Abstract
Plant diseases cause losses of approximately 16% globally. Thus, management measures must be implemented to mitigate losses and guarantee food production. In addition to traditional management measures, induced resistance and biological control have gained ground in agriculture due to their enormous potential. Endophytic fungi internally colonize plant tissues and have the potential to act as control agents, such as biological agents or elicitors in the process of induced resistance and in attenuating abiotic stresses. In this review, we list the mode of action of this group of microorganisms which can act in controlling plant diseases and describe several examples in which endophytes were able to reduce the damage caused by pathogens and adverse conditions. This is due to their arsenal of molecules generated during the interaction by which they form a kind of biological shield in the plant. Furthermore, considering that endophytic fungi can be an important tool in managing for biotic and abiotic stresses due to the large amount of biologically active substances produced, bioprospecting this class of microorganisms is tending to increase and generate valuable products for agriculture.
1. Introduction
Agricultural production and global food security face substantial challenges. The world population is expected to exceed 9 billion by 2050, and an estimated 70% increase in food production over today’s production will be needed to ensure food security [1]. In this sense, the health of cultivated plants is of vital importance for the various economic sectors, because plants also provide essential products in addition to providing food for the population, such as wood, fibers, medicines, and bioenergy, among others. Plant diseases are responsible for quantitative and qualitative reduction in production, causing significant economic losses, and occasionally can lead to disastrous social consequences [2,3,4,5,6].
Plant diseases cause losses of up to 16% on a global scale [7], and studies have already pointed to losses directed at pathogens and more specifically to performed cultivations [3,8]. The potential for losses triggered by pathogens is indisputable, and their losses may vary depending on climatic factors, the culture and aggressiveness of the causal agent [8].
Diseases are traditionally managed through the use of genetic resistance (when available), and through the use of traditional chemical pesticides. The latter is highly used and has good efficiency in most cases [9]. However, this approach has experienced difficulties over the years due to its exhaustive use, which has led to the selection of pathogen populations which are resistant to the available active ingredients [10,11,12,13]. Driven by such a scenario, the study of complementary and alternative management measures has increased in recent years and has gained significant space in integrated management programs. As a result, biological control [14,15] and induced resistance [16,17,18,19] can be highlighted among the tools which have received attention. The potential of these two tools has been studied, explored and implemented in production fields, with numerous reports of successful cases in controlling pathogens.
Plants and microorganisms in nature live in interactions among them, which can affect plant growth, development and even defense responses to biotic and abiotic stresses [20]. Endophytic fungi are among the microorganisms that live in interaction with plants and can be used in biological control and induced resistance, and comprise one of the most interesting groups with high potential for use and high diversity (Figure 1). They present advantages since they internally colonize tissues and therefore remain protected from more hostile environmental conditions which could threaten their survival [21]. Thus, endophytic fungi are increasingly being studied due to their ability to assist in plant health. For example, regarding induced resistance, Piriformospora indica is able to induce resistance in Musa spp. against Fusarium oxysporum f. sp. cubense tropical race 4 by increasing the activities of antioxidant enzymes [22]. On the other hand, the endophytic fungus Fusarium oxysporum strain EF119 sensu lato acts as a biocontrol agent for tomato plants against oomycetes such as Phytophthora infestans [23].
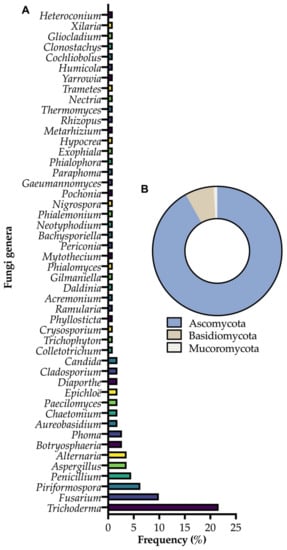
Figure 1.
(A) Frequency of endophytic fungi genera cited in this review; (B) the phyla in which these microorganisms are classified.
Endophytes have received attention both as biological control agents and as activators of the plant’s defense response to biotic and abiotic stresses. Both approaches generally have satisfactory results and have the potential to be used as auxiliary strategies to traditional control and to be implemented in integrated disease management systems. The biological pesticide market in Brazil grew more than 70% in 2018, with a turnover of around US$ 127.2 million. This value exceeded the percentage presented by the international market, where the expectation was that the sector would earn US$5 billion in 2020 and reach US$11 billion in 2025 [24].
In this review, we discuss how endophytic fungi can benefit and act in plant protection. To do so, we approach three examples of phyla and several different genera within these, although there is a predominance of endophytic fungi such as Trichoderma, Fusarium and Piriformospora (Figure 1). The use of endophytic fungi as biological control agents and resistance inducers is detailed, emphasizing some of the most recent information on this topic which has been explored. In addition, the basis of biological control and stress-induced resistance is highlighted to facilitate understanding of applying endophytic fungi in this context, and in the context of integrated management. Finally, perspectives are presented to better understand how the endophytic fungi area should evolve in the coming years. Although endophytic bacteria can also act to protect plants against biotic [25,26,27] and abiotic stresses, this is not the focus of this review.
2. Endophytic Fungi
Endophytic microorganisms were first defined as those which live inside plant tissues, whether in asymptomatic infections (or not), and either in antagonistic or symbiotic interactions [28]. Later, any microorganisms which colonize the interior of aerial plant tissues in at least one stage of their life cycle without causing apparent damage to the host plant were considered endophytes [29]. After a few years of study, Azevedo and Araújo [30] defined endophytic microorganisms as all those cultivable (or not) which inhabit the interior of plant tissues, without causing damage to the host, and which do not develop visible external structures.
More recent views have been considered conceptualizing endophytic microorganisms as those that live in healthy plant tissues without causing obvious symptoms of infection in the host plant, and their existence is characterized as being abundant in nature [31]. The long-term coexistence of endophytes and host plants makes their relationship complex, so that endophytes can produce the same or similar active secondary metabolites as plants [31].
The colonization of plant tissues does not occur by chance, but probably because they were selected and adapted to grow in this niche. This is evident due to the energy used by the plant in producing biomass for the endophyte, being compensated by adaptive improvements resulting from the presence of the microorganisms [32]. The intense chemotactic signaling in the endophytic-host interaction also suggests that these microorganisms are not merely accidental opportunists, but are the result of a co-evolutionary adaptation between them [33].
Endophytes associated with plants represent an untapped source of new natural and bioactive products, with more than 20,000 described substances [34], of which 51% have new structures and 80% have biological activity [35]. For example, some have antimicrobial, antioxidant and anti-tumor activities [36,37,38,39]. This can be explained by the ecological theory, which establishes that this metabolic production is dependent on the ecological niche in which the microorganism is inserted and the consequent biotic and abiotic interactions [40].
Endophytic fungi inhabit a similar ecological niche to that occupied by phytopathogens, thus being able to protect their environment and control them through competition, production of antagonistic substances, direct parasitism or even inducing resistance or tolerance [41]. It is important to consider that some fungi which are endophytic for one plant species may be pathogenic for another species. In the same sense, the production of compounds such as antibiotics, for example, suggests that endophytes can control plant diseases [41].
Both hosts and endophytes are benefited in the interaction among them. On the one hand, the microorganism benefits from protection, nutrition and shelter in the plant. On the other hand, endophytes also help their hosts by stimulating their growth, development, adaptation, and stress tolerance [42,43]. Protection against diseases occurs by reducing the infection levels, as well as suppressing and reducing the growth of pathogens [44,45]. In this sense, it is suggested that the presence of endophytes during the evolutionary process allowed the plants to grow better and be more resistant to insects, herbivorous animals and pathogenic organisms. The same can be inferred regarding adverse environmental conditions such as low humidity and/or high temperatures [46].
The main focus in studying endophytic organisms is on the benefits promoted in the host plant’s health, in which they can “protect” plants against pests and pathogens, increasing growth, resistance to stress, and produce chemical compounds such as enzymes, alkaloids, hormones and antibiotics [47]. In turn, these compounds can present considerable toxicity, as is the case of the alkaloids produced by these fungi [33], which can help plants in the battle against pathogens.
The beneficial effect of the plant-endophytic association has received attention, and therefore these microorganisms have become an important tool in modern agriculture [47]. In addition, endophytic fungi can be genetically altered in order to introduce characteristics of interest in host plants [48].
The plants provide an environment in their interior for a high diversity of endophytic fungi. These microorganisms can colonize leaves, branches and roots, without causing damage to the hosts [47], systemically inhabiting the apoplast, vascular tissues and in some cases the cell interior [49]. For example, in cacao grown in Bahia State, Brazil, it was observed that plants harbor endophytic fungi belonging to several groups, such as Acremonium spp., Colletotrichum gloeosporioides, Fusarium, Gliocladium, Lasiodiplodia theobromae, Pestalotiopsis spp., Trichoderma spp. and Verticillium spp. [50]. Fusarium sp. and Colletotrichum sp. were also isolated as endophytes from cacao leaves in Panama [51]. The most frequent endophytically isolated fungi include Colletotrichum, Cladosporium, Fusarium and Xylaria species [52,53,54,55].
The diversity of plant endophytes from Paeonia spp. was recently analyzed and different genera of fungi were identified. The most abundant among them were Fusarium, Phoma, Alternaria and Pestalotiopsis [56]. Other examples can also be found, such as Coniothyrium species isolated from the cortex of Picea abies branches [57], Asteromella fungus isolated from the inside of Quercus emoryi leaves [58], Phoma isolated from wheat leaves [59] and Aspergillus, Curvularia lunata, Fusarium, Penicillium and Trichoderma isolated from sunflower seeds [60]. In addition, there were a total of 60 isolates of endophytic fungi belonging to 16 different genera in the medicinal Sceletium tortuosum plant in South Africa, the most ubiquitous being the Fusarium, Aspergillus, Penicillium and Phomopsis genera [61]. Yerba mate plants (Ilex paraguariensis) are also colonized by endophytic fungi, with the main ones being Aspergillus, Penicilium, Acremonium, Fusarium and Colletotrichum [62].
The population dynamics of endophytic fungi may be related to some host properties such as chemical composition [63], physiological conditions [64], geographic distribution, plant age and ecological conditions, including altitude and precipitation [65]. Ecological or environmental conditions such as temperature, humidity, lighting, geographic location and vegetation significantly affect the distribution pattern and population structure of endophytic fungi [56]. For example, one or two species are predominantly endophytic in a given host, while others are uncommon [51,66]. The population of endophytes of a given plant can also vary according to their health state, suggesting that the microorganisms have a probable protective action [67,68].
Although endophytes are closely related to the plants, they need to overcome the defense barriers interposed. For example, secondary plant metabolites are one of these obstacles for colonization by endophytic fungi and, therefore, these organisms must secrete corresponding detoxifying enzymes. Thus, they are able to decompose the secondary metabolites so that they can enter and colonize the host plant tissues. In addition, these detoxifying compounds secreted by endophytic fungi can in turn induce production of a variety of new bioactive secondary metabolites, which can further serve as important medicinal resources [69].
With all the existing evidence, endophytes have begun to be recognized for their ability to protect their hosts from pathogens and be used as biocontrol agents. Thus, isolating and characterizing endophytic microorganisms from plants which have not yet been studied can enable the discovery of new species with the potential to produce substances of interest such as compounds with antimicrobial activity, which are extremely important for industry [40]. In addition, the ability of in vitro production of substances which inhibit the growth of other microorganism species has stimulated research regarding the bioprospecting of endophytic fungi for biological control [70].
3. Biological Control
As stated, plant pathogens always threaten world food security. In many cases, the available tools have not been enough to properly manage them and reduce losses. For example, Phytophthora infestans was the first plant pathogen successfully reported by De Barry in 1845 [28], but still constrains the production of important crops such as tomato and potato [71,72]. We can also highlight plant parasitic nematodes. A single species, the root-knot nematode (Meloidogyne incognita), presents one of the broadest host ranges among all plant pathogens, being able to parasitize more than 3000 plants [73]. It damages and imposes limitations to food and plant-resource production in both tropical and temperate areas of the world. Despite the phylogenetic differences (an oomycete and an invertebrate animal, respectively), they share some similarities from a management perspective. Together, the broad range of hosts and the rise of virulent strains/populations impose difficulties in the use of resistant cultivars and non-host crops. The use of chemical pesticides is not always efficient (insensitive strains) or viable (cost or application method). In addition, the pesticide industry has been struggling to produce novel pesticide molecules. Lastly, society not only demands security in food production, but higher quality and lower impacts on the environment [74]. Altogether, these factors have driven the search for novel, effective and eco-friendly ways to manage pests, which has enabled biopesticides to become an important asset to reduce losses from plant pathogens. In view of the above, then, what is biological control? Traditionally, biological control is defined as a decrease in a pathogen population (inoculum) or in the disease determinants by an organism which is not human or plant [75]. It is also referenced as an attempt to transport a common phenomenon from nature to the agricultural systems, taking advantage of natural and established relationships [76]. However, most (if not all) biological control agents have demonstrated the ability to closely interact and/or colonize plants in some way. They developed a complex inter-kingdom communication in which signaling occurs through a biochemical language with plants [77,78,79]. For example, plants have the ability to harbor a microbial community in the rhizosphere, being able to recruit some in unfavorable situations [80]. This current view of biological control especially mediated by endophytes opens a novel way to face microorganism–plant relationships and unveil new biotechnological tools to manage plant pathogens. We will address this subject in the present section.
Biological control is a wide and generic category which embraces relatives and distant phylogenetic organisms, as well as different suppression mechanisms of plant pathogens. There are several invertebrates (e.g., acari, predatory nematodes, parasitoids, tardigrades), fungi (e.g., avirulent strains of Fusarium, Trichoderma), bacteria (Bacillus spp., Pseudomonas spp.) and viruses among biological control agents. However, not all of them are suited to be used as biopesticides as they have to fulfill several requirements, among which we would like to highlight the following: (1) they are not harmful to plants, humans or animals; (2) Are efficient in controlling the target; (3) they survive in different conditions and in the absence of the target; (4) they are economically viable to produce on a large scale; (4) they have a long shelf-life and are infective after being stored; (5) they are compatible with different agricultural assets (pesticides, fertilizers, etc.) [81,82,83,84,85].
Taking these requirements into consideration, the most prominent agents to control plant pathogens are fungi and bacteria. As stated, most of them have the ability to colonize plants.
Regarding the action mechanisms, biological agents can suppress pathogens through predation, parasitism (sometimes referred to as hyperparasitism, the parasite of a parasite), secretion of repellent and/or toxic compounds, including volatiles (antibiosis) and competition for a specific niche (nutrient, infection site, plant tissues, etc.).
The same agent often uses several mechanisms at the same time or applies different mechanisms for different pathogens. For example, T. harzianum usually antagonizes Sclerotinia sclerotiorum through direct parasitism, in which Trichoderma coils and degrades the target’s hyphae [86]. It can also inhibit a white mold agent through antibiosis and competition for space and/or resources [87]. Another interesting example is Purpureocillium lilacinum (syn. Paecilomyces lilacinus), a fungus known for its effect against plant parasitic nematodes. P. lilacinum performs antibiosis against S. sclerotiorum, and thus antagonizes it by producing and secreting an array of extracellular enzymes which inhibit the development of the white mold agent in vitro [88]. It also parasitizes the eggs and egg-laying females of root-knot nematodes (Meloidogyne spp.) by killing and digesting them with extracellular enzymes [89,90]. These examples emphasize that the most important suppression component can change with the pathosystem: to the white mold agent hyperparasitism and antibiosis for T. harzianum and P. lilacinum, respectively. In contrast, regarding the root-knot nematode, antibiosis and hyperparasitism by T. harzianum and P. lilacinum, respectively.
Some biological agents colonize the plant, which often present biostimulating effects. Baron et al. [91] showed that P. lilacinum and Metarhizium marquandii promote growth in maize, bean and soybean plants when used as bioinoculants. They observed indoleacetic acid (IAA) production and phosphorus solubilization, showing the biostimulating effect of these endophytes in addition to their effects against plant pathogens. The biological control mediated by endophytes and their beneficial effects on plants will be further addressed in the specific section of the present review.
Although biopesticides are currently commercialized worldwide, the development and the subsequent steps (i.e., packing and shelf-life) of biological control agents are not easily carried out. A discrepancy in results obtained in controlled field conditions are often reported among the main limitations. Interference from the environment is usually overcome in laboratory conditions [92] and sometimes could lead to misleading conclusions [93]. In addition, another issue is the ineffectiveness of the biopesticide in different environmental conditions and in population variations of the plant pathogens. The effectiveness of biopesticides may vary among cultivars of a particular host.
Biological control does not follow the same pattern as chemical pesticides. The use of biopesticides is complex and is influenced by the environment and agricultural conditions. Biological agents do not aim to eradicate pathogens. Instead, their use is intended to reduce them to non-harmful levels, below the dangerous threshold [15,94]. Even so, the use of biopesticides presents several (already presented) advantages which we will further develop focusing on the potential of endophytes in agriculture.
4. Induced Resistance
Plant resistance can be defined as the ability of the host to delay and/or prevent colonization and development of the pathogen. There are several defense mechanisms involved in resistance, which can be biochemical or structural, and still classified on a temporal scale as preformed or postformed [85].
According to Kesel et al. [95], the plants have an immune system composed of constitutive and inducible defenses which can be increased through biotic and/or abiotic stimuli, providing higher defense capacity against pathogens and pests, characterizing the induced resistance. Thus, this phenomenon in plants can be seen as a possible measure for controlling plant diseases, in addition to being used as a tool for studying the resistance mechanisms and susceptibility of plants against phytopathogens [17,96,97,98].
The induced resistance has several advantages; for example, it can be effective against viruses, bacteria, fungi, phytonematodes and abiotic stresses. In addition, it exhibits stability due to the fact that different resistance mechanisms work together, highlighting the non-specificity, systemicity, persistence, and grafting transmission, among others [85].
The most desired result in induced resistance is the state of “priming”, in which the elicited plants go into a “state of alert”, and the resistance mechanisms are more intensely expressed with the arrival of the stressor, and to a lesser extent time lapse. However, this state does not result in energy expenditure due to the latent state of the mechanisms that govern resistance [85,99,100].
The term induced resistance can be used to designate a local protection only in the tissues where the treatment with the inducing agent was carried out, but it can also indicate a systemic resistance which manifests itself at a distance from the inducer application site [101,102].
Activation of plant defense can occur from elicitation by compounds present in plant extracts [103,104,105], yeast preparations [106,107], growth-promoting rhizobacteria [108], growth-promoting fungi [109], avirulent pathogens [110], endophytic fungi [47,111,112], among others.
Therefore, induced resistance consists of activating resistance through the use of external agents without any change in the plant’s genome [97], and non-specifically occurring through the activation of genes involved in several defense responses such as oxidative explosions [113], hypersensitivity responses [114], accumulation of PR-proteins [115], enzymes involved in the phenylpropanoid pathway [116,117], enzymes involved in lipid peroxidation [118], phytoalexin synthesis [119], and accumulation of phenolic compounds [120], among others.
According to the signaling pathway which promotes the expression of defenses, induced resistance can be divided into resistance induced by non-pathogenic microorganisms and biotrophic pathogens which have salicylic acid (SA) as the main signaling agent, mainly expressing PR-proteins, and designated as systemic acquired resistance (SAR). The resistance induced by rhizospheric growth-promoting microorganisms or necrotrophic pathogens, known as induced systemic resistance (ISR), has jasmonic acid (JA) and ethylene (ET) as the main signaling agents, independent of SA [121,122,123,124].
This is a generalization, since there are already reports where the pathogen Phakopsora pachyrhizi, the causal agent of Asian soybean rust, supposedly modulates the expression of target genes when penetrating the host tissue, activating the JA pathway and inhibiting the defense mediated by SA [125]. It is believed that there is a positive crosstalk between SA, JA, and ET, in addition to gene expression effectors. In a study using Arabidopsis isolated rhizobacteria, it was shown that the SA and JA pathway has additive effects on the induced resistance against the P. syringae pv. tomato pathogen. It is believed that the responses mediated by SA and JA are capable of working together to a certain degree, with the prevalence of one over the other after a certain time [126,127].
5. Endophytes as Biocontrol Agents
The biological control of plant pests has been boosted in recent years. As discussed, the agents have demonstrated the ability to colonize plants or at least to establish a close relationship with them. Thus, most biological control endophyte (BCE) agents have frequently been found among different crops and are able to suppress important pathogens (Table 1).
Several Trichoderma species are among the most studied BCE. Trichoderma species are able to colonize a wide range of crops such as soybean, wheat, corn and cotton. This fungus has shown different mechanisms involved in disease control and is widely known for its capacity to induce resistance in plants [128], although it shows a remarkable ability to parasitize very different plant pathogens. For example, Trichoderma spp. have been found to parasitize S. sclerotiorum and Rhizoctonia solani hyphae [129]. In addition, several strains have been reported penetrating and parasitizing eggs and second-stage M. javanica juveniles and Heterodera avenae cysts, a very resilient structure [130,131]. Thus, the Trichoderma species present a wide number of hosts which include both plants and the different plant pathogens as symbiotic and parasitic, respectively.
In addition to the direct parasitism, several BCE produce and release many effector compounds (e.g., antibiotics, toxins and fungitoxic metabolites) against plant pathogens. BCEs inhibit pathogens through the production of cellulase, glucanase, chitinase and lactones (volatile compounds) [132]. This kind of mechanism is also observed in other endophytes. For example, P. lilacinum is a classic biocontrol agent of plant parasitic nematodes and insects, but have demonstrated the ability to suppress different plant pathogens through deploying effectors. Wang et al. [133] showed the involvement of the leucinostatins (antibiotics) on the suppression of P. infestans and P. capsici. The culture filtrates of P. lilacinum, which contains effectors, suppressed S. sclerotiorum and induced defense responses in the common bean [88]. Furthermore, the culture filtrate and cell wall extract of Piriformospora indica reduced the infection of Heterodera schachtii in Arabidopsis based upon nematode per cm of root, syncytia length and eggs per cyst in [134]. This fungus interestingly does not parasitize the nematode. These examples highlight the potential of the cell-free filtrates of BCE to control plant pathogens in agriculture nowadays.
Another group of prominent endophytes are the non-pathogenic strains of plant pathogens. F. oxysporum sensu lato can interact with plants as a pathogen, causing root rot or wilt. However, some avirulent strains of F. oxysporum sensu lato can colonize plants as endophytes and protect the plants against soil-borne diseases (i.e., Pythium ultimum and Verticillium dahliae) [135]. F. oxysporum sensu lato can also compete for nutrient and/or root niches, which suppresses fungal plant pathogens [135,136]. However, other mechanisms may be involved such as Fusarium endophytes which controlled F. oxysporum f. sp. lycopersici in tomato plants through induced resistance mediated by SA, JA and ET [137]. Induced resistance mediated by endophytes will be further discussed in the specific section below.
Some studies have interestingly shown the potential of non-toxigenic strains of Aspergillus flavus on the control of mycotoxigenic Aspergillus in cereals [138]. The strategy to avoid aflatoxin contamination at a pre-harvest stage includes introducing the non-pathogenic A. flavus strains to compete and suppress the toxigenic Aspergillus [138]. Additionally, other biocontrol agents have similarly been used to control toxigenic strains of Fusarium in maize [138,139].
As a result, the following question arises: how can we isolate and select potential fungal endophytes to control plant pathogens? The potential answer may be the plant’s biome. The microbes associated to plants have been demonstrated to be effective to control most pathogens related to this host crop. For example, Halecker et al. [140] aimed to develop a biocontrol agent by using an endophyte fungus to control ash dieback caused by Hymenoscyphus fraxineus. A total of 340 endophytic fungi were isolated from the Fraxinus excelsior, the tree host. The fungi were further investigated and co-cultivated to find a suitable biocontrol agent. Rubini et al. [50] investigated the fungal community of cacao plants (Theobroma cacao) and addressed the biological control of Moniliophthora perniciosa, the causal agent of witches’ broom disease. A diverse number of fungal genera were found associated to cacao plants, but only one reduced the incidence of the disease: treatment with Gliocladium catenulatum reduced the incidence of witches’ broom disease in 70% of the infected plants. This highlights the potential of the phytobiome to be used in the control of plant pathogens. Additionally, despite not being the focus of the present review, the potential of bacterial endophytes is noteworthy. Similar to what has been discussed, Khaskheli et al. [141] addressed the importance of root-associated bacterial endophytes from rice plants to control its major diseases. They followed a similar approach and we recommend their work for additional material.
Thus, given the relevance of endophytic fungi for controlling plant diseases, Table 1 presents an overview of the scientific work carried out with endophytic fungi in the area of biological control regarding phytopathogens.

Table 1.
Phytopathogens affected by endophytic fungi based on mechanisms related to biological control *.
Table 1.
Phytopathogens affected by endophytic fungi based on mechanisms related to biological control *.
| Endophytic Fungi | Plants | Fungi Targets | References |
|---|---|---|---|
| Cladosporium tenuissimum | - | Uromyces appendiculatus | [142] |
| Trichoderma viride, T. harzianum, T. stromaticum, T. virens | - | Rhizopus stolonifer | [143] |
| Trichoderma viride | - | Penicillium digitatum | [144] |
| Trichoderma viride | - | Phytophthora nicotianae | [132] |
| Trichoderma viride | - | Rhizoctonia solani | [145] |
| Trichoderma viride, T. koningii | - | Verticillium dahliae | [146] |
| Fusarium oxysporum sensu lato | Solanum lycopersicum | Phytophthora infestans and P. capsici | [23] |
| Xylaria sp. | Ginkgo biloba | Penicillium expansum and Aspergillus niger | [36] |
| Heteroconium chaetospira | Brassica oleracea | Verticillium dahliae | [147] |
| Diaporthe helianthi | Leuhea divaricata | Moniliophthora perniciosa | [148] |
| Aspergillus, Penicillium and Trichoderma sp. | Eucalyptus benthamii | Botrytis cinerea | [149] |
| Trichophyton sp., Chrysosporium sp., Candida pseudotropicalis, and Candida tropicalis | Symphytum officinale | Sclerotinia sclerotiorum | [150] |
| Colletotrichum gloeosporioides and Clonostachys rosea | Theobroma cacao | Phythophthora sp. and Moniliophthora roreri | [151] |
| Gliocladium catenulatum | Theobroma cacao | Crinipellis perniciosa | [50] |
| Diaporthe terebinthifolii | Schinus terebinthifolius | Phyllosticta citricarpa | [152] |
| Fusarium solani sensu lato | Vitis labrusca | Botrytis sp. | [153] |
| Aspergillus insulicola and A. melleus | Sesuvium portulacastrum | Pythium aphanidermatum | [154] |
| Phyllosticta fallopiae | Cornus officinalis | Alternaria alternata, A. arborescens, Botryosphaeria dothidea and Colletotrichum gloeosporioides | [155] |
| Alternaria tenuissima | C. officinalis | Alternaria alternata | [155] |
| Alternaria alternata | C. officinalis | Alternaria arborescens | [155] |
| Botryosphaeria dothidea | C. officinalis | Alternaria alternata, A. arborescens, Botryosphaeria dothidea and Colletotrichum gloeosporioides | [155] |
| Colletotrichum gloeosporioides | C. officinalis | Alternaria alternata | [155] |
| Botryosphaeria berengeriana | C. officinalis | Botryosphaeria dothidea | [155] |
| Alternaria sp., Botryosphaeria ribis, Phoma medicaginis, Bionectria ochroleuca, Aureobasidium pullulans and Chaetomium spirochaete | Vitis vinifera | Botrytis cinerea | [156] |
| Ramularia pratensis, Phoma aliena and Fusarium acuminatum | Vitis riparia | Botrytis cinerea | [157] |
| Bacteria targets | |||
| Xylariales sp. | Distylium chinense | Clavibacter michiganensis, Xanthomonas citri pv. phaseoli var. fuscans and Pseudomonas syringae pv. lachrymans | [158] |
| Viruses targets | |||
| Paecilomyces variotii | Nicotiana benthamiana and N. tabacum | Potato Virus X (PVX) and Tobacco mosaic virus (TMV) | [159] |
| Nematodes targets | |||
| Acremonium implicatum | Solanum lycopersicum | Meloidogyne incognita | [160] |
| Fusarium oxysporum sensu lato | Musa spp. | Pratylenchus goodeyi | [161] |
| Chaetomium globosum | - | Meloidogyne incognita | |
| Daldinia cf. concentrica | Olea europaea | Meloidogyne javanica | [162] |
| Alternaria sp. | - | Bursaphelenchus xylophilus | [143] |
* The possible mechanisms of action are commented on in the text. “-” means that the host plant was not identified in the cited reference. The Bold is applied to divide different kinds of plant pathogens.
6. Endophytes in Induced Resistance
The presence of endophytic fungi in plants can induce them to produce compounds which act on phytopathogens or alter their plant morphology so that they may be better able to defend themselves in unfavorable situations. The action mechanisms of endophytes in inducing resistance may include increased synthesis of phytoalexins and PR-proteins, cell wall thickening through depositing lignin and glucans, increased cuticle thickness, among others, which may hinder penetration and development of the pathogen in the host plant [47].
The endophytic microorganisms have the ability to produce a large number of secondary metabolites, with this number being higher than any other microorganism [69]. It was recently revealed that the endophyte-plant interaction can go beyond the balance between virulence and defense, being much more complex and precisely controlled [163]. Among the control mechanisms provided by endophytes such as competition for space and nutrients, mycoparasitism, antibiosis and induced resistance, there is a high probability that induced resistance is one of the most important mechanisms used by endophytes in disease control [23]. Some of the compounds recognized by the plant are common among all fungi, such as certain cell wall components and enzymes such as xylanases, cellulases and chitinases [163]. Other compounds are more specific for certain species, including secreted proteins, specialized metabolites and lipids, hormonal molecules and volatile compounds [164].
Some studies report the production of bioactive molecules by endophytic microorganisms identical to those produced by the host plant [165]. These studies corroborate the theory that they adapted to the plant microenvironment during the co-evolution of the host plant with the microorganism and were able to assimilate part of their hosts’ DNA to their genome, acquiring the ability to synthesize bioactive compounds [166]. Other theories assume that the reverse is also true, so that part of the microbial DNA was assimilated to the plant’s genome during a co-evolution process, and what was exclusive to the endophyte is also passed to its host [167]. Thus, endophytic fungi can regulate biochemical routes, leading to the production of substances which are common to their hosts or vice versa, and which can have applications outside the plant in which they live [168]. Examples of endophytic microorganisms that produced the same metabolites as the host plant can be illustrated by Fusarium sp. and Myrothecium sp. fungi [169], as well as macrocyclic trichothecene producers, which were isolated from Baccharis megapotamica and B. coridifolia plants [170,171].
Gilmaniella sp. is an endophytic fungus isolated from Atractylodes lancea plants, and has been reported to produce metabolites with an elicitor effect on its hosts which can substantially improve the total volatile oil content, while in turn the fungus could effectively improve the quality of herbal medicines [172]. Endophytes isolated from Cicer arietinum plants have been identified and characterized due to their ability to induce resistance in plants by producing higher levels of defense compounds, antioxidant and phenolic enzymes, in addition to solubilizing P and Zn, and reducing infection by B. cinerea in plant tissues [173]. The moderate and constant activation of these enzymes can be a key mechanism for plant resistance [173].
The endophytic fungus P. indica has a wide range of hosts and exhibits interesting biological activities for agriculture such as promoting growth, inducing resistance against phytopathogens, water and abiotic stresses, among others [174]. For example, P. indica induces resistance against Fusarium in Hordeum vulgare [175], T. aestivum [176], Z. mays [177] and S. lycopersicum [178] plants. Endophytic fungi may present systemic distribution in the plant or be restricted to certain tissues such as the roots and stem, among others. In this sense, the inoculation of Blumeria graminis in H. vulgare plants and the pre-inoculation of P. indica in the root system reduces 58% of the symptoms of the disease, clearly demonstrating the promotion of induced resistance [175].
The SA-dependent defenses are generally effective against biotrophic pathogens, while JA/ET-dependent defenses are effective against necrotrophic pathogens [179,180,181,182]. Thus, it is assumed that if an endophyte tends to increase protection against necrotrophic fungi and makes the plant resistant, on the other hand it may become more susceptible to biotrophic fungi [183].
The suppression of plant diseases in most cases occurs by manipulating the JA and ET pathway by beneficial microorganisms leading to induced systemic resistance (ISR) [174]. Despite this, other hormones may be involved in the phenomenon of induced resistance, however, they will not be discussed here. Based on this information, it is possible to differentiate the defense mechanisms of the plant when it induces resistance to fungi or abiotic stresses (Figure 2). If a plant shows infection with biotrophic fungi, signaling will normally occur from the salicylic acid pathway (Figure 2). However, if the infection occurs from necrotrophic fungi, signaling occurs via the JA and ET pathways. Induced systemic resistance can promote local or systemic resistance of the plant against biotrophic fungi, for example, and susceptibility to necrotrophic fungi, making the plant resistant or susceptible depending on the triggered pathway (Figure 2).
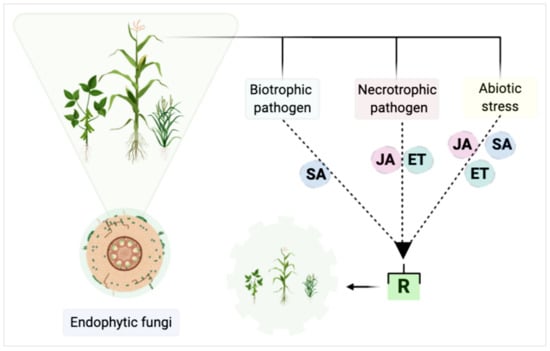
Figure 2.
Main plant pathways triggered in defense processes using fungi and abiotic stresses as models. SA—salicylic acid; JA—jasmonic acid; ET—ethylene; R—resistance. Adapted from Bastias et al. [184], with additional information from Thlaer et al., Kunkel & Brooks, and Junt et al. [179,180,183]. Created with BioRender.com.
The triggered metabolic pathways, as seen in Figure 2, are dependent upon which microorganism will affect the plant, and although the benefits of endophytic fungi in plant development are elucidated, the mechanisms involved in the plant vs. endophytic vs. pathogen/abiotic interaction are not well understood.
It should be noted that the crosstalk between SA, JA and ET signaling enables the plant to fine-tune the defense response [121]. For example, systemic resistance dependent on JA/ET has been found for some endophytes such as Piriformospora indica [185]. However, P. indica induced resistance independent of the JA/ET pathway in other pathosystems. These findings indicate that the hormonal roles and their interactions are complex, and the application of a microorganism to the plant probably alters the entire hormonal profile, depending on the host and the inducing agent.
When evaluating the compounds produced by chickpea plants inoculated with endophytes, a high production of indole acetic acid (IAA) was found [111]. It is already known that IAA levels contribute to higher growth of sprouts and roots [186], for example, mandarin plants inoculated by endophytes such as Nocardia, Nocardiopsis, Spirillospora, Microbispora and Micromonospora have higher length, number of shoots and root mass.
An avirulent isolate of F. solani sensu lato obtained from the tissues of C. acuminata bark, has been reported as a producer of the metabolite camptothecin, which guarantees its protection against this compound through specific changes in the catalytic domains of its topoisomerase I [187]. Likewise, topoisomerase I encoded by other endophytic fungi, isolated from the same tissue, but which does not produce camptothecin, contains the same changes to make it resistant to camptothecin action. This suggests that evolutionary pre-adaptation is similar in endophytes which infect the same plant, regardless of its biosynthetic capacity [188], ensuring that endophytic microorganisms have positive interactions and that their metabolites are not toxic to their hosts.
Given the above, an overview of the scientific work carried out with endophytic fungi to induce resistance can be seen in Table 2.

Table 2.
Endophytic fungi species acting through induced resistance *.
7. Endophytes in Inducing Tolerance to Abiotic Stresses
Endophytes have been used as sources of biotic elicitors because of their ability to simulate responses to diseases in plant cells. Endophytes have stood out for their ability to synthesize and accumulate secondary metabolites in the tissues of their hosts which can influence the functioning of antioxidant enzymes, in turn activating the cascade of defense signals and promoting the positive regulation of gene expression of important enzymes during the production of secondary metabolites [221]. In this sense, several studies have shown that the association of endophytes increases tolerance to abiotic stresses [221,222,223,224,225,226].
There is currently a need for new agricultural practices to maximize the efficiency of crops at elevated temperatures due to the increasing effects of global climate changes [226]. The ability of endophytes to confer heat tolerance has been observed in plants such as Adiantum capillus-veneris [227], Helianthus annuus and Glycine max [228], Cucumis sativus [226], among others.
Treatment with the thermophilic Thermomyces sp. endophytic fungus which supports high temperatures (CpE) eliminated the adverse effects of thermal stress on cucumber plants, maintaining the maximum quantum efficiency of photosystem II, the photosynthesis rate and water use efficiency. In addition, CpE treatments induced significant accumulation of total sugars, flavonoids, saponins, soluble proteins and the activities of antioxidant enzyme compared to untreated cucumber plants under heat stress conditions [226]. On the other hand, cucumber plants treated with Thermomyces sp. exhibited an improvement in root length over untreated cucumber plants. This phenological response is an essential adaptive trait in desert ecosystems, enabling the plant to better penetrate and extract soil moisture and nutrients under limited water conditions [226].
Plants under thermal stresses quickly increase stomatal conductance, thereby promoting a high transpiration rate. Even under these conditions, these plants have a slow stomatal opening and a low transpiration rate when they are treated with endophytes [226]. The endophytic Thermomyces sp. maintained water content in the leaf, increasing the water use efficiency under stress conditions. In addition, thermophilic fungi prevent excessive water losses from the plant through stomatal closure as a physiological-adaptive strategy to save water before further damage occurs due to increased temperature stresses [226]. These fungi promote an accumulation of primary and secondary metabolites [226]. The higher accumulation of sugars and flavonoids in plant tissues in many plant-microbe interactions act as reactive oxygen species (ROS) scavengers and signaling molecules, thereby enabling plant growth and tolerance to abiotic and biotic stresses [229].
The role of endophytes in providing tolerance to water stress by regulating stress-inducible genes has been reported in Cucumis sativus [230], Zea mays [231,232], Oryza sativa [233], S. lycopersicum [234], Triticum aestivum [235], Citrus reticulata [225] and Saccharum officinarum [236]. The relief of water stress due to the action of endophytes may be the result of an increase in antioxidant enzymes, bioactive compounds, chlorophyll content, carotenoid content and chlorophyll fluorescence. In addition to changing all these parameters in C. reticulata plants, Penicillium citrinum, Aureobasidium pullulans and Dothideomycetes sp. endophytes also promoted plant growth [225].
The mechanisms mediated by endophytes are reported to facilitate plant adaptation to drought tolerance by generating phytohormones, ROS, exopolysaccharides, 1-aminocyclopropane-1-carboxylate deaminase, and volatile compounds; change in root morphology; biosynthesis of anti-stress metabolites and positive regulation of stress-responsive genes in host plants [237]. In addition, the accumulation of solutes in plants with endophytes is reported in grasses when subjected to water stress [238].
One of the hypotheses for tolerance to water stresses mediated by endophytes in host plants is the use of CO2 released by endophytes to continue photosynthesis. This relieves the lack of CO2 in stressed plants due to stoma closure. It was reported that 2.7% of CO2 released in the roots by endophytes in Populus deltoides was assimilated in the host’s photosynthesis [239].
The role of endophytes in providing tolerance to heavy metal stresses has been observed in plant cultures such as Triticum aestivum [224,240], Lycopersicon esculentum [241], and Glycine max [242], among others. For example, the endophytic P. roqueforti fungus induced resistance in T. aestivum plants grown in soil contaminated with heavy metals, restricting heavy metal transfer from the soil to the plants, and secreting indole acetic acid. In addition, these wheat plants inoculated with the endophytic fungus and watered with residual water showed higher growth, nutrient absorption and low heavy metal concentrations in the shoot and roots. In contrast, wheat plants not inoculated under heavy metal stress showed stunted growth with chlorosis symptoms. The inoculation of P. roqueforti can establish a symbiotic relationship with host plants, which is useful for stabilizing heavy metals, meaning that it helps host plants to flourish in soil that is highly contaminated with heavy metals [224]. Thus, the endophytic fungi increase the host plant’s capacity to accumulate heavy metals by direct or indirect mechanisms in addition to cell detoxification by enzymatic activity. Endophytes can directly help the host plant through increased mobilization of heavy metals, thus alleviating the toxicity level of metals in plants [243], or indirectly by improving plant growth and stress tolerance.
Endophytes can benefit the host plant by increasing its ability to absorb essential nutrients from contaminated soil [244]. Furthermore, these fungi can degrade pollutants present in contaminated soil [245] and convert them to a non-toxic form. The exogenous supply of phytohormones by endophytes can bring positive physiological changes in the host plant to withstand stress conditions. In addition to phytohormones, the biofertilization capacity of endophytic fungi can increase the availability of nutrients to the host plant in soil contaminated with heavy metals through solubilization [246]. The possible mechanisms modified by the interaction with endophytic fungi under abiotic stresses can be seen in Figure 3.
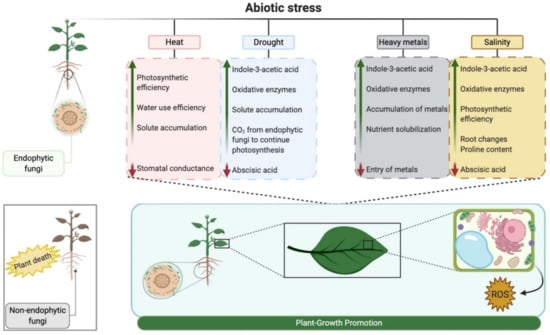
Figure 3.
Potential reactions to abiotic stresses evidenced by plants when interacting with endophytic fungi. The green arrow represents the increase and the red arrow represents the reduction of the listed characteristics. Created with BioRender.com.
The role of these microorganisms in providing tolerance to salt stress has been observed in plant cultures such as Z. mays [222], S. lycopersicum [247], O. sativa [248], T. aestivum [249], Cucumis sativus [230], and G. max [223,250], among others. For example, the endophytic fungus P. indica increased the growth and yield of S. lycopersicum under salt stress conditions, inducing a series of morphological and biochemical events which together contributed to relieve the impact of salt stress. This endophyte promoted an increase in the chlorophyll and indole acetic acid content, enzymes such as catalase and superoxide dismutase, increased the root branching, the fresh and dry mass of plants and fruit production by 65% under salt stress. In addition, tomato plants colonized with endophytes reduced abscisic acid (ABA) and proline levels when compared to non-colonized plants [247]. The ROS-sequestering enzymes appear to substantially contribute to improving salt stress tolerance [251].
Many plants produce high proline levels under salt stress; however, these proline levels can be reduced when plants are inoculated with endophytic fungi [247]. ABA controls proline biosynthesis to reduce cytoplasmic osmotic stress caused by increased salts in the root zone [252] and, therefore, for example, ABA levels are reduced by approximately 30% under saline stress conditions, and the proline content is consequently reduced [253].
Abiotic stresses, including oxidative stress, drought, flooding, salinity and heat stress are interrelated, resulting in the synthesis of ROS which cause cell damage, and consequently cell death under prolonged exposure [230]. An increase in the amount of ROS in plant cells causes oxidative degradation of RNA and DNA, lipid peroxidation and oxidative stress [254]. The ROS signal directly modifies the redox balance of regulatory proteins, transcription and translation, thereby stimulating responses in the plant which help to reduce the negative effects of stress and moderate the metabolic ROS concentration [255].
The hypothesis is that endophytes also initially secrete a small amount of ROS, for example hydrogen peroxide, which triggers the antioxidant enzymes of the infected host [256]. The constant release of ROS in small amounts prevents cell hypersensitivity to ROS, improves the absorption of nutrients (calcium, potassium, magnesium and phosphorus) by plants and increases other endosymbiotic interactions of the host [257]. One of the main responses by plant tissues to the presence of ROS produced by endophytes is to accumulate proline, methionine, flavonoids and other phenolic compounds to increase their resistance [258].
The probable mechanisms by which hypersensitivity responses and acquired systemic resistance of the hosts can occur involve the crosstalk between endophytes and host plants, as well as the generation of ROS and antioxidants [259]. While some fungal endophytes produce ROS to acquire nutrients from host cells and maintain their mutualistic interactions with plants, other fungal endophytes lower ROS concentrations to mitigate the effect of abiotic stresses on their hosts [260].
Based on the above, a general view of the scientific work carried out with endophytic fungi exhibiting effects on abiotic stresses can be seen in Table 3, together with the possible altered mechanisms outlined in Figure 3.

Table 3.
Endophytic fungi with effects on abiotic stresses in plants (induced systemic tolerance).
8. Perspectives
It is known that each of the approximately 300,000 species of plants existing on Earth includes a universe of endophytic microorganisms, especially woody plants, which may contain numerous species with potential for studies [267]. Elucidating and identifying the most active metabolite structures are essential to develop new products [268]. It is worth considering that individual substances of a crude extract often do not present relevant microbial activity, since the compounds present in this extract act synergistically with other substances produced by the microorganism [268]. Thus, elucidating the action mechanisms of endophytic fungi and their interaction in plant protection, either by the action of direct biological control, or by induced resistance and tolerance to abiotic stresses, make endophytic fungi a highly promising tool for inserting into integrated management, and widely important for the agribusiness.
During the course of evolution, the endophytes were not only able to colonize plants, but developed a complex signalization with their hosts, promoting benefits which could be explored in agricultural systems. The signalization is mediated by effector molecules, usually proteins that are delivered to the host plant and trigger beneficial effects, e.g., growth promotion and induced resistance. Several studies have been dedicating their efforts to understand this intricate network by using different approaches, notably the Omics approach (genomics, transcriptomics, proteomics, metabolomics, etc.) [269,270]. The Omics approach offers the possibility to identify and characterize proteins and genes, which could be useful to select promising strains as biopesticides, plant growth promoters, etc. In addition, metagenomic analyses have been used to investigate the microbial diversity associated with plants under several environmental conditions [271]. Furthermore, metagenomic analyses enable investigating the microbiome in plant health in crops and natural system [271]. Although the focus of the current review is not the Omics approach, it is important for understanding the endophyte–plant interactions and their possible use in agriculture. Finally, society’s pressure for food production in more sustainable ways with biotechnological approaches is encouraging exploitation of endophytic microorganisms.
9. Conclusions
Endophytic fungi can trigger innumerable mechanisms in the plant, providing protection against biotic and abiotic disorders. These fungi satisfactorily perform biological control against plant diseases with the potential to be used as a tool for bioprospecting new molecules and genetic modification of plants due to their potential for genetic modulation and interaction with the host.
Tolerance to abiotic stresses can be obtained by an association of endophytes with the target cultures, presenting promising results and making it possible to grow plants in certain places where plants without association with the endophytic agent could have difficulties to developing.
The secondary metabolites produced by endophytes exhibit important biological activity and can become valuable products. Thus, isolating and characterizing endophytic microorganisms from plants which have not yet been studied can enable discovering new species with the potential to produce substances of interest which can be used in the biological control of diseases, as elicitors in induced resistance and for inducing tolerance to abiotic stresses.
Author Contributions
Conceptualization, D.C.F., S.F.P., D.S. and D.D.N.; investigation, D.C.F., S.d.P., A.G.T. and V.H.M.d.S.; writing—original draft preparation, D.C.F., S.d.P., A.G.T. and V.H.M.d.S.; writing—review and editing, S.F.P., D.S. and D.D.N.; visualization, D.C.F. and S.d.P.; funding acquisition, D.D.N. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by Brazilian National Council for Scientific and Technological Development (CNPq), grant number 164328/2018-1, 142365/2019-0, 141975/2020-2, the Coordination for the Improvement of Higher Education Personnel (CAPES/Proex, Brazil) and The APC was funded by University of São Paulo.
Conflicts of Interest
The authors declare no conflict of interest. The research funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- FAO. How to Feed the World in 2050. Available online: http://www.fao.org/wsfs/forum2050/wsfs-forum/pt/ (accessed on 26 July 2020).
- Bergamin Filho, A.; Amorin, L.; Rezende, J.A.M. Importância das doenças de plantas. In Manual de Fitopatologia: Princípios e Conceitos; Editora Ceres LTDA: Piracicaba, Brazil, 2018; Volume 1, pp. 15–24. ISBN 978-85-318-0056-6. [Google Scholar]
- Savary, S.; Willocquet, L.; Pethybridge, S.J.; Esker, P.; McRoberts, N.; Nelson, A. The Global Burden of Pathogens and Pests on Major Food Crops. Nat. Ecol. Evol. 2019, 3, 430–439. [Google Scholar] [CrossRef]
- Goss, E.M.; Tabima, J.F.; Cooke, D.E.L.; Restrepo, S.; Fry, W.E.; Forbes, G.A.; Fieland, V.J.; Cardenas, M.; Grunwald, N.J. The Irish Potato Famine Pathogen Phytophthora Infestans Originated in Central Mexico Rather than the Andes. Proc. Natl. Acad. Sci. USA 2014, 111, 8791–8796. [Google Scholar] [CrossRef]
- Yoshida, K.; Schuenemann, V.J.; Cano, L.M.; Pais, M.; Mishra, B.; Sharma, R.; Lanz, C.; Martin, F.N.; Kamoun, S.; Krause, J.; et al. The Rise and Fall of the Phytophthora Infestans Lineage That Triggered the Irish Potato Famine. eLife 2013, 2, e00731. [Google Scholar] [CrossRef] [PubMed]
- Haverkort, A.J.; Boonekamp, P.M.; Hutten, R.; Jacobsen, E.; Lotz, L.A.P.; Kessel, G.J.T.; Visser, R.G.F.; van der Vossen, E.A.G. Societal Costs of Late Blight in Potato and Prospects of Durable Resistance Through Cisgenic Modification. Potato Res. 2008, 51, 47–57. [Google Scholar] [CrossRef]
- Oerke, E.-C. Crop Losses to Pests. J. Agric. Sci. 2006, 144, 31–43. [Google Scholar] [CrossRef]
- Ficke, A.; Cowger, C.; Bergstrom, G.; Brodal, G. Understanding Yield Loss and Pathogen Biology to Improve Disease Management: Septoria Nodorum Blotch—A Case Study in Wheat. Plant Dis. 2018, 102, 696–707. [Google Scholar] [CrossRef]
- Hirooka, T.; Ishii, H. Chemical Control of Plant Diseases. J. Gen. Plant Pathol. 2013, 79, 390–401. [Google Scholar] [CrossRef]
- Waard, M.A.; Georgopoulos, S.G.; Hollomon, D.W.; Ishii, H.; Leroux, P.; Ragsdale, N.N.; Schwinn, F.J. Chemical Control of Plant Diseases: Problems and Prospects. Annu. Rev. Phytopathol. 1993, 31, 403–421. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. ISBN 978-0-12-802275-7. [Google Scholar]
- Deising, H.B.; Reimann, S.; Pascholati, S.F. Mechanisms and Significance of Fungicide Resistance. Braz. J. Microbiol. 2008, 39, 286–295. [Google Scholar] [CrossRef] [PubMed]
- The American Phytopathological Society. Fungicide Resistance in North America, 2nd ed.; Stevenson, K.L., McGrath, M.T., Wyenandt, C.A., Eds.; The American Phytopathological Society: Saint Paul, MI, USA, 2019; ISBN 978-0-89054-622-2. [Google Scholar]
- Köhl, J.; Kolnaar, R.; Ravensberg, W.J. Mode of Action of Microbial Biological Control Agents Against Plant Diseases: Relevance Beyond Efficacy. Front. Plant Sci. 2019, 10, 845. [Google Scholar] [CrossRef]
- O’Brien, P.A. Biological Control of Plant Diseases. Australas. Plant Pathol. 2017, 46, 293–304. [Google Scholar] [CrossRef]
- Walters, D.; Walsh, D.; Newton, A.; Lyon, G. Induced Resistance for Plant Disease Control: Maximizing the Efficacy of Resistance Elicitors. Phytopathology 2005, 95, 1368–1373. [Google Scholar] [CrossRef] [PubMed]
- Llorens, E.; García-Agustín, P.; Lapeña, L. Advances in Induced Resistance by Natural Compounds: Towards New Options for Woody Crop Protection. Sci. Agric. 2017, 74, 90–100. [Google Scholar] [CrossRef]
- Hammerschmidt, R. Induced Disease Resistance: How Do Induced Plants Stop Pathogens? Physiol. Mol. Plant Physiol. 1999, 55, 77–84. [Google Scholar] [CrossRef]
- Walters, D.R.; Ratsep, J.; Havis, N.D. Controlling Crop Diseases Using Induced Resistance: Challenges for the Future. J. Exp. Bot. 2013, 64, 1263–1280. [Google Scholar] [CrossRef] [PubMed]
- Nataraja, K.N.; Suryanarayanan, T.S.; Shaanker, R.U.; Senthil-Kumar, M.; Oelmüller, R. Plant–Microbe Interaction: Prospects for Crop Improvement and Management. Plant Physiol. Rep. 2019, 24, 461–462. [Google Scholar] [CrossRef]
- Latz, M.A.C.; Jensen, B.; Collinge, D.B.; Jørgensen, H.J.L. Endophytic Fungi as Biocontrol Agents: Elucidating Mechanisms in Disease Suppression. Plant Ecol. Divers. 2018, 11, 555–567. [Google Scholar] [CrossRef]
- Cheng, C.; Li, D.; Qi, Q.; Sun, X.; Anue, M.R.; David, B.M.; Zhang, Y.; Hao, X.; Zhang, Z.; Lai, Z. The Root Endophytic Fungus Serendipita Indica Improves Resistance of Banana to Fusarium oxysporum f. sp. cubense Tropical Race 4. Eur. J. Plant Pathol. 2020, 156, 87–100. [Google Scholar] [CrossRef]
- Kim, H.-Y.; Choi, G.J.; Lee, H.B.; Lee, S.-W.; Lim, H.K.; Jang, K.S.; Son, S.W.; Lee, S.O.; Cho, K.Y.; Sung, N.D.; et al. Some Fungal Endophytes from Vegetable Crops and Their Anti-Oomycete Activities against Tomato Late Blight. Lett. Appl. Microbiol. 2007, 44, 332–337. [Google Scholar] [CrossRef]
- MAPA Mercado de Biodefensivos Cresce Mais de 70% no Brasil em um Ano. Available online: https://www.gov.br/agricultura/pt-br/assuntos/noticias/feffmercado-de-biodefensivos-cresce-em-mais-de-50-no-brasil (accessed on 5 January 2021).
- Zhang, L.; Tao, Y.; Zhao, S.; Yin, X.; Chen, J.; Wang, M.; Cai, Y.; Niu, Q. A Novel Peroxiredoxin from the Antagonistic Endophytic Bacterium Enterobacter sp. V1 Contributes to Cotton Resistance against Verticillium Dahliae. Plant Soil 2020. [Google Scholar] [CrossRef]
- Sun, X.; Wang, G.; Xiao, H.; Jiang, J.; Xiao, D.; Xing, B.; Li, A.; Zhang, Y.; Sun, K.; Xu, Y.; et al. Strepimidazoles A–G from the Plant Endophytic Streptomyces sp. PKU-EA00015 with Inhibitory Activities against a Plant Pathogenic Fungus. J. Nat. Prod. 2020, 83, 2246–2254. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, J.; Zhang, H.; Ji, G.; Zeng, L.; Li, Y.; Yu, C.; Fernando, W.G.D.; Chen, W. Bacterial Blight Induced Shifts in Endophytic Microbiome of Rice Leaves and the Enrichment of Specific Bacterial Strains with Pathogen Antagonism. Front. Plant Sci. 2020, 11, 963. [Google Scholar] [CrossRef] [PubMed]
- De Bary, A. Morphologie Und Physiologie Der Pilze, Flechten Und Myxomyceten/Handbuch der Physiologischen Botanik; W. Engelmann: Leipzig, Germany, 1866. [Google Scholar]
- Carrol, G.C. The biology of endophytism in plants with particular reference to woody perennials. In Microbiology of Phyllosphere; Cambridge University Press: London, UK, 1986; pp. 205–222. [Google Scholar]
- Azevedo, J.L.; Araújo, W.L. Diversity and applications of endophytic fungi isolated from tropical plants. In Fungi: Multifaceted Microbes; Ganguli, B.N., Deshmukh, S.K., Eds.; Anamaya Publishers: New Delhi, India, 2007. [Google Scholar]
- Tejesvi, M.V.; Pirttilä, A.M. Endophytic Fungi, Occurrence, and Metabolites. In Physiology and Genetics; Anke, T., Schüffler, A., Eds.; Springer: Cham, Switzerland, 2018; pp. 213–230. ISBN 978-3-319-71739-5. [Google Scholar]
- Backman, P.A.; Sikora, R.A. Endophytes: An Emerging Tool for Biological Control. Biol. Control 2008, 46, 1–3. [Google Scholar] [CrossRef]
- Schulz, B.; Boyle, C. The Endophytic Continuum. Mycol. Res. 2005, 109, 661–686. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic Fungal Entomopathogens with Activity against Plant Pathogens: Ecology and Evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Yang, X.-L.; Zhang, J.-Z.; Luo, D.-Q. The Taxonomy, Biology and Chemistry of the Fungal Pestalotiopsis Genus. Nat. Prod. Rep. 2012, 29, 622. [Google Scholar] [CrossRef]
- Liu, X.; Dong, M.; Chen, X.; Jiang, M.; Lv, X.; Zhou, J. Antimicrobial Activity of an Endophytic Xylaria sp.YX-28 and Identification of Its Antimicrobial Compound 7-Amino-4-Methylcoumarin. Appl. Microbiol. Biotechnol. 2008, 78, 241–247. [Google Scholar] [CrossRef]
- Aly, A.H.; Debbab, A.; Kjer, J.; Proksch, P. Fungal Endophytes from Higher Plants: A Prolific Source of Phytochemicals and Other Bioactive Natural Products. Fungal Divers. 2010, 41, 1–16. [Google Scholar] [CrossRef]
- Li, J.; Li, L.; Si, Y.; Jiang, X.; Guo, L.; Che, Y. Virgatolides A–C, Benzannulated Spiroketals from the Plant Endophytic Fungus Pestalotiopsis Virgatula. Org. Lett. 2011, 13, 2670–2673. [Google Scholar] [CrossRef] [PubMed]
- Tejesvi, M.V.; Kajula, M.; Mattila, S.; Pirttilä, A.M. Bioactivity and Genetic Diversity of Endophytic Fungi in Rhododendron Tomentosum Harmaja. Fungal Divers. 2011, 47, 97–107. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B. Bioprospecting for Microbial Endophytes and Their Natural Products. MMBR 2003, 67, 491–502. [Google Scholar] [CrossRef]
- Araújo, W.L.; Lacava, P.T.; Marcon, J.; Lima, A.O.S.; Sobral, J.K.; Pizzirani-Kleiner, A.A. Guia Prático: Isolamento e Caracterização de Microrganismos Endofíticos; Copiadora “Luiz de Queiroz”: Piracicaba, Brazil, 2010. [Google Scholar]
- Saikkonen, K. Evolution of Endophyte?Plant Symbioses. Trends Plant Sci. 2004, 9, 275–280. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, X.; Liu, L.; Xiang, M.; Wang, W.; Sun, X.; Che, Y.; Guo, L.; Liu, G.; Guo, L.; et al. Genomic and Transcriptomic Analysis of the Endophytic Fungus Pestalotiopsis Fici Reveals Its Lifestyle and High Potential for Synthesis of Natural Products. BMC Genom. 2015, 16, 28. [Google Scholar] [CrossRef]
- Symbiotic Endophytes. Soil Biology; Aroca, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; Volume 37, ISBN 978-3-642-39316-7. [Google Scholar]
- Bacon, C.W.; White, J.F. Functions, Mechanisms and Regulation of Endophytic and Epiphytic Microbial Communities of Plants. Symbiosis 2016, 68, 87–98. [Google Scholar] [CrossRef]
- Oki, Y.; Fernandes, G.W.; Correa Junior, A. Fungos: Amigos Ou Inimigos? Ciência Hoje 2008, 42, 64–66. [Google Scholar]
- Peixoto Neto, P.A.S.; Azevedo, J.L.; Araújo, W.L. Microrganismos Endofíticos: Interação Com as Plantas e Potencial Biotecnológico. Biotecnol. Ciênc. Desenvolv. 2002, 29, 62–76. [Google Scholar]
- Pamphile, J.A.; da Rocha, C.L.M.S.C.; Azevedo, J.L. Co-Transformation of a Tropical Maize Endophytic Isolate of Fusarium Verticillioides (Synonym F. Moniliforme) with GusA and Nia Genes. Genet. Mol. Biol. 2004, 27, 253–258. [Google Scholar] [CrossRef]
- Azevedo, J.L.; Maccheroni Júnior, W.; Araújo, W.L. Importância dos microorganismos endofíticos na agricultura. In RAPP: Revisão Anual de Patologia de Plantas; Luz, W.C., Ed.; Brazilian Phytopathological Society: Passo Fundo, Brazil, 2003; Volume 11, pp. 333–371. [Google Scholar]
- Rubini, M.R.; Silva-Ribeiro, R.T.; Pomella, A.W.V.; Maki, C.S.; Araújo, W.L.; Dos Santos, D.R.; Azevedo, J.L. Diversity of Endophytic Fungal Community of Cacao (Theobroma cacao L.) and Biological Control of Crinipellis Perniciosa, Causal Agent of Witches’ Broom Disease. Int. J. Biol. Sci. 2005, 1, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E.; Mejia, L.C.; Kyllo, D.; Rojas, E.I.; Maynard, Z.; Robbins, N.; Herre, E.A. Fungal Endophytes Limit Pathogen Damage in a Tropical Tree. Proc. Natl. Acad. Sci. USA 2003, 100, 15649–15654. [Google Scholar] [CrossRef]
- Araújo, W.L.; Maccheroni, W., Jr.; Aguilar-Vildoso, C.I.; Barroso, P.A.; Saridakis, H.O.; Azevedo, J.L. Variability and Interactions between Endophytic Bacteria and Fungi Isolated from Leaf Tissues of Citrus Rootstocks. Can. J. Microbiol. 2001, 47, 229–236. [Google Scholar] [CrossRef]
- Mariano, R.L.R.; Lira, R.V.I.; Silveira, E.B.; Menezes, M. Levantamento de Fungos Endofíticos e Epifíticos Em Folhas de Coqueiro No Nordeste Do Brasil. I. Frequência Da População Fúngica e Efeito Da Hospedeira. Agrotópica 1997, 9, 127–134. [Google Scholar]
- Pereira, J.O.; Vieira, M.L.C.; Azevedo, J.L. Endophytic Fungi from Musa Acuminata and Their Reintroduction into Axenic Plants. World J. Microbiol. Biotechnol. 1999, 15, 37–40. [Google Scholar] [CrossRef]
- Photita, W.; Lumyong, S.; Lumyong, P.; Hyde, K.D. Endophytic Fungi of Wild Banana (Musa Acuminata) at Doi Suthep Pui National Park, Thailand. Mycol. Res. 2001, 105, 1508–1513. [Google Scholar] [CrossRef]
- Yang, G.; Li, P.; Meng, L.; Xv, K.; Dong, F.; Qiu, Y.; He, L.; Lin, L. Diversity and Communities of Culturable Endophytic Fungi from Different Tree Peonies (Geoherbs and Non-Geoherbs), and Their Biosynthetic Potential Analysis. Braz. J. Microbiol. 2018, 49, 47–58. [Google Scholar] [CrossRef]
- Barklund, P.; Kowalski, T. Endophytic Fungi in Branches of Norway Spruce with Particular Reference to Tryblidiopsis Pinastri. Can. J. Bot. 1996, 74, 673–678. [Google Scholar] [CrossRef]
- Faeth, S.H.; Hammon, K.E. Fungal Endophytes in Oak Trees: Experimental Analyses of Interations with Leafminers. Ecology 1997, 78, 820–827. [Google Scholar] [CrossRef]
- Dingle, J.; Mcgee, P.A. Some Endophytic Fungi Reduce the Density of Pustules of Puccinia Recondita f. sp. Tritici in Wheat. Mycol. Res. 2003, 107, 310–316. [Google Scholar] [CrossRef] [PubMed]
- Paz, F.B., Jr.; Menezes, M. Fungos Endofíticos Em Sementes de Girassol e Diferenciação Morfológica e Enzimática de Espécies de Fusarium. Summa Phytopathol. 2002, 31, 87–93. [Google Scholar]
- Manganyi, M.C.; Regnier, T.; Kumar, A.; Bezuidenhout, C.C.; Ateba, C.N. Phylogenetic Analysis and Diversity of Novel Endophytic Fungi Isolated from Medicinal Plant Sceletium Tortuosum. Phytochem. Lett. 2018, 27, 36–43. [Google Scholar] [CrossRef]
- Pimentel, I.C.; Kuczkowski, F.R.; Chime, M.A.; Auer, C.G.; Grigoletti, A., Jr. Fungos Endofíticos Em Folhas de Erva-Mate (Ilex Paraguariensis A. St.-Hil.). Floresta 2006, 36, 123–128. [Google Scholar] [CrossRef]
- Espinosa-Garcia, F.J.; Langenheim, J.H. Effect of Some Leaf Essential Oil Phenotypes from Coastal Redwood on Growth of Its Predominant Endophytic Fungus, Pleuroplaconema sp. J. Chem. Ecol. 1991, 17, 1837–1857. [Google Scholar] [CrossRef] [PubMed]
- Lappalainen, J.H.; Koricheva, J.; Helander, M.L.; Haukioja, E. Densities of Endophytic Fungi and Performance of Leafminers (Lepidoptera: Eriocraniidae) on Birch along a Pollution Gradient. Environ. Pollut. 1999, 104, 99–105. [Google Scholar] [CrossRef]
- Kinkel, L. Fungal Community Dynamics. In Microbial Ecology of Leaves; Andrews, J.H., Hirano, S.S., Eds.; Springer: New York, NY, USA, 1991; pp. 253–270. ISBN 978-1-4612-7822-1. [Google Scholar]
- Carroll, G.C.; Carroll, F.E. Studies on the Incidence of Coniferous Needle Endophytes in the Pacific Northwest. Can. J. Bot. 1978, 56, 3034–3043. [Google Scholar] [CrossRef]
- Reiter, B.; Pfeifer, U.; Schwab, H.; Sessitsch, A. Response of Endophytic Bacterial Communities in Potato Plants to Infection with Erwinia Carotovora Subsp. Atroseptica. Appl. Environ. Microbiol. 2002, 68, 2261–2268. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-H.; Crowley, D.E.; Menge, J.A. 16S RDNA Fingerprinting of Rhizosphere Bacterial Communities Associated with Healthy and Phytophthora Infected Avocado Roots. FEMS Microbiol. Ecol. 2001, 35, 129–136. [Google Scholar] [CrossRef]
- Zhang, H.W.; Song, Y.C.; Tan, R.X. Biology and Chemistry of Endophytes. Nat. Prod. Rep. 2006, 23, 753. [Google Scholar] [CrossRef] [PubMed]
- Arnold, A.E. Endophytic fungi: Hidden components of tropical community ecology. In Tropical Forest Community Ecology; Carson, W.P., Schnitzer, S.A., Eds.; Wiley-Blackwell: Hoboken, NJ, USA, 2008; pp. 254–273. ISBN 978-1-4051-1897-2. [Google Scholar]
- Kamoun, S.; Furzer, O.; Jones, J.D.G.; Judelson, H.S.; Ali, G.S.; Dalio, R.J.D.; Roy, S.G.; Schena, L.; Zambounis, A.; Panabières, F.; et al. The Top 10 Oomycete Pathogens in Molecular Plant Pathology: Top 10 Oomycete Plant Pathogens. Mol. Plant Pathol. 2015, 16, 413–434. [Google Scholar] [CrossRef] [PubMed]
- Bergamin Filho, A.; Kitajima, E.W. A história da fitopatologia. In Manual de Fitopatologia: Princípios e Conceitos; Editora Agronômica Ceres Ltd.: Piracicaba, Brazil, 2018; Volume 1, pp. 3–13. ISBN 978-85-318-0056-6. [Google Scholar]
- Eves-van den Akker, S.; Jones, J.T. Sex: Not All That It’s Cracked up to Be? PLoS Genet. 2018, 14, e1007160. [Google Scholar] [CrossRef]
- Lechenet, M.; Dessaint, F.; Py, G.; Makowski, D.; Munier-Jolain, N. Reducing Pesticide Use While Preserving Crop Productivity and Profitability on Arable Farms. Nat. Plants 2017, 3, 17008. [Google Scholar] [CrossRef]
- Cook, R.J.; Baker, K.F. The Nature and Practice of Biological Control of Plant Pathogens; American Phytopathological Society: St. Paul, MI, USA, 1983; ISBN 978-0-89054-053-4. [Google Scholar]
- Mathre, D.E.; Cook, R.J.; Callan, N.W. From Discovery to Use: Traversing the World of Commercializing BioControl Agents for Plant Disease Control. Plant Dis. 1999, 83, 972–983. [Google Scholar] [CrossRef] [PubMed]
- Rosier, A.; Bishnoi, U.; Lakshmanan, V.; Sherrier, D.J.; Bais, H.P. A Perspective on Inter-Kingdom Signaling in Plant–Beneficial Microbe Interactions. Plant Mol. Biol. 2016, 90, 537–548. [Google Scholar] [CrossRef]
- Venturi, V.; Keel, C. Signaling in the Rhizosphere. Trends Plant Sci. 2016, 21, 187–198. [Google Scholar] [CrossRef]
- Mhlongo, M.I.; Piater, L.A.; Madala, N.E.; Labuschagne, N.; Dubery, I.A. The Chemistry of Plant–Microbe Interactions in the Rhizosphere and the Potential for Metabolomics to Reveal Signaling Related to Defense Priming and Induced Systemic Resistance. Front. Plant Sci. 2018, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Tena, G. Recruiting Microbial Bodyguards. Nat. Plants 2018, 4, 857. [Google Scholar] [CrossRef] [PubMed]
- Jatala, P.; Kaltenbach, R.; Rocangel, M. Multiple Application and Longterm Effect of Paecilomyces Lilacinus in Controlling Meloidogyne Incognita under Field Conditions. J. Nematol. 1981, 13, 445. [Google Scholar]
- Stirling, G.R. Biological Control of Plant Parasitic Nematodes: Progress, Problems and Prospects; Springer: Wallingford, CT, USA, 1991. [Google Scholar]
- Okra, Y.; Koltai, H.; Bar-eyal, M.; Mor, M.; Sharon, E.; Chet, I.; Spiegel, Y. New Strategies for the Control of Plant-Parasitic Nematodes. Pest Manag. Sci. 2000, 56, 983–988. [Google Scholar] [CrossRef]
- De Souza, V.H.M.; Inomoto, M.M.; Pascholati, S.F.; Roma-Almeida, R.C.C.; Melo, T.A.; Rezende, D.C. Fitonematoides: Controle biológico e indução de resistência. In Revisão Anual de Patologia de Plantas; Sociedade Brasileira de Fitopatologia: Brasília, Brazil, 2015; pp. 242–292. [Google Scholar]
- Pascholati, S.F.; Dalio, R.J.D. Fisiologia do parasitismo: Como as plantas se defendem dos patógenos. In Manual de Fitopatologia: Princípios e Conceitos; Amorim, L., Rezendo, J.A.M., Bergamim Filho, A., Eds.; Editora Agronômica Ceres Ltd.: Piracicaba, Brazil, 2018; Volume 1, pp. 424–450. ISBN 978-85-318-0056-6. [Google Scholar]
- Inbar, J.; Menendez, A.; Chet, I. Hyphal Interaction between Trichoderma harzianum and Sclerotinia sclerotiorum and Its Role in Biological Control. Soil Biol. Biochem. 1996, 28, 757–763. [Google Scholar] [CrossRef]
- Abdullah, M.T.; Ali, N.Y.; Suleman, P. Biological Control of Sclerotinia sclerotiorum (Lib.) de Bary with Trichoderma harzianum and Bacillus amyloliquefaciens. Crop Protect. 2008, 27, 1354–1359. [Google Scholar] [CrossRef]
- Elsherbiny, E.A.; Taher, M.A.; Elsebai, M.F. Activity of Purpureocillium lilacinum Filtrates on Biochemical Characteristics of Sclerotinia sclerotiorum and Induction of Defense Responses in Common Bean. Eur. J. Plant Pathol. 2019, 155, 39–52. [Google Scholar] [CrossRef]
- Silva, S.D.; Carneiro, R.M.D.G.; Faria, M.; Souza, D.A.; Monnerat, R.G.; Lopes, R.B. Evaluation of Pochonia Chlamydosporia and Purpureocillium lilacinum for Suppression of Meloidogyne Enterolobii on Tomato and Banana. J. Nematol. 2017, 49, 77–85. [Google Scholar] [CrossRef]
- Ferraz, S. Manejo Sustentável de Fitonematoides, 1st ed.; Editora UFV: Viçosa, Brazil, 2010. [Google Scholar]
- Baron, N.C.; de Souza Pollo, A.; Rigobelo, E.C. Purpureocillium lilacinum and Metarhizium marquandii as Plant Growth-Promoting Fungi. PeerJ 2020, 8, e9005. [Google Scholar] [CrossRef]
- Spadaro, D.; Gullino, M.L. Improving the Efficacy of BioControl Agents against Soilborne Pathogens. Crop Protect. 2005, 24, 601–613. [Google Scholar] [CrossRef]
- Laur, J.; Ramakrishnan, G.B.; Labbé, C.; Lefebvre, F.; Spanu, P.D.; Bélanger, R.R. Effectors Involved in Fungal-Fungal Interaction lead to a Rare Phenomenon of Hyperbiotrophy in the Tritrophic System BioControl Agent-Powdery Mildew-Plant. New Phytol. 2018, 217, 713–725. [Google Scholar] [CrossRef] [PubMed]
- De Medeiros, F.H.V.; da Silva, J.C.P.; Pascholati, S.F. Controle biológico de doenças de plantas. In Manual de Fitopatologia: Princípios e Conceitos; Amorim, L., Rezende, J.A.M., Bergamin Filho, A., Eds.; Agronômica Ceres Ltd.: Piracicaba/São Paulo, Brazil, 2018; Volume 1, pp. 261–272. ISBN 978-85-318-0056-6. [Google Scholar]
- Kesel, J.; Conrath, U.; Flors, V.; Luna, E.; Mageroy, M.H.; Mauch-Mani, B.; Pastor, V.; Pozo, M.J.; Pieterse, C.M.J.; Ton, J.; et al. The Induced Resistance Lexicon: Do’s and Don’ts. Trends Plant Sci. 2021, S1360138521000029. [Google Scholar] [CrossRef]
- Burketova, L.; Trda, L.; Ott, P.G.; Valentova, O. Bio-Based Resistance Inducers for Sustainable Plant Protection against Pathogens. Biotechnol. Adv. 2015, 33, 994–1004. [Google Scholar] [CrossRef]
- Cavalcanti, L.S.; Di Piero, R.M.; Cia, P.; Pascholati, S.F.; Resende, M.L.V.; Romeiro, R.S. Indução de Resistência em Plantas a Patógenos e Insetos, 1st ed.; Fundação de Estudos Agrários Luiz de Queiroz-FEALQ: Piracicaba, Brazil, 2005; ISBN 85-7133-035-2. [Google Scholar]
- Oliveira, M.D.M.; Varanda, C.M.R.; Félix, M.R.F. Induced Resistance during the Interaction Pathogen x Plant and the Use of Resistance Inducers. Phytochem. Lett. 2016, 15, 152–158. [Google Scholar] [CrossRef]
- Mauch-Mani, B.; Baccelli, I.; Luna, E.; Flors, V. Defense Priming: An Adaptive Part of Induced Resistance. Annu. Rev. Plant Biol. 2017, 68, 485–512. [Google Scholar] [CrossRef]
- Conrath, U.; Beckers, G.J.M.; Flors, V.; García-Agustín, P.; Jakab, G.; Mauch, F.; Newman, M.-A.; Pieterse, C.M.J.; Poinssot, B.; Pozo, M.J.; et al. Priming: Getting Ready for Battle. Mol. Plant 2006, 19, 1062–1071. [Google Scholar] [CrossRef]
- Moraes, W.B.C. Controle alternativo de fitopatógenos. Pesquisa Agropecuária Bras. 1992, 27, 175–190. [Google Scholar]
- Bonaldo, S.M.; Pascholati, S.F.; Romeiro, R.S. Indução de resistência: Noções básicas e perspectivas. In Indução de Resistência em Plantas a Patógenos e Insetos; Fealq: Piracicaba, Brazil, 2005; pp. 11–28. [Google Scholar]
- Bettiol, W.; Stadnik, M.J. Controle alternativo. In Oídios; Stadnik, M.J., Rivera, M.C., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2001; pp. 165–192. ISBN 978-85-85771-15-7. [Google Scholar]
- Daayf, F.; Schmitt, A.; Belanger, R.R. The Effects of Plant Extracts of Reynoutria Sachalinensis on Powdery Mildew Development and Leaf Physiology of Long English Cucumber. Plant Dis. 1995, 79, 577. [Google Scholar] [CrossRef]
- Stangarlin, J.R.; Schwan-Estrada, J.R.F.; Cruz, M.D.S.; Nozaki, M.H. Plantas Medicinais e Controle Alternativo de Fitopatógenos. Biotecnol. Ciênc. Desenvolv. 1999, 11, 16–21. [Google Scholar]
- Pascholati, S.F. Potencial de Saccharomyces Cerevisiae e Outros Agentes Bióticos na Proteção de Plantas Contra Patógenos; Livre Docência, University of São Paulo: Piracicaba, Brazil, 1998. [Google Scholar]
- Stadnik, M.J.; Bettiol, W. Controle biológico de oídios. In Controle Biológico; Melo, I.S., Azevedo, J.L., Eds.; Embrapa Meio Ambiente: Jaguariúna, Brazil, 2000; Volume 2, pp. 95–116. [Google Scholar]
- Murphy, J.F.; Zehnder, G.W.; Schuster, D.J.; Sikora, E.J.; Polston, J.E.; Kloepper, J.W. Plant Growth-Promoting Rhizobacterial Mediated Protection in Tomato Against Tomato Mottle Virus. Plant Dis. 2000, 84, 779–784. [Google Scholar] [CrossRef] [PubMed]
- Madi, L.; Katan, J. Penicillium Janczewskiiand Its Metabolites, Applied to Leaves, Elicit Systemic Acquired Resistance to Stem Rot Caused ByRhizoctonia Solani. Physiol. Mol. Plant Physiol. 1998, 53, 163–175. [Google Scholar] [CrossRef]
- Monot, C.; Pajot, E.; Le Corre, D.; Silué, D. Induction of Systemic Resistance in Broccoli (Brassica Oleracea Var. Botrytis) against Downy Mildew (Peronospora Parasitica) by Avirulent Isolates. Biol. Control 2002, 24, 75–81. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Srinivas, V.; Sharma, M. Deciphering the Tri-Dimensional Effect of Endophytic Streptomyces sp. on Chickpea for Plant Growth Promotion, Helper Effect with Mesorhizobium Ciceri and Host-Plant Resistance Induction against Botrytis Cinerea. Microb. Pathogenes 2018, 122, 98–107. [Google Scholar] [CrossRef]
- Nagendran, K.; Karthikeyan, G.; Mohammed Faisal, P.; Kalaiselvi, P.; Raveendran, M.; Prabakar, K.; Raguchander, T. Exploiting Endophytic Bacteria for the Management of Sheath Blight Disease in Rice. Biol. Agric. Hortic. 2014, 30, 8–23. [Google Scholar] [CrossRef]
- Lamb, C.; Dixon, R.A. The Oxidative Burst in Plant Disease Resistance. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1997, 48, 251–275. [Google Scholar] [CrossRef]
- Zimmerli, L.; Jakab, G.; Metraux, J.-P.; Mauch-Mani, B. Potentiation of Pathogen-Specific Defense Mechanisms in Arabidopsis by Beta-Aminobutyric Acid. Proc. Natl. Acad. Sci. USA 2000, 97, 12920–12925. [Google Scholar] [CrossRef]
- Nandakumar, R.; Babu, S.; Viswanathan, R.; Raguchander, T.; Samiyappan, R. Induction of Systemic Resistance in Rice against Sheath Blight Disease by Pseudomonas Fluorescens. Soil Biol. Biochem. 2001, 33, 603–612. [Google Scholar] [CrossRef]
- Campos, Â.D.; Ferreira, A.G.; Hampe, M.M.V.; Antunes, I.F.; Brancão, N.; Silveira, E.P.; da Silva, J.B.; Osório, V.A. Induction of Chalcone Synthase and Phenylalanine Ammonia-Lyase by Salicylic Acid and Colletotrichum Lindemuthianum in Common Bean. Braz. J. Plant Physiol. 2003, 15, 129–134. [Google Scholar] [CrossRef]
- Cools, H.J.; Ishii, H. Pre-Treatment of Cucumber Plants with Acibenzolar-S-Methyl Systemically Primes a Phenylalanine Ammonia Lyase Gene (PAL1) for Enhanced Expression upon Attack with a Pathogenic Fungus. Physiol. Mol. Plant Physiol. 2002, 61, 273–280. [Google Scholar] [CrossRef]
- Buzi, A.; Chilosi, G.; De Sillo, D.; Magro, P. Induction of Resistance in Melon to Didymella Bryoniae and Sclerotinia sclerotiorum by Seed Treatments with Acibenzolar-S-Methyl and Methyl Jasmonate but Not with Salicylic Acid: Induction of Resistance in Melon. J. Phytopathol. 2004, 152, 34–42. [Google Scholar] [CrossRef]
- Salles, I.I.; Blount, J.W.; Dixon, R.A.; Schubert, K. Phytoalexin Induction and β-1,3-Glucanase Activities in Colletotrichum Trifolii Infected Leaves of Alfalfa (Medicago sativa L.). Physiol. Mol. Plant Physiol. 2002, 61, 89–101. [Google Scholar] [CrossRef]
- Stadnik, M.J.; Buchenauer, H. Inhibition of Phenylalanine Ammonia-Lyase Suppresses the Resistance Induced by Benzothiadiazole in Wheat to Blumeria graminis f. sp. Tritici. Physiol. Mol. Plant Physiol. 2000, 57, 25–34. [Google Scholar] [CrossRef]
- Pieterse, C.M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, D.K.; Prakash, A.; Johri, B.N. Induced Systemic Resistance (ISR) in Plants: Mechanism of Action. Indian J. Microbiol. 2007, 47, 289–297. [Google Scholar] [CrossRef] [PubMed]
- Vallad, G.E.; Goodman, R.M. Systemic Acquired Resistance and Induced Systemic Resistance in Conventional Agriculture. Crop Sci. 2004, 44, 1920–1934. [Google Scholar] [CrossRef]
- Kamle, M.; Borah, R.; Bora, H.; Jaiswal, A.K.; Singh, R.K.; Kumar, P. Systemic Acquired Resistance (SAR) and Induced Systemic Resistance (ISR): Role and Mechanism of Action Against Phytopathogens. In Fungal Biotechnology and Bioengineering; Hesham, A.E.-L., Upadhyay, R.S., Sharma, G.D., Manoharachary, C., Gupta, V.K., Eds.; Springer: Cham, Switzerland, 2020; pp. 457–470. ISBN 978-3-030-41869-4. [Google Scholar]
- De Almeida Barros, V.; Fontes, P.P.; Barcelos de Souza, G.; Gonçalves, A.B.; de Carvalho, K.; Rincão, M.P.; de Oliveira Negrão Lopes, I.; Dal-Bianco Lamas Costa, M.; Alves, M.S.; Marcelino-Guimarães, F.C.; et al. Phakopsora Pachyrhizi Triggers the Jasmonate Signaling Pathway during Compatible Interaction in Soybean and GmbZIP89 Plays a Role of Major Component in the Pathway. Plant Physiol. Biochem. 2020, 151, 526–534. [Google Scholar] [CrossRef] [PubMed]
- Van Wees, S.C.M.; de Swart, E.A.M.; van Pelt, J.A.; van Loon, L.C.; Pieterse, C.M.J. Enhancement of Induced Disease Resistance by Simultaneous Activation of Salicylate- and Jasmonate-Dependent Defense Pathways in Arabidopsisthaliana. Proc. Natl. Acad. Sci. USA 2000, 97, 8711–8716. [Google Scholar] [CrossRef]
- Bostock, R.M. Signal Crosstalk and Induced Resistance: Straddling the Line Between Cost and Benefit. Annu. Rev. Phytopathol. 2005, 43, 545–580. [Google Scholar] [CrossRef]
- Pascholati, S.F.; De Souza, V.H.M.; Cardoso Filho, J.A. Indução de resistência por Trichoderma. In Trichoderma: Uso na Agricultura; Embrapa Soja: Brasília, Brazil, 2019; Volume 1, pp. 235–254. ISBN 978-85-7035-943-8. [Google Scholar]
- Harman, G.E.; Howell, C.R.; Viterbo, A.; Chet, I.; Lorito, M. Trichoderma Species—Opportunistic, Avirulent Plant Symbionts. Nat. Rev. Microbiol. 2004, 2, 43–56. [Google Scholar] [CrossRef]
- Sharon, E.; Chet, I.; Viterbo, A.; Bar-Eyal, M.; Nagan, H.; Samuels, G.J.; Spiegel, Y. Parasitism of Trichoderma on Meloidogyne javanica and Role of the Gelatinous Matrix. Eur. J. Plant Pathol. 2007, 118, 247–258. [Google Scholar] [CrossRef]
- Zhang, S.; Gan, Y.; Xu, B.; Xue, Y. The Parasitic and Lethal Effects of Trichoderma longibrachiatum against Heterodera avenae. Biol. Control 2014, 72, 1–8. [Google Scholar] [CrossRef]
- Stefanova, M.; Leiva, A.; Larrinaga, L.; Coronado, M.F. Actividad Metabólica de Cepas de Trichoderma Spp Para El Control de Hongos Fitopatógenos Del Suelo. Rev. Fac. Agron. 1999, 16, 509–516. [Google Scholar]
- Wang, G.; Liu, Z.; Lin, R.; Li, E.; Mao, Z.; Ling, J.; Yang, Y.; Yin, W.-B.; Xie, B. Biosynthesis of Antibiotic Leucinostatins in Bio-Control Fungus Purpureocillium lilacinum and Their Inhibition on Phytophthora Revealed by Genome Mining. PLoS Pathog. 2016, 12, e1005685. [Google Scholar] [CrossRef] [PubMed]
- Daneshkhah, R.; Cabello, S.; Rozanska, E.; Sobczak, M.; Grundler, F.M.W.; Wieczorek, K.; Hofmann, J. Piriformospora indica Antagonizes Cyst Nematode Infection and Development in Arabidopsis Roots. J. Exp. Bot. 2013, 64, 3763–3774. [Google Scholar] [CrossRef]
- De Lamo, F.J.; Takken, F.L.W. BioControl by Fusarium oxysporum Using Endophyte-Mediated Resistance. Front. Plant Sci. 2020, 11, 37. [Google Scholar] [CrossRef]
- Fadiji, A.E.; Babalola, O.O. Elucidating Mechanisms of Endophytes Used in Plant Protection and Other Bioactivities with Multifunctional Prospects. Front. Bioeng. Biotechnol. 2020, 8, 467. [Google Scholar] [CrossRef]
- Constantin, M.E.; de Lamo, F.J.; Vlieger, B.V.; Rep, M.; Takken, F.L.W. Endophyte-Mediated Resistance in Tomato to Fusarium oxysporum Is Independent of ET, JA, and SA. Front. Plant Sci. 2019, 10, 979. [Google Scholar] [CrossRef]
- Sarrocco, S.; Vannacci, G. Preharvest Application of Beneficial Fungi as a Strategy to Prevent Postharvest Mycotoxin Contamination: A Review. Crop Prot. 2018, 110, 160–170. [Google Scholar] [CrossRef]
- Sarrocco, S.; Valenti, F.; Manfredini, S.; Esteban, P.; Bernardi, R.; Puntoni, G.; Baroncelli, R.; Haidukowski, M.; Moretti, A.; Vannacci, G. Is Exploitation Competition Involved in a Multitrophic Strategy for the BioControl of Fusarium Head Blight? Phytopathology 2019, 109, 560–570. [Google Scholar] [CrossRef]
- Halecker, S.; Wennrich, J.-P.; Rodrigo, S.; Andrée, N.; Rabsch, L.; Baschien, C.; Steinert, M.; Stadler, M.; Surup, F.; Schulz, B. Fungal Endophytes for BioControl of Ash Dieback: The Antagonistic Potential of Hypoxylon Rubiginosum. Fungal Ecol. 2020, 45, 100918. [Google Scholar] [CrossRef]
- Khaskheli, M.A.; Wu, L.; Chen, G.; Chen, L.; Hussain, S.; Song, D.; Liu, S.; Feng, G. Isolation and Characterization of Root-Associated Bacterial Endophytes and Their BioControl Potential against Major Fungal Phytopathogens of Rice (Oryza sativa L.). Pathogens 2020, 9, 172. [Google Scholar] [CrossRef]
- Assante, G.; Maffi, D.; Saracchi, M.; Farina, G.; Moricca, S.; Ragazzi, A. Histological Studies on the Mycoparasitism of Cladosporium Tenuissimum on Urediniospores of Uromyces Appendiculatus. Mycol. Res. 2004, 108, 170–182. [Google Scholar] [CrossRef] [PubMed]
- Bomfim, M.P.; São José, A.R.; Rebouças, T.N.H.; Almeida, S.S.; de Souza, I.V.B.; Dias, N.O. Avaliação Antagônica in Vitro e in Vivo de Trichoderma spp. a Rhizopus stolonifer Em Maracujazeiro Amarelo. Summa Phytopathol. 2010, 36, 61–67. [Google Scholar] [CrossRef]
- Borrás, A.D.; Aguilar, R.V. Biological Control of Penicillium digitatum by Trichoderma viride on Postharvest Citrus Fruits. Int. J. Food Microbiol. 1990, 11, 179–183. [Google Scholar] [CrossRef]
- Mathivanan, N.; Prabavathy, V.R.; Vijayanandraj, V.R. Application of Talc Formulations of Pseudomonas fluorescens Migula and Trichoderma viride Pers. Ex S.F. Gray Decrease the Sheath Blight Disease and Enhance the Plant Growth and Yield in Rice. J. Phytopathol. 2005, 153, 697–701. [Google Scholar] [CrossRef]
- Martins-Corder, M.P.; Melo, I.S. Antagonismo in vitro de Trichoderma spp. a Verticilium dahliae Kleb. Sci. Agric. 1998, 55, 1–7. [Google Scholar] [CrossRef]
- Narisawa, K.; Kawamata, H.; Currah, R.S.; Hashiba, T. Supression of Verticillium Wilt in Egg Plant by Some Fungal Root Endophytes. Eur. J. Plant Pathol. 2002, 108, 103–109. [Google Scholar] [CrossRef]
- Bernardi-Wenzel, J. Bioprospecção e Caracterização Citológica e Molecular de Fungos Endofíticos Isolados de Luehea Divaricata (Martius et Zuccarini): Estudo da Interação Endófito-Planta Hospedeira. Ph.D. Thesis, Universidade Estadual de Maringá, Maringá, Brazil, 2008. [Google Scholar]
- Sbravatti Junior, J.A.; Auer, C.G.; Pimentel, I.C.; dos Santos, Á.F.; Schultz, B. Seleção in Vitro de Fungos Endofíticos Para o Controle Biológico de Botrytis Cinerea Em Eucalyptus Benthamii. Floresta 2013, 43, 145. [Google Scholar] [CrossRef][Green Version]
- Rocha, R.; da Luz, D.E.; Engels, C.; Pileggi, S.A.V.; de Souza Jaccoud Filho, D.; Matiello, R.R.; Pileggi, M. Selection of Endophytic Fungi from Comfrey (Symphytum officinale L.) for in Vitro Biological Control of the Phytopathogen Sclerotinia sclerotiorum (Lib.). Braz. J. Microbiol. 2009, 40, 73–78. [Google Scholar] [CrossRef]
- Mejía, L.C.; Rojas, E.I.; Maynard, Z.; Bael, S.V.; Arnold, A.E.; Hebbar, P.; Samuels, G.J.; Robbins, N.; Herre, E.A. Endophytic Fungi as BioControl Agents of Theobroma Cacao Pathogens. Biol. Control 2008, 46, 4–14. [Google Scholar] [CrossRef]
- Tonial, F. Atividade Antimicrobiana de Endófitos e Extratos Foliares de Schinus terebinthifolius Raddi (Aroeira). Ph.D. Thesis, Federal University of Paraná, Curitiba, Brazil, 2008. [Google Scholar]
- Brum, M.C.P. Fungos Endofíticos de Vitis labrusca L. var. Niagara Rosada e o seu Potencial Biotecnológico. Ph.D. Thesis, Universidade de Mogi das Cruzes, Mogi das Cruzes, Brazil, 2008. [Google Scholar]
- Karunasinghe, T.G.; Maharachchikumbura, S.S.N.; Velazhahan, R.; Al-Sadi, A.M. Antagonistic Activity of Endophytic and Rhizosphere Fungi Isolated from Sea Purslane (Sesuvium portulacastrum) Against Pythium Damping off of Cucumber. Plant Dis. 2020, 104, 2158–2167. [Google Scholar] [CrossRef]
- Zhao, X.; Hu, Z.; Hou, D.; Xu, H.; Song, P. Biodiversity and Antifungal Potential of Endophytic Fungi from the Medicinal Plant Cornus officinalis. Symbiosis 2020. [Google Scholar] [CrossRef]
- Cosoveanu, A.; Gimenez-Mariño, C.; Cabrera, Y.; Hernandez, G.; Cabrera, R. Endophytic Fungi from Grapevine Cultivars in Canary Islands and Their Activity against Phytopatogenic Fungi. Int. J. Agric. Crop Sci. 2014, 7, 1497–1503. [Google Scholar]
- Kusari, P.; Kusari, S.; Spiteller, M.; Kayser, O. BioControl Potential of Endophytes Harbored in Radula Marginata (Liverwort) from the New Zealand Ecosystem. Antonie van Leeuwenhoek 2014, 106, 771–788. [Google Scholar] [CrossRef]
- Zhang, X.-Q.; Qu, H.-R.; Bao, S.-S.; Deng, Z.-S.; Guo, Z.-Y. Secondary Metabolites from the Endophytic Fungus xylariales sp. and Their Antimicrobial Activity. Chem. Nat. Compd. 2020, 56, 530–532. [Google Scholar] [CrossRef]
- Peng, C.; Zhang, A.; Wang, Q.; Song, Y.; Zhang, M.; Ding, X.; Li, Y.; Geng, Q.; Zhu, C. Ultrahigh-Activity Immune Inducer from Endophytic Fungi Induces Tobacco Resistance to Virus by SA Pathway and RNA Silencing. BMC Plant Biol. 2020, 20, 169. [Google Scholar] [CrossRef]
- Yao, Y.-R.; Tian, X.-L.; Shen, B.-M.; Mao, Z.-C.; Chen, G.; Xie, B.-Y. Transformation of the Endophytic Fungus Acremonium Implicatum with GFP and Evaluation of Its BioControl Effect against Meloidogyne Incognita. World J. Microbiol. Biotechnol. 2015, 31, 549–556. [Google Scholar] [CrossRef] [PubMed]
- Mwaura, P.; Baum, T.J.; Losenge, T.; Coyne, D.; Kahangi, E. Effect of Endophytic Fusarium oxysporum on Paralysis and Mortality of Pratylenchus goodeyi. Afr. J. Biotechnol. 2010, 9, 1130–1134. [Google Scholar] [CrossRef]
- Liarzi, O.; Bar, E.; Lewinsohn, E.; Ezra, D. Use of the Endophytic Fungus Daldinia Cf. concentrica and Its Volatiles as Bio-Control Agents. PLoS ONE 2016, 11, e0168242. [Google Scholar] [CrossRef]
- Kusari, S.; Hertweck, C.; Spiteller, M. Chemical Ecology of Endophytic Fungi: Origins of Secondary Metabolites. Chem. Biol. 2012, 19, 792–798. [Google Scholar] [CrossRef]
- Druzhinina, I.S.; Seidl-Seiboth, V.; Herrera-Estrella, A.; Horwitz, B.A.; Kenerley, C.M.; Monte, E.; Mukherjee, P.K.; Zeilinger, S.; Grigoriev, I.V.; Kubicek, C.P. Trichoderma: The Genomics of Opportunistic Success. Nat. Rev. Microbiol. 2011, 9, 749–759. [Google Scholar] [CrossRef]
- Puri, S.C.; Nazir, A.; Chawla, R.; Arora, R.; Riyaz-ul-Hasan, S.; Amna, T.; Ahmed, B.; Verma, V.; Singh, S.; Sagar, R.; et al. The Endophytic Fungus Trametes Hirsuta as a Novel Alternative Source of Podophyllotoxin and Related Aryl Tetralin Lignans. J. Biotechnol. 2006, 122, 494–510. [Google Scholar] [CrossRef]
- Kour, A.; Shawl, A.S.; Rehman, S.; Sultan, P.; Qazi, P.H.; Suden, P.; Khajuria, R.K.; Verma, V. Isolation and Identification of an Endophytic Strain of Fusarium oxysporum Producing Podophyllotoxin from Juniperus Recurva. World J. Microbiol. Biotechnol. 2008, 24, 1115–1121. [Google Scholar] [CrossRef]
- Pileggi, S.A.V.; Vieira de Oliveira, S.F.; Andrade, C.W.; Vicente, V.A.; do Dalzoto, P.R.; Kniphoff da Cruz, G.; Gabardo, J.; Massola, N.S.; Tozze, H.J.; Pileggi, M.; et al. Molecular and Morphological Markers for Rapid Distinction between 2 Colletotrichum Species. Can. J. Microbiol. 2009, 55, 1076–1088. [Google Scholar] [CrossRef]
- Strobel, G.; Daisy, B.; Castillo, U.; Harper, J. Natural Products from Endophytic Microorganisms. J. Nat. Prod. 2004, 67, 257–268. [Google Scholar] [CrossRef] [PubMed]
- Trapp, S.C.; Hohn, T.M.; McCormick, S.; Jarvis, B.B. Characterization of the Gene Cluster for Biosynthesis of Macrocyclic Trichothecenes in Myrothecium Roridum. Mol. Gen. Genet. 1998, 257, 421–432. [Google Scholar] [CrossRef] [PubMed]
- Jarvis, B.B.; Midiwo, J.O.; Bean, G.A.; Aboul-Nasr, M.B.; Barnos, C.S. The Mystery of Trichothecene Antibiotics in Baccharis Species. J. Nat. Prod. 1988, 51, 736–744. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, I.; Varsavky, E.; Haidukowski, M.; Frade, H. Macrocyclic Trichothecenes in Baccharis Coridifolia Plants and Endophytes and Baccharis Artemisioides Plants. Toxicon 1997, 35, 753–757. [Google Scholar] [CrossRef]
- Wang, Y.; Dai, C.-C.; Zhao, Y.-W.; Peng, Y. Fungal Endophyte-Induced Volatile Oil Accumulation in Atractylodes Lancea Plantlets Is Mediated by Nitric Oxide, Salicylic Acid and Hydrogen Peroxide. Process Biochem. 2011, 46, 730–735. [Google Scholar] [CrossRef]
- Vijayabharathi, R.; Gopalakrishnan, S.; Sathya, A.; Vasanth Kumar, M.; Srinivas, V.; Mamta, S. Streptomyces sp. as Plant Growth-Promoters and Host-Plant Resistance Inducers against Botrytis Cinerea in Chickpea. BioControl Sci. Technol. 2018, 28, 1140–1163. [Google Scholar] [CrossRef]
- Pascholati, S.F.; Dalio, R.J.D.; Filho, J.A.C.; Brand, S.C.; Pinto, L.R.; Osswald, W. Piriformospora indica—Indutor de Resistência Em Plantas Contra Patógenos. In Anais da VI Reunião Brasileira sobre Indução de Resistencia em Plantas a Patógenos; Editora UFV: Viçosa, Brazil, 2012; pp. 79–112. [Google Scholar]
- Waller, F.; Achatz, B.; Deshmukh, S.; Baltruschat, H.; Kogel, K.H. Induction of Systemic Resistance by the Root Endophytic Fungus Piriformospora indica in Barley. Plant Cell Physiol. 2006, 47, 96. [Google Scholar]
- Serfling, A.; Wirsel, S.G.R.; Lind, V.; Deising, H.B. Performance of the BioControl Fungus Piriformospora indica on Wheat Under Greenhouse and Field Conditions. Phytopathology 2007, 97, 523–531. [Google Scholar] [CrossRef]
- Kumar, M.; Yadav, V.; Tuteja, N.; Johri, A.K. Antioxidant Enzyme Activities in Maize Plants Colonized with Piriformospora indica. Microbiology 2009, 155, 780–790. [Google Scholar] [CrossRef]
- Qiang, X.; Weiss, M.; Kogel, K.-H.; Schäfer, P. Piriformospora indica—A Mutualistic Basidiomycete with an Exceptionally Large Plant Host Range: Mutualistic Root Symbiosis. Mol. Plant Pathol. 2012, 13, 508–518. [Google Scholar] [CrossRef] [PubMed]
- Thaler, J.S.; Humphrey, P.T.; Whiteman, N.K. Evolution of Jasmonate and Salicylate Signal Crosstalk. Trends Plant Sci. 2012, 17, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Kunkel, B.N.; Brooks, D.M. Cross Talk between Signaling Pathways in Pathogen Defense. Curr. Opin. Plant Biol. 2002, 5, 325–331. [Google Scholar] [CrossRef]
- Schwartzberg, E.G.; Tumlinson, J.H. Aphid Honeydew Alters Plant Defence Responses. Funct. Ecol. 2014, 28, 386–394. [Google Scholar] [CrossRef]
- Schweiger, R.; Heise, A.-M.; Persicke, M.; Müller, C. Interactions between the Jasmonic and Salicylic Acid Pathway Modulate the Plant Metabolome and Affect Herbivores of Different Feeding Types: Phytohormone Interactions Affect Metabolome and Herbivores. Plant Cell Environ. 2014, 37, 1574–1585. [Google Scholar] [CrossRef]
- Jung, S.C.; Martinez-Medina, A.; Lopez-Raez, J.A.; Pozo, M.J. Mycorrhiza-Induced Resistance and Priming of Plant Defenses. J. Chem. Ecol. 2012, 38, 651–664. [Google Scholar] [CrossRef]
- Bastias, D.A.; Martínez-Ghersa, M.A.; Ballaré, C.L.; Gundel, P.E. Epichloë Fungal Endophytes and Plant Defenses: Not Just Alkaloids. Trends Plant Sci. 2017, 22, 939–948. [Google Scholar] [CrossRef]
- Hossain, M.M.; Sultana, F.; Kubota, M.; Hyakumachi, M. Differential Inducible Defense Mechanisms against Bacterial Speck Pathogen in Arabidopsis Thaliana by Plant-Growth-Promoting-Fungus Penicillium sp. GP16-2 and Its Cell Free Filtrate. Plant Soil 2008, 304, 227–239. [Google Scholar] [CrossRef]
- Shutsrirung, A.; Chromkaew, Y.; Pathom-Aree, W.; Choonluchanon, S.; Boonkerd, N. Diversity of Endophytic Actinomycetes in Mandarin Grown in Northern Thailand, Their Phytohormone Production Potential and Plant Growth Promoting Activity. Soil Sci. Plant Nutr. 2013, 59, 322–330. [Google Scholar] [CrossRef]
- Kusari, S.; Zühlke, S.; Spiteller, M. Effect of Artificial Reconstitution of the Interaction between the Plant Camptotheca Acuminata and the Fungal Endophyte Fusarium Solani on Camptothecin Biosynthesis. J. Nat. Prod. 2011, 74, 764–775. [Google Scholar] [CrossRef] [PubMed]
- Sirikantaramas, S.; Yamazaki, M.; Saito, K. A Survival Strategy: The Coevolution of the Camptothecin Biosynthetic Pathway and Self-Resistance Mechanism. Phytochemistry 2009, 70, 1894–1898. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, G.A.A.; de Abreu, M.S.; Pinto, F.A.M.F.; Monteiro, A.C.A.; Núñez, Á.M.P.; de Resende, M.L.V.; de Souza, J.T.; de Medeiros, F.H.V. Phialomyces Macrosporus Decreases Anthracnose Severity on Coffee Seedlings by Competition for Nutrients and Induced Resistance. Biol. Control 2016, 103, 119–128. [Google Scholar] [CrossRef]
- Alencar, M.S.R.; Solino, A.J.D.S.; Oliveira, J.S.B.; Pascholati, S.F.; Schwan-Estrada, K.R.F. Induction of Defense Mechanisms in Tomato Plants by Saprobic Fungi Filtrates against Early Blight Diseases. Rev. Caatinga 2020, 33, 671–678. [Google Scholar] [CrossRef]
- Tian, P.; Nan, Z.; Li, C.; Spangenberg, G. Effect of the Endophyte Neotyphodium Lolii on Susceptibility and Host Physiological Response of Perennial Ryegrass to Fungal Pathogens. Eur. J. Plant Pathol. 2008, 122, 593–602. [Google Scholar] [CrossRef]
- Kavroulakis, N.; Ntougias, S.; Zervakis, G.I.; Ehaliotis, C.; Haralampidis, K.; Papadopoulou, K.K. Role of Ethylene in the Protection of Tomato Plants against Soil-Borne Fungal Pathogens Conferred by an Endophytic Fusarium Solani Strain. J. Exp. Bot. 2007, 58, 3853–3864. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Medina, A.; Fernández, I.; Sánchez-Guzmán, M.J.; Jung, S.C.; Pascual, J.A.; Pozo, M.J. Deciphering the Hormonal Signalling Network behind the Systemic Resistance Induced by Trichoderma harzianum in Tomato. Front. Plant Sci. 2013, 4. [Google Scholar] [CrossRef] [PubMed]
- Chowdappa, P.; Mohan Kumar, S.P.; Jyothi Lakshmi, M.; Upreti, K.K. Growth Stimulation and Induction of Systemic Resistance in Tomato against Early and Late Blight by Bacillus Subtilis OTPB1 or Trichoderma harzianum OTPB3. Biol. Control 2013, 65, 109–117. [Google Scholar] [CrossRef]
- Da Silva, V.N.; Guzzo, S.D.; Lucon, C.M.M.; Harakava, R. Promoção de Crescimento e Indução de Resistência à Antracnose por Trichoderma spp. Em Pepineiro. Pesq. Agropec. Bras. 2011, 46, 1609–1618. [Google Scholar] [CrossRef]
- Jogaiah, S.; Abdelrahman, M.; Tran, L.-S.P.; Ito, S.-I. Different Mechanisms of Trichoderma virens-Mediated Resistance in Tomato against Fusarium Wilt Involve the Jasmonic and Salicylic Acid Pathways: T. Virens Resistance Mechanism against Fusarium. Mol. Plant Pathol. 2018, 19, 870–882. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, A.S.; Sánchez, C.P.; Candela, M.E. Evaluation of Induction of Systemic Resistance in Pepper Plants (Capsicum annuum) to Phytophthora Capsici Using Trichoderma harzianum and Its relation with Capsidiol Accumulation. Eur. J. Plant Pathol. 2000, 106, 817–824. [Google Scholar] [CrossRef]
- Varkey, S.; Anith, K.N.; Narayana, R.; Aswini, S. A Consortium of Rhizobacteria and Fungal Endophyte Suppress the Root-Knot Nematode Parasite in Tomato. Rhizosphere 2018, 5, 38–42. [Google Scholar] [CrossRef]
- Bajaj, R.; Hu, W.; Huang, Y.; Chen, S.; Prasad, R.; Varma, A.; Bushley, K.E. The Beneficial Root Endophyte Piriformospora indica Reduces Egg Density of the Soybean Cyst Nematode. Biol. Control 2015, 90, 193–199. [Google Scholar] [CrossRef]
- Lin, H.-F.; Xiong, J.; Zhou, H.-M.; Chen, C.-M.; Lin, F.-Z.; Xu, X.-M.; Oelmüller, R.; Xu, W.-F.; Yeh, K.-W. Growth Promotion and Disease Resistance Induced in Anthurium Colonized by the Beneficial Root Endophyte Piriformospora indica. BMC Plant Biol. 2019, 19, 40. [Google Scholar] [CrossRef]
- Zhou, W.; Wheeler, T.A.; Starr, J.L.; Valencia, C.U.; Sword, G.A. A Fungal Endophyte Defensive Symbiosis Affects Plant-Nematode Interactions in Cotton. Plant Soil 2018, 422, 251–266. [Google Scholar] [CrossRef]
- Amin, N. Nematicidal Activity of Root Exudates of Sengon Plant Inoculated with Endophytic Fungi Nigrospora sp. to Control of Root-Knot Nematode meloidogyne spp. J. Chem. Pharm. Res. 2015, 7, 307–310. [Google Scholar]
- Miao, G.; Han, J.; Zhang, K.; Wang, S.; Wang, C. Protection of Melon against Fusarium Wilt-Root Knot Nematode Complex by Endophytic Fungi Penicillium Brefeldianum HS-1. Symbiosis 2019, 77, 83–89. [Google Scholar] [CrossRef]
- Bogner, C.W.; Kamdem, R.S.T.; Sichtermann, G.; Matthäus, C.; Hölscher, D.; Popp, J.; Proksch, P.; Grundler, F.M.W.; Schouten, A. Bioactive Secondary Metabolites with Multiple Activities from a Fungal Endophyte. Microb. Biotechnol. 2017, 10, 175–188. [Google Scholar] [CrossRef]
- Martinuz, A.; Zewdu, G.; Ludwig, N.; Grundler, F.; Sikora, R.A.; Schouten, A. The Application of Arabidopsis Thaliana in Studying Tripartite Interactions among Plants, Beneficial Fungal Endophytes and Biotrophic Plant-Parasitic Nematodes. Planta 2015, 241, 1015–1025. [Google Scholar] [CrossRef] [PubMed]
- Dababat, A.E.-F.A.; Sikora, R.A. Induced Resistance by the Mutualistic Endophyte, Fusarium oxysporum Strain 162, toward Meloidogyne Incognita on Tomato. BioControl Sci. Technol. 2007, 17, 969–975. [Google Scholar] [CrossRef]
- Vu, T.; Sikora, R.; Hauschild, R. Fusarium oxysporum Endophytes Induced Systemic Resistance against Radopholus Similis on Banana. Nematology 2006, 8, 847–852. [Google Scholar] [CrossRef]
- Le, H.T.T.; Padgham, J.L.; Hagemann, M.H.; Sikora, R.A.; Schouten, A. Developmental and Behavioural Effects of the Endophytic Fusarium Moniliforme Fe14 towards Meloidogyne Graminicola in Rice: Tripartite Interactions between Rice, Root-Knot Nematodes and an Endophyte. Ann. Appl. Biol. 2016, 169, 134–143. [Google Scholar] [CrossRef]
- Escudero, N.; Lopez-Moya, F.; Ghahremani, Z.; Zavala-Gonzalez, E.A.; Alaguero-Cordovilla, A.; Ros-Ibañez, C.; Lacasa, A.; Sorribas, F.J.; Lopez-Llorca, L.V. Chitosan Increases Tomato Root Colonization by Pochonia Chlamydosporia and Their Combination Reduces Root-Knot Nematode Damage. Front. Plant Sci. 2017, 8, 1415. [Google Scholar] [CrossRef]
- Chu, H.; Wang, C.; Li, Z.; Wang, H.; Xiao, Y.; Chen, J.; Tang, M. The Dark Septate Endophytes and Ectomycorrhizal Fungi Effect on Pinus Tabulaeformis Carr. Seedling Growth and Their Potential Effects to Pine Wilt Disease Resistance. Forests 2019, 10, 140. [Google Scholar] [CrossRef]
- De Medeiros, H.A.; de Araújo Filho, J.V.; de Freitas, L.G.; Castillo, P.; Rubio, M.B.; Hermosa, R.; Monte, E. Tomato Progeny Inherit Resistance to the Nematode meloidogyne Javanica Linked to Plant Growth Induced by the BioControl Fungus Trichoderma atroviride. Sci. Rep. 2017, 7, 40216. [Google Scholar] [CrossRef]
- Kath, J.; Dias-Arieira, C.R.; Ferreira, J.C.A.; Homiak, J.A.; da Silva, C.R.; Cardoso, C.R. Control of Pratylenchus Brachyurus in Soybean with Trichoderma spp. and Resistance Inducers. J. Phytopathol. 2017, 165, 791–799. [Google Scholar] [CrossRef]
- Leonetti, P.; Zonno, M.C.; Molinari, S.; Altomare, C. Induction of SA-Signaling Pathway and Ethylene Biosynthesis in Trichoderma harzianum-Treated Tomato Plants after Infection of the Root-Knot Nematode meloidogyne Incognita. Plant Cell Rep. 2017, 36, 621–631. [Google Scholar] [CrossRef]
- Muvea, A.M.; Subramanian, S.; Maniania, N.K.; Poehling, H.-M.; Ekesi, S.; Meyhöfer, R. Endophytic Colonization of Onions Induces Resistance Against Viruliferous Thrips and Virus Replication. Front. Plant Sci. 2018, 9, 1785. [Google Scholar] [CrossRef]
- Kiarie, S.; Nyasani, J.O.; Gohole, L.S.; Maniania, N.K.; Subramanian, S. Impact of Fungal Endophyte Colonization of Maize (Zea mays L.) on Induced Resistance to Thrips- and Aphid-Transmitted Viruses. Plants 2020, 9, 416. [Google Scholar] [CrossRef] [PubMed]
- Vitti, A.; Pellegrini, E.; Nali, C.; Lovelli, S.; Sofo, A.; Valerio, M.; Scopa, A.; Nuzzaci, M. Trichoderma harzianum T-22 Induces Systemic Resistance in Tomato Infected by Cucumber Mosaic Virus. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Elsharkawy, M.M.; Shimizu, M.; Takahashi, H.; Ozaki, K.; Hyakumachi, M. Induction of Systemic Resistance against Cucumber Mosaic Virus in Arabidopsis Thaliana by Trichoderma asperellum SKT-1. Plant Pathol. J. 2013, 29, 193–200. [Google Scholar] [CrossRef] [PubMed]
- Yedidia, I.; Shoresh, M.; Kerem, Z.; Benhamou, N.; Kapulnik, Y.; Chet, I. Concomitant Induction of Systemic Resistance to Pseudomonas syringae Pv. lachrymans in Cucumber by Trichoderma asperellum (T-203) and Accumulation of Phytoalexins. Appl. Environ. Microbiol. 2003, 69, 7343–7353. [Google Scholar] [CrossRef]
- Konappa, N.; Krishnamurthy, S.; Siddaiah, C.N.; Ramachandrappa, N.S.; Chowdappa, S. Evaluation of Biological Efficacy of Trichoderma asperellum against Tomato Bacterial Wilt Caused by Ralstonia Solanacearum. Egypt. J. Biol. Pest Control 2018, 28, 63. [Google Scholar] [CrossRef]
- Alfano, G.; Ivey, M.L.L.; Cakir, C.; Bos, J.I.B.; Miller, S.A.; Madden, L.V.; Kamoun, S.; Hoitink, H.A.J. Systemic Modulation of Gene Expression in Tomato by Trichoderma hamatum 382. Phytopathology 2007, 97, 429–437. [Google Scholar] [CrossRef] [PubMed]
- Biswas, T.; Kalra, A.; Mathur, A.K.; Lal, R.K.; Singh, M.; Mathur, A. Elicitors’ Influenced Differential Ginsenoside Production and Exudation into Medium with Concurrent Rg3/Rh2 Panaxadiol Induction in Panax Quinquefolius Cell Suspensions. Appl. Microbiol. Biotechnol. 2016, 100, 4909–4922. [Google Scholar] [CrossRef] [PubMed]
- Jan, G.F.; Hamayun, M.; Hussain, A.; Jan, G.; Iqbal, A.; Khan, A.; Lee, I.-J. An Endophytic Isolate of the Fungus Yarrowia Lipolytica Produces Metabolites That Ameliorate the Negative Impact of Salt Stress on the Physiology of Maize. BMC Microbiol. 2019, 19, 3. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Khan, A.L.; Lee, I.-J. Endophytic Fungal Pre-Treatments of Seeds Alleviates Salinity Stress Effects in Soybean Plants. J. Microbiol. 2013, 51, 850–857. [Google Scholar] [CrossRef]
- Malik, A.; Butt, T.A.; Naqvi, S.T.A.; Yousaf, S.; Qureshi, M.K.; Zafar, M.I.; Farooq, G.; Nawaz, I.; Iqbal, M. Lead Tolerant Endophyte Trametes Hirsuta Improved the Growth and Lead Accumulation in the Vegetative Parts of Triticum aestivum L. Heliyon 2020, 6, e04188. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, F.; Samsampour, D.; Askari Seyahooei, M.; Bagheri, A.; Soltani, J. Fungal Endophytes Alleviate Drought-Induced Oxidative Stress in Mandarin (Citrus reticulata L.): Toward Regulating the Ascorbate–Glutathione Cycle. Sci. Hortic. 2020, 261, 108991. [Google Scholar] [CrossRef]
- Ali, A.H.; Abdelrahman, M.; Radwan, U.; El-Zayat, S.; El-Sayed, M.A. Effect of Thermomyces Fungal Endophyte Isolated from Extreme Hot Desert-Adapted Plant on Heat Stress Tolerance of Cucumber. Appl. Soil Ecol. 2018, 124, 155–162. [Google Scholar] [CrossRef]
- Ismail, I.; Hussain, A.; Mehmood, A.; Qadir, M.; Husna, H.; Iqbal, A.; Hamayun, M.; Khan, N. Thermal Stress Alleviating Potential of Endophytic Fungus Rhizopus Oryzae Inoculated to Sunflower (Helianthus annuus L.) and Soybean (Glycine max L.). Pak. J. Bot. 2020, 52. [Google Scholar] [CrossRef]
- Ismail; Hamayun, M.; Hussain, A.; Iqbal, A.; Khan, S.A.; Lee, I.-J. Aspergillus Niger Boosted Heat Stress Tolerance in Sunflower and Soybean via Regulating Their Metabolic and Antioxidant System. J. Plant Interact. 2020, 15, 223–232. [Google Scholar] [CrossRef]
- Abdelrahman, M.; Sawada, Y.; Nakabayashi, R.; Sato, S.; Hirakawa, H.; El-Sayed, M.; Hirai, M.Y.; Saito, K.; Yamauchi, N.; Shigyo, M. Integrating Transcriptome and Target Metabolome Variability in Doubled Haploids of Allium Cepa for Abiotic Stress Protection. Mol. Breed. 2015, 35, 195. [Google Scholar] [CrossRef]
- Waqas, M.; Khan, A.L.; Kamran, M.; Hamayun, M.; Kang, S.-M.; Kim, Y.-H.; Lee, I.-J. Endophytic Fungi Produce Gibberellins and Indoleacetic Acid and Promotes Host-Plant Growth during Stress. Molecules 2012, 17, 10754–10773. [Google Scholar] [CrossRef]
- Guler, N.S.; Pehlivan, N.; Karaoglu, S.A.; Guzel, S.; Bozdeveci, A. Trichoderma atroviride ID20G Inoculation Ameliorates Drought Stress-Induced Damages by Improving Antioxidant Defence in Maize Seedlings. Acta Physiol. Plant 2016, 38, 132. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R.; Sepehri, M. Effect of Endophytic Fungus Piriformospora indica and PEG-Induced Water Stress on Maximum Root Growth Pressure and Elongation Rate of Maize. Plant Soil 2019, 435, 423–436. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-Dependent Response of Trichoderma harzianum in Improving Drought Tolerance in Rice Genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef] [PubMed]
- Valli, P.P.S.; Muthukumar, T. Dark Septate Root Endophytic Fungus Nectria haematococca Improves Tomato Growth Under Water Limiting Conditions. Indian J. Microbiol. 2018, 58, 489–495. [Google Scholar] [CrossRef]
- Ripa, F.A.; Cao, W.; Tong, S.; Sun, J. Assessment of Plant Growth Promoting and Abiotic Stress Tolerance Properties of Wheat Endophytic Fungi. BioMed Res. Int. 2019, 2019, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Vargas, L.; Santa Brígida, A.B.; Mota Filho, J.P.; de Carvalho, T.G.; Rojas, C.A.; Vaneechoutte, D.; Van Bel, M.; Farrinelli, L.; Ferreira, P.C.G.; Vandepoele, K.; et al. Drought Tolerance Conferred to Sugarcane by Association with Gluconacetobacter Diazotrophicus: A Transcriptomic View of Hormone Pathways. PLoS ONE 2014, 9, e114744. [Google Scholar] [CrossRef] [PubMed]
- Eid, A.M.; Salim, S.S.; Hassan, S.E.-D.; Ismail, M.A.; Fouda, A. Role of Endophytes in Plant Health and Abiotic Stress Management. In Microbiome in Plant Health and Disease; Kumar, V., Prasad, R., Kumar, M., Choudhary, D.K., Eds.; Springer: Singapore, 2019; pp. 119–144. ISBN 9789811384943. [Google Scholar]
- Malinowski, D.P.; Belesky, D.P. Adaptations of Endophyte-Infected Cool-Season Grasses to Environmental Stresses: Mechanisms of Drought and Mineral Stress Tolerance. Crop Sci. 2000, 40, 923–940. [Google Scholar] [CrossRef]
- Bloemen, J.; McGuire, M.A.; Aubrey, D.P.; Teskey, R.O.; Steppe, K. Transport of Root-Respired CO 2 via the Transpiration Stream Affects Aboveground Carbon Assimilation and CO 2 Efflux in Trees. New Phytol. 2013, 197, 555–565. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Jan, F.G.; Rahman, I.U.; Iqbal, A.; Hamayun, M. IAA Producing Fungal Endophyte Penicillium Roqueforti Thom., Enhances Stress Tolerance and Nutrients Uptake in Wheat Plants Grown on Heavy Metal Contaminated Soils. PLoS ONE 2018, 13, e0208150. [Google Scholar] [CrossRef]
- Zhu, L.; Li, T.; Wang, C.; Zhang, X.; Xu, L.; Xu, R.; Zhao, Z. The Effects of Dark Septate Endophyte (DSE) Inoculation on Tomato Seedlings under Zn and Cd Stress. Environ. Sci. Pollut. Res. 2018, 25, 35232–35241. [Google Scholar] [CrossRef]
- Bilal, S.; Shahzad, R.; Imran, M.; Jan, R.; Kim, K.M.; Lee, I.-J. Synergistic Association of Endophytic Fungi Enhances Glycine max L. Resilience to Combined Abiotic Stresses: Heavy Metals, High Temperature and Drought Stress. Ind. Crops Prod. 2020, 143, 111931. [Google Scholar] [CrossRef]
- Babu, A.G.; Reddy, M.S. Dual Inoculation of Arbuscular Mycorrhizal and Phosphate Solubilizing Fungi Contributes in Sustainable Maintenance of Plant Health in Fly Ash Ponds. Water Air Soil Pollut. 2011, 219, 3–10. [Google Scholar] [CrossRef]
- Bashan, Y.; de-Bashan, L.E. Plant growth promoter. In Encyclopedia of Soils in the Environment; Elsevier: Oxford, UK, 2005; Volume 1, pp. 103–115. [Google Scholar]
- Khan, Z.; Doty, S. Endophyte-Assisted Phytoremediation. Curr. Top. Plant Biol. 2011, 12, 97–105. [Google Scholar]
- Khan, M.S.; Zaidi, A.; Ahemad, M.; Oves, M.; Wani, P.A. Plant Growth Promotion by Phosphate Solubilizing Fungi—Current Perspective. Arch. Agron. Soil Sci. 2010, 56, 73–98. [Google Scholar] [CrossRef]
- Abdelaziz, M.E.; Abdelsattar, M.; Abdeldaym, E.A.; Atia, M.A.M.; Mahmoud, A.W.M.; Saad, M.M.; Hirt, H. Piriformospora indica Alters Na+/K+ Homeostasis, Antioxidant Enzymes and LeNHX1 Expression of Greenhouse Tomato Grown under Salt Stress. Sci. Hortic. 2019, 256, 108532. [Google Scholar] [CrossRef]
- Jogawat, A.; Saha, S.; Bakshi, M.; Dayaman, V.; Kumar, M.; Dua, M.; Varma, A.; Oelmüller, R.; Tuteja, N.; Johri, A.K. Piriformospora indica Rescues Growth Diminution of Rice Seedlings during High Salt Stress. Plant Signal. Behav. 2013, 8, e26891. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of Plant-Growth-Promoting Fungi Trichoderma longibrachiatum T6 Enhances Tolerance of Wheat to Salt Stress through Improvement of Antioxidative Defense System and Gene Expression. Front. Plant Sci. 2016, 7. [Google Scholar] [CrossRef] [PubMed]
- Radhakrishnan, R.; Khan, A.L.; Kang, S.M.; Lee, I.-J. A Comparative Study of Phosphate Solubilization and the Host Plant Growth Promotion Ability of Fusarium Verticillioides RK01 and Humicola sp. KNU01 under Salt Stress. Ann. Microbiol. 2015, 65, 585–593. [Google Scholar] [CrossRef]
- Hosseini, F.; Mosaddeghi, M.R.; Dexter, A.R. Effect of the Fungus Piriformospora indica on Physiological Characteristics and Root Morphology of Wheat under Combined Drought and Mechanical Stresses. Plant Physiol. Biochem. 2017, 118, 107–120. [Google Scholar] [CrossRef]
- Cao, M.-J.; Wang, Z.; Zhao, Q.; Mao, J.-L.; Speiser, A.; Wirtz, M.; Hell, R.; Zhu, J.-K.; Xiang, C.-B. Sulfate Availability Affects ABA Levels and Germination Response to ABA and Salt Stress in Arabidopsis Thaliana. Plant J. 2014, 77, 604–615. [Google Scholar] [CrossRef] [PubMed]
- Khalid, M.; Hassani, D.; Liao, J.; Xiong, X.; Bilal, M.; Huang, D. An Endosymbiont Piriformospora indica Reduces Adverse Effects of Salinity by Regulating Cation Transporter Genes, Phytohormones, and Antioxidants in Brassica campestris ssp. Chinensis. Environ. Exp. Bot. 2018, 153, 89–99. [Google Scholar] [CrossRef]
- Mittler, R. Oxidative Stress, Antioxidants and Stress Tolerance. Trends Plant Sci. 2002, 7, 405–410. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Rivero, R.M.; Blumwald, E.; Mittler, R. Reactive Oxygen Species, Abiotic Stress and Stress Combination. Plant J. 2017, 90, 856–867. [Google Scholar] [CrossRef]
- White, J.F.; Torres, M.S. Is Plant Endophyte-Mediated Defensive Mutualism the Result of Oxidative Stress Protection? Physiol. Plant. 2010, 138, 440–446. [Google Scholar] [CrossRef] [PubMed]
- Overmyer, K.; Brosché, M.; Kangasjärvi, J. Reactive Oxygen Species and Hormonal Control of Cell Death. Trends Plant Sci. 2003, 8, 335–342. [Google Scholar] [CrossRef]
- Herrera-Carillo, Z.; Torres, M.S.; Singh, A.P.; Vorsa, N.; Gianfagna, T.; Meyer, W.; White, J.F.J. Phenolic, Flavonoid and Antioxidant Profiling for Cool-Season Grasses with and without Endophyte. In Proceedings of the 18th Annual Rutgers Turfgrass Symposium, New Brunswick, NJ, USA, 12 January 2009; p. 43. [Google Scholar]
- Tanaka, A.; Christensen, M.J.; Takemoto, D.; Park, P.; Scott, B. Reactive Oxygen Species Play a Role in Regulating a Fungus–Perennial Ryegrass Mutualistic Interaction. Plant Cell 2006, 18, 1052–1066. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, R.; Redman, R. More than 400 Million Years of Evolution and Some Plants Still Can’t Make It on Their Own: Plant Stress Tolerance via Fungal Symbiosis. J. Exp. Botany 2008, 59, 1109–1114. [Google Scholar] [CrossRef] [PubMed]
- Haruma, T.; Yamaji, K.; Ogawa, K.; Masuya, H.; Sekine, Y.; Kozai, N. Root-Endophytic Chaetomium Cupreum Chemically Enhances Aluminium Tolerance in Miscanthus Sinensis via Increasing the Aluminium Detoxicants, Chlorogenic Acid and Oosporein. PLoS ONE 2019, 14, e0212644. [Google Scholar] [CrossRef]
- Chen, T.; Li, C.; White, J.F.; Nan, Z. Effect of the Fungal Endophyte Epichloë Bromicola on Polyamines in Wild Barley (Hordeum Brevisubulatum) under Salt Stress. Plant Soil 2019, 436, 29–48. [Google Scholar] [CrossRef]
- Li, L.; Li, L.; Wang, X.; Zhu, P.; Wu, H.; Qi, S. Plant Growth-Promoting Endophyte Piriformospora indica Alleviates Salinity Stress in Medicago Truncatula. Plant Physiol. Biochem. 2017, 119, 211–223. [Google Scholar] [CrossRef]
- Lubna, A.S.; Hamayun, M.; Khan, A.L.; Waqas, M.; Khan, M.A.; Jan, R.; Lee, I.-J.; Hussain, A. Salt Tolerance of Glycine Max.L Induced by Endophytic Fungus Aspergillus Flavus CSH1, via Regulating Its Endogenous Hormones and Antioxidative System. Plant Physiol. Biochem. 2018, 128, 13–23. [Google Scholar] [CrossRef]
- Manasa, K.M.; Vasanthakumari, M.M.; Nataraja, K.N.; Uma Shaanker, R. Endophytic Fungi of Salt Adapted Ipomea Pes-Caprae L. R. Br: Their Possible Role in Inducing Salinity Tolerance in Paddy (Oryza sativa L.). Curr. Sci. 2020, 118, 1448–1453. [Google Scholar] [CrossRef]
- Bibi, N.; Jan, G.; Jan, F.G.; Hamayun, M.; Iqbal, A.; Hussain, A.; Rehman, H.; Tawab, A.; Khushdil, F. Cochliobolus sp. Acts as a Biochemical Modulator to Alleviate Salinity Stress in Okra Plants. Plant Physiol. Biochem. 2019, 139, 459–469. [Google Scholar] [CrossRef] [PubMed]
- Saikkonen, K.; Faeth, S.H.; Helander, M.; Sullivan, T.J. Fungal Endophytes: A Continuum of Interactins with Host Plants. Annu. Rev. Ecol. Syst. 1998, 29, 319–343. [Google Scholar] [CrossRef]
- Radić, N.; Štrukelj, B. Endophytic Fungi—The Treasure Chest of Antibacterial Substances. Phytomedicine 2012, 19, 1270–1284. [Google Scholar] [CrossRef]
- Lorito, M.; Woo, S.L.; Harman, G.E.; Monte, E. Translational Research on Trichoderma: From ’Omics to the Field. Annu. Rev. Phytopathol. 2010, 48, 395–417. [Google Scholar] [CrossRef]
- Mendoza-Mendoza, A.; Zaid, R.; Lawry, R.; Hermosa, R.; Monte, E.; Horwitz, B.A.; Mukherjee, P.K. Molecular Dialogues between Trichoderma and Roots: Role of the Fungal Secretome. Fung. Biol. Rev. 2018, 32, 62–85. [Google Scholar] [CrossRef]
- Crandall, S.G.; Gold, K.M.; del Jiménez-Gasco, M.M.; Filgueiras, C.C.; Willett, D.S. A Multi-Omics Approach to Solving Problems in Plant Dis. Ecology. PLoS ONE 2020, 15, e0237975. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).