High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad
Abstract
:1. Introduction
2. Materials and Methods
2.1. Invitation of Participating Laboratories
2.2. Collection of Patient Data
2.3. Laboratory Investigations
3. Results
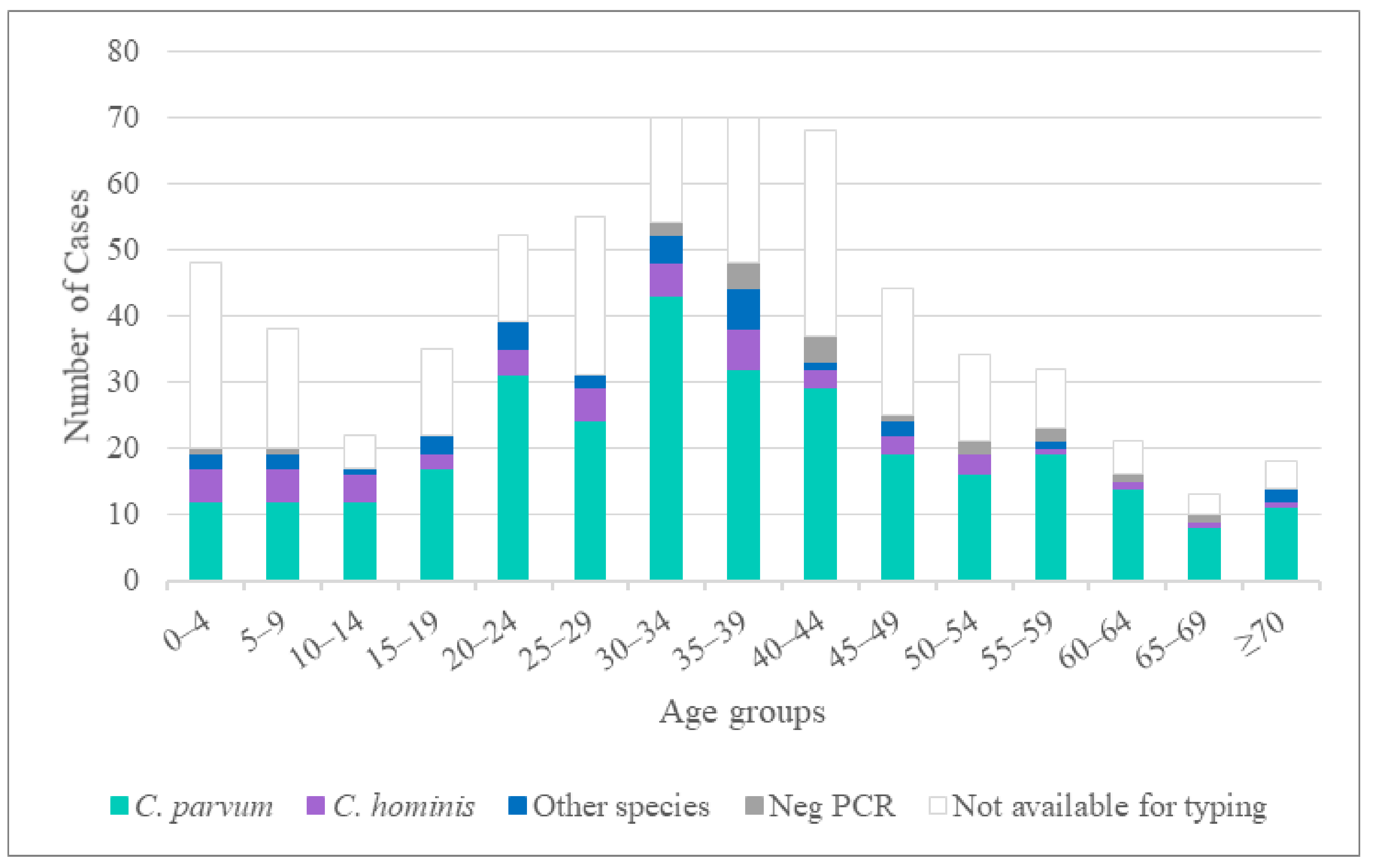
3.1. Participating Laboratories and Patient Demographics
3.2. Cryptosporidium Species Identified
3.3. Origin of Infection
3.4. Molecular Characterization of Cryptosporidium parvum
3.5. Molecular Characterization of Cryptosporidium hominis
3.6. Molecular Characterization of C. hominis/C. parvum Mixed Infection
3.7. Outbreaks and Family Clusters
3.8. Molecular Characterization of Additional Species
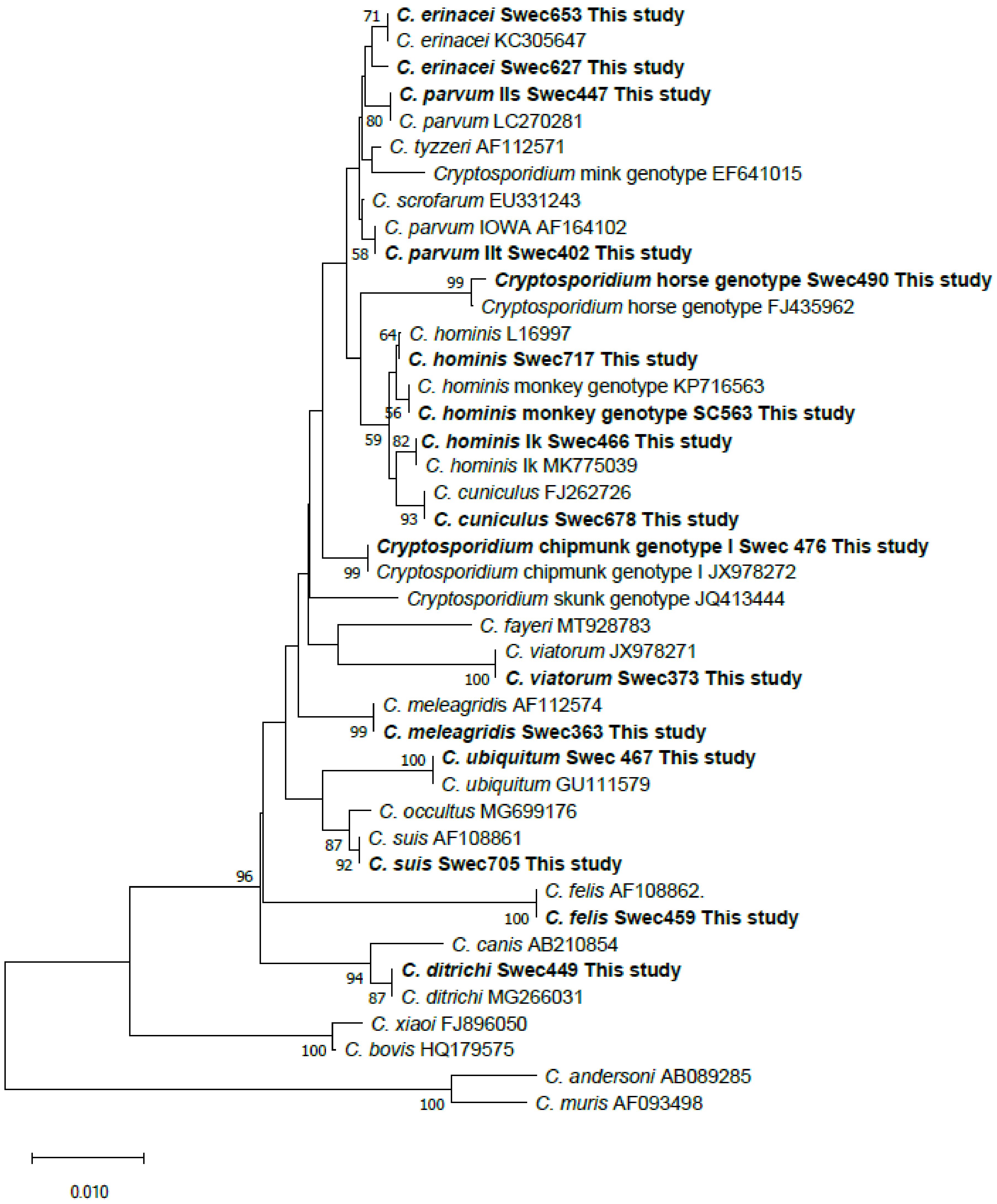
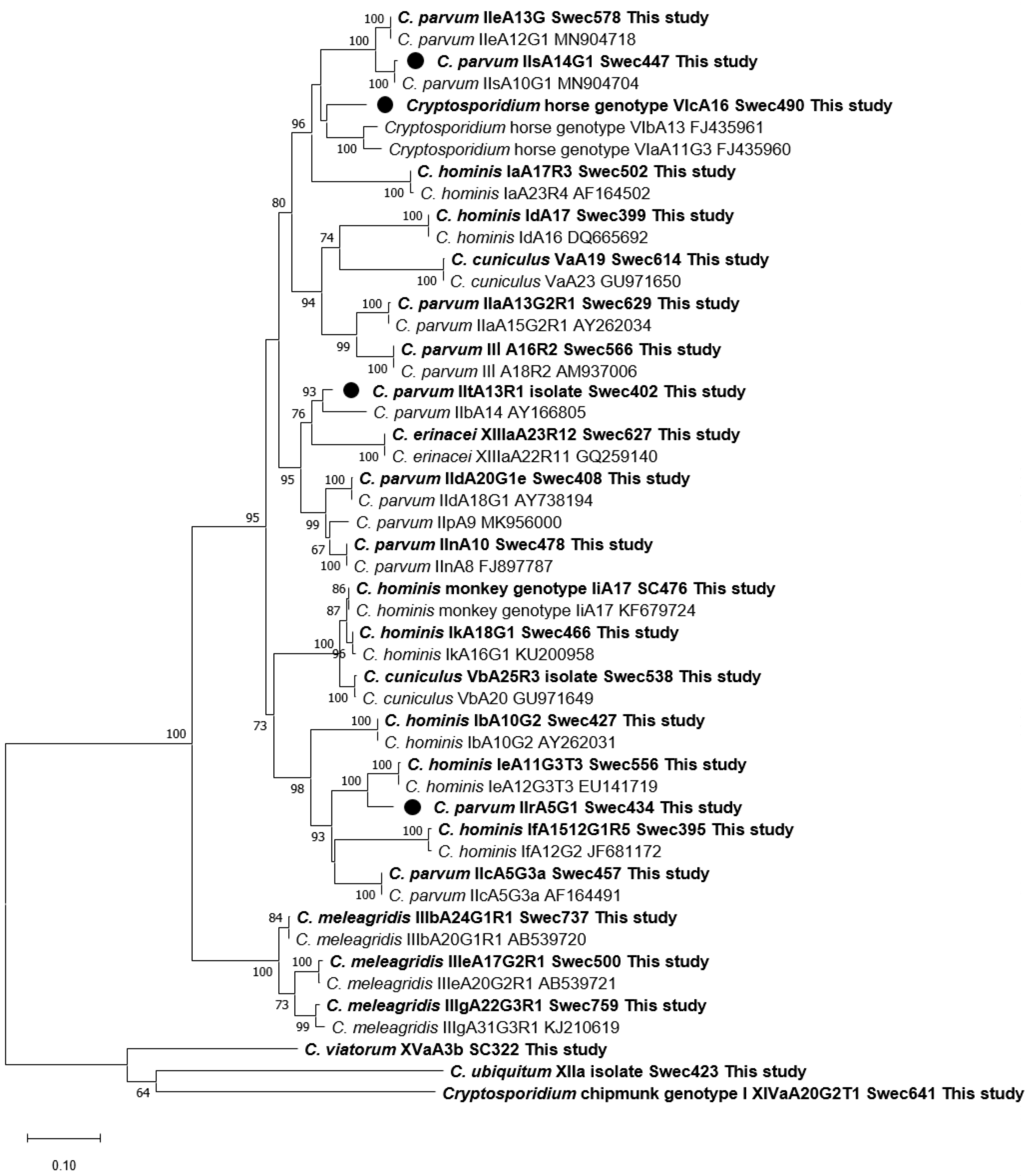
3.9. Phylogenetic Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kotloff, K.L.; Nataro, J.P.; Blackwelder, W.C.; Nasrin, D.; Farag, T.H.; Panchalingam, S.; Wu, Y.; Sow, S.O.; Sur, D.; Breiman, R.F.; et al. Burden and aetiology of diarrhoeal disease in infants and young children in developing countries (the Global Enteric Multicenter Study, GEMS): A prospective, case-control study. Lancet 2013, 382, 209–222. [Google Scholar] [CrossRef]
- Ryan, U.; Fayer, R.; Xiao, L. Cryptosporidium species in humans and animals: Current understanding and research needs. Parasitology 2014, 141, 1667–1685. [Google Scholar] [CrossRef] [Green Version]
- Feng, Y.; Ryan, U.M.; Xiao, L. Genetic diversity and population structure of Cryptosporidium. Trends Parasitol. 2018, 34, 997–1011. [Google Scholar] [CrossRef] [PubMed]
- Zahedi, A.; Ryan, U. Cryptosporidium—An update with an emphasis on foodborne and waterborne transmission. Res. Vet. Sci. 2020, 132, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Public Health Agency of Sweden. Surveillance of Communicable Diseases Sweden. Available online: https://www.folkhalsomyndigheten.se/folkhalsorapportering-statistik/statistik-a-o/sjukdomsstatistik/cryptosporidiuminfektion/ (accessed on 24 April 2021).
- Harvala, H.; Ogren, J.; Boman, P.; Riedel, H.M.; Nilsson, P.; Winiecka-Krusnell, J.; Beser, J. Cryptosporidium infections in Sweden-understanding the regional differences in reported incidence. Clin. Microbiol. Infect. 2016, 22, 1012–1013. [Google Scholar] [CrossRef] [PubMed]
- Ogren, J.; Dienus, O.; Beser, J.; Henningsson, A.J.; Matussek, A. Protozoan infections are under-recognized in Swedish patients with gastrointestinal symptoms. Eur. J. Clin. Microbiol. Infect. Dis. 2020, 39, 2153–2160. [Google Scholar] [CrossRef]
- Waldron, L.S.; Dimeski, B.; Beggs, P.J.; Ferrari, B.C.; Power, M.L. Molecular epidemiology, spatiotemporal analysis, and ecology of sporadic human cryptosporidiosis in Australia. Appl. Environ. Microbiol. 2011, 77, 7757–7765. [Google Scholar] [CrossRef] [Green Version]
- Insulander, M.; Silverlås, C.; Lebbad, M.; Karlsson, L.; Mattsson, J.; Svenungsson, B. Molecular epidemiology and clinical manifestations of human cryptosporidiosis in Sweden. Epidemiol. Infect. 2013, 141, 1009–1020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bjelkmar, P.; Hansen, A.; Schönning, C.; Bergström, J.; Löfdahl, M.; Lebbad, M.; Wallensten, A.; Allestam, G.; Stenmark, S.; Lindh, J. Early outbreak detection by linking health advice line calls to water distribution areas retrospectively demonstrated in a large waterborne outbreak of cryptosporidiosis in Sweden. BMC Public Health 2017, 17, 328. [Google Scholar] [CrossRef] [PubMed]
- Widerström, M.; Schönning, C.; Lilja, M.; Lebbad, M.; Ljung, T.; Allestam, G.; Ferm, M.; Bjorkholm, B.; Hansen, A.; Hiltula, J.; et al. Large outbreak of Cryptosporidium hominis infection transmitted through the public water supply, Sweden. Emerg. Infect. Dis. 2014, 20, 581. [Google Scholar] [CrossRef] [PubMed]
- Gherasim, A.; Lebbad, M.; Insulander, M.; Decraene, V.; Kling, A.; Hjertqvist, M.; Wallensten, A. Two geographically separated food-borne outbreaks in Sweden linked by an unusual Cryptosporidium parvum subtype, October 2010. Eurosurveillance 2012, 17, 20318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Insulander, M.; Lebbad, M.; Stenström, T.A.; Svenungsson, B. An outbreak of cryptosporidiosis associated with exposure to swimming pool water. Scand. J. Infect. Dis. 2005, 37, 354–360. [Google Scholar] [CrossRef]
- Alsmark, C.; Nolskog, P.; Angervall, A.L.; Toepfer, M.; Winiecka-Krusnell, J.; Bouwmeester, J.; Bjelkmar, P.; Troell, K.; Lahti, E.; Beser, J. Two outbreaks of cryptosporidiosis associated with cattle spring pasture events. Vet. Parasitol. Reg. Stud. Rep. 2018, 14, 71–74. [Google Scholar] [CrossRef]
- Xiao, L.; Escalante, L.; Yang, C.; Sulaiman, I.; Escalante, A.A.; Montali, R.J.; Fayer, R.; Lal, A.A. Phylogenetic analysis of Cryptosporidium parasites based on the small-subunit rRNA gene locus. Appl. Environ. Microbiol. 1999, 65, 1578–1583. [Google Scholar] [CrossRef] [Green Version]
- Xiao, L.; Bern, C.; Limor, J.; Sulaiman, I.; Roberts, J.; Checkley, W.; Cabrera, L.; Gilman, R.H.; Lal, A.A. Identification of 5 types of Cryptosporidium parasites in children in Lima, Peru. J. Infect. Dis. 2001, 183, 492–497. [Google Scholar] [CrossRef] [Green Version]
- Alves, M.; Xiao, L.; Sulaiman, I.; Lal, A.A.; Matos, O.; Antunes, F. Subgenotype analysis of Cryptosporidium isolates from humans, cattle, and zoo ruminants in Portugal. J. Clin. Microbiol. 2003, 41, 2744–2747. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morgan, U.M.; Monis, P.T.; Xiao, L.; Limor, J.; Sulaiman, I.; Raidal, S.; O′Donoghue, P.; Gasser, R.; Murray, A.; Fayer, R.; et al. Molecular and phylogenetic characterisation of Cryptosporidium from birds. Int. J. Parasitol. 2001, 31, 289–296. [Google Scholar] [CrossRef]
- Guo, Y.; Cebelinski, E.; Matusevich, C.; Alderisio, K.A.; Lebbad, M.; McEvoy, J.; Roellig, D.M.; Yang, C.; Feng, Y.; Xiao, L. Subtyping novel zoonotic pathogen Cryptosporidium chipmunk genotype I. J. Clin. Microbiol. 2015, 53, 1648–1654. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, N.; Xiao, L.; Alderisio, K.; Elwin, K.; Cebelinski, E.; Chalmers, R.; Santin, M.; Fayer, R.; Kvac, M.; Ryan, U.; et al. Subtyping Cryptosporidium ubiquitum, a zoonotic pathogen emerging in humans. Emerg. Infect. Dis. 2014, 20, 217. [Google Scholar] [CrossRef] [PubMed]
- Rojas-Lopez, L.; Elwin, K.; Chalmers, R.M.; Enemark, H.L.; Beser, J.; Troell, K. Development of a gp60-subtyping method for Cryptosporidium felis. Parasites Vectors 2020, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.; Elwin, K.; Winiecka-Krusnell, J.; Chalmers, R.; Xiao, L.; Lebbad, M. Development and application of a gp60-based typing assay for Cryptosporidium viatorum. J. Clin. Microbiol. 2015, 53, 1891–1897. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stensvold, C.R.; Beser, J.; Axén, C.; Lebbad, M. High applicability of a novel method for gp60-based subtyping of Cryptosporidium meleagridis. J. Clin. Microbiol. 2014, 52, 2311–2319. [Google Scholar] [CrossRef] [Green Version]
- Sulaiman, I.M.; Lal, A.A.; Xiao, L. Molecular phylogeny and evolutionary relationships of Cryptosporidium parasites at the actin locus. J. Parasitol. 2002, 88, 388–394. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Morgan, U.M.; Thompson, R.C.; Lal, A.A.; Xiao, L. Phylogenetic relationships of Cryptosporidium parasites based on the 70-kilodalton heat shock protein (HSP70) gene. Appl. Environ. Microbiol. 2000, 66, 2385–2391. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kumar, S.; Stecher, G.; Li, M.; Knyaz, C.; Tamura, K. MEGA X: Molecular evolutionary genetics analysis across computing platforms. Molecular Biol. Evol. 2018, 35, 1547–1549. [Google Scholar] [CrossRef] [PubMed]
- Kinross, P.; Beser, J.; Troell, K.; Axen, C.; Björkman, C.; Lebbad, M.; Winiecka-Krusnell, J.; Lindh, J.; Lofdahl, M. Cryptosporidium parvum infections in a cohort of veterinary students in Sweden. Epidemiol Infect. 2015, 143, 2748–2756. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amer, S.; Zidan, S.; Adamu, H.; Ye, J.; Roellig, D.; Xiao, L.; Feng, Y. Prevalence and characterization of Cryptosporidium spp. in dairy cattle in Nile River delta provinces, Egypt. Exp. Parasitol. 2013, 135, 518–523. [Google Scholar] [CrossRef] [PubMed]
- Lebbad, M.; Winiecka-Krusnell, J.; Insulander, M.; Beser, J. Molecular characterization and epidemiological investigation of Cryptosporidium hominis IkA18G1 and C. hominis monkey genotype IiA17, two unusual subtypes diagnosed in Swedish patients. Exp. Parasitol. 2018, 188, 7. [Google Scholar] [CrossRef] [PubMed]
- Beser, J.; Toresson, L.; Eitrem, R.; Troell, K.; Winiecka-Krusnell, J.; Lebbad, M. Possible zoonotic transmission of Cryptosporidium felis in a household. Infect. Ecol. Epidemiol. 2015, 5, 28463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beser, J.; Bujila, I.; Wittesjo, B.; Lebbad, M. From mice to men: Three cases of human infection with Cryptosporidium ditrichi. Infect. Genet. Evol. 2020, 78, 104120. [Google Scholar] [CrossRef] [PubMed]
- Nolan, M.J.; Jex, A.R.; Haydon, S.R.; Stevens, M.A.; Gasser, R.B. Molecular detection of Cryptosporidium cuniculus in rabbits in Australia. Infect. Genet. Evol. 2010, 10, 1179–1187. [Google Scholar] [CrossRef]
- Sulaiman, I.M.; Hira, P.R.; Zhou, L.; Al-Ali, F.M.; Al-Shelahi, F.A.; Shweiki, H.M.; Iqbal, J.; Khalid, N.; Xiao, L. Unique endemicity of cryptosporidiosis in children in Kuwait. J Clin. Microbiol. 2005, 43, 2805–2809. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guy, R.A.; Yanta, C.A.; Muchaal, P.K.; Rankin, M.A.; Thivierge, K.; Lau, R.; Boggild, A.K. Molecular characterization of Cryptosporidium isolates from humans in Ontario, Canada. Parasites Vectors 2021, 14, 69. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, J.K.; Blake, L.; Corcoran, G.D.; Sleator, R.D.; Lucey, B. Increased diversity and novel subtypes among clinical Cryptosporidium parvum and Cryptosporidium hominis isolates in Southern Ireland. Exp. Parasitol. 2020, 218, 107967. [Google Scholar] [CrossRef] [PubMed]
- Costa, D.; Razakandrainibe, R.; Valot, S.; Vannier, M.; Sautour, M.; Basmaciyan, L.; Gargala, G.; Viller, V.; Lemeteil, D.; Ballet, J.-J. Epidemiology of Cryptosporidiosis in France from 2017 to 2019. Microorganisms 2020, 8, 1358. [Google Scholar] [CrossRef]
- Braima, K.; Zahedi, A.; Oskam, C.; Reid, S.; Pingault, N.; Xiao, L.; Ryan, U. Retrospective analysis of Cryptosporidium species in Western Australian human populations (2015–2018), and emergence of the C. hominis IfA12G1R5 subtype. Infect. Genet. Evol. 2019, 73, 306–313. [Google Scholar] [CrossRef] [PubMed]
- De Lucio, A.; Merino, F.J.; Martínez-Ruiz, R.; Bailo, B.; Aguilera, M.; Fuentes, I.; Carmena, D. Molecular genotyping and sub-genotyping of Cryptosporidium spp. isolates from symptomatic individuals attending two major public hospitals in Madrid, Spain. Infect. Genet. Evol. 2016, 37, 49–56. [Google Scholar] [CrossRef] [PubMed]
- Nic Lochlainn, L.M.; Sane, J.; Schimmer, B.; Mooij, S.; Roelfsema, J.; Van Pelt, W.; Kortbeek, T. Risk Factors for Sporadic Cryptosporidiosis in the Netherlands: Analysis of a 3-Year Population Based Case-Control Study Coupled with Genotyping, 2013–2016. J. Infect. Dis. 2019, 219, 1121–1129. [Google Scholar] [CrossRef] [PubMed]
- Garcia-R, J.C.; Pita, A.B.; Velathanthiri, N.; French, N.P.; Hayman, D.T. Species and genotypes causing human cryptosporidiosis in New Zealand. Parasitol. Res. 2020, 119, 2317–2326. [Google Scholar] [CrossRef] [PubMed]
- Stensvold, C.R.; Ethelberg, S.; Hansen, L.; Sahar, S.; Voldstedlund, M.; Kemp, M.; Hartmeyer, G.T.; Otte, E.; Engsbro, A.L.; Nielsen, H.V.; et al. Cryptosporidium infections in Denmark, 2010–2014. Dan. Med. J. 2015, 62, A5086. [Google Scholar] [PubMed]
- Chalmers, R.; Elwin, K.; Thomas, A.; Guy, E.; Mason, B. Long-term Cryptosporidium typing reveals the aetiology and species-specific epidemiology of human cryptosporidiosis in England and Wales, 2000 to 2003. Euro Surveill. 2009, 14, 19086. [Google Scholar] [CrossRef] [PubMed]
- Loeck, B.K.; Pedati, C.; Iwen, P.C.; McCutchen, E.; Roellig, D.M.; Hlavsa, M.C.; Fullerton, K.; Safranek, T.; Carlson, A.V. Genotyping and Subtyping Cryptosporidium to Identify Risk Factors and Transmission Patterns—Nebraska, 2015–2017. Morb. Mortal. Wkly. Rep. 2020, 69, 335. [Google Scholar] [CrossRef]
- Chalmers, R.; Smith, R.; Elwin, K.; Clifton-Hadley, F.; Giles, M. Epidemiology of anthroponotic and zoonotic human cryptosporidiosis in England and Wales, 2004–2006. Epidemiol. Infect. 2011, 139, 700–712. [Google Scholar] [CrossRef] [Green Version]
- Björkman, C.; Lindström, L.; Oweson, C.; Ahola, H.; Troell, K.; Axén, C. Cryptosporidium infections in suckler herd beef calves. Parasitology 2015, 142, 1108–1114. [Google Scholar] [CrossRef] [Green Version]
- Chalmers, R.M.; Robinson, G.; Elwin, K.; Elson, R. Analysis of the Cryptosporidium spp. and gp60 subtypes linked to human outbreaks of cryptosporidiosis in England and Wales, 2009 to 2017. Parasites Vectors 2019, 12, 95. [Google Scholar] [CrossRef] [PubMed]
- Thomas-Lopez, D.; Müller, L.; Vestergaard, L.S.; Christoffersen, M.; Andersen, A.-M.; Jokelainen, P.; Agerholm, J.S.; Stensvold, C.R. Veterinary students have a higher risk of contracting cryptosporidiosis when calves with high fecal Cryptosporidium loads are used for fetotomy exercises. Appl. Environ. Microbiol. 2020, 86. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, Y. Molecular epidemiologic tools for waterborne pathogens Cryptosporidium spp. and Giardia duodenalis. Food Waterborne Parasitol. 2017, 8, 14–32. [Google Scholar] [CrossRef] [PubMed]
- Feng, Y.; Torres, E.; Li, N.; Wang, L.; Bowman, D.; Xiao, L. Population genetic characterisation of dominant Cryptosporidium parvum subtype IIaA15G2R1. Int. J. Parasitol. 2013, 43, 1141–1147. [Google Scholar] [CrossRef] [PubMed]
- Silverlås, C.; Bosaeus-Reineck, H.; Näslund, K.; Björkman, C. Is there a need for improved Cryptosporidium diagnostics in Swedish calves? Int. J. Parasitol. 2013, 43, 155–161. [Google Scholar] [CrossRef]
- Whithworth, J. Five Foodborne Outbreaks Added to Cryptosporidium Rise in Sweden. Food Safety News, 13 March 2020. Available online: https://www.foodsafetynews.com/2020/03/five-foodborne-outbreaks-added-to-cryptosporidium-rise-in-sweden/ (accessed on 24 April 2021).
- Ajjampur, S.S.; Liakath, F.B.; Kannan, A.; Rajendran, P.; Sarkar, R.; Moses, P.D.; Simon, A.; Agarwal, I.; Mathew, A.; O’Connor, R.; et al. Multisite study of cryptosporidiosis in children with diarrhea in India. J Clin. Microbiol. 2010, 48, 2075–2081. [Google Scholar] [CrossRef] [Green Version]
- Mulunda, N.R.; Hayashida, K.; Yamagishi, J.; Sianongo, S.; Munsaka, G.; Sugimoto, C.; Mutengo, M.M. Molecular characterization of Cryptosporidium spp. from patients with diarrhoea in Lusaka, Zambia. Parasite 2020, 27, 53. [Google Scholar] [CrossRef]
- Xiao, L.; Hlavsa, M.C.; Yoder, J.; Ewers, C.; Dearen, T.; Yang, W.; Nett, R.; Harris, S.; Brend, S.M.; Harris, M.; et al. Subtype analysis of Cryptosporidium specimens from sporadic cases in Colorado, Idaho, New Mexico, and Iowa in 2007: Widespread occurrence of one Cryptosporidium hominis subtype and case history of an infection with the Cryptosporidium horse genotype. J. Clin. Microbiol. 2009, 47, 3017–3020. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hlavsa, M.C.; Roellig, D.M.; Seabolt, M.H.; Kahler, A.M.; Murphy, J.L.; McKitt, T.K.; Geeter, E.F.; Dawsey, R.; Davidson, S.L.; Kim, T.N.; et al. Using Molecular Characterization to Support Investigations of Aquatic Facility-Associated Outbreaks of Cryptosporidiosis—Alabama, Arizona, and Ohio, 2016. MMWR Morb. Mortal. Wkly. Rep. 2017, 66, 493–497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jian, F.; Liu, A.; Wang, R.; Zhang, S.; Qi, M.; Zhao, W.; Shi, Y.; Wang, J.; Wei, J.; Zhang, L.; et al. Common occurrence of Cryptosporidium hominis in horses and donkeys. Infect. Genet. Evol. 2016, 43, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Lebbad, M.; Beser, J.; Insulander, M.; Karlsson, L.; Mattsson, J.G.; Svenungsson, B.; Axén, C. Unusual cryptosporidiosis cases in Swedish patients: Extended molecular characterization of Cryptosporidium viatorum and Cryptosporidium chipmunk genotype I. Parasitology 2013, 140, 1735–1740. [Google Scholar] [CrossRef] [Green Version]
- Kvác, M.; Hofmannová, L.; Bertolino, S.; Wauters, L.; Tosi, G.; Modrý, D. Natural infection with two genotypes of Cryptosporidium in red squirrels (Sciurus vulgaris) in Italy. Folia Parasitol. 2008, 55, 95. [Google Scholar] [CrossRef] [Green Version]
- Zhao, W.; Zhou, H.; Huang, Y.; Xu, L.; Rao, L.; Wang, S.; Wang, W.; Yi, Y.; Zhou, X.; Wu, Y.; et al. Cryptosporidium spp. in wild rats (Rattus spp.) from the Hainan Province, China: Molecular detection, species/genotype identification and implications for public health. Int. J. Parasitol. Parasites Wildl. 2019, 9, 317–321. [Google Scholar] [CrossRef]
- Laatamna, A.E.; Wagnerova, P.; Sak, B.; Kvetonova, D.; Aissi, M.; Rost, M.; Kvac, M. Equine cryptosporidial infection associated with Cryptosporidium hedgehog genotype in Algeria. Vet. Parasitol. 2013, 197, 350–353. [Google Scholar] [CrossRef]
- Kváč, M.; Hofmannová, L.; Hlásková, L.; Květoňová, D.; Vítovec, J.; McEvoy, J.; Sak, B. Cryptosporidium erinacei n. sp. (Apicomplexa: Cryptosporidiidae) in hedgehogs. Vet. Parasitol. 2014, 201, 9–17. [Google Scholar] [CrossRef]
- Costa, D.; Razakandrainibe, R.; Sautour, M.; Valot, S.; Basmaciyan, L.; Gargala, G.; Lemeteil, D.; Favennec, L.; Dalle, F. Human cryptosporidiosis in immunodeficient patients in France (2015–2017). Exp. Parasitol. 2018, 192, 108–112. [Google Scholar] [CrossRef] [PubMed]
- Kvac, M.; Sakova, K.; Kvetonova, D.; Kicia, M.; Wesolowska, M.; McEvoy, J.; Sak, B. Gastroenteritis caused by the Cryptosporidium hedgehog genotype in an immunocompetent man. J. Clin. Microbiol. 2014, 52, 347–349. [Google Scholar] [CrossRef] [Green Version]
- Puleston, R.L.; Mallaghan, C.M.; Modha, D.E.; Hunter, P.R.; Nguyen-Van-Tam, J.S.; Regan, C.M.; Nichols, G.L.; Chalmers, R.M. The first recorded outbreak of cryptosporidiosis due to Cryptosporidium cuniculus (formerly rabbit genotype), following a water quality incident. J Water Health 2014, 12, 41–50. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koehler, A.V.; Rashid, M.H.; Zhang, Y.; Vaughan, J.L.; Gasser, R.B.; Jabbar, A. First cross-sectional, molecular epidemiological survey of Cryptosporidium, Giardia and Enterocytozoon in alpaca (Vicugna pacos) in Australia. Parasites Vectors 2018, 11, 498. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Whipp, M.J.; Haydon, S.R.; Gasser, R.B. Cryptosporidium cuniculus--new records in human and kangaroo in Australia. Parasites Vectors 2014, 7, 492. [Google Scholar] [CrossRef]
- Elwin, K.; Hadfield, S.J.; Robinson, G.; Crouch, N.D.; Chalmers, R.M. Cryptosporidium viatorum n. sp. (Apicomplexa: Cryptosporidiidae) among travellers returning to Great Britain from the Indian subcontinent, 2007–2011. Int. J. Parasitol. 2012, 42, 675–682. [Google Scholar] [CrossRef] [PubMed]
- Koehler, A.V.; Wang, T.; Haydon, S.R.; Gasser, R.B. Cryptosporidium viatorum from the native Australian swamp rat Rattus lutreolus-an emerging zoonotic pathogen? Int. J. Parasitol. Parasites Wildl. 2018, 7, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.-W.; Zheng, W.-B.; Zhang, N.-Z.; Gui, B.-Z.; Lv, Q.-Y.; Yan, J.-Q.; Zhao, Q.; Liu, G.H. Identification of Cryptosporidium viatorum XVa subtype family in two wild rat species in China. Parasites Vectors. 2019, 12, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Burton, A.; Nydam, D.; Dearen, T.; Mitchell, K.; Bowman, D.; Xiao, L. The prevalence of Cryptosporidium, and identification of the Cryptosporidium horse genotype in foals in New York State. Vet. Parasitol. 2010, 174, 139–144. [Google Scholar] [CrossRef]
- Caffara, M.; Piva, S.; Pallaver, F.; Iacono, E.; Galuppi, R. Molecular characterization of Cryptosporidium spp. from foals in Italy. Vet. J. 2013, 198, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Robinson, G.; Elwin, K.; Chalmers, R.M. Unusual Cryptosporidium genotypes in human cases of diarrhea. Emerg. Infect. Dis. 2008, 14, 1800. [Google Scholar] [CrossRef]
- Abe, N.; Matsubara, K. Molecular identification of Cryptosporidium isolates from exotic pet animals in Japan. Vet. Parasitol. 2015, 209, 254–257. [Google Scholar] [CrossRef]
- Takaki, Y.; Takami, Y.; Watanabe, T.; Nakaya, T.; Murakoshi, F. Molecular identification of Cryptosporidium isolates from ill exotic pet animals in Japan including a new subtype in Cryptosporidium fayeri. Vet. Parasitol. Reg. Stud. Rep. 2020, 21, 100430. [Google Scholar] [CrossRef]
- Sannella, A.R.; Suputtamongkol, Y.; Wongsawat, E.; Caccio, S.M. A retrospective molecular study of Cryptosporidium species and genotypes in HIV-infected patients from Thailand. Parasites Vectors 2019, 12, 91, Epub 2019/03/15. [Google Scholar] [CrossRef] [PubMed]
- Condlova, S.; Horcickova, M.; Sak, B.; Kvetonova, D.; Hlaskova, L.; Konecny, R.; Stanko, M.; McEvoy, J.; Kvac, M. Cryptosporidium apodemi sp. n. and Cryptosporidium ditrichi sp. n. (Apicomplexa: Cryptosporidiidae) in Apodemus spp. Eur. J. Protistol. 2018, 63, 1–12. [Google Scholar] [CrossRef]
- Xiao, L.; Feng, Y. Zoonotic cryptosporidiosis. FEMS Immunol. Med Microbiol. 2008, 52, 309–323. [Google Scholar] [CrossRef] [PubMed]
- Da Cunha, M.J.R.; Cury, M.C.; Santín, M. Molecular characterization of Cryptosporidium spp. in poultry from Brazil. Res. Vet. Sci. 2018, 118, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.; Wang, T.; Koehler, A.V.; Fan, Y.; Hu, M.; Gasser, R.B. Molecular investigation of Cryptosporidium in farmed chickens in Hubei Province, China, identifies ‘zoonotic’ subtypes of C. meleagridis. Parasites Vectors 2018, 11, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Silverlås, C.; Mattsson, J.G.; Insulander, M.; Lebbad, M. Zoonotic transmission of Cryptosporidium meleagridis on an organic Swedish farm. Int. J. Parasitol. 2012, 42, 963–967. [Google Scholar] [CrossRef] [PubMed]
- Abdelsalam, I.M.; Sarhan, R.M.; Hanafy, M.A. The impact of different copro-preservation conditions on molecular detection of Cryptosporidium species. Iran. J. Parasitol. 2017, 12, 274. [Google Scholar]
- Autier, B.; Belaz, S.; Razakandrainibe, R.; Gangneux, J.P.; Robert-Gangneux, F. Comparison of three commercial multiplex PCR assays for the diagnosis of intestinal protozoa. Parasite 2018, 25, 48. [Google Scholar] [CrossRef] [PubMed]
| County | Number of Laboratories | Number of Samples | Species (Number of Samples) |
|---|---|---|---|
| Halland | 1 | 138 | C. parvum (112), C. hominis (15), C. cuniculus (5), C. erinacei (2), C. meleagridis (1), non-typeable (3) |
| Jämtland | 1 | 4 | C. parvum (4) |
| Jönköping | 1 | 76 | C. parvum (58), C. hominis (5), Cryptosporidium chipmunk genotype I (3), non-typeable (10) |
| Kronoberg | 1 | 1 | C. felis (1) |
| Skåne | 1 | 5 | C. parvum (3), C. felis (1), Cryptosporidium horse genotype (1) |
| Stockholm | 2 | 92 | C. parvum (62), C. hominis (23), C. ubiquitum (2), C. ditrichi (1), C. felis (1), C. meleagridis (1), C. viatorum (1), non-typeable (1) |
| Uppsala | 1 | 70 | C. parvum (52), C. hominis (6), C. meleagridis (6), Cryptosporidium chipmunk genotype I (2), C. felis (1), C. hominis + C. parvum (1), C. suis (1), non-typeable (1) |
| Västerbotten | 1 | 4 | C. parvum (4) |
| Västernorrland | 1 | 2 | C. parvum (2) |
| Västra Götaland | 1 | 5 | C. parvum (2), non-typeable (3) |
| Örebro | 1 | 1 | non-typeable (1) |
| Total | 12 | 398 | C. parvum (299), C. hominis (49), C. parvum + C. hominis (1), C. meleagridis (8), C. cuniculus (5), Cryptosporidium chipmunk genotype I (5), C. felis (4), C. erinacei (2), C. ubiquitum (2), C. ditrichi (1), C. suis (1), C. viatorum (1), Cryptosporidium horse genotype (1), non-typeable (19) |
| Species/Genotypes | Number of Samples (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| Total | Sweden | Other European Countries | Africa | Asia | North America | South America | Unknown | |
| C. parvum | 299 | 211 (71) | 60 (20) | 9 (3) | 5 (2) | 3 (1) | 1 | 10 (3) |
| C. hominis | 49 | 8 (16) | 10 (20) | 14 (29) | 13 (27) | 3 (6) | 1 (2) | 0 |
| C. parvum + C. hominis | 1 | 0 | 0 | 0 | 1 | 0 | 0 | 0 |
| C. meleagridis | 8 | 0 | 0 | 0 | 8 (100) | 0 | 0 | 0 |
| C. cuniculus | 5 | 3 (60) | 2 (40) | 0 | 0 | 0 | 0 | 0 |
| Cryptosporidium chipmunk genotype I | 5 | 5 (100) | 0 | 0 | 0 | 0 | 0 | 0 |
| C. felis | 4 | 3 (75) | 0 | 0 | 1 (25) | 0 | 0 | 0 |
| C. erinacei | 2 | 1 | 1 | 0 | 0 | 0 | 0 | 0 |
| C. ubiquitum | 2 | 2 | 0 | 0 | 0 | 0 | 0 | 0 |
| C. suis | 1 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| C. viatorum | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| Cryptosporidium horse genotype | 1 | 0 | 0 | 1 | 0 | 0 | 0 | 0 |
| C. ditrichi | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| None-typable 1 | 19 | 16 (84) | 1 (5) | 2 (11) | 0 | 0 | 0 | 0 |
| All cases | 398 | 250 (63) | 75 (19) | 27 (7) | 28 (7) | 6 (3) | 2 (1) | 10 (3) |
| Species (No. of Patients) | Subtype Family (No. of Patients) | Subtype (No. of Patients) | GenBank Acc. No. 1 | Origin of Infection (No. of Patients) |
|---|---|---|---|---|
| C. parvum (299) | IIa (164) | IIaA13G2R1 (2) | KU852706 | Turkey (1) |
| KU852701 | Morocco (1) | |||
| IIaA13R1 (1) | KU852702 | Sweden (1) | ||
| IIaA14G1R1 (13) | JQ030882 | Cyprus (1) Georgia (2) Sweden (3) | ||
| JX183798 (IIaA14G1R1b) | Sweden (7) | |||
| IIaA14G1R1r1 (6) | KU852703 | Sweden (4) Spain (1) Uzbekistan (1) | ||
| IIaA14G2R1 (1) | KF128738 | Greece (1) | ||
| IIaA14R1 (2) | JX183797 | Estonia (1) Sweden (1) | ||
| IIaA15G1R1 (6) | AM937012 | Sweden (4) unknown (1) | ||
| KU852704 (IIaA15G1R1_variant) | Sweden (1) | |||
| IIaA15G2R1 (31) | AF164490 | Dominican Republic (1) France (1) Mexico (1) Portugal (9) Portugal/Spain (1) Sweden (16) Venezuela (1) unknown (1) | ||
| IIaA16G1R1 (42) | EU647727 (IIaA16G1R1b) | Georgia (1) Italy (1) Serbia (1) Spain (6) Sweden (30 2) | ||
| KU852707 | unknown (1) | |||
| KT895368 (IIaA16G1R1b_variant) | Austria (1) Sweden (1) | |||
| IIaA16G2R1 (3) | DQ192505 | Sweden (3) | ||
| IIaA16G3R1 (2) | DQ192506 | Portugal (1) Sweden (1) | ||
| IIaA16R1 (1) | AM937010 | Malta (1) | ||
| IIaA17G1R1 (20) | GQ983359 | Poland (1) Sweden (1) | ||
| JX183801 (IIaA17G1R1c) | Italy (1) Sweden (11) South Africa (1) | |||
| AF403168 (IIaA17G1R1c_variant) | Sweden (5) | |||
| IIaA17R1 (1) | JX183800 | Sweden (1) | ||
| IIaA18G1R1 (12) | HQ005742 | Finland (1) | ||
| KF289038 (IIaA18G1R1b) | Sweden (2) | |||
| KT895369 (IIaA18G1R1b_variant) | Portugal (1) Sweden (3) | |||
| JX183803 (IIaA18G1R1d) | United Kingdom (1) Sweden (4) | |||
| IIaA18R1 (1) | KU852705 | Sweden (1) | ||
| IIaA19G1R1 (3) | KC679056 | Spain (1) Sweden (2) | ||
| IIaA19G2R1 (1) | DQ630514 | Mexico (1) | ||
| IIaA20G1R1 (4) | KC995127 | Croatia (1) Italy (1) Sweden (1) unknown (1) | ||
| IIaA21G1R1 (5) | FJ917373 | Sweden (5) | ||
| IIaA22G1R1 (5) | JX183806 | Sweden (4) unknown (1) | ||
| IIaA23G1R1 (2) | KC995126 | Sweden (2) | ||
| IId (118) | IIdA16G1 (5) | FJ917372 | Sweden (1) | |
| JX183808 (IIdA16G1b) | Spain (1) Sweden (3) | |||
| IIdA17G1 (4) | KU852708 | Italy (1) Norway (1) Spain (1) Sweden (1) | ||
| IIdA18G1 (3) | AY738194 | France (1) | ||
| KU852709 | Africa (1) Sweden (1) | |||
| IIdA19G1 (16) | DQ280496 | China (1) Morocco (2) Oman (1) Portugal (5) Sweden (2) Sweden/Portugal (1) | ||
| KU852711 | Sweden (3) | |||
| KU852713 | Sweden (1) | |||
| IIdA20G1 (24) | AY738185 (IIdA20G1b) | Israel (1) | ||
| JQ028866 (IIdA20G1e) | Sweden (20) | |||
| KU852711 | Croatia (1) Sweden (1) | |||
| KU852713 | Croatia (1) | |||
| IIdA21G1 (3) | DQ280497 | Portugal (1) Sweden (2) | ||
| IIdA22G1 (37) | AY166806 | Spain (1) Sweden (15) | ||
| FJ917374 (IIdA22G1c) | Estonia (1) Greece (1) Sweden (15) Sweden/US (1) unknown (1) | |||
| KR349103 | France (1) Sweden (1) | |||
| IIdA23G1 (5) | FJ917376 | Ivory Coast (1) Sweden (4) | ||
| IIdA24G1 (14) | JQ028865 | Denmark (1) Germany (1) Sweden (11 3) | ||
| JX183810 (IIdA24G1c) | Sweden (1) | |||
| IIdA25G1 (6) | JX043492 | Sweden (6) | ||
| IIdA29G1 (1) | GU458803 | Sweden (1) | ||
| IIc (2) | IIcA5G3a (1) | AF164491 | Germany (1) | |
| IIcA5G3j (1) | HQ005749 | Sweden (1) | ||
| IIe (2) | IIeA10G1 (1) | KM539058 | Guinea (1) | |
| IIeA13G1 (1) | KU852716 | Sweden (1) | ||
| IIl (1) | IIlA16R2 (1) | AM937007 | Europe/Asia (1) | |
| IIn (2) | IInA10 (2) | KU852717 | Tanzania/Sweden (1) Tanzania (1) | |
| IIr (1) | IIrA5G1 (1) | KU852719 | Sweden (1) | |
| IIs (1) | IIsA10G1 (1) | KU852720 | Sweden (1) | |
| IIt (1) | IItA13R1 (1) | KU852718 | Tanzania (1) | |
| Mixed subtypes | IIa + IIa (1) | IIaA14G2R1 + IIaA15G2R1 (1) | KF128738, KF128738 | Italy (1) |
| Mixed subtypes | IIa + IId (2) | IIaA15G2R1 + IIdA19G1 (2) | AF164490, JF691561 | Portugal (2) |
| C. parvum + C. hominis (1) | Ia + IIa (1) | IaA18R3 + IIaA16R1 (1) | KM538987, AM937010 | Syria (1) |
| neg gp60 PCR (4) | Sweden (4) |
| Species (No. of Patients) | Subtype Family (No. of Patients) | Subtype (No. of Patients) | GenBank Acc. No. 1 | Origin of Infection (No. of Patients) |
|---|---|---|---|---|
| C. hominis (49) | Ia (12) | IaA17R3 (1) | KU852723 | India (1) |
| IaA18R3 (4) | JF927190 | Sweden (2) Thailand (2) | ||
| IaA18R4 (1) | FJ153246 | Thailand (1) | ||
| IaA20R3 (2) | KU727289 | Tanzania (2) | ||
| IaA23R3 (1) | JQ798143 | India (1) | ||
| IaA26R3 (1) | KU852724 | Somalia (1) | ||
| IaA28R4 (2) | KF682373 | US (2) | ||
| Ib (26) | IbA6G3 (1) | KU852722 | Egypt (1) | |
| IbA9G3 (9) | DQ665688 | Afghanistan (1) Ethiopia (1) Malawi (1) Mozambique (1) Somalia (1) Uzbekistan (1) Zambia (1) | ||
| KF974523 | Congo Republic (1) Uganda (1) | |||
| IbA10G2 (15) | AY262031 | Estonia (1) Greece (1) United Arab Emirates (2) Guatemala (1) Peru (1) Spain (7) Sweden (2) | ||
| IbA13G3 (1) | KM539004 | Burkina Faso (1) | ||
| Id (3) | IdA16 (2) | HQ149034 | China (1) Sri Lanka (1) | |
| IdA17 (1) | KU852721 | Tanzania (1) | ||
| Ie (1) | IeA11G3T3 (1) | GU214354 | South Africa (1) | |
| If (2) | IfA12G1R5 (2) | HQ149036 | Germany (1) Sweden (1) | |
| Ik (2) | IkA18G1 (2 2) | KU727290 | Sweden (2) | |
| Ii (2) | IiA17 (2 2) | KF679724 | Thailand (2) | |
| neg gp60 PCR (1) | Sweden (1) |
| Outbreak/Cluster | Month/Year | Number of Suspected Cases | Number of Confirmed Cases | Number of Cases Typed | Information | Species | Subtype | GenBank Acc. No. |
|---|---|---|---|---|---|---|---|---|
| Outbreak 1 1 | Feb. 2013 | 13 | 6 | 6 | Sweden: veterinary students | C. parvum | IIaA16G1R1 (4 cases) 1 IIdA24G1 (2 cases) 1 | EU647727 (IIaA16G1R1b) JQ028865 |
| Outbreak 2 | Jan. 2013 | 10 | 2 | 2 | Sweden: foodborne, private dinner, salad was the suspected source of infection | C. parvum | IIaA16G2R1 | DQ192505 |
| Outbreak 3 | May 2014 | 8 | 2 | 1 | Sweden: foodborne, private dinner, no identified source of infection | C. parvum | IIaA17R1 | JX183800 |
| Outbreak 4 | March 2014 | - | 23 | 13 | Sweden: foodborne, restaurant, parsley was the suspected source | C. parvum | IIdA22G1 | AY166806 |
| Cluster 1 | July 2014 | 5 | 3 | 3 | Sweden: a family, suspected contaminated water well | C. parvum | IIaA15G2R1 | AF164490 |
| Cluster 2 | Nov. 2014 | 2 | 2 | 2 | Portugal: a couple traveling together | C. parvum | IIaA15G2R1 + IIdA19G1 | AF164490 + JF691561 |
| Cluster 3 2 | Feb. 2013 | 3 | 2 | 2 | Thailand: a father and his son traveling together 2 | C. hominis | IiA17 2 | KF679724 |
| Species (No. of Patients) | Subtype Family (No. of Patients) | Subtype (No. of Patients) | GenBank Acc. No. 1 | Origin of Infection (No of Patients) |
|---|---|---|---|---|
| C. meleagridis (8) | IIIb (3) | IIIbA23G1R1 (2) | KJ210606 (IIIbA23G1R1a) 2 | Indonesia (1) |
| KU852727 (IIIbA23G1R1c) | Malaysia (1) | |||
| IIIbA24G1R1 (1) | KU852729 | China (1) | ||
| IIIe (4) | IIIeA17G2R1 (2) | KU852726 | China (2) | |
| IIIeA19G2R1 (1) | KJ210620 2 | Uzbekistan (1) | ||
| IIIeA21G2R1 (1) | KU852728 | Indonesia (1) | ||
| IIIg (1) | IIIgA22G3R1 (1) | KU852730 | Nepal (1) | |
| C. cuniculus (5) | Va (1) | VaA19 (1) | KU852733 | Sweden (1) |
| Vb (4) | VbA20R2 (1) | KU852735 (VbA20R2b) | Sweden (1) | |
| VbA25R3 (1) | KU852731 | Spain (1) | ||
| VbA29R4 (1) | KU852734 | Sweden (1) | ||
| VbA31R4 (1) | KU852732 | Greece (1) | ||
| C. erinacei (2) | XIIIa (2) | XIIIaA23R12 (1) | KU852736 | Sweden (1) |
| XIIIaA24R9 (1) | KU852737 | Greece (1) | ||
| C. ubiquitum (2) | XIIa (2) | XIIa-1 (2) | KU852740 | Sweden (2) |
| C. viatorum (1) | XVa (1) | XVaA3b (1) | KP115937 3 | Kenya (1) |
| Cryptosporidium chipmunk genotype I (5) | XIVa (5) | XIVaA20G2T1 (5) | KP099089 | Sweden (5) |
| Cryptosporidium horse genotype (1) | VIc (1) | VIcA16 (1) | KU852738 | Kenya (1) |
| C. felis (4) 4 | XIXa (3) | XIXa-39 (1) | MH240852 4 | Indonesia (1) |
| XIXa-43 (1) | MH240856 4 | Sweden (1) | ||
| XIXa-68 (1) | MH240883 4,5 | Sweden (1) | ||
| XIXb (1) | XIXb-1 (1) | MH240901 4 | Sweden (1) | |
| C. suis (1) | XXVa (1) | XXVaR37 (1) | MH187875 | Lithuania (1) |
| C. ditrichi (1) 6 | gp60 PCR neg | Sweden (1) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lebbad, M.; Winiecka-Krusnell, J.; Stensvold, C.R.; Beser, J. High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad. Pathogens 2021, 10, 523. https://doi.org/10.3390/pathogens10050523
Lebbad M, Winiecka-Krusnell J, Stensvold CR, Beser J. High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad. Pathogens. 2021; 10(5):523. https://doi.org/10.3390/pathogens10050523
Chicago/Turabian StyleLebbad, Marianne, Jadwiga Winiecka-Krusnell, Christen Rune Stensvold, and Jessica Beser. 2021. "High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad" Pathogens 10, no. 5: 523. https://doi.org/10.3390/pathogens10050523
APA StyleLebbad, M., Winiecka-Krusnell, J., Stensvold, C. R., & Beser, J. (2021). High Diversity of Cryptosporidium Species and Subtypes Identified in Cryptosporidiosis Acquired in Sweden and Abroad. Pathogens, 10(5), 523. https://doi.org/10.3390/pathogens10050523






