Integrative Analysis of miRNA and mRNA Expression Profiles in Mammary Glands of Holstein Cows Artificially Infected with Staphylococcus aureus
Abstract
1. Introduction
2. Results
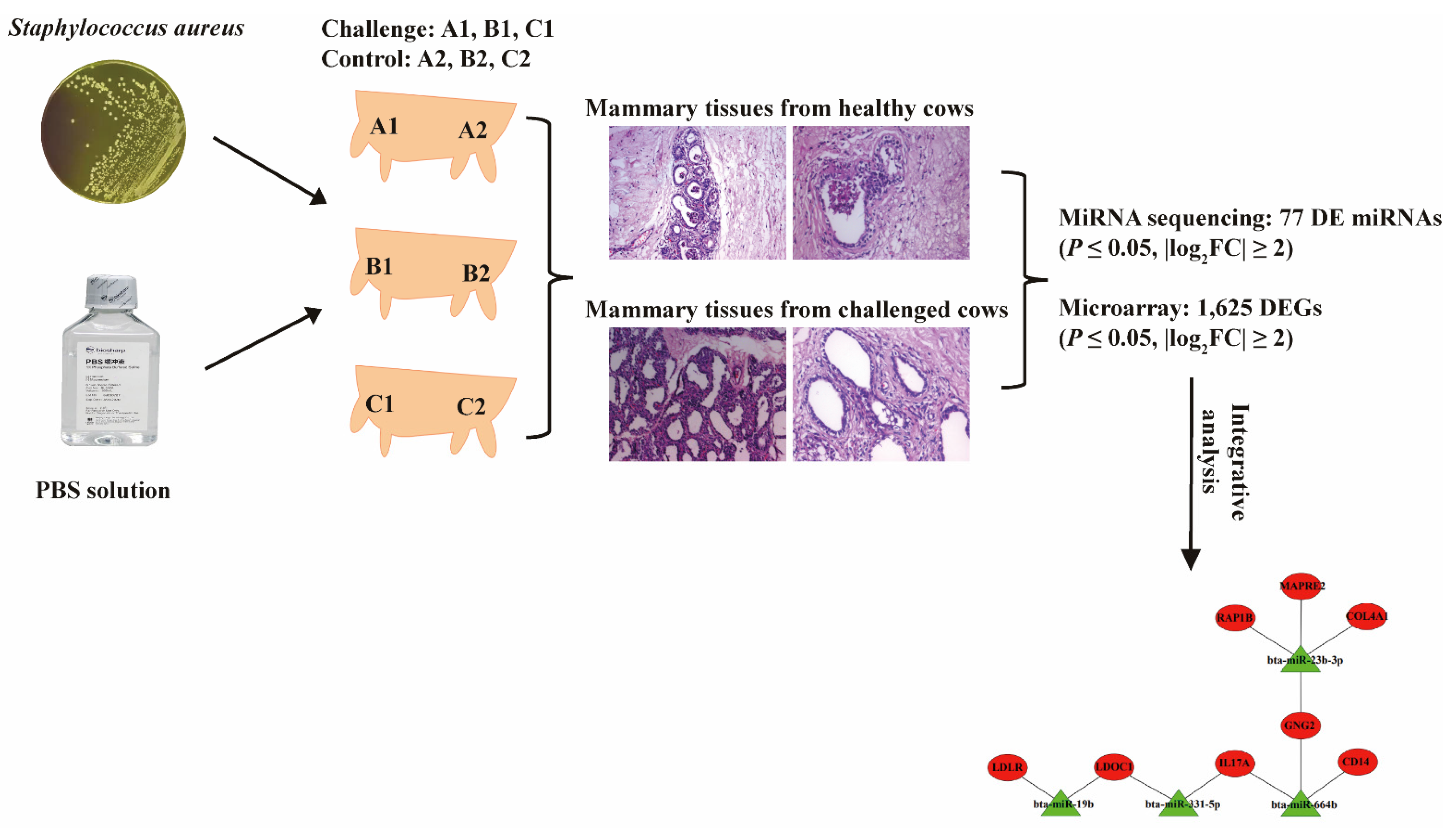
2.1. The Establishment of Bovine S. aureus-Induced Mastitis Model
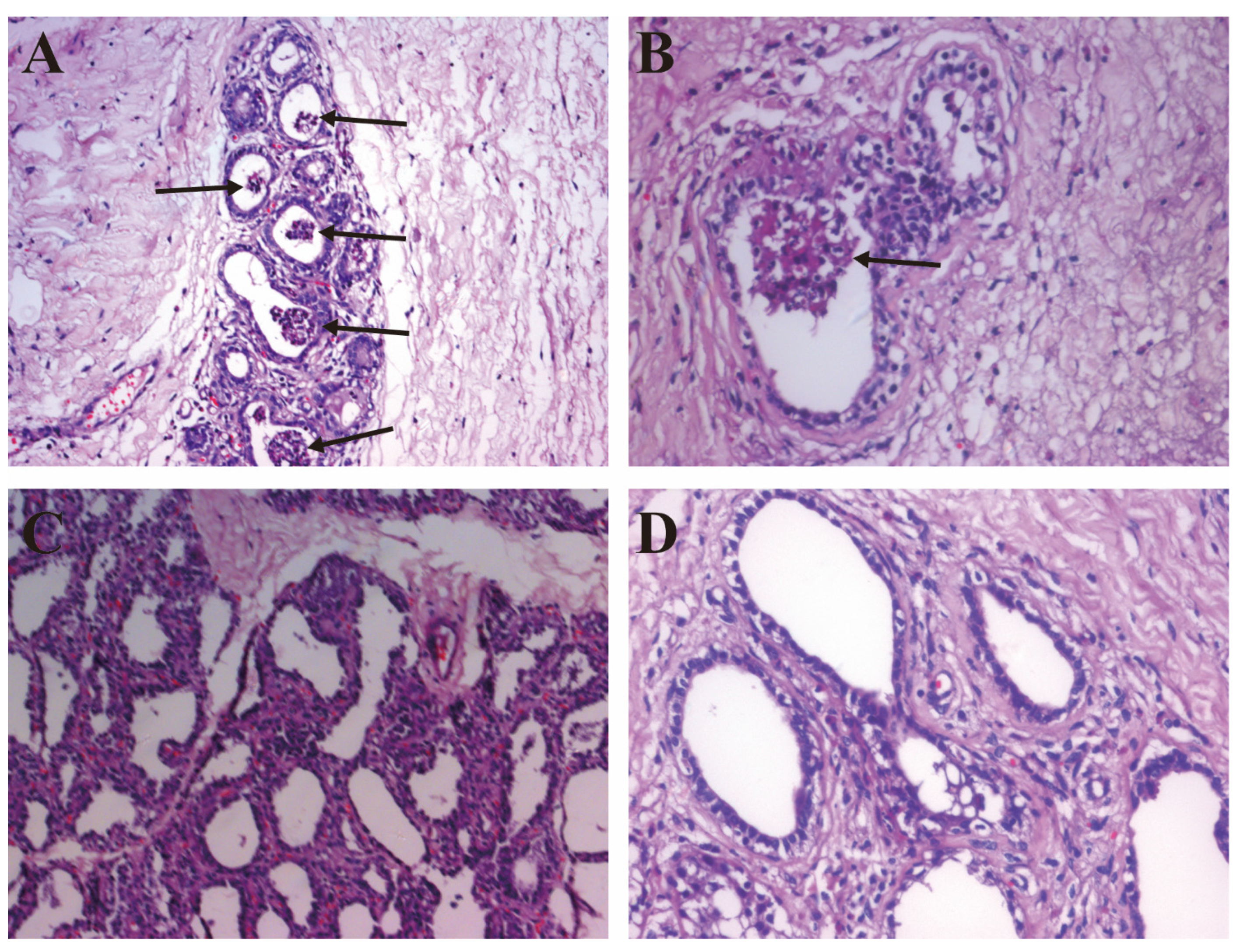
2.2. The Pathological Observation
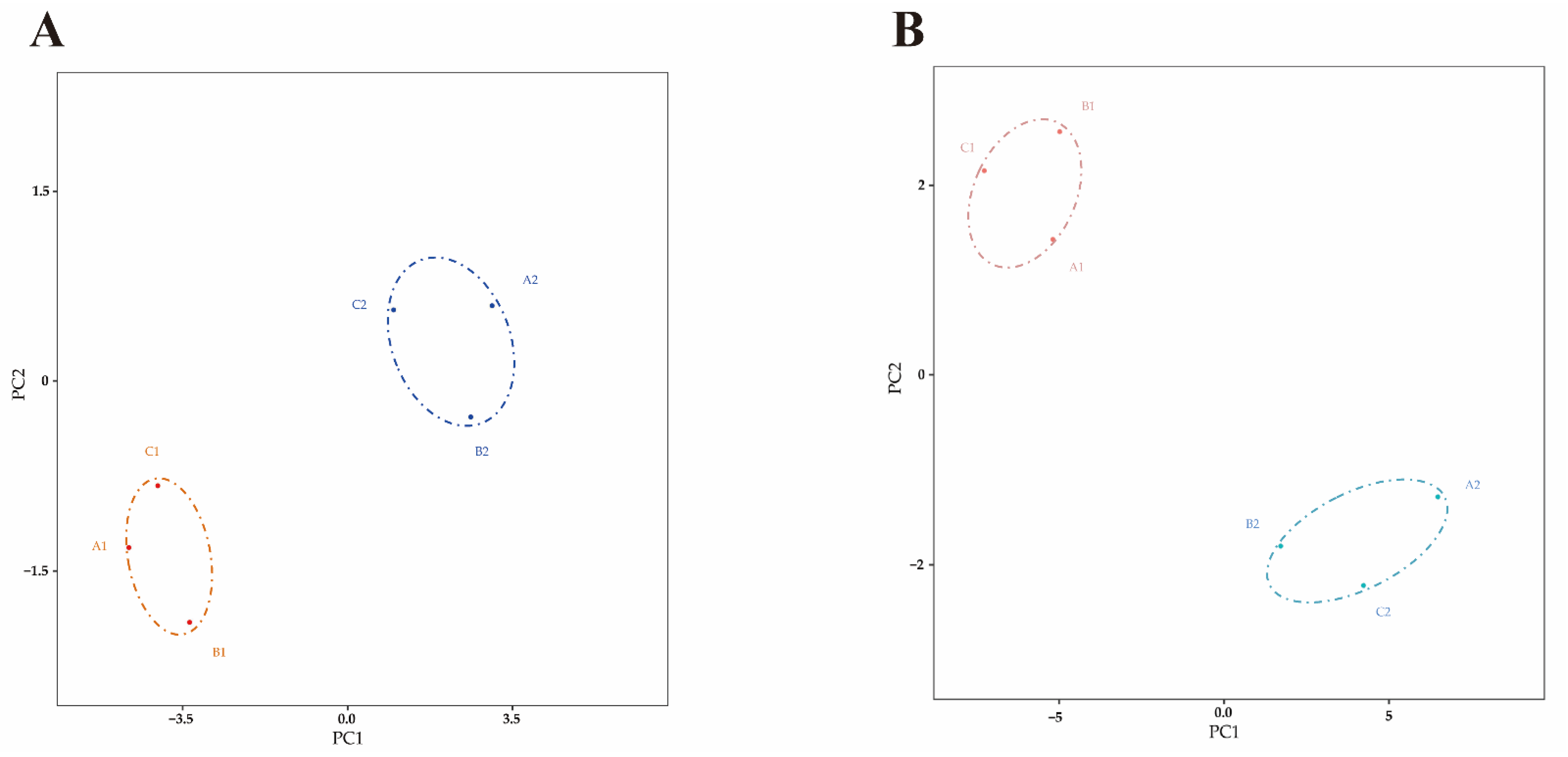
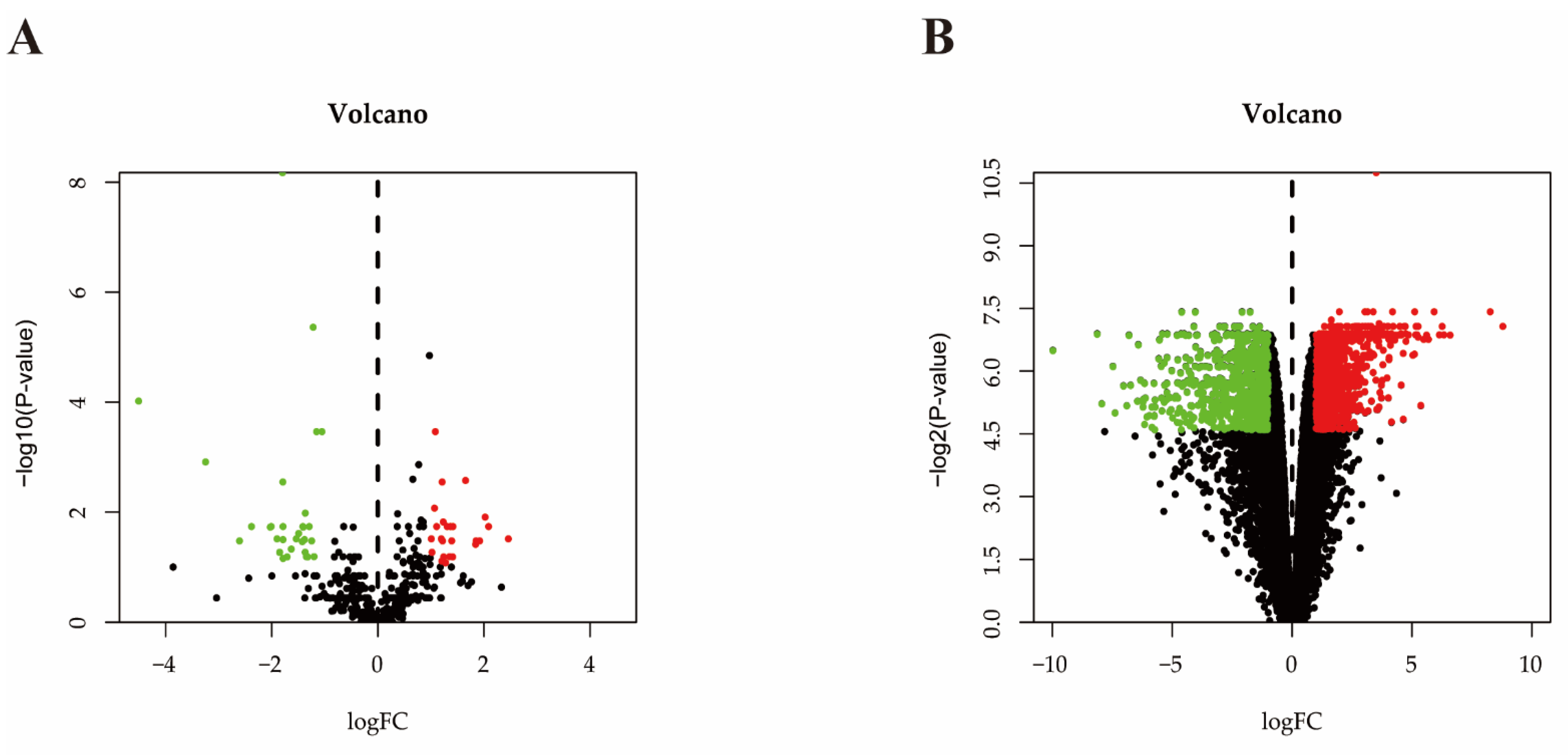
2.3. Differential Expressed miRNA Identification
2.4. Differential Expressed mRNA Identification
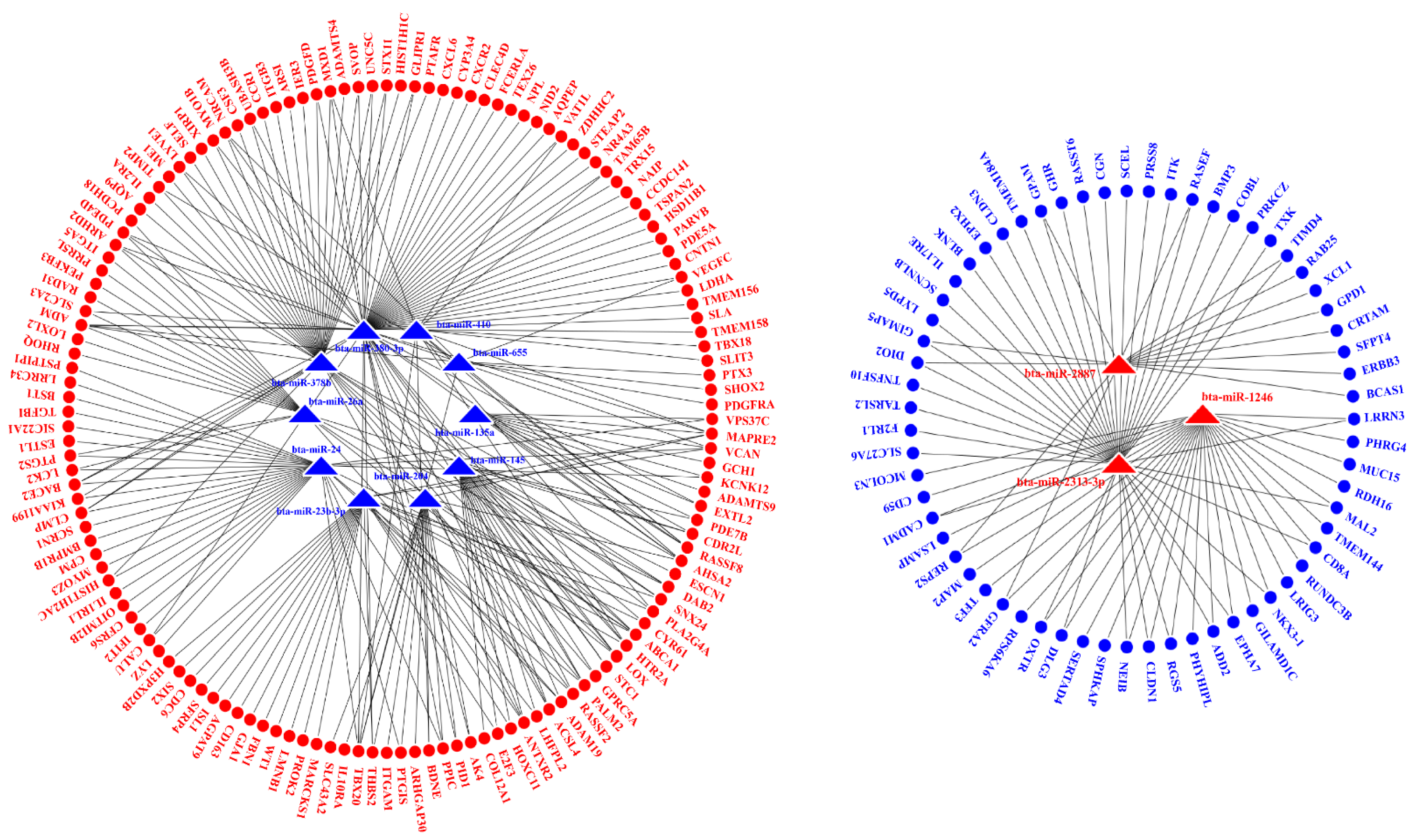
2.5. Interaction Analysis of the miRNAs and mRNAs
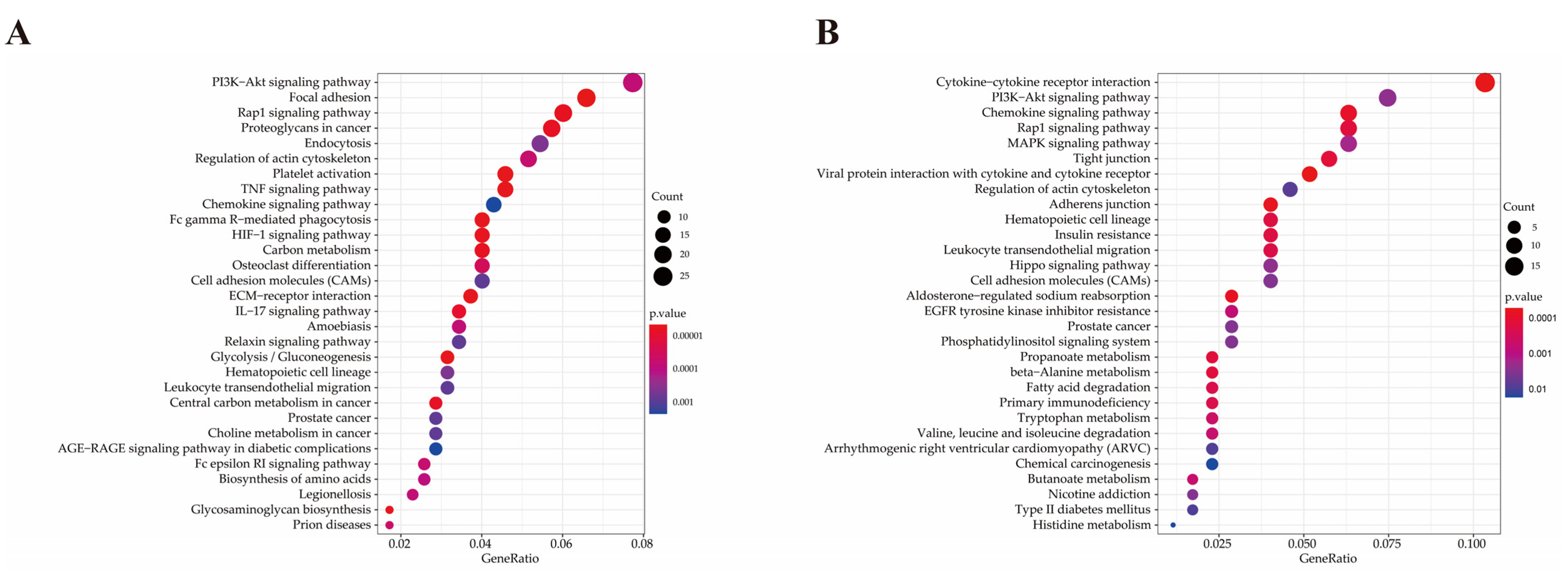
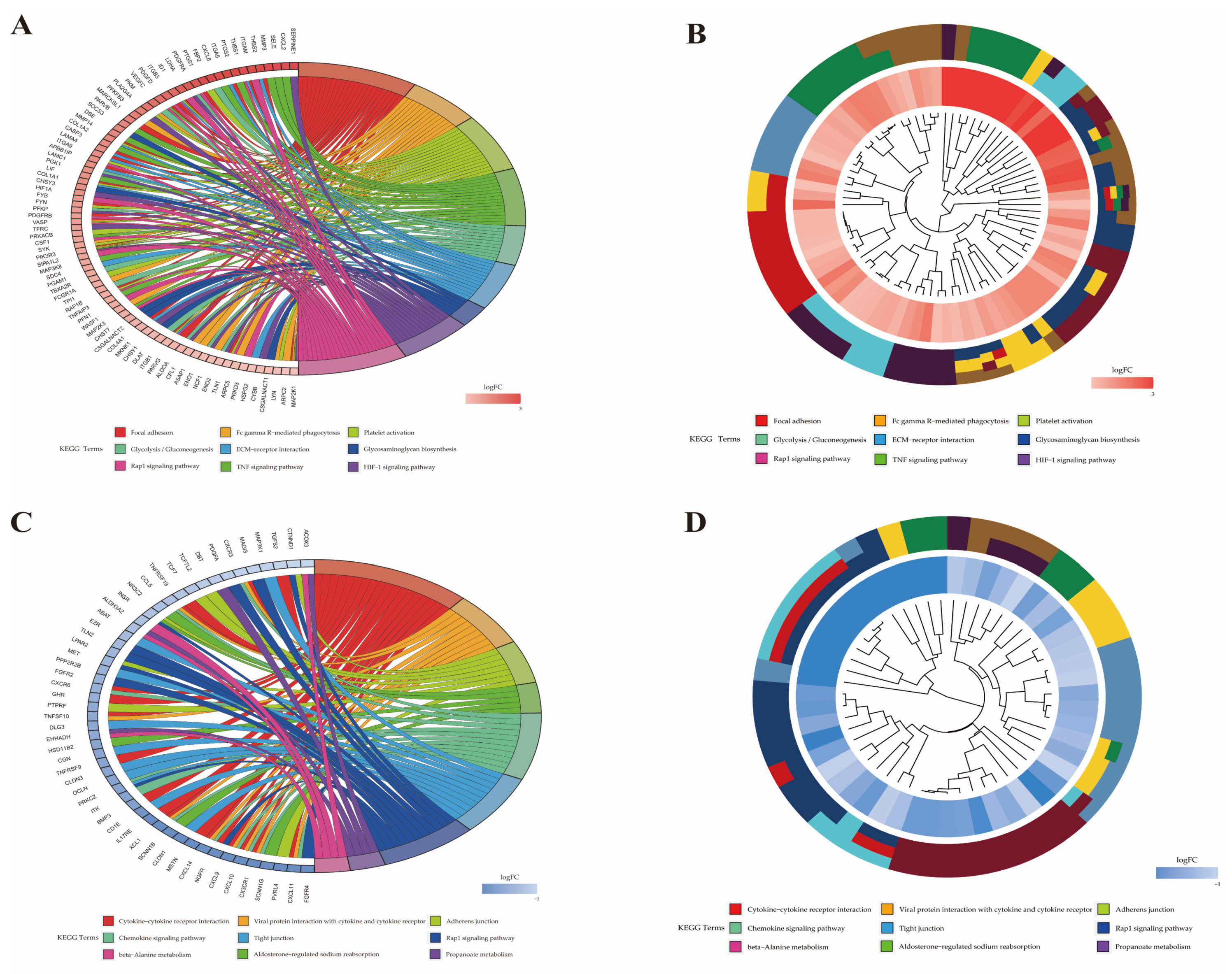
2.6. Functional Analysis of Differentially Expressed Genes
2.7. Validation of DE miRNAs and DEGs by qRT-PCR
3. Discussion
4. Materials and Methods
4.1. Ethics Statement and Animals Selection
4.2. Mastitis Model Construction
4.3. Sample Collection and Total RNA Extraction
4.4. Pathological Tests
4.5. Small RNA Sequencing and Data Analysis
4.6. mRNA Analysis and Data Process
4.7. miRNA–mRNA Interaction Network Construction
4.8. Functional Analysis of Differentially Expressed Genes
4.9. RT-qPCR Validation of DEGs and DE miRNAs
4.10. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, L.; Gao, J.; Barkema, H.W.; Ali, T.; Liu, G.; Deng, Y.; Naushad, S.; Kastelic, J.P.; Han, B. Virulence gene profiles: Alpha-hemolysin and clonal diversity in Staphylococcus aureus isolates from bovine clinical mastitis in China. BMC Vet. Res. 2018, 14, 63. [Google Scholar] [CrossRef]
- Sutra, L.; Poutrel, B. Virulence factors involved in the pathogenesis of bovine intramammary infections due to Staphylococcus aureus. J. Med. Microbiol. 1994, 40, 79–89. [Google Scholar] [CrossRef]
- Hwang, S.Y.; Park, Y.K.; Koo, H.C.; Park, Y.H. spa typing and enterotoxin gene profile of Staphylococcus aureus isolated from bovine raw milk in Korea. J. Vet. Sci. 2010, 11, 125–131. [Google Scholar] [CrossRef][Green Version]
- Nedic, S.; Vakanjac, S.; Samardzija, M.; Borozan, S. Paraoxonase 1 in bovine milk and blood as marker of subclinical mastitis caused by Staphylococcus aureus. Res. Vet. Sci. 2019, 125, 323–332. [Google Scholar] [CrossRef]
- Lutzow, Y.C.; Donaldson, L.; Gray, C.P.; Vuocolo, T.; Pearson, R.D.; Reverter, A.; Byrne, K.A.; Sheehy, P.A.; Windon, R.; Tellam, R.L. Identification of immune genes and proteins involved in the response of bovine mammary tissue to Staphylococcus aureus infection. BMC Vet. Res. 2008, 4, 18. [Google Scholar] [CrossRef]
- Oliver, J.P.; Gooch, C.A.; Lansing, S.; Schueler, J.; Hurst, J.J.; Sassoubre, L.; Crossette, E.M.; Aga, D.S. Invited review: Fate of antibiotic residues, antibiotic-resistant bacteria, and antibiotic resistance genes in US dairy manure management systems. J. Dairy Sci. 2020, 103, 1051–1071. [Google Scholar] [CrossRef]
- Saidi, R.; Kaidi, R.; Khelef, D.; Solmaz, H.; Ergun, Y.; Mimoune, N.; Cantekin, Z. Investigation of the presence of slime production, VanA gene and antiseptic resistance genes in Staphylococci isolated from bovine mastitis in Algeria. Vet. Stn. 2020, 52, 57–63. [Google Scholar] [CrossRef]
- Burović, J. Izolacija bakterijskih patogena kod klinički manifestnih mastitisa mliječnih goveda i njihova antimikrobna osjetljivost u zeničkoj regiji u 2017. godini. Vet. Stn. 2020, 51, 47–52. [Google Scholar] [CrossRef]
- Benić, M.; Maćešić, N.; Cvetnić, L.; Habrun, B.; Cvetnić, Ž.; Turk, R.; Đuričić, D.; Lojkić, M.; Dobranić, V.; Valpotić, H.; et al. Bovine mastitis: A persistent and evolving problem requiring novel approaches for its control—A review. Vet. Arh. 2018, 88, 535–557. [Google Scholar] [CrossRef]
- Huang, J.; Luo, G.; Zhang, Z.; Wang, X.; Ju, Z.; Qi, C.; Zhang, Y.; Wang, C.; Li, R.; Li, J.; et al. iTRAQ-proteomics and bioinformatics analyses of mammary tissue from cows with clinical mastitis due to natural infection with Staphylococci aureus. BMC Genom. 2014, 15, 839. [Google Scholar] [CrossRef]
- Bobbo, T.; Penasa, M.; Finocchiaro, R.; Visentin, G.; Cassandro, M. Alternative somatic cell count traits exploitable in genetic selection for mastitis resistance in Italian Holsteins. J. Dairy Sci. 2018, 101, 10001–10010. [Google Scholar] [CrossRef]
- Asselstine, V.; Miglior, F.; Suarez-Vega, A.; Fonseca, P.A.S.; Mallard, B.; Karrow, N.; Islas-Trejo, A.; Medrano, J.F.; Canovas, A. Genetic mechanisms regulating the host response during mastitis. J. Dairy Sci. 2019, 102, 9043–9059. [Google Scholar] [CrossRef]
- Strandberg, Y.; Gray, C.; Vuocolo, T.; Donaldson, L.; Broadway, M.; Tellam, R. Lipopolysaccharide and lipoteichoic acid induce different innate immune responses in bovine mammary epithelial cells. Cytokine 2005, 31, 72–86. [Google Scholar] [CrossRef]
- Gunther, J.; Koczan, D.; Yang, W.; Nurnberg, G.; Repsilber, D.; Schuberth, H.J.; Park, Z.; Maqbool, N.; Molenaar, A.; Seyfert, H.M. Assessment of the immune capacity of mammary epithelial cells: Comparison with mammary tissue after challenge with Escherichia coli. Vet. Res. 2009, 40, 31. [Google Scholar] [CrossRef]
- Griesbeck-Zilch, B.; Meyer, H.H.; Kuhn, C.H.; Schwerin, M.; Wellnitz, O. Staphylococcus aureus and Escherichia coli cause deviating expression profiles of cytokines and lactoferrin messenger ribonucleic acid in mammary epithelial cells. J. Dairy Sci. 2008, 91, 2215–2224. [Google Scholar] [CrossRef]
- Fu, Y.; Zhou, E.; Liu, Z.; Li, F.; Liang, D.; Liu, B.; Song, X.; Zhao, F.; Fen, X.; Li, D.; et al. Staphylococcus aureus and Escherichia coli elicit different innate immune responses from bovine mammary epithelial cells. Vet. Immunol. Immunopathol. 2013, 155, 245–252. [Google Scholar] [CrossRef]
- Li, R.; Zhang, C.L.; Liao, X.X.; Chen, D.; Wang, W.Q.; Zhu, Y.H.; Geng, X.H.; Ji, D.J.; Mao, Y.J.; Gong, Y.C.; et al. Transcriptome microRNA profiling of bovine mammary glands infected with Staphylococcus aureus. Int. J. Mol. Sci. 2015, 16, 4997–5013. [Google Scholar] [CrossRef] [PubMed]
- Luoreng, Z.M.; Wang, X.P.; Mei, C.G.; Zan, L.S. Expression profiling of peripheral blood miRNA using RNAseq technology in dairy cows with Escherichia coli-induced mastitis. Sci. Rep. 2018, 8, 12693. [Google Scholar] [CrossRef]
- Kosciuczuk, E.M.; Lisowski, P.; Jarczak, J.; Majewska, A.; Rzewuska, M.; Zwierzchowski, L.; Bagnicka, E. Transcriptome profiling of Staphylococci-infected cow mammary gland parenchyma. BMC Vet. Res. 2017, 13, 161. [Google Scholar] [CrossRef]
- Luoreng, Z.M.; Wang, X.P.; Mei, C.G.; Zan, L.S. Comparison of microRNA Profiles between Bovine Mammary Glands Infected with Staphylococcus aureus and Escherichia coli. Int. J. Biol. Sci. 2018, 14, 87–99. [Google Scholar] [CrossRef]
- Pisanu, S.; Cacciotto, C.; Pagnozzi, D.; Puggioni, G.M.G.; Uzzau, S.; Ciaramella, P.; Guccione, J.; Penati, M.; Pollera, C.; Moroni, P.; et al. Proteomic changes in the milk of water buffaloes (Bubalus bubalis) with subclinical mastitis due to intramammary infection by Staphylococcus aureus and by non-aureus staphylococci. Sci. Rep. 2019, 9, 15850. [Google Scholar] [CrossRef]
- Han, S.; Li, X.; Liu, J.; Zou, Z.; Luo, L.; Wu, R.; Zhao, Z.; Wang, C.; Shen, B. Bta-miR-223 Targeting CBLB Contributes to Resistance to Staphylococcus aureus Mastitis Through the PI3K/AKT/NF-kappaB Pathway. Front. Vet. Sci 2020, 7, 529. [Google Scholar] [CrossRef] [PubMed]
- Ju, Z.; Jiang, Q.; Liu, G.; Wang, X.; Luo, G.; Zhang, Y.; Zhang, J.; Zhong, J.; Huang, J. Solexa sequencing and custom microRNA chip reveal repertoire of microRNAs in mammary gland of bovine suffering from natural infectious mastitis. Anim. Genet. 2018, 49, 3–18. [Google Scholar] [CrossRef]
- Sun, J.; Aswath, K.; Schroeder, S.G.; Lippolis, J.D.; Reinhardt, T.A.; Sonstegard, T.S. MicroRNA expression profiles of bovine milk exosomes in response to Staphylococcus aureus infection. BMC Genom. 2015, 16, 806. [Google Scholar] [CrossRef]
- Jin, W.; Ibeagha-Awemu, E.M.; Liang, G.; Beaudoin, F.; Zhao, X.; Guan, L.L. Transcriptome microRNA profiling of bovine mammary epithelial cells challenged with Escherichia coli or Staphylococcus aureus bacteria reveals pathogen directed microRNA expression profiles. BMC Genom. 2014, 15, 181. [Google Scholar] [CrossRef] [PubMed]
- Islam, M.A.; Takagi, M.; Fukuyama, K.; Komatsu, R.; Albarracin, L.; Nochi, T.; Suda, Y.; Ikeda-Ohtsubo, W.; Rutten, V.; Eden, W.V.; et al. Transcriptome Analysis of The Inflammatory Responses of Bovine Mammary Epithelial Cells: Exploring Immunomodulatory Target Genes for Bovine Mastitis. Pathogens 2020, 9, 200. [Google Scholar] [CrossRef]
- Gunther, J.; Petzl, W.; Bauer, I.; Ponsuksili, S.; Zerbe, H.; Schuberth, H.J.; Brunner, R.M.; Seyfert, H.M. Differentiating Staphylococcus aureus from Escherichia coli mastitis: S. aureus triggers unbalanced immune-dampening and host cell invasion immediately after udder infection. Sci. Rep. 2017, 7, 4811. [Google Scholar] [CrossRef]
- Gilbert, F.B.; Cunha, P.; Jensen, K.; Glass, E.J.; Foucras, G.; Robert-Granie, C.; Rupp, R.; Rainard, P. Differential response of bovine mammary epithelial cells to Staphylococcus aureus or Escherichia coli agonists of the innate immune system. Vet. Res. 2013, 44, 40. [Google Scholar] [CrossRef] [PubMed]
- Gunther, J.; Esch, K.; Poschadel, N.; Petzl, W.; Zerbe, H.; Mitterhuemer, S.; Blum, H.; Seyfert, H.M. Comparative kinetics of Escherichia coli- and Staphylococcus aureus-specific activation of key immune pathways in mammary epithelial cells demonstrates that S. aureus elicits a delayed response dominated by interleukin-6 (IL-6) but not by IL-1A or tumor necrosis factor alpha. Infect. Immun. 2011, 79, 695–707. [Google Scholar] [CrossRef]
- Wang, D.; Liu, L.; Augustino, S.M.A.; Duan, T.; Hall, T.J.; MacHugh, D.E.; Dou, J.; Zhang, Y.; Wang, Y.; Yu, Y. Identification of novel molecular markers of mastitis caused by Staphylococcus aureus using gene expression profiling in two consecutive generations of Chinese Holstein dairy cattle. J. Anim. Sci. Biotechnol. 2020, 11, 98. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.D.; Ganaie, F.; Bankar, K.; Velu, D.; Mani, B.; Vasudevan, M.; Shome, R.; Rahman, H.; Kumar Ghosh, S.; Shome, B.R. Genome-wide analysis of mammary gland shows modulation of transcriptome landscape with alternative splice variants in Staphylococcus aureus mastitis in mice. Gene 2020, 735, 144278. [Google Scholar] [CrossRef]
- Yang, Y.; Peng, Y.; Jiang, J.; Gong, Z.; Zhu, H.; Wang, K.; Zhou, Q.; Tian, Y.; Qin, A.; Yang, Z.; et al. Isolation and characterization of multidrug-resistant Klebsiella pneumoniae from raw cow milk in Jiangsu and Shandong provinces, China. Transbound. Emerg. Dis. 2020. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Xia, H.; Shen, H.; Xu, X.; Arbab, A.A.I.; Li, M.; Zhang, H.; Mao, Y.; Yang, Z. Pathological Features of Staphylococcus aureus Induced Mastitis in Dairy Cows and Isobaric-Tags-for-Relative-and-Absolute-Quantitation Proteomic Analyses. J. Agric. Food Chem. 2018, 66, 3880–3890. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Jiang, H.; Fan, Y.; Chen, Z.; Li, M.; Mao, Y.; Karrow, N.A.; Loor, J.J.; Moore, S.; Yang, Z. Transcriptomics and iTRAQ-Proteomics Analyses of Bovine Mammary Tissue with Streptococcus agalactiae-Induced Mastitis. J. Agric. Food Chem. 2018, 66, 11188–11196. [Google Scholar] [CrossRef]
- Podolska, A.; Anthon, C.; Bak, M.; Tommerup, N.; Skovgaard, K.; Heegaard, P.M.; Gorodkin, J.; Cirera, S.; Fredholm, M. Profiling microRNAs in lung tissue from pigs infected with Actinobacillus pleuropneumoniae. BMC Genom. 2012, 13, 459. [Google Scholar] [CrossRef][Green Version]
- Lahouassa, H.; Moussay, E.; Rainard, P.; Riollet, C. Differential cytokine and chemokine responses of bovine mammary epithelial cells to Staphylococcus aureus and Escherichia coli. Cytokine 2007, 38, 12–21. [Google Scholar] [CrossRef] [PubMed]
- Bannerman, D.D.; Paape, M.J.; Goff, J.P.; Kimura, K.; Lippolis, J.D.; Hope, J.C. Innate immune response to intramammary infection with Serratia marcescens and Streptococcus uberis. Vet. Res. 2004, 35, 681–700. [Google Scholar] [CrossRef]
- Yajima, I.; Kumasaka, M.Y.; Tamura, H.; Ohgami, N.; Kato, M. Functional analysis of GNG2 in human malignant melanoma cells. J. Dermatol. Sci. 2012, 68, 172–178. [Google Scholar] [CrossRef]
- Yajima, I.; Kumasaka, M.Y.; Yamanoshita, O.; Zou, C.; Li, X.; Ohgami, N.; Kato, M. GNG2 inhibits invasion of human malignant melanoma cells with decreased FAK activity. Am. J. Cancer Res. 2014, 4, 182–188. [Google Scholar] [PubMed]
- Yajima, I.; Kumasaka, M.Y.; Naito, Y.; Yoshikawa, T.; Takahashi, H.; Funasaka, Y.; Suzuki, T.; Kato, M. Reduced GNG2 expression levels in mouse malignant melanomas and human melanoma cell lines. Am. J. Cancer Res. 2012, 2, 322–329. [Google Scholar]
- Campos-Viguri, G.E.; Peralta-Zaragoza, O.; Jimenez-Wences, H.; Longinos-Gonzalez, A.E.; Castanon-Sanchez, C.A.; Ramirez-Carrillo, M.; Camarillo, C.L.; Castaneda-Saucedo, E.; Jimenez-Lopez, M.A.; Martinez-Carrillo, D.N.; et al. MiR-23b-3p reduces the proliferation, migration and invasion of cervical cancer cell lines via the reduction of c-Met expression. Sci. Rep. 2020, 10, 3256. [Google Scholar] [CrossRef]
- Zaman, M.S.; Thamminana, S.; Shahryari, V.; Chiyomaru, T.; Deng, G.; Saini, S.; Majid, S.; Fukuhara, S.; Chang, I.; Arora, S.; et al. Inhibition of PTEN gene expression by oncogenic miR-23b-3p in renal cancer. PLoS ONE 2012, 7, e50203. [Google Scholar] [CrossRef]
- Chen, D.; Wu, X.; Xia, M.; Wu, F.; Ding, J.; Jiao, Y.; Zhan, Q.; An, F. Upregulated exosomic miR23b3p plays regulatory roles in the progression of pancreatic cancer. Oncol. Rep. 2017, 38, 2182–2188. [Google Scholar] [CrossRef]
- Grieco, F.A.; Sebastiani, G.; Juan-Mateu, J.; Villate, O.; Marroqui, L.; Ladriere, L.; Tugay, K.; Regazzi, R.; Bugliani, M.; Marchetti, P.; et al. MicroRNAs miR-23a-3p, miR-23b-3p, and miR-149-5p Regulate the Expression of Proapoptotic BH3-Only Proteins DP5 and PUMA in Human Pancreatic beta-Cells. Diabetes 2017, 66, 100–112. [Google Scholar] [CrossRef]
- Nakada, T.A.; Russell, J.A.; Boyd, J.H.; Walley, K.R. IL17A genetic variation is associated with altered susceptibility to Gram-positive infection and mortality of severe sepsis. Crit. Care 2011, 15, R254. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Villar, M.; Hafler, D.A. Immunology. An innate role for IL-17. Science 2011, 332, 47–48. [Google Scholar] [CrossRef] [PubMed]
- Iwakura, Y.; Ishigame, H.; Saijo, S.; Nakae, S. Functional specialization of interleukin-17 family members. Immunity 2011, 34, 149–162. [Google Scholar] [CrossRef] [PubMed]
- Zhang, G.; Zou, X.; Liu, Q.; Xie, T.; Huang, R.; Kang, H.; Lai, C.; Zhu, J. MiR-193a-3p functions as a tumour suppressor in human aldosterone-producing adrenocortical adenoma by down-regulating CYP11B2. Int. J. Exp. Pathol. 2018, 99, 77–86. [Google Scholar] [CrossRef]
- Hayden, M.S.; Ghosh, S. NF-kappaB in immunobiology. Cell Res. 2011, 21, 223–244. [Google Scholar] [CrossRef] [PubMed]
- Inoue, M.; Takahashi, K.; Niide, O.; Shibata, M.; Fukuzawa, M.; Ra, C. LDOC1, a novel MZF-1-interacting protein, induces apoptosis. FEBS Lett. 2005, 579, 604–608. [Google Scholar] [CrossRef]
- Mizutani, K.; Koike, D.; Suetsugu, S.; Takenawa, T. WAVE3 functions as a negative regulator of LDOC1. J. Biochem. 2005, 138, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Salemi, M.; Barone, N.; La Vignera, S.; Condorelli, R.A.; Recupero, D.; Galia, A.; Fraggetta, F.; Aiello, A.M.; Pepe, P.; Castiglione, R.; et al. Leucine zipper, down regulated in cancer-1 gene expression in prostate cancer. Oncol. Lett. 2016, 12, 2796–2800. [Google Scholar] [CrossRef][Green Version]
- Liu, X.; Zhang, H.; Zhang, B.; Zhang, X. Expression and Role of MicroRNA-663b in Childhood Acute Lymphocytic Leukemia and its Mechanism. Open Med. 2019, 14, 863–871. [Google Scholar] [CrossRef]
- He, J.; Kang, Y.; Lian, C.; Wu, J.; Zhou, H.; Ye, X. Effect of miR-19b on the protective effect of Exendin-4 on islet cells in non-obese diabetic mice. Exp. Ther. Med. 2019, 18, 503–508. [Google Scholar] [CrossRef]
- Kiugel, M.; Hellberg, S.; Kakela, M.; Liljenback, H.; Saanijoki, T.; Li, X.G.; Tuomela, J.; Knuuti, J.; Saraste, A.; Roivainen, A. Evaluation of [(68)Ga]Ga-DOTA-TCTP-1 for the Detection of Metalloproteinase 2/9 Expression in Mouse Atherosclerotic Plaques. Molecules 2018, 23, 3168. [Google Scholar] [CrossRef]
- Ramadori, G.; Ljubicic, S.; Ricci, S.; Mikropoulou, D.; Brenachot, X.; Veyrat-Durebex, C.; Aras, E.; Ioris, R.M.; Altirriba, J.; Malle, E.; et al. S100A9 extends lifespan in insulin deficiency. Nat. Commun. 2019, 10, 3545. [Google Scholar] [CrossRef]
- Li, H.; Huang, X.; Chang, X.; Yao, J.; He, Q.; Shen, Z.; Ji, Y.; Wang, K. S100-A9 protein in exosomes derived from follicular fluid promotes inflammation via activation of NF-kappaB pathway in polycystic ovary syndrome. J. Cell Mol. Med. 2020, 24, 114–125. [Google Scholar] [CrossRef] [PubMed]
- Qiu, H.; Kelly, P.J.; Zhang, J.; Luo, Q.; Yang, Y.; Mao, Y.; Yang, Z.; Li, J.; Wu, H.; Wang, C. Molecular Detection of Anaplasma spp. and Ehrlichia spp. in Ruminants from Twelve Provinces of China. Can. J. Infect. Dis. Med. Microbiol. 2016, 2016, 9183861. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Kelly, P.; Zhang, J.; Xu, C.; Wang, C. Development of a pan-Babesia FRET-qPCR and a survey of livestock from five Caribbean islands. BMC Vet. Res. 2015, 11, 246. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Mao, Y.; Kelly, P.; Yang, Z.; Luan, L.; Zhang, J.; Li, J.; El-Mahallawy, H.S.; Wang, C. A pan-Theileria FRET-qPCR survey for Theileria spp. in ruminants from nine provinces of China. Parasites Vectors 2014, 7, 413. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Yang, Y.; Fan, W.; Mao, Y.; Yang, Z.; Lu, G.; Zhang, R.; Zhang, H.; Szeto, C.; Wang, C. Bovine leukemia virus infection in cattle of China: Association with reduced milk production and increased somatic cell score. J. Dairy Sci. 2016, 99, 3688–3697. [Google Scholar] [CrossRef] [PubMed]
- Fang, L.; Hou, Y.; An, J.; Li, B.; Song, M.; Wang, X.; Sorensen, P.; Dong, Y.; Liu, C.; Wang, Y.; et al. Genome-Wide Transcriptional and Post-transcriptional Regulation of Innate Immune and Defense Responses of Bovine Mammary Gland to Staphylococcus aureus. Front. Cell Infect. Microbiol. 2016, 6, 193. [Google Scholar] [CrossRef] [PubMed]
- Pu, J.; Li, R.; Zhang, C.; Chen, D.; Liao, X.; Zhu, Y.; Geng, X.; Ji, D.; Mao, Y.; Gong, Y.; et al. Expression profiles of miRNAs from bovine mammary glands in response to Streptococcus agalactiae-induced mastitis. J. Dairy Res. 2017, 84, 300–308. [Google Scholar] [CrossRef]
- Chen, Z.; Chu, S.; Wang, X.; Fan, Y.; Zhan, T.; Arbab, A.A.I.; Li, M.; Zhang, H.; Mao, Y.; Loor, J.J.; et al. MicroRNA-106b Regulates Milk Fat Metabolism via ATP Binding Cassette Subfamily A Member 1 (ABCA1) in Bovine Mammary Epithelial Cells. J. Agric. Food Chem. 2019, 67, 3981–3990. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Fan, Y.; Yang, Z.; Loor, J.J.; Yang, Y. Mammary Transcriptome Profile during Peak and Late Lactation Reveals Differentially Expression Genes Related to Inflammation and Immunity in Chinese Holstein. Animals 2020, 10, 510. [Google Scholar] [CrossRef]

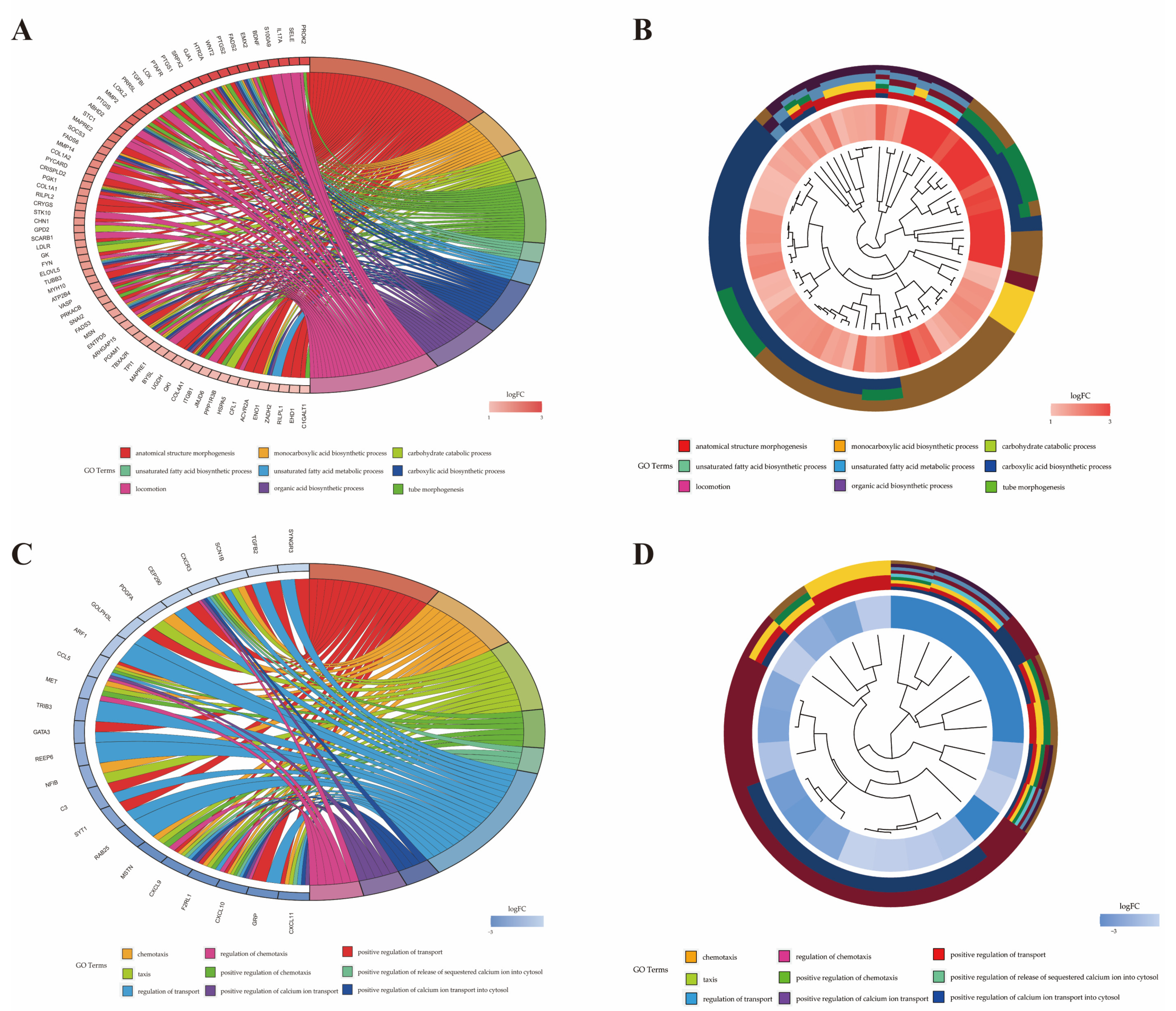
| Term ID | Term | P-Value | Gene Name | Number |
|---|---|---|---|---|
| GO:0071310 | cellular response to organic substance | 0.009 | CXCR1a, GFPT2, CSF3, IL17A, PTGS2, WNT2, CXCL5, IL2RA, OAS2, PTAFR, PTGIS, ABHD2, RIPOR2, SOCS3, COL1A2, GNG2, COL1A1, SCARB1, LDLR, FYN, ATP2B4, SNAI2, MSN, IRAK2, RAP1B, WASF1, CD14, COL4A1, DERL1, HSPA5, ACVR2A, LDOC1, EHD1, UFM1 | 34 |
| GO:0051345 | positive regulation of hydrolase activity | 0.010 | SELE, S100A9, HTR2A, MAPRE2, AHSA2, PYCARD, ABR, CHN1, DNAJB4, ARHGAP15, SEC23A, ATP1B3, AGFG1, ASAP1 | 14 |
| GO:1901701 | cellular response to oxygen-containing compound | 0.011 | CXCL5, PTAFR, COL1A2, GNG2, COL1A1, SCARB1, LDLR, FYN, ATP2B4, TXN, SNAI2, MSN, IRAK2, RAP1B, CD14, COL4A1, LDOC1, NCF1, SOD2 | 19 |
| GO:0071216 | cellular response to biotic stimulus | 0.015 | CXCL5, PTAFR, SCARB1, IRAK2, CD14, HSPA5, LDOC1 | 7 |
| GO:0071222 | cellular response to lipopolysaccharide | 0.015 | CXCL5, PTAFR, SCARB1, IRAK2, CD14, LDOC1 | 6 |
| GO:0072676 | lymphocyte migration | 0.016 | RIPOR2, PYCARD, STK10, MSN | 4 |
| GO:0032496 | response to lipopolysaccharide | 0.020 | CXCL5, PTAFR, SCARB1, IRAK2, TBXA2R, CD14, LDOC1 | 7 |
| GO:0071219 | cellular response to molecule of bacterial origin | 0.021 | CXCL5, PTAFR, SCARB1, IRAK2, CD14, LDOC1 | 6 |
| GO:0030334 | regulation of cell migration | 0.023 | SRPX2, PRR5L, ABHD2, RIPOR2, STC1, MAPRE2, MMP14, PYCARD, COL1A1, STK10, SNAI2, MSN, TBXA2R, ITGB1, HSPA5 | 15 |
| GO:0071229 | cellular response to acid chemical | 0.028 | COL1A2, GNG2, COL1A1, LDLR, COL4A1 | 5 |
| GO:0032729 | positive regulation of interferon gamma production | 0.028 | PYCARD, FAM49B, CD14 | 3 |
| GO:0050900 | leukocyte migration | 0.030 | SELE, IL17A, S100A9, CXCL5, RIPOR2, PYCARD, STK10, MSN | 8 |
| GO:0002237 | response to molecule of bacterial origin | 0.031 | CXCL5, PTAFR, SCARB1, IRAK2, TBXA2R, CD14, LDOC1 | 7 |
| GO:0030593 | neutrophil chemotaxis | 0.034 | S100A9, CXCL5, RIPOR2 | 3 |
| GO:0072678 | T cell migration | 0.034 | RIPOR2, PYCARD, MSN | 3 |
| GO:0006954 | inflammatory response | 0.038 | IL17A, S100A9, THBS1, PTGS2, ALOX5AP, CD163, PTGS1, PTAFR, PTGIS, SOCS3, PYCARD, LDLR, IRAK2, CYBB | 14 |
| GO:0030203 | glycosaminoglycan metabolic process | 0.040 | LYVE1, DSE, SLC35D1, UGDH | 4 |
| GO:0050954 | sensory perception of mechanical stimulus | 0.040 | RIPOR2, COL1A1, FYN, SNAI2 | 4 |
| GO:0071230 | cellular response to amino acid stimulus | 0.041 | COL1A2, COL1A1, COL4A1 | 3 |
| GO:0006935 | chemotaxis | <0.001 | CXCL11b, CXCL10, F2RL1, CXCL9, MSTN, NFIB, MET, CCL5, PDGFA, CXCR3, SCN1B | 11 |
| GO:0050921 | positive regulation of chemotaxis | <0.001 | CXCL10, F2RL1, MSTN, MET, CCL5, CXCR3 | 6 |
| GO:0050920 | regulation of chemotaxis | <0.001 | CXCL10, F2RL1, MSTN, MET, CCL5, CXCR3 | 6 |
| GO:0032103 | positive regulation of response to external stimulus | 0.001 | CXCL10, F2RL1, MSTN, C3, MET, CCL5, CXCR3 | 7 |
| GO:0050900 | leukocyte migration | 0.001 | CXCL11, CXCL10, F2RL1, MSTN, GATA3, CCL5, CXCR3 | 7 |
| GO:0060326 | cell chemotaxis | 0.004 | CXCL11, CXCL10, MSTN, MET, CCL5 | 5 |
| GO:0002690 | positive regulation of leukocyte chemotaxis | 0.005 | CXCL10, MSTN, CCL5 | 3 |
| GO:1990868 | response to chemokine | 0.005 | CX3CR1, CCL5, CXCR3 | 3 |
| GO:1990869 | cellular response to chemokine | 0.005 | CX3CR1, CCL5, CXCR3 | 3 |
| GO:0032101 | regulation of response to external stimulus | 0.006 | CXCL10, F2RL1, MSTN, S100B, C3, GATA3, MET, CCL5, PDGFA, CXCR3 | 10 |
| GO:0002688 | regulation of leukocyte chemotaxis | 0.010 | CXCL10, MSTN, CCL5 | 3 |
| GO:0002685 | regulation of leukocyte migration | 0.012 | CXCL10, MSTN, CCL5, CXCR3 | 4 |
| GO:0030595 | leukocyte chemotaxis | 0.013 | CXCL11, CXCL10, MSTN, CCL5 | 4 |
| GO:0002687 | positive regulation of leukocyte migration | 0.016 | CXCL10, MSTN, CCL5 | 3 |
| GO:0007606 | sensory perception of chemical stimulus | 0.027 | SCNN1G, SCNN1B | 2 |
| GO:0036230 | granulocyte activation | 0.027 | F2RL1, CCL5 | 2 |
| GO:0071622 | regulation of granulocyte chemotaxis | 0.027 | MSTN, CCL5 | 2 |
| GO:1905517 | macrophage migration | 0.027 | MSTN, CCL5 | 2 |
| GO:0002673 | regulation of acute inflammatory response | 0.032 | S100B, C3 | 2 |
| GO:0050918 | positive chemotaxis | 0.034 | CXCL10, MET, CCL5 | 3 |
| GO:0009605 | response to external stimulus | 0.039 | CXCL11, CXCL10, F2RL1, CXCL9, MSTN, S100B, C3, NFIB, REEP6, GATA3, AQP3, MET, IKZF3, CCL5, PDGFA, CXCR3, SCN1B | 17 |
| GO:0072678 | T cell migration | 0.043 | CXCL11, CXCL10 | 2 |
| GO:2000401 | regulation of lymphocyte migration | 0.048 | CXCL10, CCL5 | 2 |
| GO:1904062 | regulation of cation transmembrane transport | 0.048 | CXCL11, CXCL10, CXCL9, CXCR3 | 4 |
| GO:0042379 | chemokine receptor binding | <0.001 | CXCL11, CXCL10, CXCL9, CCL5 | 4 |
| Pathway ID | Pathway | P-Value | Gene Name | Number |
|---|---|---|---|---|
| bta04666 | Fc gamma R-mediated phagocytosis | <0.001 | PLA2G4Aa, MARCKSL1, VASP, SYK, PIK3R3, FCGR1A, WASF1, CFL1, ASAP1, NCF1, ARPC5, LYN, ARPC2, MAP2K1 | 14 |
| bta04668 | TNF signaling pathway | <0.001 | CXCL2, SELE, MMP3, PTGS2, CXCL6, VEGFC, SOCS3, MMP14, CASP3, LIF, CSF1, PIK3R3, MAP3K8, TNFAIP3, MAP2K3, MAP2K1 | 16 |
| bta04066 | HIF-1 signaling pathway | <0.001 | SERPINE1, LDHA, PFKFB3, PGK1, HIF1A, PFKP, TFRC, PIK3R3, MKNK1, ALDOA, ENO1, ENO2, CYBB, MAP2K1 | 14 |
| bta04015 | Rap1 signaling pathway | <0.001 | ITGAM, THBS1, PDGFRA, ID1, ITGB3, PDGFD, VEGFC, APBB1IP, FYB, PDGFRB, VASP, CSF1, PIK3R3, SIPA1L2, RAP1B, PFN1, MAP2K3, ITGB1, TLN1, PRKD3, MAP2K1 | 21 |
| bta04657 | IL-17 signaling pathway | <0.001 | CXCL2, CSF3, IL17A, MMP3, S100A9, FOSL1, PTGS2, CXCL6, MMP1, CASP3, TNFAIP3, MAPK6 | 12 |
| bta05020 | Prion diseases | 0.001 | NCAM1, LAMC1, FYN, PRKACB, HSPA5, MAP2K1 | 6 |
| bta04664 | Fc epsilon RI signaling pathway | 0.002 | ALOX5AP, FCER1A, PLA2G4A, FYN, SYK, PIK3R3, MAP2K3, LYN, MAP2K1 | 9 |
| bta04151 | PI3K–Akt signaling pathway | 0.002 | CSF3, THBS2, BDNF, THBS1, ITGA5, IL2RA, PDGFRA, EPOR, ITGB3, PDGFD, VEGFC, COL1A2, LAMA4, ITGA9, LAMC1, GNG2, COL1A1, PDGFRB, CSF1, SYK, PIK3R3, YWHAG, GNB4, COL4A1, ITGB1, CDK2, MAP2K1 | 27 |
| bta05134 | Legionellosis | 0.002 | CXCL2, ITGAM, NAIP, CASP3, PYCARD, HSPA8, CD14, SAR1A | 8 |
| bta05146 | Amoebiasis | 0.002 | SERPINB4, CXCL2, ITGAM, COL1A2, CASP3, LAMA4, LAMC1, COL1A1, PRKACB, PIK3R3, CD14, COL4A1 | 12 |
| bta04670 | Leukocyte transendothelial migration | 0.005 | ITGAM, MMP2, JAM3, VASP, PIK3R3, MSN, RAP1B, PTPN11, ITGB1, NCF1, CYBB | 11 |
| bta04062 | Chemokine signaling pathway | 0.007 | CXCR2, CXCL2, CCR1, CXCL6, CCL16, PREX1, GNG2, ARRB2, PRKACB, PIK3R3, RAP1B, GNB4, NCF1, LYN, MAP2K1 | 15 |
| bta05100 | Bacterial invasion of epithelial cells | 0.008 | ITGA5, CBL, PIK3R3, WASF1, DNM3, ITGB1, ARPC5, ARPC2 | 8 |
| bta04145 | Phagosome | 0.008 | THBS2, ITGAM, THBS1, ITGA5, ITGB3, SCARB1, TUBB3, TFRC, FCGR1A, CD14, ITGB1, ATP6V1C1, NCF1, CYBB | 14 |
| bta05165 | Human papillomavirus infection | 0.011 | THBS2, THBS1, PTGS2, WNT2, ITGA5, ITGB3, PKM, COL1A2, CASP3, LAMA4, ITGA9, LAMC1, COL1A1, PDGFRB, PRKACB, NOTCH2, PIK3R3, COL4A1, MX2, ITGB1, ATP6V1C1, CDK2, MAP2K1 | 23 |
| bta05167 | Kaposi sarcoma-associated herpesvirus infection | 0.023 | CXCL2, CCR1, PTGS2, E2F3, CASP3, PREX1, GNG2, HIF1A, RCAN1, SYK, PIK3R3, GNB4, MAPKAPK2, LYN, MAP2K1 | 15 |
| bta05323 | Rheumatoid arthritis | 0.023 | CXCL2, IL17A, MMP3, CXCL6, MMP1, CD80, CSF1, ATP6V1C1, IL11 | 9 |
| bta04392 | Hippo signaling pathway- multiple species | 0.026 | RASSF2, WTIP, TEAD3, WWTR1 | 4 |
| bta04014 | Ras signaling pathway | 0.030 | BDNF, PDGFRA, PDGFD, VEGFC, PLA2G4A, GNG2, PDGFRB, PRKACB, CSF1, PIK3R3, RAP1B, GNB4, ABL1, PTPN11, ABL2, MAP2K1 | 16 |
| bta04061 | Viral protein interaction with cytokine and cytokine receptor | 0.033 | CXCR2, CXCL2, CCR1, CXCL6, IL2RA, CCL16, IL10RA, CSF1 | 8 |
| bta05140 | Leishmaniasis | 0.033 | ITGAM, PTGS2, MARCKSL1, FCGR1A, ITGB1, NCF1, CYBB | 7 |
| bta05145 | Toxoplasmosis | 0.035 | IL10RA, CASP3, LAMA4, LAMC1, LDLR, SOCS1, MAP2K3, HSPA8, ITGB1 | 9 |
| bta04060 | Cytokine–cytokine receptor interaction | <0.001 | CXCL11b, CX3CR1, CXCL10, CXCL9, NGFR, CXCL14, MSTN, XCL1, IL17RE, BMP3, TNFRSF9, TNFSF10, GHR, CXCR6, CCL5, TNFRSF19, CXCR3, TGFB2 | 18 |
| bta04061 | Viral protein interaction with cytokine and cytokine receptor | <0.001 | CXCL11, CX3CR1, CXCL10, CXCL9, CXCL14, XCL1, TNFSF10, CCL5, CXCR3 | 9 |
| bta04062 | Chemokine signaling pathway | 0.001 | CXCL11, CX3CR1, CXCL10, CXCL9, CXCL14, XCL1, ITK, PRKCZ, CXCR6, CCL5, CXCR3 | 11 |
| bta04015 | Rap1 signaling pathway | 0.004 | FGFR4, NGFR, PRKCZ, FGFR2, MET, LPAR2, TLN2, INSR, PDGFA, MAGI3, CTNND1 | 11 |
| bta04670 | Leukocyte trans endothelial migration | 0.007 | CLDN1, ITK, OCLN, CLDN3, TXK, EZR, CTNND1 | 7 |
| bta05340 | Primary immunodeficiency | 0.009 | CD8A, BLNK, CIITA, TAP1 | 4 |
| bta01521 | EGFR tyrosine kinase inhibitor resistance | 0.020 | ERBB3, FGFR2, MET, PDGFA, GAB1 | 5 |
| bta04010 | MAPK signaling pathway | 0.028 | FGFR4, ERBB3, NGFR, RPS6KA6, FGFR2, MET, INSR, MAP3K13, PDGFA, MAP3K1, TGFB2 | 11 |
| bta04390 | Hippo signaling pathway | 0.034 | RASSF6, PRKCZ, DLG3, PPP2R2B, TCF7, TCF7L2, TGFB2 | 7 |
| bta04151 | PI3K–Akt signaling pathway | 0.035 | FGFR4, ERBB3, NGFR, GHR, FGFR2, PPP2R2B, MET, LPAR2, INSR, ITGA6, LAMC2, PDGFA, ITGA3 | 13 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, X.; Fan, Y.; He, Y.; Han, Z.; Gong, Z.; Peng, Y.; Meng, Y.; Mao, Y.; Yang, Z.; Yang, Y. Integrative Analysis of miRNA and mRNA Expression Profiles in Mammary Glands of Holstein Cows Artificially Infected with Staphylococcus aureus. Pathogens 2021, 10, 506. https://doi.org/10.3390/pathogens10050506
Wang X, Fan Y, He Y, Han Z, Gong Z, Peng Y, Meng Y, Mao Y, Yang Z, Yang Y. Integrative Analysis of miRNA and mRNA Expression Profiles in Mammary Glands of Holstein Cows Artificially Infected with Staphylococcus aureus. Pathogens. 2021; 10(5):506. https://doi.org/10.3390/pathogens10050506
Chicago/Turabian StyleWang, Xiaolong, Yongliang Fan, Yifan He, Ziyin Han, Zaicheng Gong, Yalan Peng, Yining Meng, Yongjiang Mao, Zhangping Yang, and Yi Yang. 2021. "Integrative Analysis of miRNA and mRNA Expression Profiles in Mammary Glands of Holstein Cows Artificially Infected with Staphylococcus aureus" Pathogens 10, no. 5: 506. https://doi.org/10.3390/pathogens10050506
APA StyleWang, X., Fan, Y., He, Y., Han, Z., Gong, Z., Peng, Y., Meng, Y., Mao, Y., Yang, Z., & Yang, Y. (2021). Integrative Analysis of miRNA and mRNA Expression Profiles in Mammary Glands of Holstein Cows Artificially Infected with Staphylococcus aureus. Pathogens, 10(5), 506. https://doi.org/10.3390/pathogens10050506






