||
| ST4248 | 1 (1.4%) | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Klaper, K.; Hammerl, J.A.; Rau, J.; Pfeifer, Y.; Werner, G. Genome-Based Analysis of Klebsiella spp. Isolates from Animals and Food Products in Germany, 2013–2017. Pathogens 2021, 10, 573. https://doi.org/10.3390/pathogens10050573
Klaper K, Hammerl JA, Rau J, Pfeifer Y, Werner G. Genome-Based Analysis of Klebsiella spp. Isolates from Animals and Food Products in Germany, 2013–2017. Pathogens. 2021; 10(5):573. https://doi.org/10.3390/pathogens10050573
Chicago/Turabian StyleKlaper, Kathleen, Jens Andre Hammerl, Jörg Rau, Yvonne Pfeifer, and Guido Werner. 2021. "Genome-Based Analysis of Klebsiella spp. Isolates from Animals and Food Products in Germany, 2013–2017" Pathogens 10, no. 5: 573. https://doi.org/10.3390/pathogens10050573
APA StyleKlaper, K., Hammerl, J. A., Rau, J., Pfeifer, Y., & Werner, G. (2021). Genome-Based Analysis of Klebsiella spp. Isolates from Animals and Food Products in Germany, 2013–2017. Pathogens, 10(5), 573. https://doi.org/10.3390/pathogens10050573






