Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain
Abstract
1. Introduction
2. Results and Discussion
2.1. Hypervirulent and MDR A. baumannii Transcriptome Response to Human Fluids
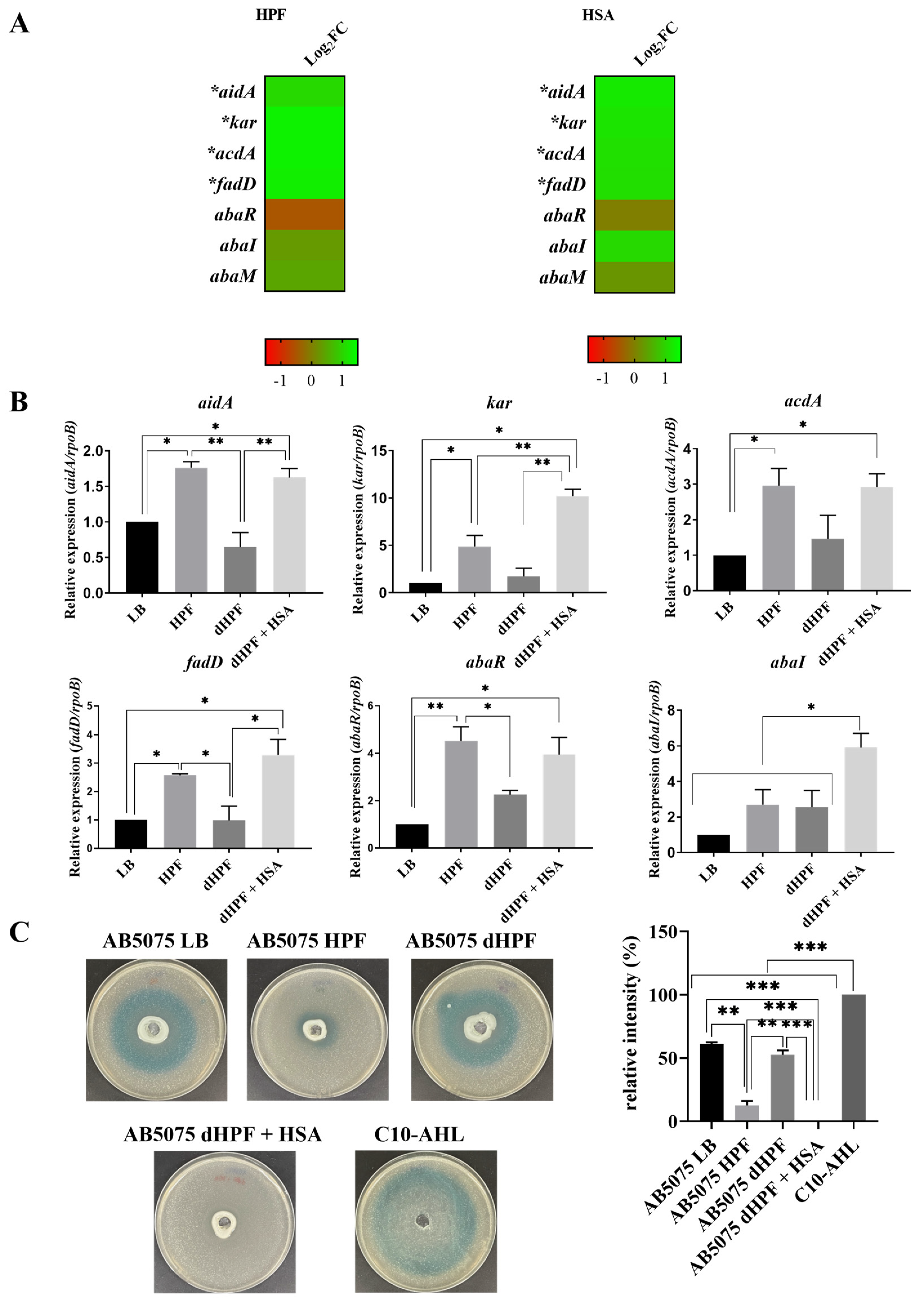
2.2. Human Fluids Enhance the Expression of Genes Involved in Quorum Sensing and Quorum Quenching in a Hypervirulent and MDR A. baumannii Strain
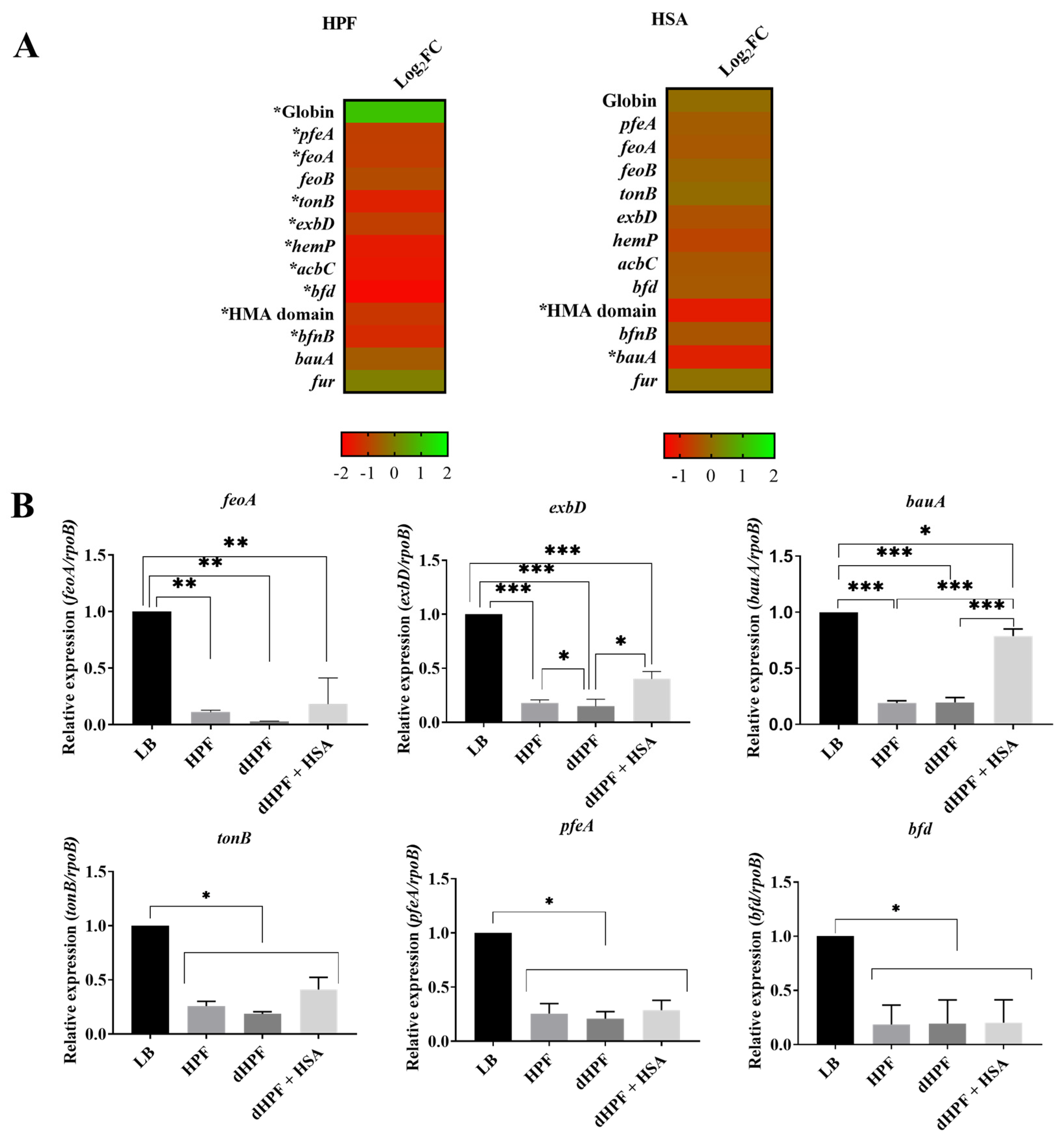
2.3. Iron Acquisition Is Modulated in Presence of Human Fluids in the Hypervirulent, MDR A. baumannii Strain AB5075
2.4. Human Fluids Shift Fatty Acid Metabolic Gene Expression in A. baumannii AB5075
2.5. The Catabolism of Acetoin Is Altered in the Presence of Human Fluids in A. baumannii AB5075
2.6. Human Fluids Affect the Expression of Others Important Genes
3. Materials and Methods
3.1. Bacterial Strains
3.2. RNA Extraction, Sequencing, and Transcriptomic Analysis
3.3. HSA Depletion
3.4. Quantitative Reverse Transcription Polymerase Chain Reaction (qRT-PCR)
3.5. N-Acyl Homoserine Lactone (AHL) Detection
3.6. Growth in the Presence of Acetoin
3.7. Biofilm Assays
3.8. Determination of Total iron Concentration
3.9. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wong, D.; Nielsen, T.B.; Bonomo, R.A.; Pantapalangkoor, P.; Luna, B.; Spellberg, B. Clinical and Pathophysiological Overview of Acinetobacter Infections: A Century of Challenges. Clin. Microbiol. Rev. 2016, 30, 409–447. [Google Scholar] [CrossRef] [PubMed]
- Peleg, A.Y.; Seifert, H.; Paterson, D.L. Acinetobacter baumannii: Emergence of a Successful Pathogen. Clin. Microbiol. Rev. 2008, 21, 538–582. [Google Scholar] [CrossRef] [PubMed]
- CDC. Antibiotic Resistance Threats in the United States; Department of Health and Human Services: Atlanta, GA, USA, 2019.
- De Oliveira, D.M.P.; Forde, B.M.; Kidd, T.J.; Harris, P.N.A.; Schembri, M.A.; Beatson, S.A.; Paterson, D.L.; Walker, M.J. Antimicrobial Resistance in ESKAPE Pathogens. Clin. Microbiol. Rev. 2020, 33. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.-R.; Lee, J.H.; Park, M.; Park, K.S.; Bae, I.K.; Kim, Y.B.; Cha, C.-J.; Jeong, B.C.; Lee, S.H. Biology of Acinetobacter baumannii: Pathogenesis, Antibiotic Resistance Mechanisms, and Prospective Treatment Options. Front. Cell. Infect. Microbiol. 2017, 7, 55. [Google Scholar] [CrossRef] [PubMed]
- Breijyeh, Z.; Karaman, R. Comprehensive Review on Alzheimer’s Disease: Causes and Treatment. Molecules 2020, 25, 5789. [Google Scholar] [CrossRef]
- Juttukonda, L.J.; Chazin, W.J.; Skaar, E.P. Acinetobacter baumannii Coordinates Urea Metabolism with Metal Import To Resist Host-Mediated Metal Limitation. mBio 2016, 7, e01475-16. [Google Scholar] [CrossRef]
- Rodman, N.; Martinez, J.; Fung, S.; Nakanouchi, J.; Myers, A.L.; Harris, C.M.; Dang, E.; Fernandez, J.S.; Liu, C.; Mendoza, A.M.; et al. Human Pleural Fluid Elicits Pyruvate and Phenylalanine Metabolism in Acinetobacter baumannii to Enhance Cytotoxicity and Immune Evasion. Front. Microbiol. 2019, 10, 1581. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Fernandez, J.S.; Liu, C.; Hoard, A.; Mendoza, A.; Nakanouchi, J.; Rodman, N.; Courville, R.; Tuttobene, M.R.; Lopez, C.; et al. Human pleural fluid triggers global changes in the transcriptional landscape of Acinetobacter baumannii as an adaptive response to stress. Sci. Rep. 2019, 9, 17251. [Google Scholar] [CrossRef]
- Quinn, B.; Rodman, N.; Jara, E.; Fernandez, J.S.; Martinez, J.; Traglia, G.M.; Montaña, S.; Cantera, V.; Place, K.; Bonomo, R.A.; et al. Human serum albumin alters specific genes that can play a role in survival and persistence in Acinetobacter baumannii. Sci. Rep. 2018, 8, 14741. [Google Scholar] [CrossRef]
- Ohneck, E.J.; Arivett, B.A.; Fiester, S.E.; Wood, C.R.; Metz, M.L.; Simeone, G.M.; Actis, L.A. Mucin acts as a nutrient source and a signal for the differential expression of genes coding for cellular processes and virulence factors in Acinetobacter baumannii. PLoS ONE 2018, 13, e0190599. [Google Scholar] [CrossRef]
- Murray, G.L.; Tsyganov, K.; Kostoulias, X.P.; Bulach, D.M.; Powell, D.; Creek, D.J.; Boyce, J.D.; Paulsen, I.T.; Peleg, A.Y. Global Gene Expression Profile of Acinetobacter baumannii During Bacteremia. J. Infect. Dis. 2017, 215, S52–S57. [Google Scholar] [CrossRef] [PubMed]
- López, M.; Blasco, L.; Gato, E.; Perez, A.; Fernández-Garcia, L.; Martínez-Martinez, L.; Fernández-Cuenca, F.; Rodríguez-Baño, J.; Pascual, A.; Bou, G.; et al. Response to Bile Salts in Clinical Strains of Acinetobacter baumannii Lacking the AdeABC Efflux Pump: Virulence Associated with Quorum Sensing. Front. Cell. Infect. Microbiol. 2017, 7, 143. [Google Scholar] [CrossRef] [PubMed]
- Quinn, B.; Traglia, G.M.; Nguyen, M.; Martinez, J.; Liu, C.; Fernandez, J.S.; Ramirez, M.S. Effect of Host Human Products on Natural Transformation in Acinetobacter baumannii. Curr. Microbiol. 2019, 76, 950–953. [Google Scholar] [CrossRef] [PubMed]
- Martinez, J.; Razo-Gutierrez, C.; Le, C.; Courville, R.; Pimentel, C.; Liu, C.; Fung, S.E.; Tuttobene, M.R.; Phan, K.; Vila, A.J.; et al. Cerebrospinal fluid (CSF) augments metabolism and virulence expression factors in Acinetobacter baumannii. Sci. Rep. 2021, 11, 1–13. [Google Scholar] [CrossRef]
- Jacobs, A.C.; Thompson, M.G.; Black, C.C.; Kessler, J.L.; Clark, L.P.; McQueary, C.N.; Gancz, H.Y.; Corey, B.W.; Moon, J.K.; Si, Y.; et al. AB5075, a Highly Virulent Isolate of Acinetobacter baumannii, as a Model Strain for the Evaluation of Pathogenesis and Antimicrobial Treatments. mBio 2014, 5, e01076-14. [Google Scholar] [CrossRef]
- López, M.; Mayer, C.; Fernández-García, L.; Blasco, L.; Muras, A.; Ruiz, F.M.; Bou, G.; Otero, A.; Tomás, M.; on behalf of the GEIH-GEMARA (SEIMC). Quorum sensing network in clinical strains of A. baumannii: AidA is a new quorum quenching enzyme. PLoS ONE 2017, 12, e0174454. [Google Scholar] [CrossRef]
- Cha, C.; Gao, P.; Chen, Y.-C.; Shaw, P.D.; Farrand, S.K. Production of Acyl-Homoserine Lactone Quorum-Sensing Signals by Gram-Negative Plant-Associated Bacteria. Mol. Plant Microbe Interact. 1998, 11, 1119–1129. [Google Scholar] [CrossRef]
- McClean, K.H.; Winson, M.K.; Fish, L.; Taylor, A.; Chhabra, S.R.; Camara, M.; Daykin, M.; Lamb, J.H.; Swift, S.; Bycroft, B.W.; et al. Quorum sensing and Chromobacterium violaceum: Exploitation of violacein production and inhibition for the detection of N-acylhomoserine lactones. Microbiology 1997, 143, 3703–3711. [Google Scholar] [CrossRef]
- Niu, C.; Clemmer, K.M.; Bonomo, R.A.; Rather, P.N. Isolation and Characterization of an Autoinducer Synthase from Acinetobacter baumannii. J. Bacteriol. 2008, 190, 3386–3392. [Google Scholar] [CrossRef]
- González, R.H.; Dijkshoorn, L.; Barselaar, M.V.D.; Nudel, C. Quorum sensing signal profile of Acinetobacter strains from nosocomial and environmental sources. Rev. Argent. Microbiol. 2009, 41, 73–78. [Google Scholar]
- López-Martín, M.; Dubern, J.-F.; Alexander, M.R.; Williams, P. AbaM Regulates Quorum Sensing, Biofilm Formation, and Virulence in Acinetobacter baumannii. J. Bacteriol. 2021, 203. [Google Scholar] [CrossRef]
- Elhosseiny, N.M.; Attia, A.S. Acinetobacter: An emerging pathogen with a versatile secretome. Emerg. Microbes Infect. 2018, 7, 1–15. [Google Scholar] [CrossRef]
- Wooldridge, K.G.; Williams, P.H. Iron uptake mechanisms of pathogenic bacteria. FEMS Microbiol. Rev. 1993, 12, 325–348. [Google Scholar] [CrossRef] [PubMed]
- Miethke, M.; Marahiel, M.A. Siderophore-Based Iron Acquisition and Pathogen Control. Microbiol. Mol. Biol. Rev. 2007, 71, 413–451. [Google Scholar] [CrossRef] [PubMed]
- Braun, V. Iron uptake mechanisms and their regulation in pathogenic bacteria. Int. J. Med. Microbiol. 2001, 291, 67–79. [Google Scholar] [CrossRef] [PubMed]
- Di Lorenzo, M.; Stork, M. Plasmid-Encoded Iron Uptake Systems. Microbiol. Spectr. 2014, 2. [Google Scholar] [CrossRef] [PubMed]
- Gaddy, J.A.; Arivett, B.A.; McConnell, M.J.; López-Rojas, R.; Pachón, J.; Actis, L.A. Role of Acinetobactin-Mediated Iron Acquisition Functions in the Interaction of Acinetobacter baumannii Strain ATCC 19606Twith Human Lung Epithelial Cells, Galleria mellonella Caterpillars, and Mice. Infect. Immun. 2012, 80, 1015–1024. [Google Scholar] [CrossRef]
- Kehl-Fie, T.E.; Skaar, E.P. Nutritional immunity beyond iron: A role for manganese and zinc. Curr. Opin. Chem. Biol. 2010, 14, 218–224. [Google Scholar] [CrossRef]
- Hood, M.I.; Skaar, E.P. Nutritional immunity: Transition metals at the pathogen–host interface. Nat. Rev. Genet. 2012, 10, 525–537. [Google Scholar] [CrossRef] [PubMed]
- Cuajungco, M.; Ramirez, M.; Tolmasky, M. Zinc: Multidimensional Effects on Living Organisms. Biomedicines 2021, 9, 208. [Google Scholar] [CrossRef]
- Gerós, A.S.; Simmons, A.; Drakesmith, H.; Aulicino, A.; Frost, J.N. The battle for iron in enteric infections. Immunology 2020, 161, 186–199. [Google Scholar] [CrossRef]
- Weinberg, E.D. Iron depletion: A defense against intracellular infection and neoplasia. Life Sci. 1992, 50, 1289–1297. [Google Scholar] [CrossRef]
- Sheldon, J.R.; Skaar, E.P. Acinetobacter baumannii can use multiple siderophores for iron acquisition, but only acinetobactin is required for virulence. PLoS Pathog. 2020, 16, e1008995. [Google Scholar] [CrossRef]
- Martínez-Guitián, M.; Vázquez-Ucha, J.C.; Álvarez-Fraga, L.; Conde-Pérez, K.; Vallejo, J.A.; Perina, A.; Bou, G.; Poza, M.; Beceiro, A. Global Transcriptomic Analysis During Murine Pneumonia Infection Reveals New Virulence Factors in Acinetobacter baumannii. J. Infect. Dis. 2020. [Google Scholar] [CrossRef]
- Funahashi, T.; Tanabe, T.; Maki, J.; Miyamoto, K.; Tsujibo, H.; Yamamoto, S. Identification and characterization of a cluster of genes involved in biosynthesis and transport of acinetoferrin, a siderophore produced by Acinetobacter haemolyticus ATCC 17906T. Microbiology 2013, 159, 678–690. [Google Scholar] [CrossRef] [PubMed]
- Runci, F.; Gentile, V.; Frangipani, E.; Rampioni, G.; Leoni, L.; Lucidi, M.; Visaggio, D.; Harris, G.; Chen, W.; Stahl, J.; et al. Contribution of Active Iron Uptake to Acinetobacter baumannii Pathogenicity. Infect. Immun. 2019, 87, e00755-18. [Google Scholar] [CrossRef] [PubMed]
- Sato, T.; Nonoyama, S.; Kimura, A.; Nagata, Y.; Ohtsubo, Y.; Tsuda, M. The Small Protein HemP Is a Transcriptional Activator for the Hemin Uptake Operon in Burkholderia multivorans ATCC 17616. Appl. Environ. Microbiol. 2017, 83, 83. [Google Scholar] [CrossRef]
- Soldano, A.; Yao, H.; Hewage, A.N.D.P.; Meraz, K.; Annor-Gyamfi, J.K.; Bunce, R.A.; Battaile, K.P.; Lovell, S.; Rivera, M. Small Molecule Inhibitors of the Bacterioferritin (BfrB)–Ferredoxin (Bfd) Complex Kill Biofilm-Embedded Pseudomonas aeruginosa Cells. ACS Infect. Dis. 2021, 7, 123–140. [Google Scholar] [CrossRef]
- Fournier, P.-E.; Vallenet, D.; Barbe, V.; Audic, S.; Ogata, H.; Poirel, L.; Richet, H.; Robert, C.; Mangenot, S.; Abergel, C.; et al. Comparative Genomics of Multidrug Resistance in Acinetobacter baumannii. PLoS Genet. 2006, 2, e7. [Google Scholar] [CrossRef]
- Ramirez, M.S.; Penwell, W.F.; Traglia, G.M.; Zimbler, D.L.; Gaddy, J.A.; Nikolaidis, N.; Arivett, B.A.; Adams, M.D.; Bonomo, R.A.; Actis, L.A.; et al. Identification of Potential Virulence Factors in the Model Strain Acinetobacter baumannii A118. Front. Microbiol. 2019, 10, 1599. [Google Scholar] [CrossRef]
- Dorsey, C.W.; Tomaras, A.P.; Connerly, P.L.; Tolmasky, M.E.; Crosa, J.H.; Actis, L.A. The siderophore-mediated iron acquisition systems of Acinetobacter baumannii ATCC 19606 and Vibrio anguillarum 775 are structurally and functionally related. Microbiology 2004, 150, 3657–3667. [Google Scholar] [CrossRef]
- Klockars, M.; Weber, T.; Tanner, P.; Hellström, P.E.; Pettersson, T. Pleural fluid ferritin concentrations in human disease. J. Clin. Pathol. 1985, 38, 818–824. [Google Scholar] [CrossRef] [PubMed]
- Zhao, J.; Zhu, Y.; Han, J.; Lin, Y.-W.; Aichem, M.; Wang, J.; Chen, K.; Velkov, T.; Schreiber, F.; Li, J. Genome-Scale Metabolic Modeling Reveals Metabolic Alterations of Multidrug-Resistant Acinetobacter baumannii in a Murine Bloodstream Infection Model. Microorganisms 2020, 8, 1793. [Google Scholar] [CrossRef]
- Jiang, J.-H.; Hassan, K.A.; Begg, S.L.; Rupasinghe, T.W.T.; Naidu, V.; Pederick, V.G.; Khorvash, M.; Whittall, J.J.; Paton, J.C.; Paulsen, I.T.; et al. Identification of Novel Acinetobacter baumannii Host Fatty Acid Stress Adaptation Strategies. mBio 2019, 10, e02056-18. [Google Scholar] [CrossRef] [PubMed]
- Liebergesell, M.; Sonomoto, K.; Madkour, M.; Mayer, F.; Steinbüchel, A. Purification and Characterization of the Poly(Hydroxyalkanoic Acid) Synthase from Chromatium vinosum and Localization of the Enzyme at the Surface of Poly(Hydroxyalkanoic Acid) Granules. JBIC J. Biol. Inorg. Chem. 1994, 226, 71–80. [Google Scholar] [CrossRef]
- Choi, A.H.K.; Slamti, L.; Avci, F.Y.; Pier, G.B.; Maira-Litrán, T. The pgaABCD Locus of Acinetobacter baumannii Encodes the Production of Poly-β-1-6-N-Acetylglucosamine, Which Is Critical for Biofilm Formation. J. Bacteriol. 2009, 191, 5953–5963. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-R.; Oh, D.-K. Production of hydroxy fatty acids by microbial fatty acid-hydroxylation enzymes. Biotechnol. Adv. 2013, 31, 1473–1485. [Google Scholar] [CrossRef] [PubMed]
- Altabe, S.G.; Mansilla, M.C.; de Mendoza, D. Remodeling of Membrane Phospholipids by Bacterial Desaturases. In Stearoyl-CoA Desaturase Genes in Lipid Metabolism; Ntambi, M.J., Ed.; Springer: New York, NY, USA, 2013; pp. 209–231. [Google Scholar]
- Peng, Q.; Zhao, X.; Wen, J.; Huang, M.; Zhang, J.; Song, F. Transcription in the acetoin catabolic pathway is regulated by AcoR and CcpA in Bacillus thuringiensis. Microbiol. Res. 2020, 235, 126438. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Z.; Xu, P. Acetoin Metabolism in Bacteria. Crit. Rev. Microbiol. 2007, 33, 127–140. [Google Scholar] [CrossRef]
- Khandekar, S.; Liebens, V.; Fauvart, M.; Tulkens, P.M.; Michiels, J.; Van Bambeke, F. The Putative De-N-acetylase DnpA Contributes to Intracellular and Biofilm-Associated Persistence of Pseudomonas aeruginosa Exposed to Fluoroquinolones. Front. Microbiol. 2018, 9, 1455. [Google Scholar] [CrossRef]
- Sepahvand, S.; Doudi, M.; Davarpanah, M.A.; Bahador, A.; Ahmadi, M. Analyzing pmrA and pmrB genes in Acinetobacter baumannii resistant to colistin in Shahid Rajai Shiraz, Iran Hospital by PCR: First report in Iran. Pak. J. Pharm. Sci. 2016, 29, 1401–1406. [Google Scholar]
- Gallagher, L.A.; Ramage, E.; Weiss, E.J.; Radey, M.; Hayden, H.S.; Held, K.G.; Huse, H.K.; Zurawski, D.V.; Brittnacher, M.J.; Manoil, C. Resources for Genetic and Genomic Analysis of Emerging Pathogen Acinetobacter baumannii. J. Bacteriol. 2015, 197, 2027–2035. [Google Scholar] [CrossRef]
- Moshiri, J.; Kaur, D.; Hambira, C.M.; Sandala, J.L.; Koopman, J.A.; Fuchs, J.R.; Gunn, J.S. Identification of a Small Molecule Anti-biofilm Agent Against Salmonella enterica. Front. Microbiol. 2018, 9, 2804. [Google Scholar] [CrossRef]
- Morimoto, Y.V.; Nakamura, S.; Hiraoka, K.D.; Namba, K.; Minamino, T. Distinct Roles of Highly Conserved Charged Residues at the MotA-FliG Interface in Bacterial Flagellar Motor Rotation. J. Bacteriol. 2012, 195, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Sun, D.; Crowell, S.A.; Harding, C.M.; De Silva, P.M.; Harrison, A.; Fernando, D.M.; Mason, K.M.; Santana, E.; Loewen, P.C.; Kumar, A.; et al. KatG and KatE confer Acinetobacter resistance to hydrogen peroxide but sensitize bacteria to killing by phagocytic respiratory burst. Life Sci. 2016, 148, 31–40. [Google Scholar] [CrossRef] [PubMed]
- Schramm, S.T.J.; Place, K.; Montaña, S.; Almuzara, M.; Fung, S.; Fernandez, J.S.; Tuttobene, M.R.; Golic, A.; Altilio, M.; Traglia, G.M.; et al. Genetic and Phenotypic Features of a Novel Acinetobacter Species, Strain A47, Isolated From the Clinical Setting. Front. Microbiol. 2019, 10, 1375. [Google Scholar] [CrossRef] [PubMed]
- Tomaras, A.P.; Flagler, M.J.; Dorsey, C.W.; Gaddy, J.A.; Actis, L.A. Characterization of a two-component regulatory system from Acinetobacter baumannii that controls biofilm formation and cellular morphology. Microbiology 2008, 154, 3398–3409. [Google Scholar] [CrossRef] [PubMed]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A flexible trimmer for Illumina sequence data. Bioinformatics 2014, 30, 2114–2120. [Google Scholar] [CrossRef]
- Li, H.; Durbin, R. Fast and accurate long-read alignment with Burrows–Wheeler transform. Bioinformatics 2010, 26, 589–595. [Google Scholar] [CrossRef]
- Thorvaldsdóttir, H.; Robinson, J.T.; Mesirov, J.P. Integrative Genomics Viewer (IGV): High-performance genomics data visualization and exploration. Brief. Bioinform. 2012, 14, 178–192. [Google Scholar] [CrossRef]
- Liao, Y.; Smyth, G.K.; Shi, W. featureCounts: An efficient general purpose program for assigning sequence reads to genomic features. Bioinformatics 2013, 30, 923–930. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef] [PubMed]
- Tierney, A.R.P.; Rather, P.N. Methods for Detecting N-Acyl Homoserine Lactone Production in Acinetobacter baumannii. Methods Mol. Biol. 2019, 253–258. [Google Scholar] [CrossRef]
- Martinez, J.; Liu, C.; Rodman, N.; Fernandez, J.S.; Barberis, C.; Sieira, R.; Perez, F.; Bonomo, R.A.; Ramirez, M.S. Human fluids alter DNA-acquisition in Acinetobacter baumannii. Diagn. Microbiol. Infect. Dis. 2019, 93, 183–187. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pimentel, C.; Le, C.; Tuttobene, M.R.; Subils, T.; Martinez, J.; Sieira, R.; Papp-Wallace, K.M.; Keppetipola, N.; Bonomo, R.A.; Actis, L.A.; et al. Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain. Pathogens 2021, 10, 471. https://doi.org/10.3390/pathogens10040471
Pimentel C, Le C, Tuttobene MR, Subils T, Martinez J, Sieira R, Papp-Wallace KM, Keppetipola N, Bonomo RA, Actis LA, et al. Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain. Pathogens. 2021; 10(4):471. https://doi.org/10.3390/pathogens10040471
Chicago/Turabian StylePimentel, Camila, Casin Le, Marisel R. Tuttobene, Tomas Subils, Jasmine Martinez, Rodrigo Sieira, Krisztina M. Papp-Wallace, Niroshika Keppetipola, Robert A. Bonomo, Luis A. Actis, and et al. 2021. "Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain" Pathogens 10, no. 4: 471. https://doi.org/10.3390/pathogens10040471
APA StylePimentel, C., Le, C., Tuttobene, M. R., Subils, T., Martinez, J., Sieira, R., Papp-Wallace, K. M., Keppetipola, N., Bonomo, R. A., Actis, L. A., Tolmasky, M. E., & Ramirez, M. S. (2021). Human Pleural Fluid and Human Serum Albumin Modulate the Behavior of a Hypervirulent and Multidrug-Resistant (MDR) Acinetobacter baumannii Representative Strain. Pathogens, 10(4), 471. https://doi.org/10.3390/pathogens10040471










