Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields
Abstract
1. Introduction
2. Materials and Methods
2.1. Farms, Pests, and Sampling Site Description
2.2. Soil Sampling
2.3. Insect Rearing and Baiting
2.4. Fungal Isolation and Screening
2.5. Fungal Identification
2.6. Soil Chemical Analyses
2.7. Data Analyses
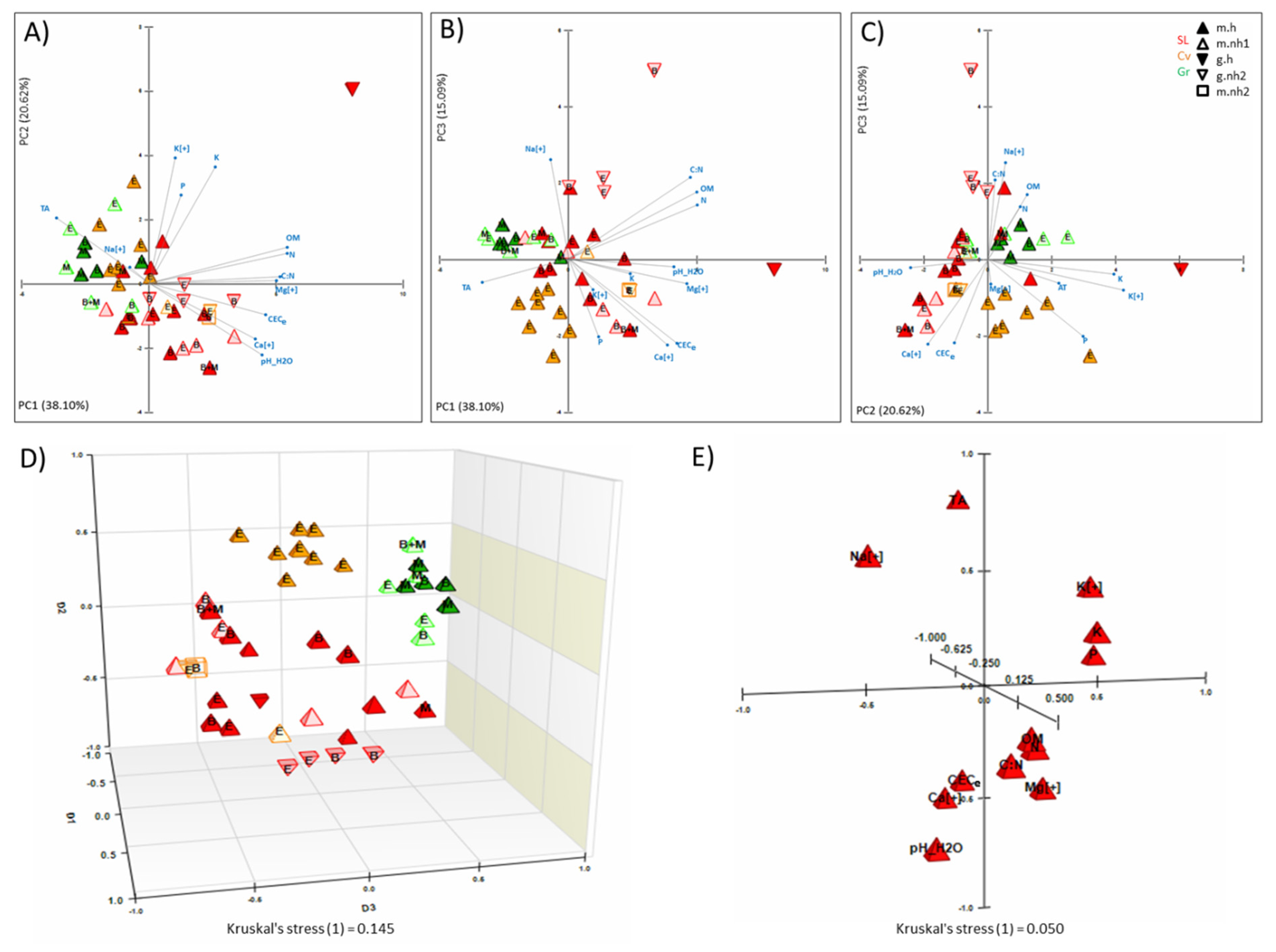
3. Results
3.1. Effects of Soils Chemical Properties, OM, and Herbicide Usage on EPF
3.2. Biological Proximities and Related Factors
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ghaley, B.B.; Porter, J.R.; Sandhu, H.S. Soil-based ecosystem services: A synthesis of nutrient cycling and carbon sequestration assessment methods. Int. J. Biodivers. Sci. Ecosyst. Serv. Manag. 2014, 10, 177–186. [Google Scholar] [CrossRef]
- Barker, C.W.; Barker, G.M. Generalist entomopathogens as biological indicators of deforestation and agricultural land use impacts on waikato soils. N. Z. J. Ecol. 1998, 22, 189–196. [Google Scholar]
- Barrios, E. Soil biota, ecosystem services and land productivity. Ecol. Econ. 2007, 64, 269–285. [Google Scholar] [CrossRef]
- Lugtenberg, B.J.J.; Caradus, J.R.; Johnson, L.J. Fungal endophytes for sustainable crop production. FEMS Microbiol. Ecol. 2016, 92, fiw194. [Google Scholar] [CrossRef]
- Mantzoukas, S.; Eliopoulos, P.A. Endophytic entomopathogenic fungi: A valuable biological control tool against plant pests. Appl. Sci. 2020, 10, 360. [Google Scholar] [CrossRef]
- Turlings, T.C.J.; Tumlinson, J.H.; Lewis, W.J. Exploitation of herbivore-induced plant odors by host-seeking parasitic wasps. Science 1990, 250, 1251. [Google Scholar] [CrossRef]
- De Moraes, C.M.; Lewis, W.J.; Paré, P.W.; Alborn, H.T.; Tumlinson, J.H. Herbivore-infested plants selectively attract parasitoids. Nature 1998, 393, 570–573. [Google Scholar] [CrossRef]
- Rasmann, S.; Köllner, T.G.; Degenhardt, J.; Hiltpold, I.; Toepfer, S.; Kuhlmann, U.; Gershenzon, J.; Turlings, T.C.J. Recruitment of entomopathogenic nematodes by insect-damaged maize roots. Nature 2005, 434, 732–737. [Google Scholar] [CrossRef]
- Delory, B.M.; Delaplace, P.; Fauconnier, M.-L.; du Jardin, P. Root-emitted volatile organic compounds: Can they mediate belowground plant-plant interactions? Plant Soil 2016, 402, 1–26. [Google Scholar] [CrossRef]
- Heuskin, S.; Béra, F.; Lorge, S.; Leroy, P.; Haubruge, E.; Wathelet, J.-P.; Brostaux, Y.; Lognay, G. A Semiochemical slow-release formulation in a biological control approach to attract hoverflies. J. Environ. Ecol. 2012, 3, 72–85. [Google Scholar] [CrossRef]
- Crespo, R.; Pedrini, N.; Juárez, M.P.; Dal Bello, G.M. Volatile organic compounds released by the entomopathogenic fungus Beauveria bassiana. Microbiol. Res. 2008, 163, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Cotes, B.; Rännbäck, L.-M.; Björkman, M.; Norli, H.; Meyling, N.; Rämert, B.; Anderson, P. Habitat selection of a parasitoid mediated by volatiles informing on host and intraguild predator densities. Oecologia 2015, 179, 151–162. [Google Scholar] [CrossRef]
- Bojke, A.; Tkaczuk, C.; Stepnowski, P.; Gołębiowski, M. Comparison of volatile compounds released by entomopathogenic fungi. Microbiol. Res. 2018, 214, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Lozano-Soria, A.; Picciotti, U.; Lopez-Moya, F.; Lopez-Cepero, J.; Porcelli, F.; Lopez-Llorca, L.V. Volatile organic compounds from entomopathogenic and nematophagous fungi, repel banana black weevil (Cosmopolites sordidus). Insects 2020, 11, 509. [Google Scholar] [CrossRef] [PubMed]
- Khoja, S.; Eltayef, K.M.; Baxter, I.; Myrta, A.; Bull, J.C.; Butt, T. Volatiles of the entomopathogenic fungus, Metarhizium brunneum, attract and kill plant parasitic nematodes. Biol. Control 2021, 152, 104472. [Google Scholar] [CrossRef]
- Jaronski, S.T. Soil ecology of the entomopathogenic ascomycetes: A critical examination of what we (think) we know. In Use of Entomopathogenic Fungi in Biological Pest Management; Maniana, K., Ekesi, S., Eds.; Research SignPosts: Trivandrum, India, 2007; pp. 91–144. [Google Scholar]
- Quesada-Moraga, E.; Navas-Cortés, J.A.; Maranhao, E.A.A.; Ortiz-Urquiza, A.; Santiago-Álvarez, C. Factors affecting the occurrence and distribution of entomopathogenic fungi in natural and cultivated soils. Mycol. Res. 2007, 111, 947–966. [Google Scholar] [CrossRef]
- Jabbour, R.; Barbercheck, M.E. Soil management effects on entomopathogenic fungi during the transition to organic agriculture in a feed grain rotation. Biol. Control 2009, 51, 435–443. [Google Scholar] [CrossRef]
- Clifton, E.H.; Jaronski, S.T.; Hodgson, E.W.; Gassmann, A.J. Abundance of soil-borne entomopathogenic fungi in organic and conventional fields in the midwestern USA with an emphasis on the effect of herbicides and fungicides on fungal persistence. PLoS ONE 2015, 10, e0133613. [Google Scholar] [CrossRef]
- Garrido-Jurado, I.; Torrent, J.; Barrón, V.; Corpas, A.; Quesada-Moraga, E. Soil properties affect the availability, movement, and virulence of entomopathogenic fungi conidia against puparia of Ceratitis capitata (Diptera: Tephritidae). Biol. Control 2011, 58, 277–285. [Google Scholar] [CrossRef]
- Asensio, L.; Carbonell, T.; Lopez Jimenez, J.; López Llorca, L. Entomopathogenic fungi in soils from alicante province. Span. J. Agric. Res. 2003, 1, 37–45. [Google Scholar] [CrossRef]
- Rath, A.C.; Koen, T.B.; Yip, H.Y. The influence of abiotic factors on the distribution and abundance of Metarhizium anisopliae in Tasmanian pasture soils. Mycol. Res. 1992, 96, 378–384. [Google Scholar] [CrossRef]
- Oddsdottir, E.S.; Nielsen, C.; Sen, R.; Harding, S.; Eilenberg, J.; Halldorsson, G. Distribution patterns of soil entomopathogenic and birch symbiotic ectomycorrhizal fungi across native woodland and degraded habitats in Iceland. Icel. Agric. Sci. 2010, 23, 37–49. [Google Scholar]
- Uzman, D.; Pliester, J.; Leyer, I.; Entling, M.H.; Reineke, A. Drivers of entomopathogenic fungi presence in organic and conventional vineyard soils. Appl. Soil Ecol. 2019, 133, 89–97. [Google Scholar] [CrossRef]
- Hallouti, A.; Ait Hamza, M.; Zahidi, A.; Ait Hammou, R.; Bouharroud, R.; Ait Ben Aoumar, A.; Boubaker, H. Diversity of entomopathogenic fungi associated with Mediterranean fruit fly (Ceratitis capitata (Diptera: Tephritidae)) in Moroccan Argan forests and nearby area: Impact of soil factors on their distribution. BMC Ecol. 2020, 20, 64. [Google Scholar] [CrossRef]
- Moloinyane, S.; Addison, P.; Achiano, K.A.; Nchu, F. Association between chemical properties of vineyard soils and occurrence of entomopathogenic fungi causing different levels of mortality in Planococcus ficus. BioControl 2020, 65, 197–209. [Google Scholar] [CrossRef]
- Sharma, L.; Oliveira, I.; Torres, L.; Marques, G. Entomopathogenic fungi in Portuguese vineyards soils: Suggesting a ‘Galleria-Tenebrio-bait method’ as bait-insects Galleria and Tenebrio significantly underestimate the respective recoveries of Metarhizium (robertsii) and Beauveria (bassiana). MycoKeys 2018, 38, 1–23. [Google Scholar] [CrossRef]
- Sharma, L.; Oliveira, I.; Raimundo, F.; Torres, L.; Marques, G. Soil chemical properties barely perturb the abundance of entomopathogenic Fusarium oxysporum: A case study using a generalized linear mixed model for microbial pathogen occurrence count data. Pathogens 2018, 7, 89. [Google Scholar] [CrossRef]
- Carlos, C.G.F.; Sousa, S.; Salvação, J.; Sharma, L.; Soares, R.; Manso, J.; Nóbrega, M.; Lopes, A.; Soares, S.; Aranha, J.; et al. Environmentally safe strategies to control the European grapevine moth, Lobesia botrana (Den. & Schiff.) in the Douro demarcated region. Cienc. Tec. Vitivinic 2013, 28, 1006–1011. [Google Scholar]
- Sharma, L.; Marques, G. Fusarium, an entomopathogen—a myth or reality? Pathogens 2018, 7, 93. [Google Scholar] [CrossRef]
- Zazzerini, A.; Quaglia, M.; Davolio Marani, O. First report of Clonostachys rhizophaga as a pathogen of Dendrocalamus giganteus in Mozambique. Plant Dis. 2010, 94, 372. [Google Scholar] [CrossRef]
- Imoulan, A.; Alaoui, A.; El Meziane, A. Natural occurrence of soil-borne entomopathogenic fungi in the Moroccan endemic forest of Argania spinosa and their pathogenicity to Ceratitis capitata. World J. Microbiol. Biotechnol. 2011, 27, 2619–2628. [Google Scholar] [CrossRef]
- Keyser, C.A.; De Fine Licht, H.H.; Steinwender, B.M.; Meyling, N.V. Diversity within the entomopathogenic fungal species Metarhizium flavoviride associated with agricultural crops in Denmark. BMC Microbiol. 2015, 15, 249. [Google Scholar] [CrossRef] [PubMed]
- Meyling, N.V.; Eilenberg, J. Occurrence and distribution of soil borne entomopathogenic fungi within a single organic agroecosystem. Agric. Ecosyst. Environ. 2006, 113, 336–341. [Google Scholar] [CrossRef]
- Goble, T.A.; Dames, J.F.; Hill, M.P.; Moore, S.D. The effects of farming system, habitat type and bait type on the isolation of entomopathogenic fungi from citrus soils in the Eastern Cape Province, South Africa. BioControl 2010, 55, 399–412. [Google Scholar] [CrossRef]
- Posadas, J.B.; Comerio, R.M.; Mini, J.I.; Nussenbaum, A.L.; Lecuona, R.E. A novel dodine-free selective medium based on the use of cetyl trimethyl ammonium bromide (CTAB) to isolate Beauveria bassiana, Metarhizium anisopliae sensu lato and Paecilomyces lilacinus from soil. Mycologia 2012, 104, 974–980. [Google Scholar] [CrossRef] [PubMed]
- Ali-Shtayeh, M.S.; Mara’i, A.-B.B.M.; Jamous, R.M. Distribution, occurrence and characterization of entomopathogenic fungi in agricultural soil in the Palestinian area. Mycopathologia 2003, 156, 235–244. [Google Scholar] [CrossRef]
- Sun, B.-D.; Liu, X.-Z. Occurrence and diversity of insect-associated fungi in natural soils in China. Appl. Soil Ecol. 2008, 39, 100–108. [Google Scholar] [CrossRef]
- Steinwender, B.M.; Enkerli, J.; Widmer, F.; Eilenberg, J.; Thorup-Kristensen, K.; Meyling, N.V. Molecular diversity of the entomopathogenic fungal Metarhizium community within an agroecosystem. J. Invertebr. Pathol. 2014, 123, 6–12. [Google Scholar] [CrossRef]
- Möller, E.M.; Bahnweg, G.; Sandermann, H.; Geiger, H.H. A simple and efficient protocol for isolation of high molecular weight DNA from filamentous fungi, fruit bodies, and infected plant tissues. Nucleic Acids Res. 1992, 20, 6115–6116. [Google Scholar] [CrossRef]
- Sharma, L.; Gonçalves, F.; Oliveira, I.; Torres, L.; Marques, G. Insect-associated fungi from naturally mycosed vine mealybug Planococcus ficus (Signoret) (Hemiptera: Pseudococcidae). Biocontrol. Sci. Technol. 2018, 28, 122–141. [Google Scholar] [CrossRef]
- Yurkov, A.; Guerreiro, M.A.; Sharma, L.; Carvalho, C.; Fonseca, Á. Correction: Multigene Assessment of the Species Boundaries and Sexual Status of the Basidiomycetous Yeasts Cryptococcus flavescens and C. terrestris (Tremellales). PLoS ONE 2015, 10, e0126996. [Google Scholar] [CrossRef] [PubMed]
- Bremner, J.M. Nitrogen-total. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 1085–1121. [Google Scholar]
- Bower, C.A.; Reitemeier, R.F.; Fireman, M. Exchangeable cation analysis of saline and alkali soils. Soil Sci. 1952, 73, 251–262. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis Part 3—Chemical Methods; Sparks, D.L., Page, A.L., Helmke, P.A., Loeppert, R.H., Eds.; Soil Science Society of America, American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Faul, F.; Erdfelder, E.; Buchner, A.; Lang, A.-G. Statistical power analyses using G*Power 3.1: Tests for correlation and regression analyses. Behav. Res. Methods 2009, 41, 1149–1160. [Google Scholar] [CrossRef] [PubMed]
- Jaronski, S.T. Ecological factors in the inundative use of fungal entomopathogens. BioControl 2010, 55, 159–185. [Google Scholar] [CrossRef]
- Reeleder, R.D.; Miller, J.J.; Ball Coelho, B.R.; Roy, R.C. Impacts of tillage, cover crop, and nitrogen on populations of earthworms, microarthropods, and soil fungi in a cultivated fragile soil. Appl. Soil Ecol. 2006, 33, 243–257. [Google Scholar] [CrossRef]
- Rousk, J.; Bååth, E. Fungal and bacterial growth in soil with plant materials of different C/N ratios. FEMS Microbiol. Ecol. 2007, 62, 258–267. [Google Scholar] [CrossRef]
- Birkhofer, K.; Bezemer, T.M.; Bloem, J.; Bonkowski, M.; Christensen, S.; Dubois, D.; Ekelund, F.; Fließbach, A.; Gunst, L.; Hedlund, K.; et al. Long-term organic farming fosters below and aboveground biota: Implications for soil quality, biological control and productivity. Soil Biol. Biochem. 2008, 40, 2297–2308. [Google Scholar] [CrossRef]
- Koorem, K.; Gazol, A.; Öpik, M.; Moora, M.; Saks, Ü.; Uibopuu, A.; Sõber, V.; Zobel, M. Soil nutrient content influences the abundance of soil microbes but not plant biomass at the small-scale. PLoS ONE 2014, 9, e91998. [Google Scholar] [CrossRef]
- Bednarek, A.; Gaugler, R. Compatibility of soil amendments with entomopathogenic nematodes. J. Nematol. 1997, 29, 220–227. [Google Scholar]
- Rangel, D.E.; Alston, D.G.; Roberts, D.W. Effects of physical and nutritional stress conditions during mycelial growth on conidial germination speed, adhesion to host cuticle, and virulence of Metarhizium anisopliae, an entomopathogenic fungus. Mycol. Res. 2008, 112, 1355–1361. [Google Scholar] [CrossRef]
- Barelli, L.; Moonjely, S.; Behie, S.W.; Bidochka, M.J. Fungi with multifunctional lifestyles: Endophytic insect pathogenic fungi. Plant Mol. Bio. 2016, 90, 657–664. [Google Scholar] [CrossRef] [PubMed]
- Morjan, W.E.; Pedigo, L.P.; Lewis, L.C. Fungicidal effects of glyphosate and glyphosate formulations on four species of entomopathogenic fungi. Environ. Entomol. 2002, 31, 1206–1212. [Google Scholar] [CrossRef]
- Andaló, V.; Jr, A.; Santa-Cecília, L.; Souza, G. Compatibility of Beauveria bassiana with chemical pesticides for the control of the coffee root mealybug Dysmicoccus texensis Tinsley (Hemiptera: Pseudococcidae). Neotrop. Entomol. 2004, 33, 463–467. [Google Scholar] [CrossRef]
- Meyling, N.V.; Eilenberg, J. Ecology of the entomopathogenic fungi Beauveria bassiana and Metarhizium anisopliae in temperate agroecosystems: Potential for conservation biological control. Biol. Control 2007, 43, 145–155. [Google Scholar] [CrossRef]
- Klingen, I.; Haukeland, S. The soil as a reservoir for natural enemies of pest insects and mites with emphasis on fungi and nematodes. In An Ecological and Societal Approach to Biological Control; Eilenberg, J., Hokkanen, H.M.T., Eds.; Springer: Dordrecht, The Netherlands, 2006; pp. 145–211. [Google Scholar] [CrossRef]
- Bidochka, M.J.; Kasperski, J.E.; Wild, G.A.M. Occurrence of the entomopathogenic fungi metarhizium anisopliae and Beauveria bassiana in soils from temperate and near-northern habitats. Can. J. Bot. 1998, 76, 1198–1204. [Google Scholar]
- Cohen, E.; Joseph, T. Photostabilization of Beauveria bassiana conidia using anionic dyes. Appl. Clay Sci. 2009, 42, 569–574. [Google Scholar] [CrossRef]
- Cozzi, G.; Somma, S.; Haidukowski, M.; Logrieco, A.F. Ochratoxin A management in vineyards by Lobesia botrana biocontrol. Toxins 2013, 5, 49–59. [Google Scholar] [CrossRef]
- Sharma, L.; Bohra, N.; Singh, R.K.; Marques, G. Potential of Entomopathogenic Bacteria and Fungi. In Microbes for Sustainable Insect Pest Management: An Eco-Friendly Approach; Khan, M.A., Ahmad, W., Eds.; Springer International Publishing: Cham, Switzerland, 2019; Volume 1, pp. 115–149. [Google Scholar] [CrossRef]
- Ownley, B.H.; Gwinn, K.D.; Vega, F.E. Endophytic fungal entomopathogens with activity against plant pathogens: Ecology and evolution. BioControl 2010, 55, 113–128. [Google Scholar] [CrossRef]
- Lacey, L.A.; Grzywacz, D.; Shapiro-Ilan, D.I.; Frutos, R.; Brownbridge, M.; Goettel, M.S. Insect pathogens as biological control agents: Back to the future. J. Invertebr. Pathol. 2015, 132, 1–41. [Google Scholar] [CrossRef]
- Scheepmaker, J.W.A.; Butt, T.M. Natural and released inoculum levels of entomopathogenic fungal biocontrol agents in soil in relation to risk assessment and in accordance with EU regulations. Biocontrol Sci. Technol. 2010, 20, 503–552. [Google Scholar] [CrossRef]

| Soil Properties | Beauveria bassiana | Purpureocillium lilacinum | Metarhizium robertsii | Clonostachys rosea f. rosea | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MW-U a | Mean for Absence | Mean for Presence | pa | MW-U a | Mean for Absence | Mean for Presence | pa | MW-U a | Mean for Absence | Mean for Presence | pa | MW-U a | Mean for Absence | Mean for Presence | pa | |
| P (mg/kg) | 113 | 49.37 ± 52.57 | 20.38 ± 17.79 | 0.02 | 60 | 43.43 ± 48.61 | 17.95 ± 14.10 | 0.11 | 87 | 44.28 ± 49.48 | 20.99 ± 19.55 | 0.121 | 208 | 40.13 ± 47.55 | 40.6 ± 46.75 | 0.419 |
| K (mg/kg) | 140.5 | 119.59 ± 133.11 | 80.48 ± 37.29 | 0.097 | 43 | 113.90 ± 119.40 | 60 ± 11.22 | 0.027 | 78.5 | 106.81 ± 123.19 | 110.86 ± 0.77 | 0.07 | 182 | 118.98 ± 165.95 | 99.01 ± 48.01 | 0.193 |
| OM content (%) | 168 | 3.69 ± 3.28 | 3.7 ± 3.47 | 0.295 | 85 | 3.75 ± 3.37 | 3.24 ± 3.01 | 0.396 | 54.5 | 4.08 ± 3.45 | 1.77 ± 1.34 | 0.009 | 207 | 3.81 ± 3.79 | 3.6 ± 2.96 | 0.409 |
| N (g/kg) | 169 | 1.88 ± 1.26 | 1.83 ± 1.32 | 0.305 | 82 | 1.89 ± 1.28 | 1.64 ± 1.26 | 0.353 | 51.5 | 2.02 ± 1.29 | 1.08 ± 0.77 | 0.007 | 200.5 | 1.85 ± 1.44 | 1.88 ± 1.14 | 0.347 |
| C:N | 168 | 0.0104 ± 0.0017 | 0.01 ± 0.002 | 0.999 | 78 | 0.01 ± 0.001 | 0.01 ± 0.0016 | 0.298 | 60 | 0.01 ± 0.0018 | 0.01 ± 0.0011 | 0.017 | 165 | 0.01 ± 0.001 | 0.01 ± 0.001 | 0.097 |
| pH_H2O | 114.5 | 5.55 ± 0.67 | 6.04 ± 0.83 | 0.021 | tb = −1.06 df b = 40 | 5.66 ± 0.77 | 6.03 ± 0.5 | 0.147 b | tb = 1.336 df b = 40 | 5.77 ± 0.68 | 5.36 ± 1.03 | 0.094 b | tb = 0.596 df b = 40 | 5.78 ± 0.76 | 5.64 ± 0.75 | 0.277 b |
| pH_KCl | tb = −1.688 df b = 40 | 4.41 ± 0.71 | 4.82 ± 0.77 | 0.049 b | tb = −0.57 df b = 40 | 4.52 ± 0.77 | 4.72 ± 0.54 | 0.286 b | 110 | 4.55 ± 0.72 | 4.49 ± 0.91 | 0.346 | tb = 1.294 df b = 40 | 4.71 ± 0.68 | 4.41 ± 0.78 | 0.101 b |
| TA | 125 | 0.40 ± 0.45 | 0.15 ± 0.22 | 0.043 | 65.5 | 0.35 ± 0.42 | 0.12 ± 022 | 0.152 | 109 | 0.31 ± 0.41 | 0.38 ± 0.4 | 0.334 | 175 | 0.22 ± 0.29 | 0.4 ± 0.46 | 0.137 |
| DBS (%) | 133 | 91.90 ± 9.52 | 96.41 ± 5.76 | 0.068 | 65.5 | 92.67 ± 9.03 | 97.92 ± 3.96 | 0.152 | 103 | 93.87 ± 8.38 | 90.42 ± 10.47 | 0.265 | 192 | 94.49 ± 7.53 | 92.41 ± 9.57 | 0.261 |
| Ca2+ (cmol/kg) | 154.5 | 3.7 ± 2.06 | 4.81 ± 2.95 | 0.18 | 91 | 4.07 ± 2.47 | 3.85 ± 1.89 | 0.485 | 83 | 4.14 ± 2.18 | 3.57 ± 3.47 | 0.096 | 160 | 3.66 ± 2.33 | 4.33 ± 2.45 | 0.077 |
| Mg2+ (cmol/kg) | 162.5 | 1.28 ± 0.790 | 1.27 ± 0.49 | 0.242 | 55 | 1.24 ± 0.71 | 1.56 ± 0.57 | 0.077 | 65 | 1.36 ± 0.72 | 0.83 ± 0.40 | 0.026 | 201.5 | 1.43 ± 0.9 | 1.16 ± 0.5 | 0.356 |
| K+ (cmol/kg) | 110 | 0.33 ± 0.18 | 0.22 ± 0.10 | 0.016 | 35.5 | 0.31 ± 0.17 | 0.19 ± 0.06 | 0.011 | 115 | 0.30 ± 0.17 | 0.3 ± 0.14 | 0.408 | 142 | 0.26 ± 0.17 | 0.33 ± 0.15 | 0.03 |
| Na+ (cmol/kg) | 154 | 0.14 ± 0.12 | 0.21 ± 0.23 | 0.18 | 76 | 0.17 ± 0.17 | 0.09 ± 0.05 | 0.273 | 45 | 0.13 ± 0.15 | 0.28 ± 0.14 | 0.003 | 208 | 0.14 ± 0.11 | 0.18 ± 0.19 | 0.419 |
| CEC (cmol/kg) | 168 | 5.84 ± 2.41 | 6.69 ± 0.86 | 0.295 | 90 | 6.15 ± 2.75 | 5.80 ± 1.79 | 0.47 | 82 | 6.25 ± 2.48 | 5.36 ± 3.49 | 0.09 | 160 | 5.71 ± 2.91 | 6.4 ± 2.44 | 0.077 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sharma, L.; Oliveira, I.; Gonçalves, F.; Raimundo, F.; Singh, R.K.; Torres, L.; Marques, G. Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields. Pathogens 2021, 10, 137. https://doi.org/10.3390/pathogens10020137
Sharma L, Oliveira I, Gonçalves F, Raimundo F, Singh RK, Torres L, Marques G. Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields. Pathogens. 2021; 10(2):137. https://doi.org/10.3390/pathogens10020137
Chicago/Turabian StyleSharma, Lav, Irene Oliveira, Fátima Gonçalves, Fernando Raimundo, Rupesh Kumar Singh, Laura Torres, and Guilhermina Marques. 2021. "Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields" Pathogens 10, no. 2: 137. https://doi.org/10.3390/pathogens10020137
APA StyleSharma, L., Oliveira, I., Gonçalves, F., Raimundo, F., Singh, R. K., Torres, L., & Marques, G. (2021). Effect of Soil Chemical Properties on the Occurrence and Distribution of Entomopathogenic Fungi in Portuguese Grapevine Fields. Pathogens, 10(2), 137. https://doi.org/10.3390/pathogens10020137







