Abstract
Vancomycin is frequently prescribed in pediatrics, especially in intensive care unit settings, to treat Gram-positive bacterial infections. This work aims to collect the top-cited articles of pediatric and infectious diseases areas to gather the current evidence and gaps of knowledge on the use of vancomycin in these populations. The most relevant journals reported in the “pediatrics” and “infectious diseases” categories of the 2019 edition of Journal Citation Reports were browsed. Articles with more than 30 citations and published over the last three decades were collected. A bibliometric analysis was performed and 115 articles were retrieved. They were published in 21 journals, with a median impact factor of 4.6 (IQR 2.9–5.4). Sixty-eight of them (59.1%) belonged to “infectious diseases” journals. The most relevant topic was “bloodstream/complicated/invasive infections”, followed by “antibiotic resistance/MRSA treatment”. As for population distribution, 27 articles were on children only and 27 on neonates, most of which were from intensive care unit (ICU) settings. The current literature mainly deals with vancomycin as a treatment for severe infections and antibiotic resistance, especially in neonatal ICU settings. Lately, attention to new dosing strategies in the neonatal and pediatric population has become a sensible topic.
1. Introduction
Vancomycin is one of the most frequently prescribed glycopeptides, especially in children and newborns in intensive care unit (ICU) settings for the treatment of Gram-positive bacterial infections by coagulase-negative Staphylococci (CoNS), Enterococci spp, methicillin-resistant Staphylococcus aureus (MRSA), and C. difficile. It inhibits cell wall synthesis by binding to the D-Ala-D-Ala terminal of the peptide chain and has a volume of distribution of 0.4–1 L/kg [1]. There is significant variability in protein binding, which is believed to be up to 50% [2]. Its bactericidal activity is related to the area under the curve (AUC) and minimal inhibitory concentration (MIC) ratio (AUC/MIC ratio), which, as stated in the literature, has to be higher than 400 to guarantee standard efficacy [1]. Serum levels monitoring is recommended, with a target trough concentration goal of 15–20 µg/mL for severe infections. Based on these pharmacodynamics data, continuous infusion of vancomycin has been proposed for severe infections, guaranteeing higher steady-state concentrations. However, there is little consensus on choosing the optimal dosing regimen and administration schedule (intermittent vs. continuous infusion) in children and even more in neonates, especially in preterm and extremely preterm patients.
Another concern is the emergent antibiotic resistance issues, with the appearance of vancomycin-intermediate strains in methicillin-resistant S. aureus (MRSA) infections after the exposure to prolonged vancomycin therapy, due to the selection of resistant subpopulations under the pressure of antimicrobial exposure [3]. More recently, increasing MICs and decreased susceptibility have been reported for CoNS as well, for the same reason, with subsequent treatment failures [4,5].
The pediatric infectious diseases consultant is often involved in choosing the most appropriate antibiotic therapy in complex cases and implementing antibiotic stewardship programs. Pediatric infectious diseases as a clinical sub-specialty is accessible by pediatricians and infectious diseases specialists who share the management of severe infections and choice of therapy in pediatric patients and, therefore, require extensive knowledge and experience. Most scientific papers address mainly or exclusively the adult population, and evidence on children or neonates is often scattered in journals of both pediatric and infectious diseases areas. This might increase the difficulty in finding the most relevant sources. Their collection might help trainees and consultants improve their knowledge and monitor what is new in this field.
This bibliometric analysis aims to collect the top-cited articles in the last three decades related to vancomycin and its use in the pediatric and neonatal population. Secondary aims are to identify the most relevant scientific journals dealing with pediatric infectious diseases and summarize the most important topics regarding new dosing regimens, especially for neonates, in order to underline relevant gaps of knowledge to date.
2. Materials and Methods
The 2019 edition of Journal Citation Reports (JCR): Science Edition was browsed by category to identify highly indexed journals. All the journals of the first quartile belonging to the “pediatrics” and “infectious diseases” categories were considered in order to include those with the highest impact factor (IF). Overall, 55 journals were searched for the study. Thirty-two belonged to the “pediatrics” category, and the remaining 23 to “infectious diseases”. A complete list of the journals, ranked by their IF is provided in the Supplementary Materials (Supplementary Tables S2 and S3).
The Web of Science–Science Citation Index Expanded database was searched on September 10th, 2021. The inclusion criteria were the English language, a minimum of 30 citations, publication dates ranging from January 1990 to September 2021, the pertinence with vancomycin in general, and its use in children and neonates, assessed by the authors, excluding all studies only addressing the adult population. The articles meeting the inclusion criteria were ranked by citation number. If two or more articles were equally cited, the IF of the journal was considered the discriminating factor. A top-cited list was produced.
The articles were further classified according to article type, topic, population, ICU vs. non-ICU setting, and country of origin. The topics were classified by the Authors, starting from the keywords of the articles, and were included in the following categories: adverse reactions, MIC interpretative criteria, ototoxicity, pharmacokinetics and pharmacodynamics, antibiotic stewardship, healthcare epidemiology, nephrotoxicity, C. difficile treatment, dosing strategies/intermittent vs. continuous infusion, state of the art, antibiotic resistance/MRSA treatment, bloodstream/complicated/invasive infection treatment.
Among the evaluated parameters in our bibliometric analysis, we made a gender description of the first and last authors. The PubMed/MEDLINE database was searched for further information on these topics. Continuous data were reported as median and inter-quartile range (IQR), while non-continuous data were reported as numbers.
3. Results
3.1. Scientific Journals
One hundred and fifteen top-cited articles were retrieved in total, published in 21/55 journals, with a median IF of 4.6 (IQR 2.9–5.4). The excluded articles only addressing adults were 227. The journal with the highest IF was Lancet Infectious Diseases (IF 24.446), and the one with the lowest was Pediatric Nephrology (IF 2.676). Table 1 shows the main features of the manuscripts.

Table 1.
Main features of the top-cited articles.
Sixty-eight articles (59.1%) were published in “infectious diseases” category journals.
The median number of top-cited articles per journal was 2 (IQR 1.5–3), and the journal with the highest number of top-cited articles was “clinical infectious diseases” with 46 manuscripts. As for gender description of first and last authors, male authors were predominant (81 vs. 34 and 96 vs. 19, respectively). Only in the pediatrics area subgroup, was there an almost equal number of male and female first authors (Table 1).
3.2. Top-Cited Articles
The median number of citations was 94 (IQR 51–178), ranging from 30 to 2796. The complete list of the top-cited articles in the “infectious diseases” and “pediatrics” categories is displayed in Supplementary Materials (Supplementary Table S1).
As for article type, 57 top-cited articles were original articles, 38 were guidelines and/or Reviews of the literature, and only nine papers were clinical trials.
As shown in Figure 1, the most productive country of origin was the USA, with 71 manuscripts. However, 12 papers were multicenter, involving more than two countries.
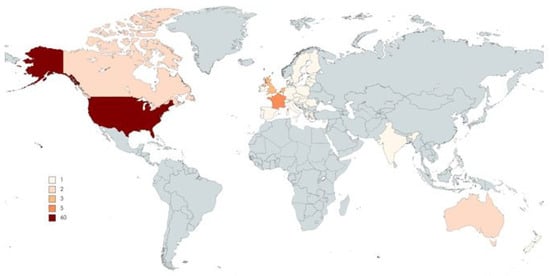
Figure 1.
Top-cited articles by country/region of origin.
Topics are displayed in detail in Table 2. The most treated topic was “bloodstream/complicated/invasive infections”, with 30 articles, followed by “antibiotic resistance/MRSA treatment”, with 27 papers, and “state of the art” papers (13 papers). Eleven articles were about “dosing strategies/continuous vs. intermittent infusion” and ten about C. difficile treatment (Table 2).

Table 2.
Top-cited articles by Topic: main findings.
As regards continuous versus intermittent infusion, most studies addressed the neonatal population. Some presented simplified schedules for continuous infusion in neonates, according to body weight and serum creatinine, that led to adequate serum vancomycin levels and a good efficacy profile, also reducing prescription error rates in this population (Table 2) [58,59,60,61,62,63,64,65,66,67,68,69,70,71,72,73,74,75,76,77,78,79,80,81,82,83,84,85,86,87,88,89,90,91,92,93,94,95,96,97,98,99,100,101,102,103,104,105,106,107,108,109,110,111,112,113,114,115,116,117,118,119,120].
Concerning the treatment of C. difficile infection, the oral administration of vancomycin is recommended in children (10 mg/kg/dose four times a day for ten days) for the initial episode, severe or non-severe, with a slow décalage in case of recurrence [104,105,106,107,108,109,110,111,112,113].
Other topics were “nephrotoxicity”, with six articles, “healthcare epidemiology” and “antibiotic stewardship” (four papers each). Papers dealing with nephrotoxicity mostly identified high vancomycin trough levels (>15 mg/L) and concomitant furosemide use as risk factors for the development of kidney injury [69,70].
Studies on epidemiology mainly focused on hospital-acquired and MRSA infections in Neonatal Intensive Care Units (NICUs), antibiotic exposure, and inflammatory bowel disease development [9,10,11,12]. Papers reporting antibiotic stewardship experiences were from NICU and Pediatric Critical Care units, showing the widespread use of vancomycin, considered inappropriate in large proportion in critically ill children and neonates [114,115].
The least reported topics were respectively “pharmacokinetics and pharmacodynamics” (PK/PD) and “adverse reactions” (three papers each), “ototoxicity”, in relation to hearing screening in newborns, and “MIC interpretative criteria”, with two articles each (Table 2).
In particular, only one of the retrieved articles on PK/PD was specific for the pediatric population, while the other two dealt with PK/PD features in general and about drug penetration in biofilm [1,14]. The most useful parameters for the evaluation of vancomycin PK/PD correlation are the AUC and MIC. As reported by Rybak et al., an AUC/MIC ratio higher than 400 is related to a plasma trough level above 15 µg/mL, assuming 1 mg/L MIC or less [1]. Model studies have reported that the current empiric recommended vancomycin dose in children of 40 mg/kg/day would unlikely achieve the recommended pharmacodynamic target of AUC 24/MIC >400 in case of methicillin-resistant S. aureus (MRSA) with MIC of 1.0 µg/mL or greater, proposing an increase of the dose to 60 mg/kg/day [120,121].
Twenty-seven papers dealt with neonates, 18 of which in NICU contexts, dealing with vancomycin for the treatment of neonatal infections, especially late-onset sepsis due to Gram-positive bacteria, mainly CoNS and MRSA [11]. Major topics were antibiotic resistance and dosing strategies, which reflect the main issues in this population to date. Ototoxicity and nephrotoxicity were addressed as the most important aspects to consider in the follow-up of neonates after vancomycin treatment [66,73]. The recommended intravenous dose for treating sepsis or severe infections in neonates is 10–15 mg/kg, 15 mg/kg for central nervous system infections, with varying intervals according to gestational and postnatal age (once every 18/12/8 h accordingly). According to most studies, for infants older than one month and children up to 12 years with a normal renal function, the advised intravenous daily dose is 60 mg/kg in four divided doses. During treatment of serious infections, including those related to MRSA, trough levels should be monitored, with a target concentration goal of 15–20 μg/mL [120].
Key points on vancomycin use in pediatric patients emerging from our analysis, including what is already known and gaps of knowledge for the development of future studies, are summarized in Table 3.

Table 3.
Key points on vancomycin use in pediatric patients.
4. Discussion
To our knowledge, this is the first bibliometric analysis with a special interest in vancomycin in the pediatric and neonatal populations.
We chose evaluative bibliometrics and the method of citation analysis to evaluate research performance in this particular field and to highlight gaps of knowledge for the development of future studies. We decided to consider the last three decades as a definite search period, because especially in those years vancomycin was extensively used, with a consequent selection pressure, determining the emergence of resistance [5,26] (Figure 2).
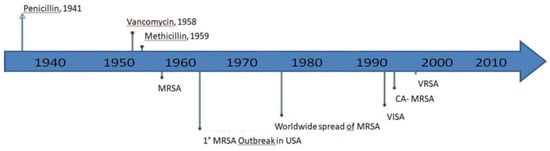
Figure 2.
S. aureus drug resistance development through the years (adapted from McGuinnes et al. [5]). MRSA: Methicillin-resistant S. aureus; VISA: vancomycin-intermediate S. aureus; CA: community-acquired; VRSA: vancomycin-resistant S. aureus.
Not surprisingly, the journal with the highest number of top-cited articles was in the infectious diseases and not the pediatric area, being ranked third among the journals of the same area. Among the top-ranked journals of the pediatric area, there were no pediatric infectious diseases sub-specialty journals. This may suggest that the search for the most relevant articles in pediatric infectious diseases should be performed not only among pediatric journals but mainly on the most impacted journals treating topics of infectious diseases.
As for the article types, the second most cited, after original articles, were guidelines and/or reviews of the literature. According to the hierarchy provided by the Center for Evidence-Based Medicine, the latter presents the highest level of evidence [122]. They mostly were clinical practice guidelines by Infectious Diseases societies or dealt with current evidence about prescription and dosing in children and neonates. The most represented topics were antibiotic resistance, complicated infections, and C. difficile infections, correctly reflecting the main fields and emerging issues involving vancomycin and its use in pediatric patients.
The two most treated topics in the top-cited articles were complicated infections and antibiotic resistance. Again, this is not surprising, thinking of the extensive use of vancomycin to treat invasive infections in critical patients, leading to antibiotic selection pressure after prolonged exposure, with an increased risk of treatment failure [3,4,28,29,30,31,32,33,34]. Selection pressure by indiscriminate use of vancomycin, linked to at least four genes (Van A-D), has led to the emergence of vancomycin-resistant Enterococci (VRE). As regards S. aureus, vancomycin-intermediate (VISA), and vancomycin-resistant S. aureus (VRSA) strains are described, together with the resistance of S. epidermidis, mainly linked to biofilm [3]. As highlighted by van Hal et al in a systematic review and meta-analysis, emerging data show that vancomycin may be less effective to treat serious MRSA infections with higher MICs, with treatment failure concerns. An association of high MICs and higher mortality rates in MRSA bloodstream infections has been demonstrated. [119]. Therefore, prospective studies are needed to assess if optimizing vancomycin treatment can improve outcomes without toxicity issues. This opens the chapter on second-line treatments for vancomycin treatment failures.
Among the 115 retrieved articles, 27 were about children as a selected population, dealing above all with the management of invasive infections and C. difficile infections. Two papers had the implementation of antibiotic stewardship programs as a primary focus, one of which in the context of a pediatric critical care unit [114,115]. This is indeed of paramount importance, considering the worldwide efforts to implement the appropriate use of antibiotics, especially in ICU settings. Vancomycin has undergone indiscriminate use for many years also in the pediatric population and the stewardship interventions aim at early therapy stop in the absence of microbiological isolates.
Despite the widespread use of vancomycin since its introduction on the market, only three top-cited papers dealt with PK/PD and one only specifically on the pediatric population. PK/PD studies and RCTs are lacking for the neonatal population, especially for preterm and extremely preterm infants, as reported by the many reviews retrieved in this study.
Dosing and safety in the pediatric and neonatal population are challenging, especially concerning continuous infusion, because of pharmacokinetics changes throughout the different ages. In general, ototoxicity and nephrotoxicity pathophysiological mechanisms are still unclear. The relation to dose exposure and treatment duration has not been proved, and focused studies are still lacking.
One central question is still open regarding the administration of vancomycin by continuous versus intermittent infusion in children and neonates. Adult evidence suggests that continuous infusion of vancomycin decreases nephrotoxicity and the incidence of infusion-related adverse events, while also diminishing time to therapeutic concentrations and drug costs [123,124]. Studies on preterm neonates and patients under three years of age on this topic are few to date, and thus evidence is limited, with data from the adult population being not completely applicable. Despite a general lack of consensus, continuous infusion regimens are already used in clinical practice in many centers, especially in the UK. Prolonged or continuous infusions strategies of time-dependent antibiotics are elsewhere still considered on a case-to-case basis in the pediatric population, and available data seem to indicate a higher probability of reaching target trough levels in children, with reported good clinical outcomes and safety profile [61,62,65,121,125].
Regarding gender description, the highest number of female first authors belonged to the Pediatrics area and were almost as represented as their male counterparts. Nevertheless, we identified a gender gap in medical research. Male authors were predominant as both first and last authors in the Infectious Diseases area and generally more represented in senior authorship in both areas. We decided to include this description as we believe it could be an interesting bibliometric parameter to be evaluated, as an added value for the readers. Any assumption related to a minor involvement of women in research teams or a less frequent presence as senior faculty members cannot be made based on our data and goes beyond the focus of our research.
Last, limitations to our study are intrinsic to the analysis type. Above all, the most recent articles may not have reached 30 citations due to a mere matter of time, and all relevant studies published in languages other than English may have been missed.
5. Conclusions and Future Perspectives
To conclude, top-cited articles about vancomycin use in children and neonates were almost equally distributed among journals of the “infectious diseases” and “pediatrics” areas. The most productive journal was Clinical Infectious Diseases. The most treated topics were bloodstream or complicated infections and antibiotic resistance/MRSA treatment. The pediatric area is indeed a critical one in the field of antibiotic therapy. In the last three decades, though less reported, also the role of antibiotic stewardship and attention to new dosing strategies in the neonatal and pediatric population have become sensible topics, which need to be further explored. As a widely used drug, data from studies properly conducted in the pediatric population, and not derived from adult studies, are needed. Moreover, the efficacy and safety of higher doses due to increases in MIC should be studied. Among the least represented topics, PK/PD, antibiotic stewardship, and dosing and infusion strategies, especially in neonates, represent a critical gap of knowledge, with a florid literature pointing out a lack of studies in the pediatric population since at least 30 years. This underlines the need for randomized-controlled trials to evaluate the clinical impact, safety, and acceptability of continuous infusion of vancomycin compared to intermittent infusion as a main challenge in the neonatal and pediatric population.
Supplementary Materials
The following are available online at https://www.mdpi.com/article/10.3390/pathogens10101343/s1, Document Supplementary 1: retrieved articles list, Document Supplementary 2: First quartile Infectious Diseases ranked journals list, Document Supplementary3: First quartile Pediatrics ranked journals list.
Author Contributions
Conceptualization and writing—original draft preparation: C.M., review and editing: C.M., D.D., E.B.; supervision: D.D., C.G. All authors have read and agreed to the published version of the manuscript.
Funding
This article received no funding.
Institutional Review Board Statement
Not Applicable.
Informed Consent Statement
Not Applicable.
Data Availability Statement
The data presented in this study are available in the Supplementary Material (S1–S3).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Rybak, M.J. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin. Infect. Dis. 2006, 42 (Suppl. S1), S35–S39. [Google Scholar] [CrossRef] [PubMed]
- Butterfield, J.M.; Patel, N.; Pai, M.P.; Rosano, T.G.; Drusano, G.L.; Lodise, T.P. Refining vancomycin protein binding estimates: Identification of clinical factors that influence protein binding. Antimicrob. Agents Chemother. 2011, 55, 4277–4282. [Google Scholar] [CrossRef] [PubMed]
- Gardete, S.; Tomasz, A. Mechanisms of vancomycin resistance in Staphylococcus aureus. J. Clin. Invest. 2014, 124, 2836–2840. [Google Scholar] [CrossRef] [PubMed]
- Center, K.J.; Reboli, A.C.; Hubler, R.; Rodgers, G.L.; Long, S.S. Decreased Vancomycin Susceptibility of Coagulase-Negative Staphylococci in a Neonatal Intensive Care Unit: Evidence of Spread of Staphylococcus warneri. J. Clin. Microbiol. 2003, 41, 4660–4665. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, W.A.; Malachowa, N.; DeLeo, F.R. Vancomycin Resistance in Staphylococcus Aureus. Yale J. Biol. Med. 2017, 90, 269–281. [Google Scholar] [PubMed]
- Le, J.; Nguyen, T.; Law, A.; Hodding, J. Adverse Drug Reactions among Children over a 10-Year Period. Pediatrics 2006, 118, 555–562. [Google Scholar] [CrossRef]
- Polk, R.E. Anaphylactoid reactions to glycopeptide antibiotics. J. Antimicrob. Chemother. 1991, 27 (Suppl. B), 17–29. [Google Scholar] [CrossRef]
- Wilson, A. Comparative safety of teicoplanin and vancomycin. Int. J. Antimicrob. Agents 1998, 10, 143–152. [Google Scholar] [CrossRef]
- McMullan, B.J.; Bowen, A.; Blyth, C.; van Hal, S.; Korman, T.; Buttery, J.; Voss, L.; Roberts, S.; Cooper, C.; Tong, S.; et al. Epidemiology and Mortality of Staphylococcus aureus Bacteremia in Australian and New Zealand Children. JAMA Pediatr. 2016, 170, 979–986. [Google Scholar] [CrossRef] [PubMed]
- Carey, A.J.; Saiman, L.; Polin, R.A. Hospital-Acquired Infections in the NICU: Epidemiology for the New Millennium. Clin. Perinatol. 2008, 35, 223–249. [Google Scholar] [CrossRef] [PubMed]
- Nelson, M.U.; Gallagher, P.G. Methicillin-Resistant Staphylococcus aureus in the Neonatal Intensive Care Unit. Semin. Perinatol. 2012, 36, 424–430. [Google Scholar] [CrossRef] [PubMed]
- Kronman, M.P.; Zaoutis, T.E.; Haynes, K.; Feng, R.; Coffin, S.E. Antibiotic Exposure and IBD Development among Children: A Population-Based Cohort Study. Pediatrics 2012, 130, e794–e803. [Google Scholar] [CrossRef]
- Grimsley, C.; Thomson, A.H. Pharmacokinetics and dose requirements of vancomycin in neonates. Arch. Dis. Child. Fetal Neonatal Ed. 1999, 81, F221–F227. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.; Ray, P.; Das, A.; Sharma, M. Penetration of antibiotics through Staphylococcus aureus and Staphylococcus epidermidis biofilms. J. Antimicrob. Chemother. 2010, 65, 1955–1958. [Google Scholar] [CrossRef] [PubMed]
- Blaskovich, M.A.T.; Hansford, K.A.; Butler, M.S.; Jia, Z.; Mark, A.E.; Cooper, M.A. Developments in Glycopeptide Antibiotics. ACS Infect. Dis. 2018, 4, 715–735. [Google Scholar] [CrossRef]
- Boyce, J.M.; Cookson, B.; Christiansen, K.; Hori, S.; Vuopio-Varkila, J.; Kocagöz, S.; Öztop, A.Y.; Vandenbroucke-Grauls, C.M.; Harbarth, S.; Pittet, D. Meticillin-resistant Staphylococcus aureus. Lancet Infect. Dis. 2005, 5, 653–663. [Google Scholar] [CrossRef]
- Deresinski, S. Counterpoint: Vancomycin and Staphylococcus aureus--An Antibiotic Enters Obsolescence. Clin. Infect. Dis. 2007, 44, 1543–1548. [Google Scholar] [CrossRef] [PubMed]
- Herigon, J.; Hersh, A.L.; Gerber, J.S.; Zaoutis, T.E.; Newland, J.G. Antibiotic Management of Staphylococcus aureus Infections in US Children’s Hospitals, 1999–2008. Pediatrics 2010, 125, e1294–e1300. [Google Scholar] [CrossRef]
- Jarvis, W.R. Epidemiology, Appropriateness, and Cost of Vancomycin Use. Clin. Infect. Dis. 1998, 26, 1200–1203. [Google Scholar] [CrossRef] [PubMed]
- Jones, R.N. Microbiological Features of Vancomycin in the 21st Century: Minimum Inhibitory Concentration Creep, Bactericidal/Static Activity, and Applied Breakpoints to Predict Clinical Outcomes or Detect Resistant Strains. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S13–S24. [Google Scholar] [CrossRef]
- Stevens, D.L. The Role of Vancomycin in the Treatment Paradigm. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S51–S57. [Google Scholar] [CrossRef] [PubMed]
- Moellering, J.R.C. Vancomycin: A 50-Year Reassessment. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S3–S4. [Google Scholar] [CrossRef] [PubMed]
- Mohr, J.F.; Murray, B.E. Point: Vancomycin is not obsolete for the treatment of infection caused by methicillin-resistant Staphylococcus aureus. Clin. Infect. Dis. 2007, 44, 1536–1542. [Google Scholar] [CrossRef] [PubMed]
- Patel, N.; Pai, M.P.; Rodvold, K.A.; Lomaestro, B.; Drusano, G.L.; Lodise, T.P. Vancomycin: We Can’t Get There From Here. Clin. Infect. Dis. 2011, 52, 969–974. [Google Scholar] [CrossRef]
- Rubin, L.G.; Sánchez, P.J.; Siegel, J.; Levine, G.; Saiman, L.; Jarvis, W.R. Evaluation and treatment of neonates with suspected late-onset sepsis: A survey of neonatologists’ practices. Pediatrics 2002, 110, e42. [Google Scholar] [CrossRef] [PubMed]
- Levine, D.P. Vancomycin: A History. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S5–S12. [Google Scholar] [CrossRef] [PubMed]
- Tice, A.D.; Rehm, S.J.; Dalovisio, J.R.; Bradley, J.S.; Martinelli, L.P.; Graham, D.R.; Gainer, R.B.; Kunkel, M.J.; Yancey, R.W.; Williams, D.N. Practice Guidelines for Outpatient Parenteral Antimicrobial Therapy. Clin. Infect. Dis. 2004, 38, 1651–1671. [Google Scholar] [CrossRef] [PubMed]
- Courvalin, P. Vancomycin Resistance in Gram-Positive Cocci. Clin. Infect. Dis. 2006, 42 (Suppl. 1), S25–S34. [Google Scholar] [CrossRef]
- Falagas, M.; Makris, G.; Dimopoulos, G.; Matthaiou, D. Heteroresistance: A concern of increasing clinical significance? Clin. Microbiol. Infect. 2008, 14, 101–104. [Google Scholar] [CrossRef]
- Stryjewski, M.; Corey, G.R. Methicillin-Resistant Staphylococcus aureus: An Evolving Pathogen. Clin. Infect. Dis. 2014, 58 (Suppl. 1), S10–S19. [Google Scholar] [CrossRef]
- Healy, C.M.; Hulten, K.; Palazzi, D.L.; Campbell, J.R.; Baker, C.J. Emergence of New Strains of Methicillin-Resistant Staphylococcus aureus in a Neonatal Intensive Care Unit. Clin. Infect. Dis. 2004, 39, 1460–1466. [Google Scholar] [CrossRef]
- Cosgrove, S.E. The Relationship between Antimicrobial Resistance and Patient Outcomes: Mortality, Length of Hospital Stay, and Health Care Costs. Clin. Infect. Dis. 2006, 42 (Suppl. 2), S82–S89. [Google Scholar] [CrossRef] [PubMed]
- Gold, H.S. Vancomycin-Resistant Enterococci: Mechanisms and Clinical Observations. Clin. Infect. Dis. 2001, 33, 210–219. [Google Scholar] [CrossRef]
- Weinstein, R.A.; Fridkin, S.K. Vancomycin-Intermediate and -Resistant Staphylococcus aureus: What the Infectious Disease Specialist Needs to Know. Clin. Infect. Dis. 2001, 32, 108–115. [Google Scholar] [CrossRef] [PubMed]
- Arthur, M.; Reynolds, P.; Depardieu, F.; Evers, S.; Dutka-Malen, S.; Quintiliani, R.; Courvalin, P. Mechanisms of glycopeptide resistance in enterococci. J. Infect. 1996, 32, 11–16. [Google Scholar] [CrossRef]
- Bizzarro, M.J.; Gallagher, P.G. Antibiotic-Resistant Organisms in the Neonatal Intensive Care Unit. Semin. Perinatol. 2007, 31, 26–32. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, D.W.; Auld, D.B.; Mermel, L.A. Community-Acquired Methicillin-Resistant Staphylococcus aureus in Southern New England Children. Pediatrics 2004, 113, e347–e352. [Google Scholar] [CrossRef] [PubMed]
- Donskey, C.J. The Role of the Intestinal Tract as a Reservoir and Source for Transmission of Nosocomial Pathogens. Clin. Infect. Dis. 2004, 39, 219–226. [Google Scholar] [CrossRef]
- French, G.L. Enterococci and Vancomycin Resistance. Clin. Infect. Dis. 1998, 27 (Suppl. 1), S75–S83. [Google Scholar] [CrossRef] [PubMed]
- Kalima, P.; Masterton, R.; Roddie, P.; Thomas, A. Lactobacillus rhamnosus infection in a child following bone marrow transplant. J. Infect. 1996, 32, 165–167. [Google Scholar] [CrossRef]
- Kollef, M.H. Limitations of Vancomycin in the Management of Resistant Staphylococcal Infections. Clin. Infect. Dis. 2007, 45, S191–S195. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Dutka-Malen, S.; Brisson-Noël, A.; Molinas, C.; Derlot, E.; Arthur, M.; Duval, J.; Courvalin, P. Resistance of Enterococci to Aminoglycosides and Glycopeptides. Clin. Infect. Dis. 1992, 15, 495–501. [Google Scholar] [CrossRef] [PubMed]
- Leclercq, R.; Courvalin, P. Resistance to Glycopeptides in Enterococci. Clin. Infect. Dis. 1997, 24, 545–554, quiz 555–556. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Bayer, A.; Cosgrove, S.E.; Daum, R.S.; Fridkin, S.K.; Gorwitz, R.J.; Kaplan, S.L.; Karchmer, A.W.; Levine, D.P.; Murray, B.E.; et al. Clinical Practice Guidelines by the Infectious Diseases Society of America for the Treatment of Methicillin-Resistant Staphylococcus aureus Infections in Adults and Children. Clin. Infect. Dis. 2011, 52, e18–e55. [Google Scholar] [CrossRef]
- Moellering, J.R.C. Vancomycin-Resistant Enterococci. Clin. Infect. Dis. 1998, 26, 1196–1199. [Google Scholar] [CrossRef] [PubMed]
- Molton, J.; Tambyah, P.A.; Ang, B.S.P.; Ling, M.L.; Fisher, D.A. The Global Spread of Healthcare-Associated Multidrug-Resistant Bacteria: A Perspective From Asia. Clin. Infect. Dis. 2013, 56, 1310–1318. [Google Scholar] [CrossRef]
- Rehm, S.J.; Tice, A. Staphylococcus aureus: Methicillin-SusceptibleS. aureusto Methicillin-ResistantS. aureusand Vancomycin-Resistant S. aureus. Clin. Infect. Dis. 2010, 51 (Suppl. 2), S176–S182. [Google Scholar] [CrossRef]
- Rodvold, K.A.; McConeghy, K.W. Methicillin-Resistant Staphylococcus aureus Therapy: Past, Present, and Future. Clin. Infect. Dis. 2014, 58 (Suppl. 1), S20–S27. [Google Scholar] [CrossRef]
- Rubinstein, E.; Kollef, M.H.; Nathwani, D. Pneumonia Caused by Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S378–S385. [Google Scholar] [CrossRef]
- Stryjewski, M.E.; Chambers, H.F. Skin and Soft-Tissue Infections Caused by Community-Acquired Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2008, 46 (Suppl. 5), S368–S377. [Google Scholar] [CrossRef]
- Tacconelli, E.; Carrara, E.; Savoldi, A.; Harbarth, S.; Mendelson, M.; Monnet, D.L.; Pulcini, C.; Kahlmeter, G.; Kluytmans, J.; Carmeli, Y.; et al. Discovery, research, and development of new antibiotics: The WHO priority list of antibiotic-resistant bacteria and tuberculosis. Lancet Infect. Dis. 2018, 18, 318–327. [Google Scholar] [CrossRef]
- Talbot, G.H.; Bradley, J.; Edwards, J.J.E.; Gilbert, D.; Scheld, M.; Bartlett, J.G. Bad Bugs Need Drugs: An Update on the Development Pipeline from the Antimicrobial Availability Task Force of the Infectious Diseases Society of America. Clin. Infect. Dis. 2006, 42, 657–668. [Google Scholar] [CrossRef]
- Zaoutis, T.E.; Prasad, P.A.; Localio, A.R.; Coffin, S.E.; Bell, L.M.; Walsh, T.J.; Gross, R. Risk Factors and Predictors for Candidemia in Pediatric Intensive Care Unit Patients: Implications for Prevention. Clin. Infect. Dis. 2010, 51, e38–e45. [Google Scholar] [CrossRef] [PubMed]
- Zembower, T.R.; A Noskin, G.; Postelnick, M.J.; Nguyen, C.; Peterson, L.R. The utility of aminoglycosides in an era of emerging drug resistance. Int. J. Antimicrob. Agents 1998, 10, 95–105. [Google Scholar] [CrossRef]
- de Hoog, M.; Mouton, J.W.; Anker, J.N.V.D. New dosing strategies for antibacterial agents in the neonate. Semin. Fetal Neonatal Med. 2005, 10, 185–194. [Google Scholar] [CrossRef]
- Downes, K.J.; Hahn, A.; Wiles, J.; Courter, J.D.; Vinks, A.A. Dose optimisation of antibiotics in children: Application of pharmacokinetics/pharmacodynamics in paediatrics. Int. J. Antimicrob. Agents 2014, 43, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Harskamp-van Ginkel, M.W.; Hill, K.D.; Becker, K.C.; Testoni, D.; Cohen-Wolkowiez, M.; Gonzalez, D.; Barrett, J.S.; Benjamin, D.J.; Siegel, D.A.; Banks, P.; et al. Drug Dosing and Pharmacokinetics in Children With Obesity A Systematic Review. JAMA Pediatr. 2015, 169, 678–685. [Google Scholar] [CrossRef]
- Jacqz-Aigrain, E.; Zhao, W.; Sharland, M.; van den Anker, J.N. Use of antibacterial agents in the neonate: 50 years of experience with vancomycin administration. Semin. Fetal Neonatal Med. 2013, 18, 28–34. [Google Scholar] [CrossRef]
- Leroux, S.; Zhao, W.; Bétrémieux, P.; Pladys, P.; Saliba, E.; Jacqz-Aigrain, E. Therapeutic guidelines for prescribing antibiotics in neonates should be evidence-based: A French national survey. Arch. Dis. Child. 2015, 100, 394–398. [Google Scholar] [CrossRef]
- Moellering, J.R.C. Editorial: Monitoring Serum Vancomycin Levels: Climbing the Mountain Because It Is There? Clin. Infect. Dis. 1994, 18, 544–546. [Google Scholar] [CrossRef]
- Oudin, C.; Vialet, R.; Boulamery, A.; Martin, C.; Simon, N. Vancomycin prescription in neonates and young infants: Toward a simplified dosage. Arch. Dis. Child.-Fetal Neonatal Ed. 2011, 96, F365–F370. [Google Scholar] [CrossRef] [PubMed]
- Plan, O.; Cambonie, G.; Barbotte, E.; Meyer, P.; Devine, C.; Milesi, C.; Pidoux, O.; Badr, M.; Picaud, J.C. Continuous-infusion vancomycin therapy for preterm neonates with suspected or documented Gram-positive infections: A new dosage schedule. Arch. Dis. Child. Fetal Neonatal Ed. 2008, 93, F418–F421. [Google Scholar] [CrossRef] [PubMed]
- Rubin, L.G.; Papsin, B. Committee on Infectious Diseases and Section on Otolaryngology-Head and Neck Surgery Cochlear Implants in Children: Surgical Site Infections and Prevention and Treatment of Acute Otitis Media and Meningitis. Pediatrics 2010, 126, 381–391. [Google Scholar] [CrossRef] [PubMed]
- Tobin, C.M.; Darville, J.M.; Thomson, A.H.; Sweeney, G.; Wilson, J.F.; MacGowan, A.P.; White, L.O. Vancomycin therapeutic drug monitoring: Is there a consensus view? The results of a UK National External Quality Assessment Scheme (UK NEQAS) for Antibiotic Assays questionnaire. J. Antimicrob. Chemother. 2002, 50, 713–718. [Google Scholar] [CrossRef] [PubMed]
- Zhao, W.; Lopez, E.; Biran, V.; Durrmeyer, X.; Fakhoury, M.; Jacqz-Aigrain, E. Vancomycin continuous infusion in neonates: Dosing optimisation and therapeutic drug monitoring. Arch. Dis. Child. 2013, 98, 449–453. [Google Scholar] [CrossRef] [PubMed]
- Berg, A.L. Newborn Hearing Screening in the NICU: Profile of Failed Auditory Brainstem Response/Passed Otoacoustic Emission. Pediatrics 2005, 116, 933–938. [Google Scholar] [CrossRef]
- de Hoog, M.; van Zanten, B.A.; Hop, W.C.; Overbosch, E.; Weisglas-Kuperus, N.; Anker, J.N.V.D. Newborn hearing screening: Tobramycin and vancomycin are not risk factors for hearing loss. J. Pediatr. 2003, 142, 41–46. [Google Scholar] [CrossRef]
- Downes, K.J.; Cowden, C.; Laskin, B.L.; Huang, Y.-S.; Gong, W.; Bryan, M.; Fisher, B.T.; Goldstein, S.L.; Zaoutis, T.E. Association of Acute Kidney Injury With Concomitant Vancomycin and Piperacillin/Tazobactam Treatment Among Hospitalized Children. JAMA Pediatr. 2017, 171, e173219. [Google Scholar] [CrossRef]
- Hammond, D.; Smith, M.N.; Li, C.; Hayes, S.M.; Lusardi, K.; Bookstaver, P.B. Systematic Review and Meta-Analysis of Acute Kidney Injury Associated with Concomitant Vancomycin and Piperacillin/tazobactam. Clin. Infect. Dis. 2016, 64, 666–674. [Google Scholar] [CrossRef]
- McKamy, S.; Hernandez, E.; Jahng, M.; Moriwaki, T.; Deveikis, A.L.J. Incidence and risk factors influencing the development of vancomycin nephrotoxicity in children. J. Pediatr. 2011, 158, 422–426. [Google Scholar] [CrossRef]
- Narendra, A.; White, M.P.; A Rolton, H.; I Alloub, Z.; Wilkinson, G.; McColl, J.; Beattie, J. Nephrocalcinosis in preterm babies. Arch. Dis. Child.-Fetal Neonatal Ed. 2001, 85, F207–F213. [Google Scholar] [CrossRef] [PubMed]
- Rhodin, M.M.; Anderson, B.J.; Peters, A.M.; Coulthard, M.G.; Wilkins, B.; Cole, M.; Chatelut, E.; Grubb, A.; Veal, G.; Keir, M.J.; et al. Human renal function maturation: A quantitative description using weight and postmenstrual age. Pediatr. Nephrol. 2009, 24, 67–76. [Google Scholar] [CrossRef] [PubMed]
- Stojanović, V.; Barisic, N.; Milanović, B.; Doronjski, A. Acute kidney injury in preterm infants admitted to a neonatal intensive care unit. Pediatr. Nephrol. 2014, 29, 2213–2220. [Google Scholar] [CrossRef]
- Boucher, H.; Miller, L.G.; Razonable, R.R. Serious Infections Caused by Methicillin-Resistant Staphylococcus aureus. Clin. Infect. Dis. 2010, 51, S183–S197. [Google Scholar] [CrossRef]
- Cometta, A.; Kern, W.V.; De Bock, R.; Paesmans, M.; Vandenbergh, M.; Crokaert, F.; Engelhard, D.; Marchetti, O.; Akan, H.; Skoutelis, A.; et al. Vancomycin versus Placebo for Treating Persistent Fever in Patients with Neutropenic Cancer Receiving Piperacillin-Tazobactam Monotherapy. Clin. Infect. Dis. 2003, 37, 382–389. [Google Scholar] [CrossRef]
- Creel, A.M.; Durham, S.H.; Benner, K.W.; Alten, J.A.; Winkler, M.K. Severe invasive community-associated methicillin-resistant Staphylococcus aureus infections in previously healthy children *. Pediatr. Crit. Care Med. 2009, 10, 323–327. [Google Scholar] [CrossRef]
- Darouiche, R.O.; Dhir, A.; Miller, A.J.; Landon, G.C.; Raad, I.I.; Musher, D.M. Vancomycin Penetration Into Biofilm Covering Infected Prostheses And Effect On Bacteria. J. Infect. Dis. 1994, 170, 720–723. [Google Scholar] [CrossRef]
- Davies, Y.K.; Cox, K.M.; A Abdullah, B.; Safta, A.; Terry, A.B.; Cox, K.L. Long-term Treatment of Primary Sclerosing Cholangitis in Children With Oral Vancomycin: An Immunomodulating Antibiotic. J. Pediatr. Gastroenterol. Nutr. 2008, 47, 61–67. [Google Scholar] [CrossRef] [PubMed]
- Denniston, S.; I Riordan, F.A. Staphylococcus aureus bacteraemia in children and neonates: A 10 year retrospective review. J. Infect. 2006, 53, 387–393. [Google Scholar] [CrossRef]
- Dubnov-Raz, G.; Scheuerman, O.; Chodick, G.; Finkelstein, Y.; Samra, Z.; Garty, B.Z. Invasive Kingella kingae Infections in Children: Clinical and Laboratory Characteristics. Pediatrics 2008, 122, 1305–1309. [Google Scholar] [CrossRef] [PubMed]
- Falagas, M.E.; Siempos, I.I.; Vardakas, K.Z. Linezolid versus glycopeptide or β-lactam for treatment of Gram-positive bacterial infections: Meta-analysis of randomised controlled trials. Lancet Infect. Dis. 2008, 8, 53–66. [Google Scholar] [CrossRef]
- Garland, J.S.; Alex, C.P.; Henrickson, K.J.; McAuliffe, T.L.; Maki, D.G. A Vancomycin-Heparin Lock Solution for Prevention of Nosocomial Bloodstream Infection in Critically Ill Neonates With Peripherally Inserted Central Venous Catheters: A Prospective, Randomized Trial. Pediatrics 2005, 116, e198–e205. [Google Scholar] [CrossRef]
- Hiramatsu, K.; Hanaki, H.; Ino, T.; Yabuta, K.; Oguri, T.; Tenover, F.C. Methicillin-resistant Staphylococcus aureus clinical strain with reduced vancomycin susceptibility. J. Antimicrob. Chemother. 1997, 40, 135–136. [Google Scholar] [CrossRef]
- Kacica, M.A.; Horgan, M.J.; Ochoa, L.; Sandler, R.; Lepow, M.L.; Venezia, R.A. Prevention of gram-positive sepsis in neonates weighing less than 1500 grams. J. Pediatr. 1994, 125, 253–258. [Google Scholar] [CrossRef]
- Karlowicz, M.G.; Buescher, E.S.; Surka, A.E. Fulminant Late-Onset Sepsis in a Neonatal Intensive Care Unit, 1988-1997, and the Impact of Avoiding Empiric Vancomycin Therapy. Pediatrics 2000, 106, 1387–1390. [Google Scholar] [CrossRef] [PubMed]
- Makhoul, I.R.; Kassis, I.; Smolkin, T.; Tamir, A.; Sujov, P. Review of 49 neonates with acquired fungal sepsis: Further characterization. Pediatrics 2001, 107, 61–66. [Google Scholar] [CrossRef] [PubMed]
- Nichols, R.L. Optimal treatment of complicated skin and skin structure infections. J. Antimicrob. Chemother. 1999, 44, 19–23. [Google Scholar] [CrossRef] [PubMed]
- Pammi, M.; Liang, R.; Hicks, J.M.; Barrish, J.; Versalovic, J. Farnesol Decreases Biofilms of Staphylococcus epidermidis and Exhibits Synergy With Nafcillin and Vancomycin. Pediatr. Res. 2011, 70, 578–583. [Google Scholar] [CrossRef]
- Rackoff, W.R.; Weiman, M.; Jakobowski, D.; Hirschl, R.; Stallings, V.; Bilodeau, J.; Danz, P.; Bell, L.; Lange, B. A randomized, controlled trial of the efficacy ofheparin and vancomycin solution in preventing central venous catheter infections in children. J. Pediatr. 1995, 127, 147–151. [Google Scholar] [CrossRef]
- Safdar, N.; Maki, D.G. Use of Vancomycin-Containing Lock or Flush Solutions for Prevention of Bloodstream Infection Associated with Central Venous Access Devices: A Meta-Analysis of Prospective, Randomized Trials. Clin. Infect. Dis. 2006, 43, 474–484. [Google Scholar] [CrossRef]
- Salzman, M.B.; Isenberg, H.D.; Shapiro, J.F.; Lipsitz, P.J.; Rubin, L.G. A Prospective Study of the Catheter Hub as the Portal of Entry for Microorganisms Causing Catheter-Related Sepsis in Neonates. J. Infect. Dis. 1993, 167, 487–490. [Google Scholar] [CrossRef]
- Sejvar, J.J.; Tenover, F.C.; Stephens, D.S. Management of anthrax meningitis. Lancet Infect. Dis. 2005, 5, 287–295. [Google Scholar] [CrossRef]
- Seltz, L.B.; Smith, J.; Durairaj, V.D.; Enzenauer, R.; Todd, J. Microbiology and Antibiotic Management of Orbital Cellulitis. Pediatrics 2011, 127, e566–e572. [Google Scholar] [CrossRef]
- Shane, A.L.; Stoll, B.J. Neonatal sepsis: Progress towards improved outcomes. J. Infect. 2014, 68, S24–S32. [Google Scholar] [CrossRef]
- Siu, Y.K.; Ng, P.C.; Fung, S.C.K.; Lee, C.H.; Wong, M.Y.; Fok, T.F.; So, K.W.; Cheung, K.L.; Wong, W.; Cheng, A.F.B. Double blind, randomised, placebo controlled study of oral vancomycin in prevention of necrotising enterocolitis in preterm, very low birthweight infants. Arch. Dis. Child.-Fetal Neonatal Ed. 1998, 79, F105–F109. [Google Scholar] [CrossRef] [PubMed]
- Spafford, P.S.; Sinkin, R.A.; Cox, C.; Reubens, L.; Powell, K.R. Prevention of central venous catheter-related coagulase-negative staphylococcal sepsis in neonates. J. Pediatr. 1994, 125, 259–263. [Google Scholar] [CrossRef]
- Tunkel, A.R.; Hasbun, R.; Bhimraj, A.; Byers, K.; Kaplan, S.L.; Scheld, W.M.; van de Beek, D.; Bleck, T.P.; Garton, H.J.; Zunt, J.R. 2017 Infectious Diseases Society of America’s Clinical Practice Guidelines for Healthcare-Associated Ventriculitis and Meningitis *. Clin. Infect. Dis. 2017, 64, e34–e65. [Google Scholar] [CrossRef] [PubMed]
- Van den Hoogen, A.; Gerards, L.J.; Verboon-Maciolek, M.A.; Fleer, A.; Krediet, T.G. Long-Term Trends in the Epidemiology of Neonatal Sepsis and Antibiotic Susceptibility of Causative Agents. Neonatology 2010, 97, 22–28. [Google Scholar] [CrossRef] [PubMed]
- Van Hal, S.J.; Fowler, J.V.G. Is It Time to Replace Vancomycin in the Treatment of Methicillin-Resistant Staphylococcus aureus Infections? Clin. Infect. Dis. 2013, 56, 1779–1788. [Google Scholar] [CrossRef][Green Version]
- Van Vliet, M.J.; Tissing, W.J.E.; Dun, C.A.J.; Meessen, N.E.L.; Kamps, W.A.; de Bont, E.S.J.M.; Harmsen, H.J.M. Chemotherapy Treatment in Pediatric Patients with Acute Myeloid Leukemia Receiving Antimicrobial Prophylaxis Leads to a Relative Increase of Colonization with Potentially Pathogenic Bacteria in the Gut. Clin. Infect. Dis. 2009, 49, 262–270. [Google Scholar] [CrossRef] [PubMed]
- Weisman, L.E.; Thackray, H.M.; Steinhorn, R.H.; Walsh, W.F.; Lassiter, H.A.; Dhanireddy, R.; Brozanski, B.S.; Palmer, K.G.H.; Trautman, M.S.; Escobedo, M.; et al. A Randomized Study of a Monoclonal Antibody (Pagibaximab) to Prevent Staphylococcal Sepsis. Pediatrics 2011, 128, 271–279. [Google Scholar] [CrossRef]
- Wisplinghoff, H.; Bischoff, T.; Tallent, S.M.; Seifert, H.; Wenzel, R.P.; Edmond, M.B. Nosocomial Bloodstream Infections in US Hospitals: Analysis of 24,179 Cases from a Prospective Nationwide Surveillance Study. Clin. Infect. Dis. 2004, 39, 309–317. [Google Scholar] [CrossRef] [PubMed]
- Wong, K.S.; Lin, T.Y.; Huang, Y.C.; Hsia, S.H.; Yang, P.H.; Chu, S.M. Clinical and radiographic spectrum of septic pulmonary embolism. Arch. Dis. Child. 2002, 87, 312–315. [Google Scholar] [CrossRef] [PubMed]
- El Feghaly, R.E.; Stauber, J.L.; Tarr, P.I.; Haslam, D.B. Intestinal Inflammatory Biomarkers and Outcome in Pediatric Clostridium difficile Infections. J. Pediatr. 2013, 163, 1697–1704.e2. [Google Scholar] [CrossRef]
- Bartlett, J.G. Clostridium difficile: History of Its Role as an Enteric Pathogen and the Current State of Knowledge About the Organism. Clin. Infect. Dis. 1994, 18, S265–S272. [Google Scholar] [CrossRef]
- Cohen, M.B. Clostridium difficile Infections: Emerging Epidemiology and New Treatments. J. Pediatr. Gastroenterol. Nutr. 2009, 2, S63–S65. [Google Scholar] [CrossRef] [PubMed]
- Debast, S.B.; Bauer, M.P.; Kuijper, E.J. European Society of Clinical Microbiology and Infectious Diseases: Update of the treatment guidance document for Clostridium difficile infection. Clin. Microbiol. Infect 2014, 20, 1–26. [Google Scholar] [CrossRef]
- McDonald, L.C.; Gerding, D.N.; Johnson, S.; Bakken, J.S.; Carroll, K.C.; Coffin, S.E.; Dubberke, E.R.; Garey, K.W.; Gould, C.V.; Kelly, C.; et al. Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA). Clin Infect Dis. 2018, 66, e1–e48. [Google Scholar] [CrossRef] [PubMed]
- Mezoff, E.; Mann, E.A.; Hart, K.W.; Lindsell, C.J.; Cohen, M.B. Clostridium difficile Infection and Treatment in the Pediatric Inflammatory Bowel Disease Population. J. Pediatr. Gastroenterol. Nutr. 2011, 52, 437–441. [Google Scholar] [CrossRef]
- Pépin, J.; Routhier, S.; Gagnon, S.; Brazeau, I. Management and Outcomes of a First Recurrence of Clostridium difficile-Associated Disease in Quebec, Canada. Clin. Infect. Dis. 2006, 42, 758–764. [Google Scholar] [CrossRef]
- Russell, G.; Kaplan, J.; Ferraro, M.; Michelow, I.C. Fecal Bacteriotherapy for Relapsing Clostridium difficile Infection in a Child: A Proposed Treatment Protocol. Pediatrics 2010, 126, e239–e242. [Google Scholar] [CrossRef]
- Schutze, G.E.; Willoughby, R.E.; Committee on Infectious Diseases; American Academy of Pediatrics. Clostridium difficile Infection in Infants and Children. Pediatrics 2013, 131, 196–200. [Google Scholar] [CrossRef]
- Youngster, I.; Sauk, J.; Pindar, C.; Wilson, R.G.; Kaplan, J.L.; Smith, M.B.; Alm, E.J.; Gevers, D.; Russell, G.H.; Hohmann, E.L. Fecal Microbiota Transplant for Relapsing Clostridium difficile Infection Using a Frozen Inoculum From Unrelated Donors: A Randomized, Open-Label, Controlled Pilot Study. Clin. Infect. Dis. 2014, 58, 1515–1522. [Google Scholar] [CrossRef]
- Blinova, E.; Lau, E.; Bitnun, A.; Cox, P.; Schwartz, S.; Atenafu, E.; Yau, Y.; Streitenberger, L.; Parshuram, C.S.; Marshall, J.; et al. Point Prevalence Survey of Antimicrobial Utilization in the Cardiac and Pediatric Critical Care Unit. Pediatr. Crit. Care Med. 2013, 14, e280–e288. [Google Scholar] [CrossRef] [PubMed]
- Clark, R.H.; Bloom, B.T.; Spitzer, A.R.; Gerstmann, D.R. Reported Medication Use in the Neonatal Intensive Care Unit: Data From a Large National Data Set. Pediatrics 2006, 117, 1979–1987. [Google Scholar] [CrossRef] [PubMed]
- Dellit, T.H.; Owens, R.C.; McGowan, J.E., Jr.; Gerding, D.N.; Weinstein, R.A.; Burke, J.P.; Huskins, W.C.; Paterson, D.L.; Fishman, N.O.; Carpenter, C.F.; et al. Infectious Diseases Society of America and the Society for Healthcare Epidemiology of America Guidelines for Developing an Institutional Program to Enhance Antimicrobial Stewardship. Clin. Infect. Dis. 2007, 44, 159–177. [Google Scholar] [CrossRef] [PubMed]
- Hersh, A.L.; De Lurgio, S.A.; Thurm, C.; Lee, B.R.; Weissman, S.J.; Courter, J.D.; Brogan, T.V.; Shah, S.S.; Kronman, M.P.; Gerber, J.S.; et al. Antimicrobial Stewardship Programs in Freestanding Children’s Hospitals. Pediatrics 2014, 135, 33–39. [Google Scholar] [CrossRef]
- Tenover, F.C.; Moellering, J.R.C. The Rationale for Revising the Clinical and Laboratory Standards Institute Vancomycin Minimal Inhibitory Concentration Interpretive Criteria for Staphylococcus aureus. Clin. Infect. Dis. 2007, 44, 1208–1215. [Google Scholar] [CrossRef]
- Van Hal, S.J.; Lodise, T.P.; Paterson, D. The Clinical Significance of Vancomycin Minimum Inhibitory Concentration in Staphylococcus aureus Infections: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2012, 54, 755–771. [Google Scholar] [CrossRef]
- Frymoyer, A.; Hersh, A.L.; Benet, L.Z.; Guglielmo, J. Current recommended dosing of vancomycin for children with invasive methicillin-resistant Staphylococcus aureus infections is inadequate. Pediatr. Infect. Dis. J. 2009, 28, 398–402. [Google Scholar] [CrossRef] [PubMed]
- Germovsek, E.; Osborne, L.; Gunaratnam, F.; A Lounis, S.; Busquets, F.B.; Standing, J.F.; Sinha, A.K. Development and external evaluation of a population pharmacokinetic model for continuous and intermittent administration of vancomycin in neonates and infants using prospectively collected data. J. Antimicrob. Chemother. 2019, 74, 1003–1011. [Google Scholar] [CrossRef]
- Burns, P.B.; Rohrich, R.J.; Chung, K.C. The Levels of Evidence and Their Role in Evidence-Based Medicine. Plast. Reconstr. Surg. 2011, 128, 305–310. [Google Scholar] [CrossRef]
- Cataldo, M.A.; Tacconelli, E.; Grilli, E.; Pea, F.; Petrosillo, N. Continuous versus intermittent infusion of vancomycin for the treatment of Gram-positive infections: Systematic review and meta-analysis. J. Antimicrob. Chemother. 2011, 67, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Hao, J.-J.; Chen, H.; Zhou, J.-X. Continuous versus intermittent infusion of vancomycin in adult patients: A systematic review and meta-analysis. Int. J. Antimicrob. Agents 2016, 47, 28–35. [Google Scholar] [CrossRef] [PubMed]
- Costenaro, P.; Minotti, C.; Cuppini, E.; Barbieri, E.; Giaquinto, C.; Donà, D. Optimizing Antibiotic Treatment Strategies for Neonates and Children: Does Implementing Extended or Prolonged Infusion Provide any Advantage? Antibiotics 2020, 9, 329. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).