Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems
Abstract
1. Introduction
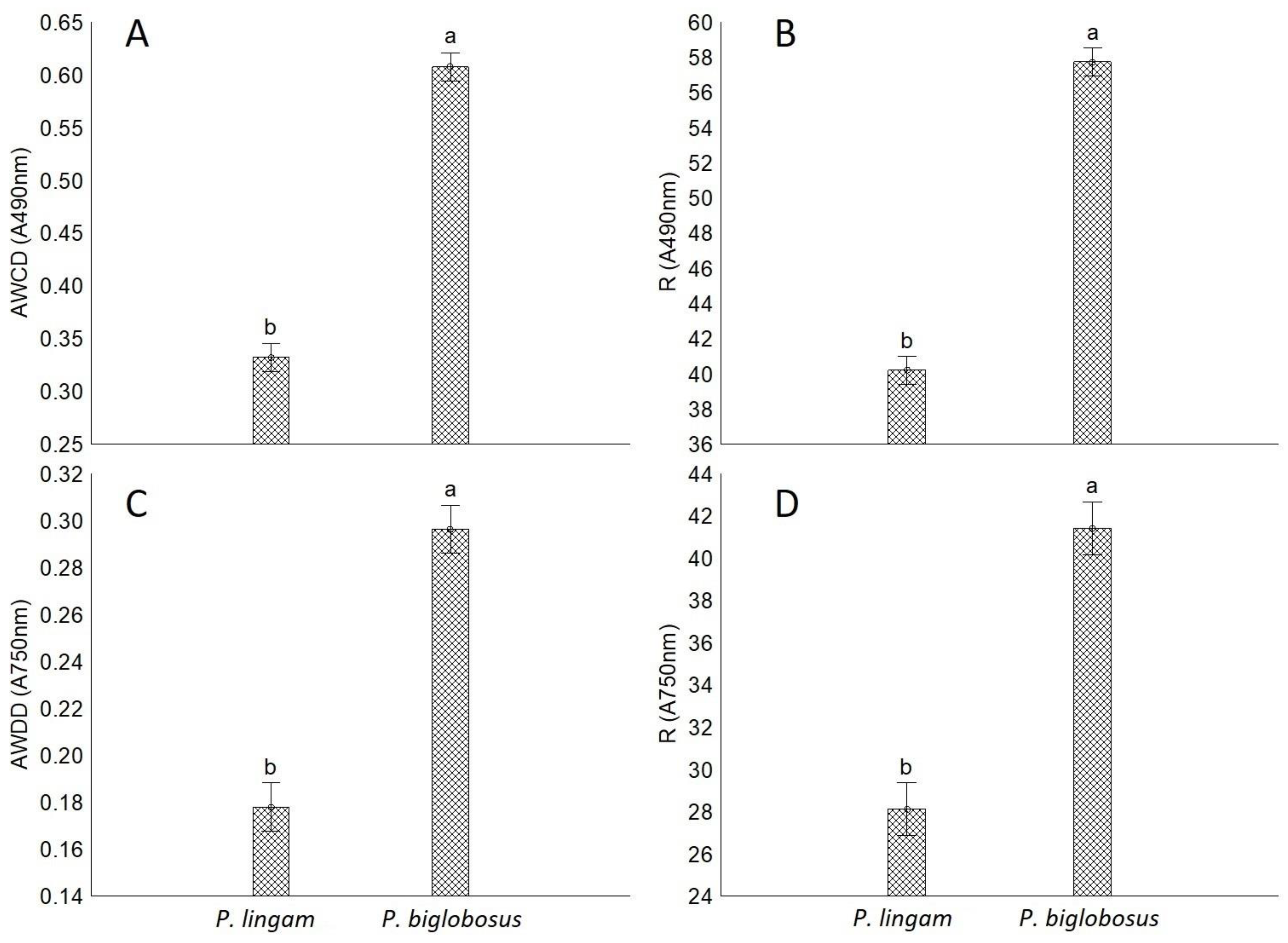
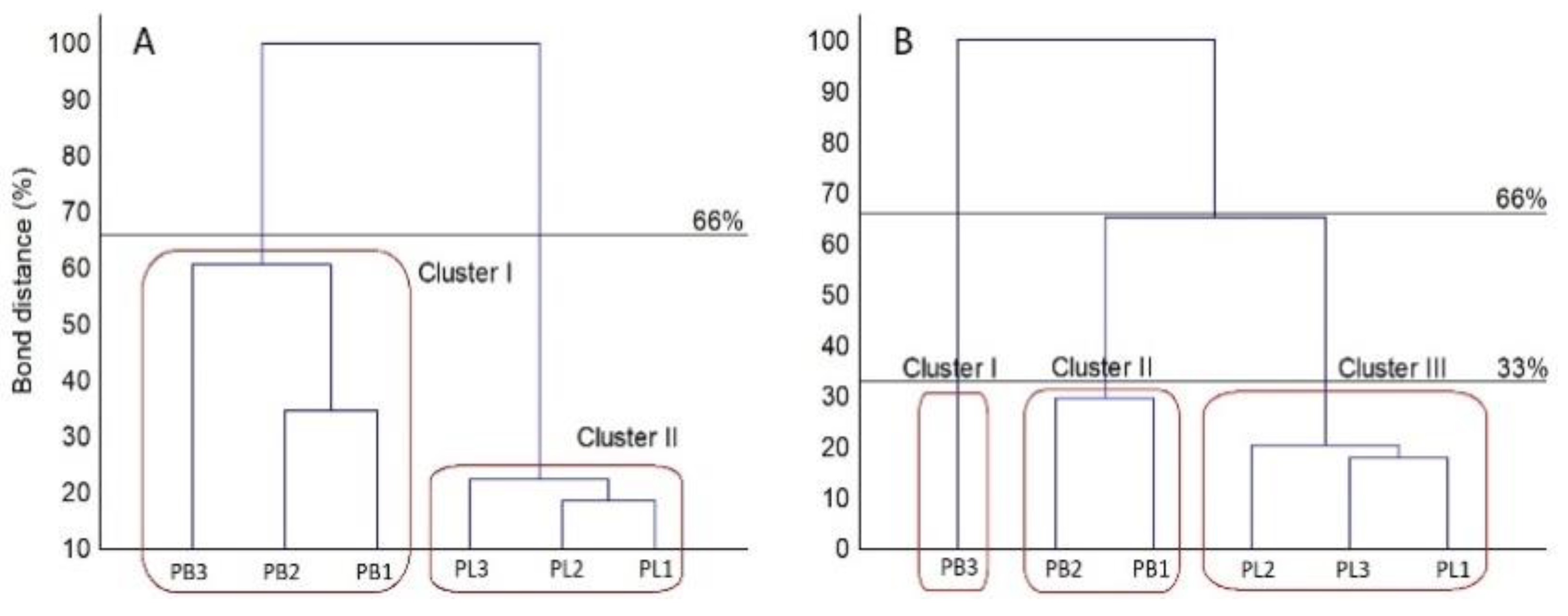
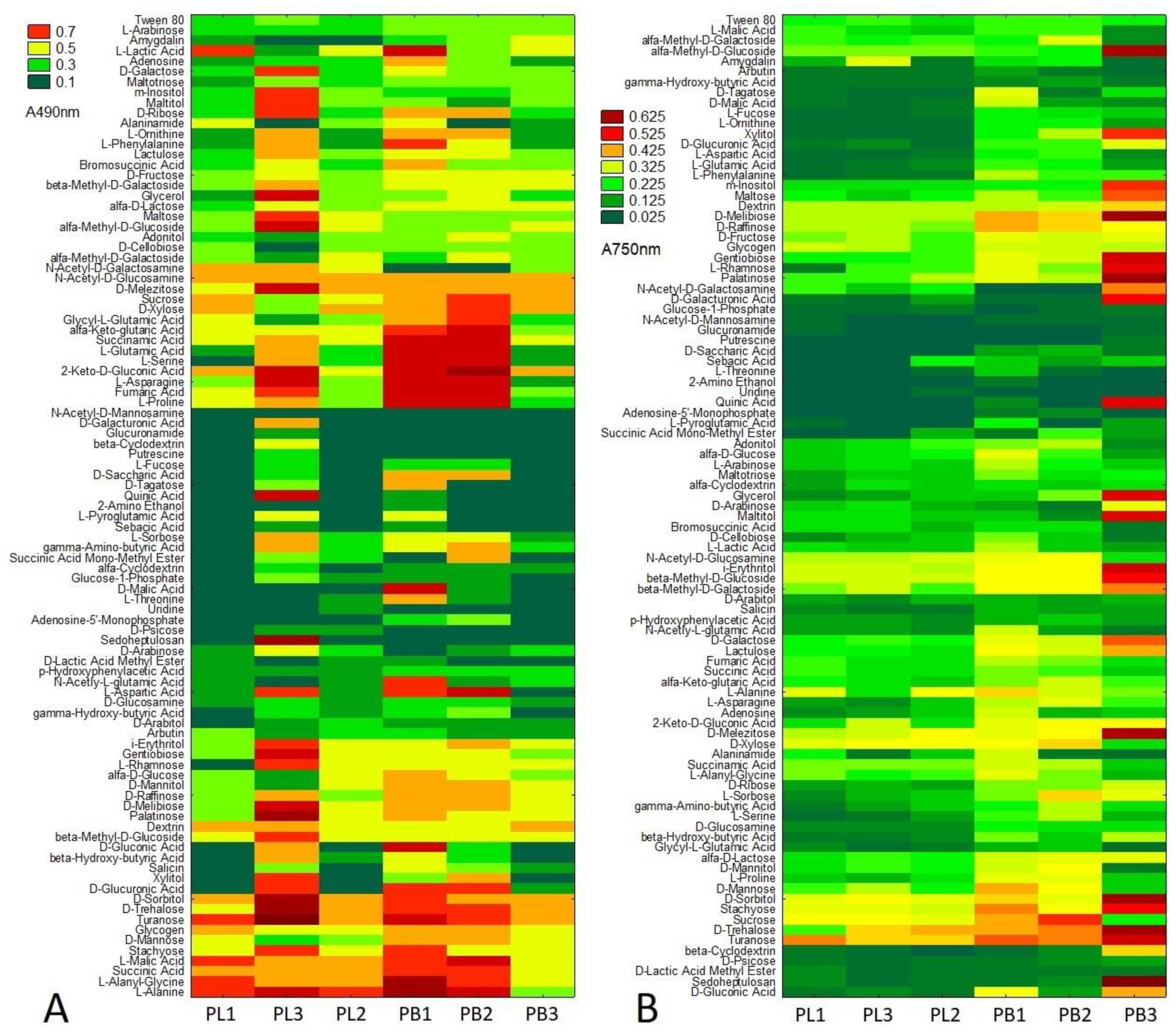
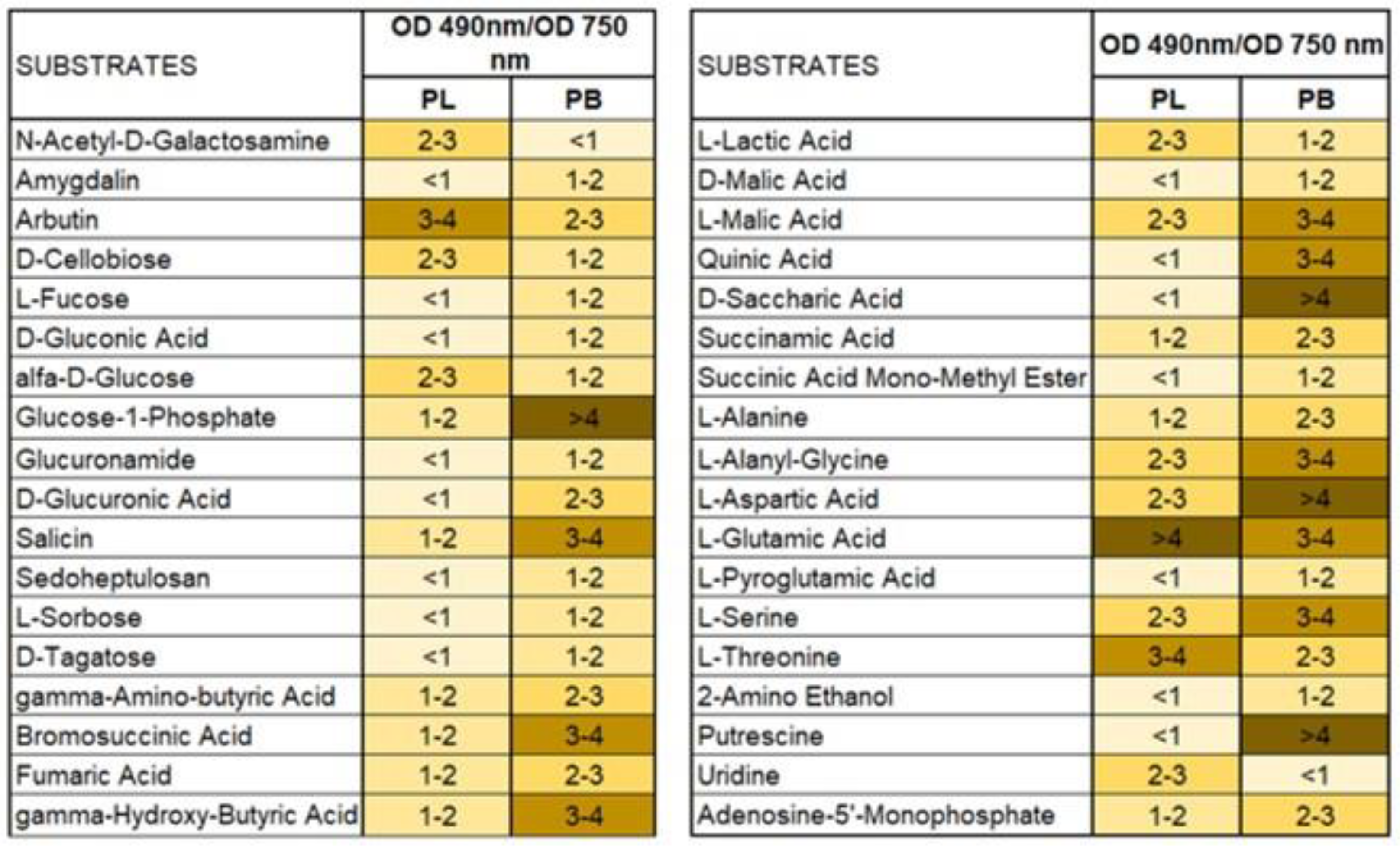
2. Results
3. Discussion
4. Materials and Methods
4.1. Fungal Strains
4.2. Filamentous Fungi (FF) Plates Assay
4.3. Metabolic Capacity, Fungal Growth and Group of Substrates Use
4.4. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gugel, R.K.; Petrie, G.A. History, occurrence, impact and control of blackleg of rapeseed. Can. J. Plant Pathol. 1992, 14, 36–45. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Huang, Y.J.; van den Bosch, F.; West, J.S. Coexistence of related pathogen species on arable crops in space and time. Annu. Rev. Phytopathol. 2006, 44, 163–182. [Google Scholar] [CrossRef] [PubMed]
- Kaczmarek, J.; Jedryczka, M. Characterization of two coexisting pathogen populations of Leptosphaeria spp., the cause of stem canker of brassicas. Acta Agrobot. 2011, 64, 3–14. [Google Scholar] [CrossRef]
- Cunningham, G.H. Dry rot of swedes and turnips: Its cause and control. N. Z. J. Agric. 1927, 35, 1–14. [Google Scholar]
- Koch, E.; Badawy, H.M.A.; Hoppe, H.H. Differences between aggressive and non-aggressive single spore lines of Leptosphaeria maculans in cultural characteristics and phytotoxin production. J. Phytopathol. 1989, 124, 52–62. [Google Scholar] [CrossRef]
- Williams, R.H.; Fitt, B.D.L. Differentiating A and B groups of Leptosphaeria maculans, causal agent of stem canker (blackleg) of oilseed rape. Plant Pathol. 1999, 48, 161–175. [Google Scholar] [CrossRef]
- Voigt, K.; Jędryczka, M.; Wöstemeyer, J. Strain typing of Polish Leptosphaeria maculans isolates supports at the genomic level the multi-species concept of aggressive and non-aggressive strains. Microbiol. Res. 2001, 156, 169–177. [Google Scholar] [CrossRef] [PubMed]
- Taylor, J.L.; Borgmann, I.; Séguin-Swartz, G. Electrophoretic karyotyping of Leptosphaeria maculans differentiates highly virulent from weakly virulent isolates. Curr. Genet. 1991, 19, 273–277. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Séguin-Swartz, G. The blackleg fungus: Phytotoxins and phytoalexins. Can. J. Plant Pathol. 1992, 1, 67–75. [Google Scholar] [CrossRef]
- Balesdent, M.-H.; Gall, C.; Robin, P.; Rouxel, T. Intraspecific variation in soluble mycelia protein and esterase patterns of Leptosphaeria maculans French isolates. Mycol. Res. 1992, 96, 677–684. [Google Scholar] [CrossRef]
- Jedryczka, M.; Fitt, B.D.L.; Kachlicki, P.; Lewartowska, E.; Balesdent, M.H.; Rouxel, T. Comparison between Polish and United Kingdom populations of Leptosphaeria maculans, cause of stem canker of winter oilseed rape. J. Plant Dis. Prot. 1999, 106, 608–617. [Google Scholar]
- West, J.S.; Balesdent, M.-H.; Rouxel, T.; Narcy, J.P.; Huang, Y.-J.; Roux, J.; Steed, J.M.; Fitt, B.D.L.; Schmit, J. Colonization of winter oilseed rape tissues by A/Tox+ and B/Tox0 Leptosphaeria maculans (phoma stem canker) in France and England. Plant Pathol. 2002, 51, 311–321. [Google Scholar] [CrossRef]
- Pedras, M.S.C.; Chumala, P.; Yu, Y. The phytopathogenic fungi Leptosphaeria maculans and Leptosphaeria biglobosa: Chemotaxonomical characterization of isolates and metabolite production in different culture media. Can. J. Microbiol. 2007, 53, 364–371. [Google Scholar] [CrossRef] [PubMed]
- Sippell, D.W.; Hall, R. Glucose Phosphate Isomerase polymorphisms distinguish weakly virulent from highly virulent-strains of Leptosphaeria maculans. Can. J. Plant Pathol. 1995, 17, 1–6. [Google Scholar] [CrossRef]
- Kachlicki, P.; Stobiecki, M.; Jędryczka, M. The benzoic acid—The phytotoxic metabolite of Tox0 strain of the fungus Phoma lingam. Oilseed Crop. 1996, 17, 193–198. (In Polish) [Google Scholar]
- Howlett, B.J.; Idnurm, A.; Pedras, M.S.C. Leptosphaeria maculans, the causal agent of blackleg disease of Brassicas. Fungal Genet. Biol. 2001, 33, 1–14. [Google Scholar] [CrossRef]
- Shoemaker, R.A.; Brun, H. The teleomorph of the weakly aggressive segregate of Leptosphaeria maculans. Can. J. Bot. 2001, 79, 412–419. [Google Scholar] [CrossRef]
- de Gruyter, J.; Woudenberg, J.H.C.; Aveskamp, M.M.; Verkley, G.J.M.; Groenewald, J.Z.; Crous, P.W. Redisposition of Phoma-like anamorphs in Pleosporales. Stud. Mycol. 2013, 75, 1–36. [Google Scholar] [CrossRef]
- Mendes-Pereira, E.; Balesdent, M.-H.; Brun, H.; Rouxel, T. Molecular phylogeny of the Leptosphaeria maculans–L. biglobosa species complex. Mycol. Res. 2003, 107, 1287–1304. [Google Scholar] [CrossRef]
- Voigt, K.; Cozijnsen, A.J.; Kroymann, J.; Pöggeler, S.; Howlett, B.J. Phylogenetic relationships between members of the crucifer pathogenic Leptosphaeria maculans species complex as shown by mating type (MAT1-2), actin, and β-tubulin sequences. Mol. Phylogenet. Evol. 2005, 37, 541–557. [Google Scholar] [CrossRef]
- Vincenot, L.; Balesdent, M.H.; Li, H.; Barbetti, M.J.; Sivasithamparam, K.; Gout, L.; Rouxel, T. Occurrence of a new subclade of Leptosphaeria biglobosa in Western Australia. Phytopathology 2008, 98, 321–329. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhang, X.H.; Parks, P.; du Toit, L.J.; Van de Wouw, P.; Fernando, W.G.D. A new subclade of Leptosphaeria biglobosa identified from Brassica rapa. Int. J. Mol. Sci. 2019, 20, 1668. [Google Scholar] [CrossRef] [PubMed]
- Balesdent, M.H.; Barbetti, M.J.; Li, H.; Sivasithamparam, K.; Gout, L.; Rouxel, T. Analysis of Leptosphaeria maculans race structure in a worldwide collection of isolates. Phytopathology 2005, 95, 1061–1071. [Google Scholar] [CrossRef]
- Dilmaghani, A.; Balesdent, M.H.; Didier, J.P.; Wu, C.; Davey, J.; Barbetti, M.J.; Li, H.; Moreno-Rico, O.; Phillips, D.; Despeghel, J.P.; et al. The Leptosphaeria maculans–Leptosphaeria biglobosa species complex in the American continent. Plant Pathol. 2009, 58, 1044–1058. [Google Scholar] [CrossRef]
- Liu, Z.; Latunde-Dada, A.O.; Hall, A.M.; Fitt, B.D.L. Phoma stem canker disease on oilseed rape (Brassica napus) in China is caused by Leptosphaeria biglobosa ‘brassicae’. Eur. J. Plant Pathol. 2014, 140, 841–857. [Google Scholar] [CrossRef]
- Luo, T.; Li, G.; Yang, L. First report of Leptosphaeria biglobosa ‘canadensis’ causing blackleg on oilseed rape (Brassica napus) in China. Plant Dis. 2021. [Google Scholar] [CrossRef]
- Punja, Z.K.; Chandanie, W.A.; Chen, X.; Rodríguez, G. Phoma leaf spot of wasabi (Wasabia japonica) caused by Leptosphaeria biglobosa. Plant Pathol. 2017, 66, 480–489. [Google Scholar] [CrossRef]
- King, K.M.; West, J.S. Detection of the Phoma pathogens Plenodomus biglobosus subclades ‘brassicae’ and ‘canadensis’ on wasabi, and ‘canadensis’ in Europe. Eur. J. Plant Pathol. 2021. [Google Scholar] [CrossRef]
- Brachaczek, A.; Kaczmarek, J.; Jędryczka, M. Monitoring blackleg (Leptosphaeria spp.) ascospore release timing and quantity enables optimal fungicide application to improved oilseed rape yield and seed quality. Eur. J. Plant Pathol. 2016, 145, 643–657. [Google Scholar] [CrossRef][Green Version]
- McCredden, J.A.; Cowley, A.R.B.; Marcroft, B.S.J.; Van de Wouw, A.P. Changes in farming practices impact on spore release patterns of the blackleg pathogen, Leptosphaeria maculans. Crop Pasture Sci. 2017, 69, 1–8. [Google Scholar] [CrossRef]
- West, J.S.; Kharbanda, P.D.; Barbetti, M.J.; Fitt, B.D.L. Epidemiology and management of Leptosphaeria maculans (phoma stem canker) on oilseed rape in Australia, Canada and Europe. Plant Pathol. 2001, 50, 10–27. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Kedziora, A.; Brachaczek, A.; Latunde-Dada, A.O.; Dakowska, S.; Karg, G.; Jedryczka, M. Effect of climate change on sporulation of the teleomorphs of Leptosphaeria species causing stem canker of brassicas. Aerobiologia 2016, 32, 39–51. [Google Scholar] [CrossRef]
- Fitt, B.D.L.; Brun, H.; Barbetti, M.J.; Rimmer, S.R. World-wide importance of phoma stem canker (Leptosphaeria maculans and L. biglobosa) on oilseed rape (Brassica napus). Eur. J. Plant Pathol. 2006, 114, 3–15. [Google Scholar] [CrossRef]
- Huang, Y.J.; Karandeni-Dewage, C.S.; Fitt, B.D.L. Importance of Leptosphaeria biglobosa as a cause of phoma stem canker on winter oilseed rape in the UK. Asp. App Biol. 2014, 127, 117–122. [Google Scholar]
- West, J.S.; Evans, N.; Liu, S.; Hu, B.; Peng, L. Leptosphaeria maculans causing stem canker of oilseed rape in China. Plant Pathol. 2000, 49, 800. [Google Scholar] [CrossRef][Green Version]
- Zhang, X.; White, R.P.; Demir, E.; Jedryczka, M.; Lange, R.M.; Islam, M.; Li, Q.; Huang, Y.J.; Hall, A.M.; Zhou, G.; et al. Leptosphaeria spp., phoma stem canker and potential spread of L. maculans on oilseed rape crops in China. Plant Pathol. 2014, 63, 598–612. [Google Scholar] [CrossRef]
- Raman, H.; Raman, R.; Coombes, N.; Song, J.; Diffey, S.; Kilian, A.; Lindbeck, K.; Barbulescu, D.M.; Batley, J.; Edwards, D.; et al. Genome-wide association study identifies new loci for resistance to Leptosphaeria maculans in canola. Front. Plant Sci. 2016, 7, 1513. [Google Scholar] [CrossRef]
- Fu, F.; Zhang, X.; Liu, F.; Peng, G.; Yu, F.; Fernando, F. Identification of resistance loci in Chinese and Canadian canola/rapeseed varieties against Leptosphaeria maculans based on genome-wide association studies. BMC Genom. 2020, 21, 501. [Google Scholar] [CrossRef]
- Plummer, K.M.; Dunse, K.; Howlett, B.J. Non-aggressive strains of the blackleg fungus, Leptosphaeria maculans, are present in Australia and can be distinguished from aggressive strains by molecular analysis. Aust. J. Bot. 1994, 42, 1–8. [Google Scholar] [CrossRef]
- Eckert, M.; Gout, L.; Rouxel, T.; Blaise, F.; Jędryczka, M.; Fitt, B.D.L.; Balesdent, M.H. Identification, molecular characterization and polymorphism of five minisatellites in the phytopathogenic ascomycete Leptosphaeria maculans. Curr. Genet. 2005, 47, 37–48. [Google Scholar] [CrossRef] [PubMed]
- Stachowiak, A.; Olechnowicz, J.; Jedryczka, M.; Rouxel, T.; Balesdent, M.H.; Happstadius, I.; Gladders, P.; Latunde-Dada, A.; Evans, N. Frequency of avirulence alleles in field populations of Leptosphaeria maculans in Europe. Eur. J. Plant Pathol. 2006, 114, 67–75. [Google Scholar] [CrossRef]
- Rouxel, T.; Balesdent, M.H. Life, death and rebirth of avirulence effectors in a fungal pathogen of Brassica crops, Leptosphaeria maculans. New Phytol. 2017, 214, 526–532. [Google Scholar] [CrossRef]
- Kutcher, H.R.; Balesdent, M.H.; Rimmer, S.R.; Rouxel, T.; Chèvre, A.M.; Delourme, R.; Brun, H. Frequency of avirulence genes in Leptosphaeria maculans in western Canada. Can. J. Plant Pathol. 2010, 32, 77–85. [Google Scholar] [CrossRef]
- Jedryczka, M. Epidemiologia i szkodliwość suchej zgnilizny kapustnych na rzepaku ozimym w Polsce. Rozpr. Monogr. IGR PAN 2006, 17, 1–150. (In Polish) [Google Scholar]
- Liu, S.Y.; Liu, Z.; Fitt, B.D.L.; Evans, N.; Foster, S.J.; Huang, Y.J.; Latunde-Dada, A.O.; Lucas, J.A. Resistance to Leptosphaeria maculans (phoma stem canker) in Brassica napus (oilseed rape) induced by L. biglobosa and chemical defense activators in field and controlled environments. Plant Pathol. 2006, 55, 401–412. [Google Scholar] [CrossRef]
- Travadon, R.; Marquer, B.; Ribulé, A.; Sache, I.; Masson, P.; Brun, H.; Delourme, R.; Bousset, L. Systemic growth of Leptosphaeria maculans from cotyledons to hypocotyls in oilseed rape: Influence of number of infection sites, competitive growth and host polygenic resistance. Plant Pathol. 2009, 58, 461–469. [Google Scholar] [CrossRef]
- Kuswinanti, T.; Koopmann, B.; Hoppe, H.H. Virulence pattern of aggressive isolates of Leptosphaeria maculans on an extended set of Brassica differentials. J. Plant Dis. Prot. 1999, 106, 12–20. [Google Scholar]
- Dawidziuk, A.; Kaczmarek, J.; Podlesna, A.; Kasprzyk, I.; Jedryczka, M. Influence of meteorological parameters on Leptosphaeria maculans and L. biglobosa spore release in central and eastern Poland. Grana 2012, 51, 240–248. [Google Scholar] [CrossRef]
- Toscano-Underwood, C.; Huang, Y.J.; Fitt, B.D.L.; Hall, A.M. Effects of temperature on maturation of pseudothecia of Leptosphaeria maculans and L. biglobosa on oilseed rape stem debris. Plant Pathol. 2003, 52, 726–736. [Google Scholar] [CrossRef]
- Brachaczek, A.; Kaczmarek, J.; Jedryczka, M. Warm and wet autumns favour yield losses of oilseed rape caused by phoma stem canker. Agronomy 2021, 11, 1171. [Google Scholar] [CrossRef]
- Barbetti, M.J. Role of pycnidiospores of Leptosphaeria maculans in spread of blackleg disease in rape. Aust. J. Environ. Res. 1976, 16, 911–914. [Google Scholar] [CrossRef]
- Guo, X.W.; Fernando, W.G.D. Seasonal and diurnal patterns of spore dispersal by Leptosphaeria maculans from canola stubble in relation to environmental conditions. Plant Dis. 2005, 89, 97–104. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Jędryczka, M.; Fitt, B.D.L.; Lucas, J.A.; Latunde-Dada, A.O. Analyses of air samples for ascospores of Leptosphaeria maculans and L. biglobosa with light microscopic and molecular techniques. J. Appl. Genet. 2009, 50, 411–419. [Google Scholar] [CrossRef]
- Eckert, M.; Rossall, S.; Selley, A.; Fitt, B.D.L. Effects of fungicides on in vitro spore germination and mycelial growth of the phytopathogens Leptosphaeria maculans and L. biglobosa (phoma stem canker of oilseed rape). Pest Manag. Sci. 2010, 66, 396–405. [Google Scholar] [CrossRef]
- Fortune, J.A.; Qi, A.; Ritchie, F.; Karandeni Dewage, C.S.; Fitt, B.D.L.; Huang, Y.-J. Effects of cultivar resistance and fungicide application on stem canker of oilseed rape (Brassica napus) and potential interseasonal transmission of Leptosphaeria spp. inoculum. Plant Pathol. 2021, 70, 2115–2124. [Google Scholar] [CrossRef]
- Kaczmarek, J.; Latunde-Dada, A.O.; Irzykowski, W.; Cools, H.J.; Stonard, J.F.; Brachaczek, A.; Jedryczka, M. Molecular screening for avirulence alleles AvrLm1 and AvrLm6 in airborne inoculum of Leptosphaeria maculans and winter oilseed rape (Brassica napus) plants from Poland and the UK. J. Appl. Genet. 2014, 55, 529–539. [Google Scholar] [CrossRef]
- Mitrović, P.; Jeromela, A.M.; Trkulja, V.; Milovac, Ž.; Terzić, S. The first occurrence of stem canker on oilseed rape caused by Leptosphaeria biglobosa in Serbia. Ratar. Povrt. 2016, 53, 53–56. [Google Scholar] [CrossRef]
- Zhang, X.; Fernando, W.G.D. Insights into fighting against blackleg disease of Brassica napus in Canada. Crop Pasture Sci. 2018, 69, 40–47. [Google Scholar] [CrossRef]
- Li, H.; Sivasithamparam, K.; Barbetti, M.J. Breakdown of a Brassica rapa subsp. sylvestris single dominant blackleg resistance gne in B. napus rapeseed by Leptosphaeria maculans field isolates in Australia. Plant Dis. 2003, 87, 752. [Google Scholar] [CrossRef]
- Rouxel, T.; Penaud, A.; Pinochet, X.; Brun, H.; Gout, L.; Delourme, R.; Schmit, J.; Balesdent, M.H. A 10-year survey of populations of Leptosphaeria maculans in France indicates a rapid adaptation towards the Rlm1 resistance gene of oilseed rape. Eur. J. Plant Pathol. 2003, 109, 871–881. [Google Scholar] [CrossRef]
- Liban, S.H.; Cross, D.J.; Kutcher, H.R.; Peng, G.; Fernando, W.G.D. Race structure and frequency of avirulence genes in the western Canadian Leptosphaeria maculans pathogen population, the causal agent of blackleg in brassica species. Plant Pathol. 2016, 65, 1161–1169. [Google Scholar] [CrossRef]
- Zhang, X.; Peng, G.; Kutcher, H.R.; Balesdent, M.H.; Delourme, R.; Fernando, W.G.D. Breakdown of Rlm3 resistance in the Brassica napus–Leptosphaeria maculans pathosystem in western Canada. Eur. J. Plant Pathol. 2016, 145, 659–674. [Google Scholar] [CrossRef]
- Delourme, R.; Piel, N.; Horvais, R.; Pouilly, N.; Domin, C.; Vallée, P.; Falentin, C.; Manzanares-Dauleux, M.J.; Renard, M. Molecular and phenotypic characterization of near isogenic lines at QTL for quantitative resistance to Leptosphaeria maculans in oilseed rape (Brassica napus L.). Theor Appl Genet. 2008, 117, 1055–1067. [Google Scholar] [CrossRef]
- Rouxel, T.; Willner, E.; Coudard, L.; Balesdent, M.H. Screening and identification of resistance to Leptosphaeria maculans in Brassica napus accessions. Euphytica 2003, 133, 219–231. [Google Scholar] [CrossRef]
- Brun, H.; Chèvre, A.-M.; Fitt, B.D.; Powers, S.; Besnard, A.-L.; Ermel, M.; Huteau, V.; Marquer, B.; Eber, F.; Renard, M.; et al. Quantitative resistance increases the durability of qualitative resistance to Leptosphaeria maculans in Brassica napus. New Phytol. 2010, 185, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Raman, H.; Raman, R.; Diffey, S.; Qiu, Y.; McVittie, B.; Barbulescu, D.M.; Salisbury, P.A.; Marcroft, S.; Delourme, R. Stable quantitative resistance loci to blackleg disease in canola (Brassica napus L.) over continents. Front Plant Sci. 2018, 9, 1622. [Google Scholar] [CrossRef]
- Kumar, V.; Paillard, S.; Fopa-Fomeju, B.; Falentin, C.; Deniot, G.; Baron, C.; Vallee, P.; Manzanares-Dauleux, M.J.; Delourme, R. Multi-year linkage and association mapping confirm the high number of genomic regions involved in oilseed rape quantitative resistance to blackleg. Theor. Appl. Genet. 2018, 131, 1627–1643. [Google Scholar] [CrossRef] [PubMed]
- Van de Wouw, A.P.; Marcroft, S.J.; Ware, A.; Lindbeck, K.; Khangura, R.; Howlett, B.J. Breakdown of resistance to the fungal disease, blackleg, is averted in commercial canola (Brassica napus) crops in Australia. Field Crops Res. 2014, 166, 144–151. [Google Scholar] [CrossRef]
- Pietravalle, S.; Lemarié, S.; van den Bosch, F. Durability of resistance and cost of virulence. Eur. J. Plant Pathol. 2006, 114, 107–116. [Google Scholar] [CrossRef]
- Jedryczka, M.; Burzyński, A.; Brachaczek, A.; Langwiński, W.; Song, P.; Kaczmarek, J. Loop-mediated Isothermal Amplification as a good tool to study changing Leptosphaeria populations in oilseed rape plants and air samples. Acta Agrobot. 2014, 67, 93–100. [Google Scholar] [CrossRef][Green Version]
- Frąc, M. Mycological evaluation of dairy sewage sludge and its influence on functional diversity of soil microorganisms. Acta Agrophysica Monogr. 2012, 1, 152. [Google Scholar]
- Oszust, K.; Panek, J.; Pertile, G.; Siczek, A.; Oleszek, M.; Frac, M. Metabolic and genetic properties of Petriella setifera precultured on waste. Front. Microb. 2018, 9, 115. [Google Scholar] [CrossRef]
- Jeszka-Skowron, M.; Oszust, K.; Zgoła-Grzeskowiak, A.; Frąc, M. Quality assessment of goji fruits, cranberries, and raisins using selected markers. Eur. Food Res. Technol. 2018, 244, 2159–2168. [Google Scholar] [CrossRef]
- Atanasova, L.; Druzhinina, I.S. Review: Global nutrient profiling by Phenotype MicroArrays: A tool complementing genomic and proteomic studies in conidial fungi. J. Zhejiang Univ. Sci. B 2010, 11, 151–168. [Google Scholar] [CrossRef] [PubMed]
- Pawlik, A.; Ruminowicz-Stefaniuk, M.; Frąc, M.; Mazur, A.; Wielbo, J.; Janusz, G. The wood decay fungus Cerrena unicolor adjusts its metabolism to grow on various types of wood and light conditions. PLoS ONE 2019, 14, e0211744. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How do Trichoderma genus fungi win a nutritional competition battle against soft fruit pathogens? A Report on niche overlap nutritional potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Pinzari, F.; Ceci, A.; Abu-Samra, N.; Canfora, L.; Maggi, O.; Persiani, A. Phenotype MicroArray system in the study of fungal functional diversity and catabolic versatility. Res. Microb. 2016, 167, 710–722. [Google Scholar] [CrossRef]
- Pinzari, F.; Maggi, O.; Lunghini, D.; Di Lonardo, D.P.; Persiani, A.M. A simple method for measuring fungal metabolic quotient and comparing carbon use efficiency of different isolates: Application to Mediterranean leaf litter fungi. Plant Biol. 2017, 151, 371–376. [Google Scholar] [CrossRef][Green Version]
- Ward, J.H. Hierarchical groupings to optimize an objective function. J. Am. Stat. Assoc. 1963, 58, 236–244. [Google Scholar] [CrossRef]
- Sneath, P.H.A.; Sokal, R.R. Numerical Taxonomy: The Principles and Practice of Numerical Classification, 1st ed.; W.H. Freeman & Co.: San Francisco, CA, USA, 1973. [Google Scholar]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąc, M.; Kaczmarek, J.; Jędryczka, M. Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems. Pathogens 2022, 11, 50. https://doi.org/10.3390/pathogens11010050
Frąc M, Kaczmarek J, Jędryczka M. Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems. Pathogens. 2022; 11(1):50. https://doi.org/10.3390/pathogens11010050
Chicago/Turabian StyleFrąc, Magdalena, Joanna Kaczmarek, and Małgorzata Jędryczka. 2022. "Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems" Pathogens 11, no. 1: 50. https://doi.org/10.3390/pathogens11010050
APA StyleFrąc, M., Kaczmarek, J., & Jędryczka, M. (2022). Metabolic Capacity Differentiates Plenodomus lingam from P. biglobosus Subclade ‘brassicae’, the Causal Agents of Phoma Leaf Spotting and Stem Canker of Oilseed Rape (Brassica napus) in Agricultural Ecosystems. Pathogens, 11(1), 50. https://doi.org/10.3390/pathogens11010050








