Retention in Care, Mortality, Loss-to-Follow-Up, and Viral Suppression among Antiretroviral Treatment-Naïve and Experienced Persons Participating in a Nationally Representative HIV Pre-Treatment Drug Resistance Survey in Mexico
Abstract
:1. Introduction
2. Results
2.1. Study Population Description
2.2. Characteristics of Participants without Information in the National HIV Database
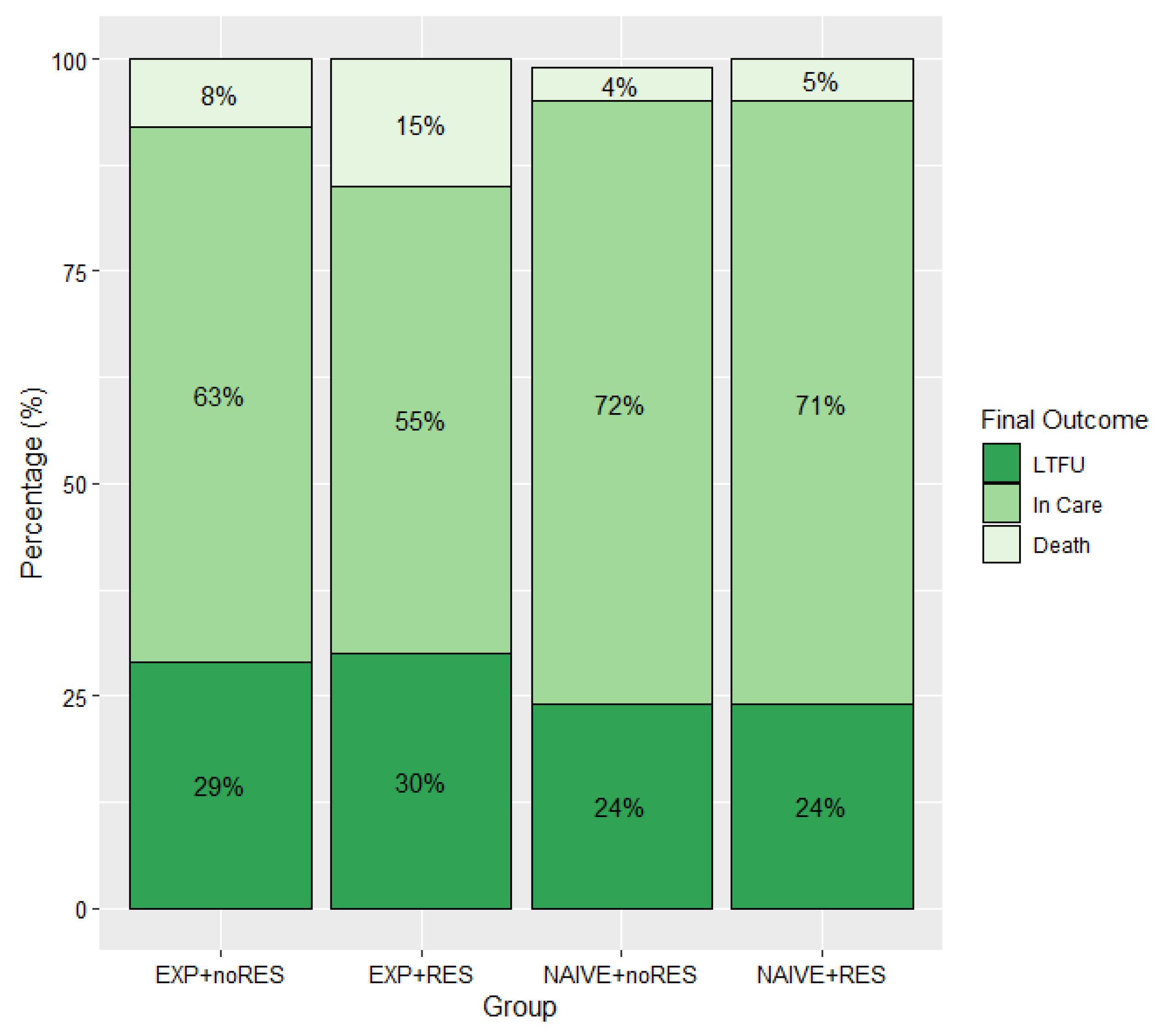
2.3. Final Outcomes
2.3.1. Viral Suppression
2.3.2. Change in Antiretroviral Treatment Regimen
2.3.3. Viral Suppression Outcome with Lost to Follow-Up and Death as Competing Events
3. Discussion
4. Materials and Methods
4.1. Data Source
4.2. Sample Selection
4.3. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guidelines on the Public Health Response to Pretreatment HIV Drug Resistance. July 2017. Available online: https://apps.who.int/iris/bitstream/handle/10665/255880/9789241550055-eng.pdf;jsessionid=1FA5DAAEDF96CC18BAB5E3AAD2DCA704?sequence=1 (accessed on 28 November 2021).
- Bertagnolio, S.; Hermans, L.; Jordan, M.R.; Avila-Rios, S.; Iwuji, C.; Derache, A.; Delaporte, E.; Wensing, A.; Aves, T.; Borhan, A.S.M.; et al. Clinical Impact of Pretreatment Human Immunodeficiency Virus Drug Resistance in People Initiating Nonnucleoside Reverse Transcriptase Inhibitor-Containing Antiretroviral Therapy: A Systematic Review and Meta-analysis. J. Infect. Dis. 2021, 224, 377–388. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.K.; Gregson, J.; Parkin, N.; Haile-Selassie, H.; Tanuri, A.; Andrade Forero, L.; Kaleebu, P.; Watera, C.; Aghokeng, A.; Mutenda, N.; et al. HIV-1 drug resistance before initiation or re-initiation of first-line antiretroviral therapy in low-income and middle-income countries: A systematic review and meta-regression analysis. Lancet Infect. Dis. 2018, 18, 346–355. [Google Scholar] [CrossRef] [Green Version]
- UNAIDS. 90-90-90 An Ambitious Treatment Target to Help End the AIDS Epidemic. 2014. Available online: https://www.unaids.org/sites/default/files/media_asset/90-90-90_en.pdf (accessed on 15 July 2018).
- García-Morales, C.; Tapia-Trejo, D.; Quiroz-Morales, V.S.; Navarro-Álvarez, S.; Barrera-Arellano, C.A.; Casillas-Rodríguez, J.; Romero-Mora, K.A.; Gómez-Palacio-Schjetnan, M.; Murakami-Ogasawara, A.; Ávila-Ríos, S.; et al. HIVDR MexNet Group. HIV pretreatment drug resistance trends in three geographic areas of Mexico. J. Antimicrob. Chemother. 2017, 72, 3149–3158. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. HIV Drug Resistance Report 2019; (WHO/CDS/HIV/19.21). Licence: CC BY-NC-SA 3.0 IGO; World Health Organization: Geneva, Switzerland, 2019; Available online: https://www.who.int/hiv/pub/drugresistance/hivdr-report-2019/en/ (accessed on 28 November 2021).
- Ávila-Ríos, S.; García-Morales, C.; Valenzuela-Lara, M.; Chaillon, A.; Tapia-Trejo, D.; Pérez-García, M.; López-Sánchez, D.M.; Maza-Sánchez, L.; del Arenal-Sánchez, S.J.; Paz-Juárez, H.E.; et al. HIV-1 drug resistance before initiation or re-initiation of first-line ART in eight regions of Mexico: A sub-nationally representative survey. J. Antimicrob. Chemother. 2019, 74, 1044–1055. [Google Scholar] [CrossRef] [PubMed]
- Guía de Manejo Antirretroviral de las Personas con VIH. México: Censida/Secretaría de Salud Décima Edición. 2019. Available online: https://www.gob.mx/cms/uploads/attachment/file/569287/GUIA_DE_MANEJO_ANTIRRETROVIRAL_DE_LAS_PERSONAS_CON_VIH_2019_-_VERSI_N_COMPLETA1.pdf (accessed on 28 November 2021).
- WHO. HIV Drug Resistance Surveillance Concept Notes. Available online: https://www.who.int/hiv/topics/drugresistance/protocols/en/ (accessed on 28 November 2021).
- Ávila-Ríos, S.; García-Morales, C.; Matías-Florentino, M.; Romero-Mora, K.A.; Tapia-Trejo, D.; Quiroz-Morales, V.S.; Reyes-Gopar, H.; Ji, H.; Sandstrom, P.; Casillas-Rodríguez, J.; et al. HIVDR MexNet Group. Pretreatment HIV-drug resistance in Mexico and its impact on the effectiveness of first-line antiretroviral therapy: A nationally representative 2015 WHO survey. Lancet HIV 2016, 12, e579–e591. [Google Scholar] [CrossRef]
- Avila-Rios, S.; Sued, O.; Rhee, S.Y.; Shafer, R.W.; Reyes-Teran, G.; Ravasi, G. Surveillance of HIV Transmitted Drug Resistance in Latin America and the Caribbean: A Systematic Review and Meta-Analysis. PLoS ONE 2016, 11, e0158560. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bautista-Arredondo, S.; Servan-Mori, E.; Beynon, F.; González, A.; Volkow, P. A tale of two epidemics: Gender differences in socio-demographic characteristics and sexual behaviors among HIV positive individuals in Mexico City. Int. J. Equity Health 2015, 14, 147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boender, T.S.; Hoenderboom, B.M.; Sigaloff, K.C.; Hamers, R.F.; Wellington, M.; Shamu, T.; Siwale, M.; Labib Maksimos, E.E.F.; Nankya, I.; Kityo, C.M.; et al. Pretreatment HIV drug resistance increases regimen switches in sub-Saharan Africa. Clin. Infect. Dis. 2015, 61, 1749–1758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aguilar-Zapata, D.; Piñeirúa-Menéndez, A.; Volkow-Fernández, P.; Rodríguez-Zulueta, P.; Ramos-Alamillo, U.; Cabrera-López, T.; Martin-Onraet, A. Sociodemographic differences among HIV-positive and HIV-negative recently pregnant women in Mexico City. Medicine 2017, 96, e7305. [Google Scholar] [CrossRef] [PubMed]
- Ramírez, R. HIV/HEP 2019. P012. Barriers to access HIV-healthcare services for women living with HIV in Mexico. J. Int. AIDS Soc. 2019, 22, e25263. [Google Scholar]
- De la Torre-Rosas, A. Estado Actual de la Epidemia en México ¿Cómo van las Metas? 2021. Available online: https://simposiovih-sida.mx/assets/pdf/PROGRAMA_SIMPOSIO_VIH_SIDA2021.pdf (accessed on 28 November 2021).
- Sierra-Madero, J.G.; Belaunzaran-Zamudio, P.F.; Crabtree-Ramírez, B.; Magis-Rodriguez, C. Mexico’s fragmented health system as a barrier to HIV care. Lancet HIV 2019, 2, e74–e75. [Google Scholar] [CrossRef]
- Piñeirúa-Menendez, A.; Del Hoyo, M.; Osorno González de León, F.; Badial Hernández, F.; Niño-Vargas, R. (Mexico City, Mexico). P035. HIV/HEP2018. The Continuum of Care among Very Immunosuppressed (Less Than 100 CD4+ Cells) Patients in an Outpatient Clinic in Mexico City: Gaps in Diagnosis, Linkage and Retention in Care. Available online: http://www.hivhepamericas.org/wp-content/uploads/2018/05/P035.pdf (accessed on 28 November 2021).
| Experienced -Resistant N = 20 | Experienced -Non-Resistant N = 211 | Naïve -Resistant N = 165 | Naïve -Non-Resistant N = 1427 | p-Value 1 | |
|---|---|---|---|---|---|
| Female; n (%) | 9 (45%) | 69 (33%) | 32 (19%) | 223 (16%) | <0.01 |
| Median age (years); (IQR) | 34 (28–39) | 34 (27–42) | 30 (25–41) | 29 (25–38) | <0.01 |
| Transmission risk *; n (%) Heterosexual cisgender women MSM Heterosexual cisgender men PWID | 8 (40%) 5 (20%) 5 (20%) 1 (0.5%) | 62 (29%) 83 (39%) 34 (16%) 13 (6%) | 30 (18%) 82 (49%) 35 (21%) 3 (1.8%) | 204 (14%) 838 (59%) 252 (18%) 26 (1.8%) | < 0.01 |
| Mean CD4+ T cell count; cells/mm3 (IQR) | 223 (58–410) | 143 (53–343) | 244 (94–459) | 237 (91–413) | 0.31 |
| Education; n (%) Elementary or lower High school or higher Unknown | 8 (40%) 12 (60%) 0 (0%) | 62 (29%) 141 (67%) 8 (4%) | 31 (19%) 128 (77%) 6 (4%) | 217 (15%) 1159 (81%) 51 (3.5%) | <0.01 |
| Occupation; n (%) Employed Unemployed Student | 4 (20%) 16 (80%) 0 (0%) | 81 (40%) 107 (53%) 15 (7%) | 88 (51%) 63 (37%) 21 (12%) | 824 (54%) 532 (35%) 154 (10%) | <0.01 |
| Median time of follow-up (years); (IQR) | 1.93 (0.85–2.08) | 1.91 (1.51–2.04) | 1.86 (1.66–2.05) | 1.87 (1.60–2.01) | 0.74 |
| First ART regimen group; n (%) Based on EFV Integrase Inhibitors Protease Inhibitors Other | 9 (45%) 3 (15%) 7 (35%) 1 (5%) | 120 (58%) 15 (7%) 68 (33%) 4 (2%) | 100 (64%) 50 (33%) 4 (2%) 1 (1%) | 907 (67%) 366 (27%) 70 (5%) 3 (0.2%) | <0.01 |
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| Characteristics | OR; IC95% | p-Value | OR; IC95% | p-Value | OR; IC95% | p-Value |
| Group | ||||||
| Naïve + non-resistant | 1 | 1 | 1 | |||
| Naïve + resistant | 0.74; 0.50–1.09 | 0.14 | 0.79; 0.53–1.17 | 0.25 | 0.71; 0.47–1.07 | 0.10 |
| Experienced + non-resistant | 0.46; 0.32–0.66 | <0.001 | 0.49; 0.34–0.70 | <0.001 | 0.37; 0.24–0.53 | <0.001 |
| Experienced + resistant | 0.28; 0.09–0.87 | 0.02 | 0.39; 0.13–1.15 | 0.09 | 0.26; 0.08–0.83 | 0.02 |
| CD4+ T cell count at the time of HIV drug resistance test (cells/mm3) | 0.16 | 0.27 | 0.04 | |||
| 100 | 1 | 1 | 1 | |||
| 200 300 400 | 1.04; 0.89–1.21 1.03; 0.82–1.31 0.98; 0.77–1.25 | 1.04; 0.90–1.21 1.05; 0.84–1.32 1.00; 0.78–1.28 | 0.99; 0.85–1.16 0.95; 0.74–1.22 0.88; 0.67–1.14 | |||
| Age at the time of HIV drug resistance test (years) 1 | 0.17 | 0.77 | ||||
| 30 | 1 | 1 | 1 | 0.03 | ||
| 40 | 1.25; 0.89–1.48 | 1.09; 0.97–1.24 | 1.10; 0.84–1.44 | |||
| 50 | 1.45; 1.04–2.04 | 1.29; 0.93–1.79 | 1.52; 1.05–2.19 | |||
| Transmission Risk | ||||||
| MSM | 1 | 1 | 1 | |||
| Heterosexual cisgender men | 0.77: 0.56–1.06 | 0.11 | 0.80; 0.58–1.07 | 0.13 | 0.75; 0.54–1.05 | 0.09 |
| Heterosexual cisgender women | 0.74; 0.53–1.03 | 0.82 | 0.73; 0.53–1.00 | 0.69 | 0.76; 0.53–1.08 | 0.97 |
| Education level | 0.56 | 0.73 | ||||
| Elementary or lower | 0.90; 0.64–1.27 | 0.94; 0.68–0.30 | 0.89; 0.62–1.28 | 0.55 | ||
| High school or higher | 1 | 1 | 1 | |||
| Employment status | 0.73 | 0.99 | ||||
| Unemployed | 0.96; 0.75–1.22 | 1.00; 0.79–1.27 | 0.76; 0.59–0.99 | 0.004 | ||
| Employed | 1 | 1 | 1 | |||
| Change in ART regimen | NA | NA | NA | NA | 1.78; 1.15–2.75 | 0.009 |
| HR 1 | 95% CI | p-Value | |
|---|---|---|---|
| Group | |||
| Naïve + non-resistant | 1 | ||
| Naïve + resistant | 0.97 | (0.78–1.22) | 0.81 |
| Experienced + non-resistant | 0.68 | (0.53–0.86) | <0.001 |
| Experienced + resistant | 0.37 | (0.17–0.84) | 0.01 |
| CD4+ T cell count at the time of HIV drug resistance test (per 100 cells/mm3) | 0.99 | (0.96–1.03) | 0.78 |
| Age at the time of HIV drug resistance test | 1.03 | (0.97–1.10) | 0.40 |
| Transmission risk | |||
| MSM | 1 | ||
| Heterosexual cisgender women | 0.84 | (0.70–1.01) | 0.06 |
| Heterosexual cisgender men | 0.91 | (0.76–1.09) | 0.29 |
| Education level | |||
| Elementary or lower | 1 | ||
| High school or higher | 1.14 | (0.99–1.31) | 0.07 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Caro-Vega, Y.; Alarid-Escudero, F.; Enns, E.A.; Sosa-Rubí, S.; Chivardi, C.; Piñeirúa-Menendez, A.; García-Morales, C.; Reyes-Terán, G.; Sierra-Madero, J.G.; Ávila-Ríos, S. Retention in Care, Mortality, Loss-to-Follow-Up, and Viral Suppression among Antiretroviral Treatment-Naïve and Experienced Persons Participating in a Nationally Representative HIV Pre-Treatment Drug Resistance Survey in Mexico. Pathogens 2021, 10, 1569. https://doi.org/10.3390/pathogens10121569
Caro-Vega Y, Alarid-Escudero F, Enns EA, Sosa-Rubí S, Chivardi C, Piñeirúa-Menendez A, García-Morales C, Reyes-Terán G, Sierra-Madero JG, Ávila-Ríos S. Retention in Care, Mortality, Loss-to-Follow-Up, and Viral Suppression among Antiretroviral Treatment-Naïve and Experienced Persons Participating in a Nationally Representative HIV Pre-Treatment Drug Resistance Survey in Mexico. Pathogens. 2021; 10(12):1569. https://doi.org/10.3390/pathogens10121569
Chicago/Turabian StyleCaro-Vega, Yanink, Fernando Alarid-Escudero, Eva A. Enns, Sandra Sosa-Rubí, Carlos Chivardi, Alicia Piñeirúa-Menendez, Claudia García-Morales, Gustavo Reyes-Terán, Juan G. Sierra-Madero, and Santiago Ávila-Ríos. 2021. "Retention in Care, Mortality, Loss-to-Follow-Up, and Viral Suppression among Antiretroviral Treatment-Naïve and Experienced Persons Participating in a Nationally Representative HIV Pre-Treatment Drug Resistance Survey in Mexico" Pathogens 10, no. 12: 1569. https://doi.org/10.3390/pathogens10121569
APA StyleCaro-Vega, Y., Alarid-Escudero, F., Enns, E. A., Sosa-Rubí, S., Chivardi, C., Piñeirúa-Menendez, A., García-Morales, C., Reyes-Terán, G., Sierra-Madero, J. G., & Ávila-Ríos, S. (2021). Retention in Care, Mortality, Loss-to-Follow-Up, and Viral Suppression among Antiretroviral Treatment-Naïve and Experienced Persons Participating in a Nationally Representative HIV Pre-Treatment Drug Resistance Survey in Mexico. Pathogens, 10(12), 1569. https://doi.org/10.3390/pathogens10121569






