Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China
Abstract
:1. Introduction
2. Results
2.1. Disease Symptoms Identified in the Field
2.2. Isolation of Fungi
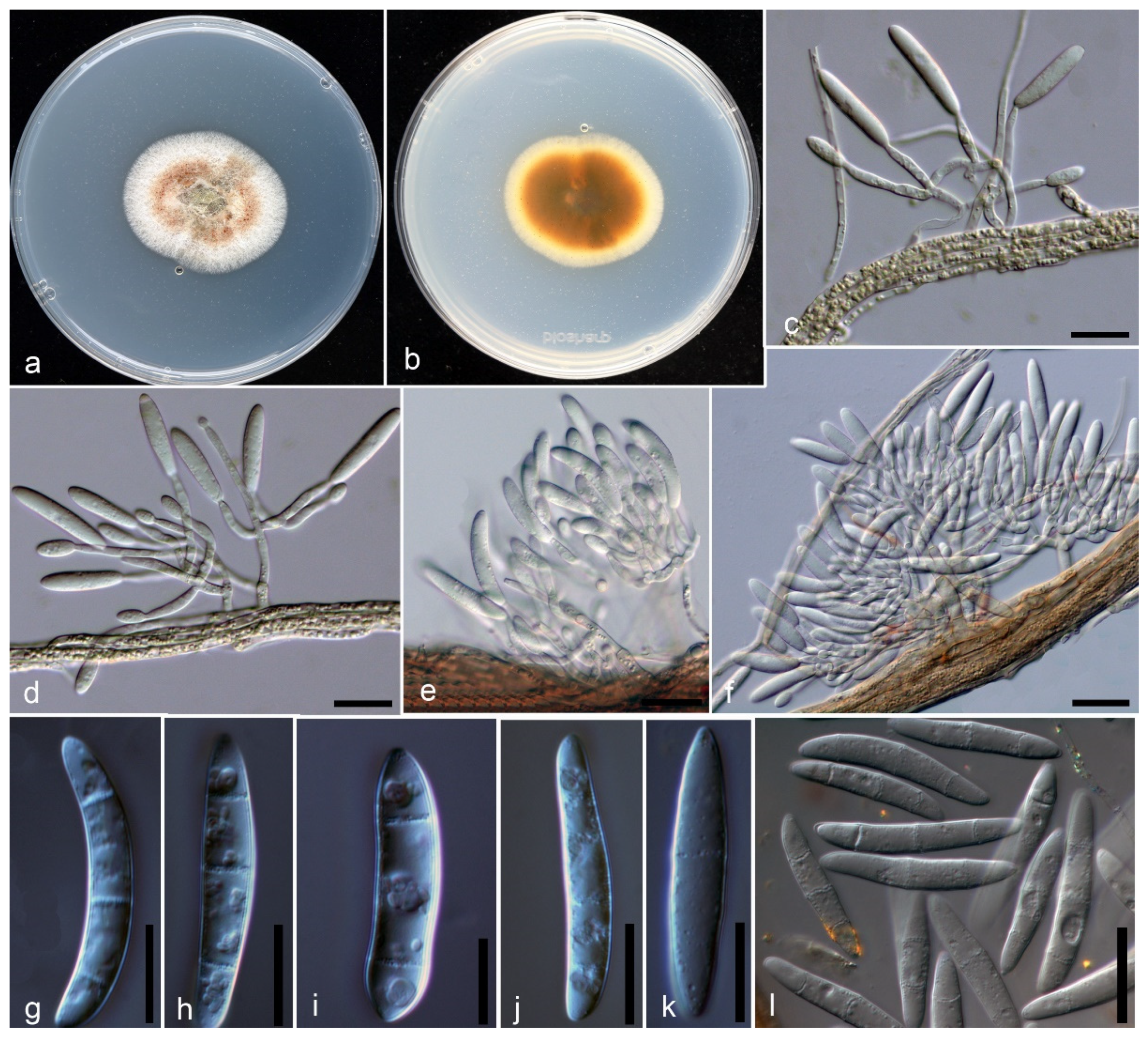
2.3. Morphological Characterization
Taxonomy
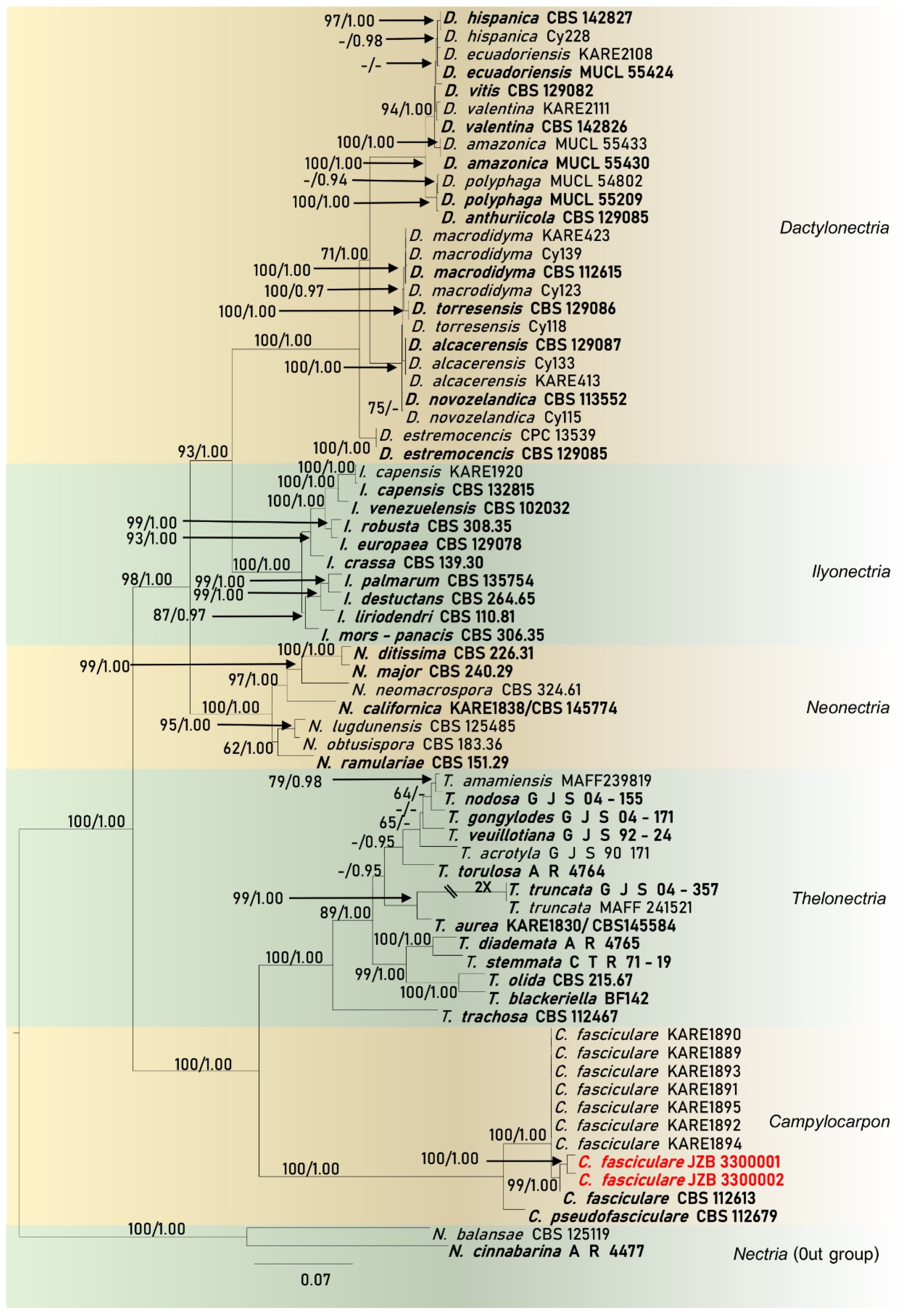
2.4. Molecular Characterization and Multi-marker Analysis
2.5. Pathogenicity Study
3. Discussion
Investigations of Grapevine Trunk Diseases (GTD) in China
4. Materials and Methods
4.1. Field Surveys and Fungal Isolation
4.2. Morphological Characterization
4.3. Molecular Characterization and Multi-Marker Analysis
Phylogenetic Analysis
4.4. Pathogenicity Study
4.4.1. Inoculum Preparation
4.4.2. Plant Material Preparation
4.4.3. Pot Preparation and Inoculation
5. Conclusions and Future Prospects
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Shao-Hua, L. Grape production in China. In Grape Production in the Asia-Pacific Region; Papademetriou, M.K., Dent, F.J., Eds.; FAO: Rome, Italy, 2001; pp. 19–27. [Google Scholar]
- Hua, L.; Hua, W.; Huanmei, L.; Goodman, S.; van der Lee, P.; Zhimin, X.; Fortunato, A.; Ping, Y. The worlds of wine: Old, new and ancient. Wine Econ. Policy 2018, 7, 178–182. [Google Scholar]
- OIV. 2019 Statistical Report on World Vitiviniculture; International Organization of Vine and Wine: Paris, France, 2019. [Google Scholar]
- Halleen, F.; Schroers, H.J.; Groenewald, J.Z.; Crous, P.W. Novel species of Cylindrocarpon (Neonectria) and Campylocarpon gen. nov. associated with black foot disease of grapevines (Vitis spp.). Stud. Mycol. 2004, 50, 431–455. [Google Scholar]
- Halleen, F.; Fourie, P.H.; Crous, P.W. A review of black foot disease of grapevine. Phytopathol. Mediterr. 2006, 45, 55–67. [Google Scholar]
- Petit, E.; Gubler, W.D. Characterization of Cylindrocarpon species, the cause of black foot disease of grapevine in California. Plant Dis. 2005, 1051–1059. [Google Scholar] [CrossRef] [Green Version]
- Scheck, H.J.; Vasquez, S.J.; Gubler, W.D.; Fogle, D. First report of black foot disease, caused by Cylindrocarpon obtusisporum, of grapevine in California. Plant Dis. 1998, 82, 448. [Google Scholar] [CrossRef]
- Whitelaw-Weckert, M.A.; Nair, N.G.; Lamont, R.; Alonso, M.; Priest, M.; Huang, R. Root infection of Vitis vinifera by Cylindrocarpon liriodendri in Australia. Australas. Plant Pathol. 2007, 36, 403–406. [Google Scholar] [CrossRef]
- Petit, E.; Gubler, W.D. First report of Cylindrocarpon liriodendri causing black foot disease of grapevine in California. Plant Dis. 2007, 91, 1060. [Google Scholar] [CrossRef]
- Auger, J.; Esterio, M.; Pérez, I. First report of black foot disease of grapevine caused by Cylindrocarpon macrodidymum in Chile. Plant Dis. 2007, 91, 470. [Google Scholar] [CrossRef]
- Grasso, S.; Magnano Di San Lio, G. Infections of Cylindrocarpon obtusisporum on grapevines in Sicily. Vitis 1975, 14, 36–39. [Google Scholar]
- Choueiri, E.; Jreijiri, E.; El Amil, R.; Chela, P.; Bugaret, Y.; Liminana, J.M.; Mayet, V.; Lecomte, P. First report of black foot disease associated with Cylindrocarpon sp. in Lebanon. J. Plant Pathol. 2009, 91, 237. [Google Scholar]
- Rego, C.; Oliveira, H.; Carvalho, A.; Oliveira, H. Involvement of Phaeoacremonium spp. and Cylindrocarpon destructans with grapevine decline in Portugal. Phytopathol. Mediterr. 2000, 39, 76–79. [Google Scholar]
- Alaniz, S.; Armengol, J.; León, M.; García-Jiménez, J.; Abad-Campos, P. Analysis of genetic and virulence diversity of Cylindrocarpon liriodendri and C. macrodidymum associated with black foot disease of grapevine. Mycol. Res. 2009, 113, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Abreo, E.S.; Martínez, L.; Bettucci, L.; Lupo, S. Morphological and molecular characterization of Campylocarpon and Cylindrocarpon spp. associated with black foot disease of grapevines in Uruguay. Australas. Plant Pathol. 2010, 39, 446–452. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; Armengol, J. Black-foot disease of grapevine: An update on taxonomy, epidemiology and management strategies. Phytopathol. Mediterr. 2013, 1, 245–261. [Google Scholar]
- Lombard, L.; Van Der Merwe, N.A.; Groenewald, J.Z.; Crous, P.W. Lineages in Nectriaceae: Re-evaluating the generic status of Ilyonectria and allied genera. Phytopathol. Mediterr. 2014, 1, 515–532. [Google Scholar]
- Carlucci, A.; Lops, F.; Mostert, L.; Halleen, F.; Raimondo, M.L. Occurrence fungi causing black foot on young grapevines and nursery rootstock plants in Italy. Phytopathol. Mediterr. 2017, 56, 10–39. [Google Scholar]
- Alaniz, S.; León, M.; Vicent, A.; García-Jiménez, J.; Abad-Campos, P.; Armengol, J. Characterization of Cylindrocarpon species associated with black foot disease of grapevine in Spain. Plant Dis. 2007, 91, 1187–1193. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, H.; Alaniz, S.; Banihashemi, Z.; Armengol, J. Characterization of Cylindrocarpon liriodendri associated with black foot disease of grapevine in Iran. J. Phytopathol. 2009, 157, 642–645. [Google Scholar] [CrossRef]
- Petit, E.J.; Barriault, E.; Baumgartner, K.; Wilcox, W.F.; Rolshausen, P.E. Cylindrocarpon species associated with black-foot of grapevine in northeastern United States and southeastern Canada. Am. J. Enol. Vitic. 2011, 62, 177–183. [Google Scholar] [CrossRef]
- Úrbez-Torres, J.R.; Haag, P.; Bowen, P.; O’Gorman, D.T. Grapevine trunk diseases in British Columbia: Incidence and characterization of the fungal pathogens associated with black foot disease of grapevine. Plant Dis. 2014, 98, 456–468. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.; Groenewald, J.Z.; Rego, M.C.N.F.; Oliveira, H.; Crous, P.W. Cylindrocarpon root rot: Multi-gene analysis reveals novel species within the Ilyonectria radicicola species complex. Mycol. Prog. 2012, 11, 655–688. [Google Scholar] [CrossRef] [Green Version]
- Cabral, A.; Rego, C.; Nascimento, T.; Oliveira, H.; Groenewald, J.Z.; Crous, P.W. Multi-gene analysis and morphology reveal novel Ilyonectria species associated with black foot disease of grapevines. Fungal Biol. 2012, 116, 62–80. [Google Scholar] [CrossRef] [PubMed]
- Bleach, C.M. Management of Cylindrocarpon Black Foot Disease in New Zealand Nurseries and Vineyards. Ph.D. Thesis, Lincoln University, Lincoln, New Zealand, 2013. [Google Scholar]
- Salgado-Salazar, C.; Rossman, A.Y.; Chaverri, P. The genus Thelonectria (Nectriaceae, Hypocreales, Ascomycota) and closely related species with cylindrocarpon-like asexual states. Fungal Divers. 2016, 80, 411–455. [Google Scholar] [CrossRef]
- Lawrence, D.P.; Nouri, M.T.; Trouillas, F.P. Taxonomy and multi-locus phylogeny of cylindrocarpon-like species associated with diseased roots of grapevine and other fruit and nut crops in California. Fungal Syst. Evol. 2019, 4, 59–75. [Google Scholar] [CrossRef]
- Manawasinghe, I.S.; Dissanayake, A.; Li, X.; Liu, M.; Wanasinghe, D.; Xu, J.; Zhao, W.; Wei, Z.; Zhou, Y.; Hyde, K.D.; et al. High genetic diversity and species complexity of Diaporthe associated with Grapevine Dieback in China. Front. Microbiol. 2019, 10, 1936. [Google Scholar] [CrossRef]
- Yan, J.-Y.; Xie, Y.; Zhang, W.; Wang, Y.; Liu, J.-K.; Hyde, K.D.; Seem, R.C.; Zhang, G.-Z.; Wang, Z.-Y.; Yao, S.-W.; et al. Species of Botryosphaeriaceae involved in grapevine dieback in China. Fungal Divers. 2013, 61, 221–236. [Google Scholar] [CrossRef]
- Yan, J.Y.; Zhao, W.S.; Chen, Z. Comparative genome and transcriptome analyses reveal adaptations to opportunistic infections in woody plant degrading pathogens of Botryosphaeriaceae. DNA Res. 2018, 25, 87–102. [Google Scholar] [CrossRef] [Green Version]
- Dissanayake, A.J.; Liu, M.; Zhang, W.; Chen, Z.; Udayanga, D.; Chukeatirote, E.; Li, X.; Yan, J.; Hyde, K.D. Morphological and molecular characterization of Diaporthe species associated with grapevine trunk disease in China. Fungal Biol. 2015, 119, 283–294. [Google Scholar] [CrossRef]
- Ye, Q.T.; Manawasinghe, I.S.; Wei, Z.; Mugnai, L.; Hyde, K.D.; Xing, H.L.; Ji, Y.Y. First report of Phaeoacremonium minimum associated with grapevine trunk diseases in China. Plant Dis. 2020, 104, 1259. [Google Scholar] [CrossRef]
- Ye, Q.; Jia, J.; Manawasinghe, I.S.; Li, X.; Zhang, W.; Mugnai, L.; Wu, X.; Hyde, K.D.; Yan, J. Fomitiporia punicata and Phaeoacremonium minimum associated with Esca complex of grapevine in China. Phytopathol. Res. 2021, 3, 11. [Google Scholar] [CrossRef]
- Li, H.; Li, R.Y.; Wang, H. New disease for winemaking grape-eutypa dieback. Liquor.-Mak. Sci. Technol. 2007, 155, 48–50. (In Chinese) [Google Scholar]
- Ye, Q.; Li, Y.; Zhou, Y.; Li, X.; Zhang, W.; Sun, Q.; Han, C.; Yan, J. Occurrence of grapevine trunk diseases caused by fungal pathogens in the domestic and overseas. J. Fruit Sci. 2021, 38, 278–292. (In Chinese) [Google Scholar]
- Farr, D.F.; Rossman, A.Y. Fungal Databases, U.S. National Fungus Collections, ARS, USDA. Available online: https://nt.ars-grin.gov/fungaldatabases/ (accessed on 8 August 2021).
- Jayawardena, R.; Purahong, W.; Zhang, W.; Wubet, T.; Li, X.; Liu, M.; Zhao, W.; Hyde, K.D.; Liu, J.; Yan, J. Biodiversity of fungi on Vitis vinifera L. revealed by traditional and high-resolution culture-independent approaches. Fungal Divers. 2018, 90, 1–84. [Google Scholar] [CrossRef] [Green Version]
- Sweetingham, M. Studies on the Nature and Pathogenicity of Soil-Borne Cylindrocarpon spp. Ph.D. Thesis, University of Tasmania, Hobart, Australia, 1983. [Google Scholar]
- Gubler, W.D.; Baumgartner, K.; Browne, G.T.; Eskalen, A.; Rooney Latham, S.; Petit, E.; Bayramian, L.A. Root diseases of grapevine in California and their control. Australas. Plant Pathol. 2004, 33, 157–165. [Google Scholar] [CrossRef]
- Jeewon, R.; Liew, E.C.Y.; Hyde, K.D. Phylogenetic relationships of Pestalotiopsis and allied genera inferred from ribosomal DNA sequences and morphological characters. Mol. Phylogenetics Evol. 2002, 25, 378–392. [Google Scholar] [CrossRef]
- Suwannarach, N.; Bussaban, B.; Hyde, K.D.; Lumyong, S. Muscodor cinnamomi, a new endophytic species from Cinnamomum bejolghota. Mycotaxon 2010, 114, 15–23. [Google Scholar] [CrossRef]
- Katoh, K.; Rozewicki, J.; Yamada, K.D. MAFFT online service: Multiple sequence alignment, interactive sequence choice and visualization. Brief. Bioinform. 2019, 20, 1160–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, T.A. Bio-Edit: A user-friendly biological sequence alignment editor and analysis program for Windows 95/98/NT. Nucleic Acids Symp. Ser. 1999, 41, 95–98. [Google Scholar]
- Posada, D.; Crandall, K.A. MODELTEST: Testing the model of DNA substitution. Bioinformatics 1998, 14, 817–818. [Google Scholar] [CrossRef] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef]
- Stamatakis, A.; Hoover, P.; Rougemont, J. A rapid bootstrap algorithm for the RAxML web servers. Syst. Biol. 2008, 57, 758–771. [Google Scholar] [CrossRef] [PubMed]
- Rannala, B.; Yang, Z. Probability distribution of molecular evolutionary trees: A new method of phylogenetic inference. J. Mol. Evol. 1996, 43, 304–311. [Google Scholar] [CrossRef] [PubMed]
- Ronquist, F.; Huelsenbeck, J.P. MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 2003, 19, 1572–1574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rambaut, A. FigTree Version 1.4. 2012. Available online: http://tree.bio.ed.ac.uk/software/figtree (accessed on 5 May 2021).



| Genus | Species | Geographical Distribution |
|---|---|---|
| Campylocarpon | C. fasciculare | Brazil, South Africa, Spain, Turkey, United States |
| C. pseudofasciculare | Brazil, Peru, South Africa, Uruguay | |
| Cylindrocarpon | C. cylindroides | Canada, United States |
| C. destructans—(Ilyonectria destructans) | Argentina, Canada, France, Iran, South Africa, Spain | |
| C. destructans var. crassum—(Ilyonectria crassa) | Uruguay | |
| C. destructans var. destructans—(Ilyonectria destructans) | Canada | |
| C. liriodendri—(Ilyonectria liriodendra) | Australia, Canada, France, Iran, Portugal, Spain, Switzerland, Uruguay, United States | |
| C. macrodidymum—(Dactylonectria macrodidyma) | Australia, Canada, Chile, New Zealand, Slovenia, South Africa, Spain, Turkey, Uruguay, United States | |
| C. obtusisporum—(Neonectria obtusispora) | United States | |
| C. olidum—(Thelonectria olida) | Uruguay | |
| C. olidum var. crassum | Uruguay | |
| C. pauciseptatum—(Dactylonectria pauciseptata) | Canada, New Zealand, Portugal, Slovenia, Spain, Uruguay | |
| Cylindrocarpon spp. | Australia, Canada, Florida, Lebanon, New Zealand, Portugal, South Africa, Spain, Switzerland, Tasmania, United States | |
| C. tonkinense | India | |
| Cylindrocladiella | C. lageniformis | South Africa, United States |
| C. parva | New Zealand, South Africa, Spain | |
| C. peruviana | Peru, South Africa, Spain, United States | |
| C. pseudoparva | New Zealand | |
| Cylindrocladiella spp. | New Zealand | |
| C. viticola | South Africa | |
| C. vitis | New Zealand | |
| Dactylonectria | D. alcacerensis | Portugal, South Africa, Spain, United States |
| D. estremocensis | Portugal | |
| D. hordeicola | France | |
| D. macrodidyma | France, Portugal, South Africa, Spain, United States | |
| D. novozelandica | New Zealand, South Africa, United States | |
| D. pauciseptata | Bulgaria, France, Slovenia, South Africa, Spain | |
| D. pinicola | Portugal | |
| D. torresensis | Czech Republic, France, Italy, New Zealand, Portugal, South Africa, Spain | |
| D. vitis | Portugal | |
| Ilyonectria | I. europaea | Portugal |
| I. liriodendri | Australia, Brazil, Canada, France, Iran, Italy, New Zealand, Portugal, South Africa, Spain, Switzerland, Turkey, United States, Uruguay | |
| I. lusitanica | Portugal | |
| I. pseudodestructans | Portugal | |
| I. robusta | Brazil, British Columbia, Canada, France, Portugal, Spain, United States | |
| Ilyonectria sp | Australia, Portugal | |
| Neonectria | N. macrodidymum | Canada, South Africa, United States |
| N. mammoidea | United States | |
| N. microconidia | Japan | |
| N. radicola | Canada | |
| Thelonectria | T. aurea | United States |
| T. blackeriella | Italy |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abeywickrama, P.D.; Zhang, W.; Li, X.; Jayawardena, R.S.; Hyde, K.D.; Yan, J. Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China. Pathogens 2021, 10, 1555. https://doi.org/10.3390/pathogens10121555
Abeywickrama PD, Zhang W, Li X, Jayawardena RS, Hyde KD, Yan J. Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China. Pathogens. 2021; 10(12):1555. https://doi.org/10.3390/pathogens10121555
Chicago/Turabian StyleAbeywickrama, Pranami D., Wei Zhang, Xinghong Li, Ruvishika S. Jayawardena, Kevin D. Hyde, and Jiye Yan. 2021. "Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China" Pathogens 10, no. 12: 1555. https://doi.org/10.3390/pathogens10121555
APA StyleAbeywickrama, P. D., Zhang, W., Li, X., Jayawardena, R. S., Hyde, K. D., & Yan, J. (2021). Campylocarpon fasciculare (Nectriaceae, Sordariomycetes); Novel Emergence of Black-Foot Causing Pathogen on Young Grapevines in China. Pathogens, 10(12), 1555. https://doi.org/10.3390/pathogens10121555







