Surveillance along the Rio Grande during the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies
Abstract
:1. Introduction
2. Results
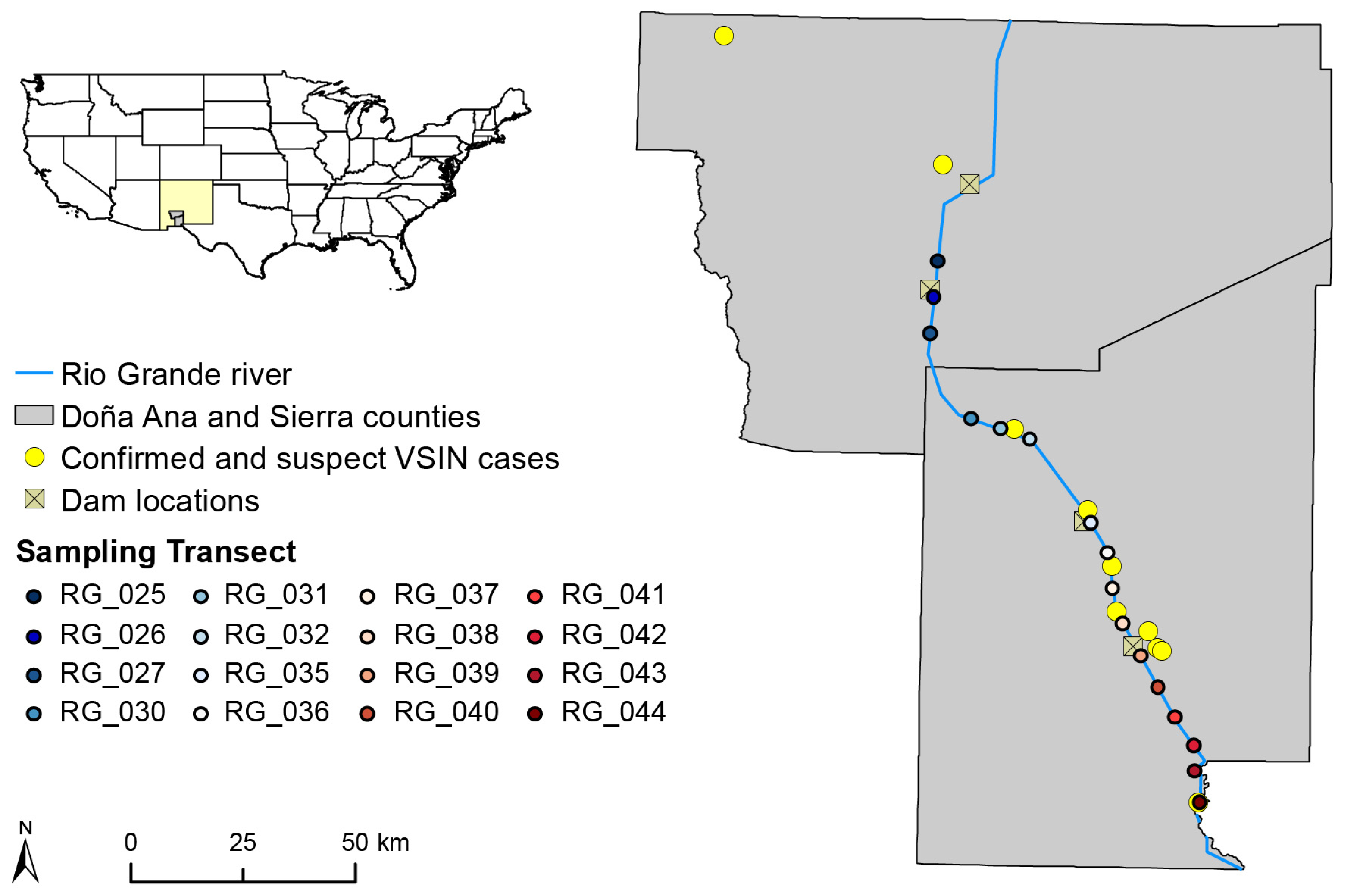
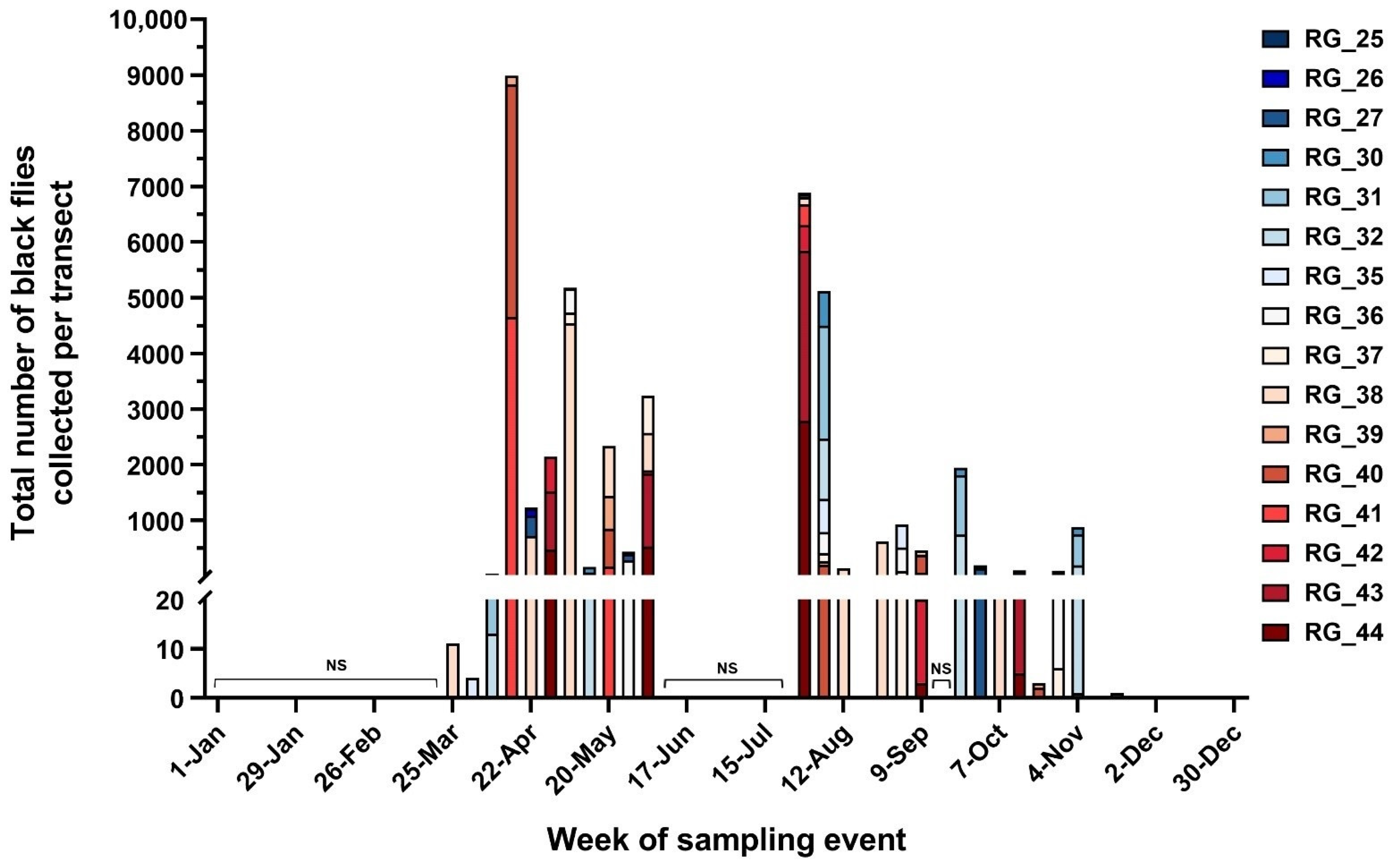
2.1. Spatiotemporal Abundance of Black Flies and Relationship to Environmental Conditions
2.2. Black Fly Identifications Using Molecular Barcoding
2.3. Detection of VSV RNA in Black Flies along the Rio Grande
3. Discussion
4. Materials and Methods
4.1. Study Location and Study Sites
4.2. Black Fly Collections
4.3. Environmental Data Collection
4.4. Black Fly Morphological Identification
4.5. DNA and RNA Isolation from Black Flies
4.6. Black Fly Molecular Barcoding
4.7. VSV Screening in Black Flies
4.8. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rodríuez, L.L. Emergence and re-emergence of vesicular stomatitis in the United States. Virus Res. 2002, 85, 211–219. [Google Scholar] [CrossRef]
- Letchworth, G.J.; Rodriguez, L.L.; Barrera, J.D.C. Vesicular stomatitis. Vet. J. 1999, 157, 239–260. [Google Scholar] [CrossRef]
- Pelzel-McCluskey, A.M. Vesicular stomatitis in large animals. In Merck Veterinary Manual, online edition; Merck & Co Inc.: Kenilworth, NJ, USA, 2020; Available online: https://www.merckvetmanual.com/generalized-conditions/vesicular-stomatitis/vesicular-stomatitis-in-large-animals (accessed on 23 September 2021).
- Alderink, F.J. Vesicular stomatitis epidemic in Colorado: Clinical observations and financial losses reported by dairymen. Prev. Veter. Med. 1984, 3, 29–44. [Google Scholar] [CrossRef]
- Goodger, W.J.; Thurmond, M.; Nehay, J.; Mitchell, J.; Smith, P. Economic impact of an epizootic of bovine vesicular stomatitis in California. J. Am. Vet. Med. Assoc. 1985, 186, 370–373. [Google Scholar] [PubMed]
- Kibler, M.L. Selected Aspects of the Economics Surrounding Equine Disease. Ph.D. Thesis, Colorado State University, Fort Collins, CO, USA, 2016. [Google Scholar]
- Hayek, A.M.; McCluskey, B.J.; Chavez, G.T.; Salman, M.D. Financial impact of the 1995 Outbreak of Vesicular Stomatitis on 16 beef ranches in Colorado. J. Am. Vet. Med. Assoc. 1998, 212, 820–823. [Google Scholar] [PubMed]
- Peters, D.P.C.; McVey, D.S.; Elias, E.H.; Pelzel-McCluskey, A.M.; Derner, J.D.; Burruss, N.D.; Schrader, T.S.; Yao, J.; Pauszek, S.J.; Lombard, J.; et al. Big data–model integration and AI for vector-borne disease prediction. Ecosphere 2020, 11, e03157. [Google Scholar] [CrossRef]
- Rodriguez, L.L.; Bunch, T.A.; Fraire, M.; Llewellyn, Z.N. Re-emergence of vesicular stomatitis in the Western United States is associated with distinct viral genetic lineages. Virology 2000, 271, 171–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velazquez-Salinas, L.; Pauszek, S.J.; Zarate, S.; Basurto-Alcantara, F.J.; Verdugo-Rodriguez, A.; Perez, A.M.; Rodriguez, L.L. Phylogeographic characteristics of vesicular stomatitis New Jersey viruses circulating in Mexico from 2005 to 2011 and their relationship to epidemics in the United States. Virology 2014, 449, 17–24. [Google Scholar] [CrossRef] [Green Version]
- Perez, A.M.; Pauszek, S.J.; Jimenez, D.; Kelley, W.N.; Whedbee, Z.; Rodriguez, L.L. Spatial and phylogenetic analysis of vesicular stomatitis virus over-wintering in the United States. Prev. Veter. Med. 2010, 93, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Rainwater-Lovett, K.; Pauszek, S.J.; Kelley, W.N.; Rodriguez, L.L. Molecular epidemiology of vesicular stomatitis New Jersey virus from the 2004–2005 US outbreak indicates a common origin with Mexican strains. J. Gen. Virol. 2007, 88, 2042–2051. [Google Scholar] [CrossRef] [PubMed]
- Rozo-Lopez, P.; Drolet, B.S.; Londoño-Renteria, B. Vesicular stomatitis virus transmission: A comparison of incriminated vectors. Insects 2018, 9, 190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elias, E.; McVey, D.S.; Peters, D.; Derner, J.; Pelzel-McCluskey, A.; Schrader, T.S.; Rodriguez, L. Contributions of hydrology to vesicular stomatitis virus emergence in the Western USA. Ecosystems 2019, 22, 416–433. [Google Scholar] [CrossRef]
- Hurd, H.S.; McCluskey, B.J.; Mumford, E.L. Management factors affecting the risk for vesicular stomatitis in livestock operations in the Western United States. J. Am. Vet. Med. Assoc. 1999, 215, 1263–1268. [Google Scholar]
- Navarro Lopez, R.; Vazquez Salinas, L.; Arellano Chavez, S.; Lopez Gonzalez, I.; Villarreal Chavez, C.L.; Montano Hirose, J.A. Epidemiological characterization of vesicular stomatitis in Mexico (1981–2012). Rev. Mex. De Cienc. Pecu. 2015, 6, 277–294. [Google Scholar] [CrossRef] [Green Version]
- Francy, D.B.; Moore, C.G.; Smith, G.C.; Jakob, W.L.; Taylor, S.A.; Calisher, C.H. Epizoötic vesicular stomatitis in Colorado, 1982: Isolation of virus from insects collected along the northern Colorado Rocky Mountain Front Range. J. Med. Entomol. 1988, 25, 343–347. [Google Scholar] [CrossRef] [Green Version]
- Schnitzlein, W.M.; Reichmann, M.E. Characterization of New Jersey vesicular stomatitis virus isolates from horses and black flies during the 1982 outbreak in Colorado. Virology 1985, 142, 426–431. [Google Scholar] [CrossRef]
- Walton, T.E.; Webb, P.A.; Kramer, W.L.; Smith, G.C.; Davis, T.; Holbrook, F.R.; Moore, C.G.; Schiefer, T.J.; Jones, R.H.; Janney, G.C. Epizootic vesicular stomatitis in Colorado, 1982: Epidemiologic and entomologic studies. Am. J. Trop. Med. Hyg. 1987, 36, 166–176. [Google Scholar] [CrossRef]
- McGregor, B.L.; Rozo-Lopez, P.; Davis, T.M.; Drolet, B.S. Detection of vesicular stomatitis virus Indiana from insects collected during the 2020 outbreak in Kansas, USA. Pathogens 2021, 10, 1126. [Google Scholar] [CrossRef]
- Drolet, B.S.; Reeves, W.K.; Bennett, K.E.; Pauszek, S.J.; Bertram, M.R.; Rodriguez, L.L. Identical viral genetic sequence found in black flies (Simulium bivittatum) and the equine index case of the 2006 U.S. vesicular stomatitis outbreak. Pathogens 2021, 10, 929. [Google Scholar] [CrossRef]
- Tesh, R.B.; Boshell, J.; Modi, G.B.; Morales, A.; Young, D.G.; Corredor, A.; Ferro de Carrasquilla, C.; de Rodriguez, C.; Walters, L.L.; Gaitan, M.O. Natural infection of humans, animals, and Phlebotomine sand flies with the Alagoas serotype of vesicular stomatitis virus in Colombia. Am. J. Trop. Med. Hyg. 1987, 36, 653–661. [Google Scholar] [CrossRef]
- Kramer, W.L.; Jones, R.H.; Holbrook, F.R.; Walton, T.E.; Calisher, C.H. Isolation of arboviruses from Culicoides midges (Diptera: Ceratopogonidae) in Colorado during an epizootic of vesicular stomatitis New Jersey. J. Med. Entomol. 1990, 27, 487–493. [Google Scholar] [CrossRef]
- Sudia, W.D.; Fields, B.N.; Calisher, C.H. The isolation of vesicular stomatitis virus (Indiana strain) and other viruses from mosquitoes in New Mexico, 1965. Am. J. Epidemiol. 1967, 86, 598–602. [Google Scholar] [CrossRef] [PubMed]
- McCreadie, J.W.; Adler, P.H.; Beard, C.E. Ecology of symbiotes of larval black flies (Diptera: Simuliidae): Distribution, diversity, and scale. Environ. Entomol. 2011, 40, 289–302. [Google Scholar] [CrossRef]
- Ciadamidaro, S.; Mancini, L.; Rivosecchi, L. Black Flies (Diptera, Simuliidae) as ecological indicators of stream ecosystem health in an urbanizing area (Rome, Italy). Ann. Dell’istituto Super. Sanita 2016, 52, 269–276. [Google Scholar]
- Adler, P.H.; Currie, D.C.; Wood, D.M. The black flies (Simuliidae) of North America; Cornell University Press: Ithaca, NY, USA, 2004; ISBN 0801424984. [Google Scholar]
- Bridges, V.E.; McCluskey, B.J.; Salman, M.D.; Hurd, H.S.; Dick, J. Review of the 1995 vesicular stomatitis outbreak in the Western United States. J. Am. Vet. Med. Assoc. 1997, 211, 556–560. [Google Scholar]
- Hanson, R.P. The natural history of vesicular stomatitis. Bacteriol. Rev. 1952, 16, 179–204. [Google Scholar] [CrossRef]
- Jenney, E.W. Vesicular stomatitis in the United States during the last five years (1963–1967). Proc. Annu. Meet. United States Anim. Health Assoc. 1967, 71, 371–385. [Google Scholar]
- Nichol, S.T.; Rowe, J.E.; Fitch, W.M. Punctuated equilibrium and positive Darwinian evolution in vesicular stomatitis virus. Proc. Natl. Acad. Sci. USA 1993, 90, 10424–10428. [Google Scholar] [CrossRef] [Green Version]
- Schmidtmann, E.T.; Craig, M.E.; English, L.M.; Herrero, M.V. Sampling for sand flies (Diptera: Psychodidae) among prairie dog colonies on ranches with histories of vesicular stomatitis in New Mexico and Colorado. J. Med. Entomol. 2002, 39, 680–684. [Google Scholar] [CrossRef] [Green Version]
- McCluskey, B.J.; Pelzel-McCluskey, A.M.; Creekmore, L.; Schiltz, J. Vesicular stomatitis outbreak in the Southwestern United States, 2012. J. Vet. Diagn. Investig. 2013, 25, 608–613. [Google Scholar] [CrossRef] [Green Version]
- Meyer, N.L.; Moulton, W.M.; Jenny, E.W.; Rodgers, R.J. Outbreaks of vesicular stomatitis in Oklahoma and Texas. Proc. US Livest. Sanit. Assoc. 1960, 64, 324–332. [Google Scholar]
- Clewley, J.P.; Bishop, D.H.; Kang, C.Y.; Coffin, J.; Schnitzlein, W.M.; Reichmann, M.E.; Shope, R.E. Oligonucleotide fingerprints of RNA species obtained from Rhabdoviruses belonging to the vesicular stomatitis virus subgroup. J. Virol. 1977, 23, 152–166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- APHIS-USDA. Vesicular Stomatitis Virus (VSV) Situation Report-June 24, 2019; APHIS-USDA: Riverdale, MD, USA, 2019.
- Peterson, B.V. The black flies of the genus Simulium, subgenus Psilopelmia (Diptera: Simuliidae), in the contiguous United States. J. N. Y. Entomol. Soc. 2009, 101, 301–390. [Google Scholar]
- APHIS-USDA. Vesicular Stomatitis Virus (VSV) Situation Report–April 13, 2020; APHIS-USDA: Riverdale, MD, USA, 2020.
- NOAA/NWS. Review of the 2020 Monsoon across the Southwest; NOAA-NWS: Phoenix, AZ, USA, 2021.
- Rivera, J.; Currie, D.C. Identification of Nearctic black flies using DNA barcodes (Diptera: Simuliidae). Mol. Ecol. Resour. 2009, 9, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Pramual, P.; Adler, P.H. DNA barcoding of tropical black flies (Diptera: Simuliidae) of Thailand. Mol. Ecol. Resour. 2014, 14, 262–271. [Google Scholar] [CrossRef]
- Conflitti, I.M.; Pruess, K.P.; Cywinska, A.; Powers, T.O.; Currie, D.C. DNA Barcoding distinguishes pest species of the black fly genus Cnephia (Diptera: Simuliidae). J. Med. Entomol. 2013, 50, 1250–1260. [Google Scholar] [CrossRef]
- Ruiz-Arrondo, I.; Hernández-Triana, L.M.; Ignjatović-Ćupina, A.; Nikolova, N.; Garza-Hernández, J.A.; Rodríguez-Pérez, M.A.; Oteo, J.A.; Fooks, A.R.; Lucientes Curdi, J. DNA Barcoding of blackflies (Diptera: Simuliidae) as a tool for species identification and detection of hidden diversity in the Eastern Regions of Spain. Parasites Vectors 2018, 11, 463. [Google Scholar] [CrossRef]
- Onder, Z.; Yildirim, A.; Duzlu, O.; Arslan, M.O.; Sari, B.; Tasci, G.T.; Ciloglu, A.; Aydin, N.P.; Inci, A.; Adler, P.H. Molecular characterization of black flies (Diptera: Simuliidae) in areas with pest outbreaks and Simuliotoxicosis in Northeast Anatolia Region, Turkey. Acta Trop. 2019, 199, 105149. [Google Scholar] [CrossRef]
- Hernández-Triana, L.M.; Chaverri, L.G.; Rodríguez-Pérez, M.A.; Prosser, S.W.J.; Hebert, P.D.N.; Gregory, T.R.; Johnson, N. DNA barcoding of Neotropical black flies (Diptera: Simuliidae): Species identification and discovery of cryptic diversity in Mesoamerica. Zootaxa 2015, 3936, 93–114. [Google Scholar] [CrossRef] [Green Version]
- Hole, K.; Clavijo, A.; Pineda, L.A. Detection and serotype-specific differentiation of vesicular stomatitis virus using a multiplex, real-time, reverse transcription-polymerase chain reaction Assay. J. Vet. Diagn. Investig. 2006, 18, 139–146. [Google Scholar] [CrossRef] [Green Version]
- Hole, K.; Velazques-Salinas, L.; Clavijo, A. Improvement and optimization of a multiplex real-time reverse transcription polymerase chain reaction assay for the detection and typing of vesicular stomatitis virus. J. Vet. Diagn. Investig. 2010, 22, 428–433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Donnell, V.K.; Pauszek, S.J.; Xu, L.; Moran, K.; Vierra, D.; Boston, T.; Dodd, K.A.; Faburay, B.; Barrette, R.W. Genome sequences of vesicular stomatitis Indiana viruses from the 2019 outbreak in the Southwest United States. Microbiol. Resour. Announc. 2020, 9, e00894-20. [Google Scholar] [CrossRef] [PubMed]
- McCluskey, B.J.; Beaty, B.J.; Salman, M.D. Climatic factors and the occurrence of vesicular stomatitis in New Mexico, United States of America. Rev. Sci. Tech. 2003, 22, 849–856. [Google Scholar] [CrossRef] [PubMed]
- Schmidtmann, E.T.; Tabachnick, W.J.; Hunt, G.J.; Thompson, L.H.; Hurd, H.S. 1995 Epizootic of vesicular stomatitis (New Jersey Serotype) in the Western United States: An entomologic perspective. J. Med. Entomol. 1999, 36, 1–7. [Google Scholar] [CrossRef]
- Jenney, E.W.; Erickson, G.A.; Snyder, M.L. Vesicular Stomatitis outbreaks and surveillance in the United States, January 1980 through May 1984. Proc. United States Anim. Health Assoc. 1984, 88, 337–345. [Google Scholar]
- Urie, N.J.; Lombard, J.E.; Marshall, K.L.; Digianantonio, R.; Pelzel-McCluskey, A.M.; McCluskey, B.J.; Traub-Dargatz, J.L.; Kopral, C.A.; Swenson, S.L.; Schiltz, J.J. Risk factors associated with clinical signs of vesicular stomatitis and seroconversion without clinical disease in Colorado horses during the 2014 outbreak. Prev. Vet. Med. 2018, 156, 28–37. [Google Scholar] [CrossRef]
- Herrero, M.V.; Yarnell, E.W. Schmidtmann, E.T. Landscape associations of the sand fly, Lutzomyia (Heleocyrtomyia) apache (Diptera: Psychodidae), in the Southwestern United States: A geographic information system analysis. J. Vector Ecol. 2004, 29, 205–211. [Google Scholar]
- Carlsson, G. Environmental factors influencing blackfly populations. Bull. World Health Organ. 1967, 37, 139–150. [Google Scholar] [PubMed]
- Zhang, Y.; Malmqvist, B.; Englund, G. Ecological processes affecting community structure of blackfly larvae in regulated and unregulated rivers: A regional study. J. Appl. Ecol. 1998, 35, 673–686. [Google Scholar] [CrossRef]
- Cilek, J.E.; Schaediger, J.F. Regional occurrence of a severe infestation of Simulium Slossonae (Diptera: Simuliidae) associated with an El Nino event in Florida. Fla. Entomol. 2004, 87, 169–172. [Google Scholar] [CrossRef]
- Grillet, M.E.; Barrera, R. Spatial and temporal abundance, substrate partitioning and species co-occurrence in a guild of Neotropical blackflies (Diptera: Simuliidae). Hydrobiologia 1997, 345, 197–208. [Google Scholar] [CrossRef]
- Martínez-de la Puente, J.; Merino, S.; Lobato, E.; Rivero-de Aguilar, J.; Del Cerro, S.; Ruiz-de-Castañeda, R.; Moreno, J. Does weather affect biting fly abundance in avian nests? J. Avian Biol. 2009, 40, 653–657. [Google Scholar] [CrossRef] [Green Version]
- Lamarre, V.; Legagneux, P.; Franke, A.; Casajus, N.; Currie, D.C.; Berteaux, D.; Bêty, J. Precipitation and ectoparasitism reduce reproductive success in an Arctic-Nesting top-predator. Sci. Rep. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Hanson, R.P.; Estupiñan, J.; Castañeda, J. vesicular stomatitis in the Americas. Bull.-Off. Int. Des. Epizoot. 1968, 70, 37–47. [Google Scholar]
- Hanson, R.P.; Brandly, C.A. Epizootiology of vesicular stomatitis. Am. J. Public Health Nations Health 1957, 47, 205–209. [Google Scholar] [CrossRef] [PubMed]
- Wilson-Ounekeo, R.C.; Lamp, W.O. Environmental and spatial predictors of the distribution patterns of the host-seeking black fly, Simulium Jenningsi (Diptera: Simuliidae). Environ. Entomol. 2021, nvab064. [Google Scholar] [CrossRef] [PubMed]
- Witter, L.A.; Johnson, C.J.; Croft, B.; Gunn, A.; Poirier, L.M. Gauging climate change effects at local scales: Weather-based indices to monitor insect harassment in Caribou. Ecol. Appl. 2012, 22, 1838–1851. [Google Scholar] [CrossRef] [PubMed]
- Hauer, F.R.; Benke, A.C. Influence of temperature and river hydrograph on black fly growth rates in a Subtropical Blackwater River. J. N. Am. Benthol. Soc. 1987, 6, 251–261. [Google Scholar] [CrossRef]
- Jitklang, S.; Sawangproh, W.; Kuvangkadilok, C.; Baimai, V.; Adler, P.H. Ecology of black flies (Diptera: Simuliidae) in streams of Northern and Southern Thailand: Factors associated with larval and pupal distributions. Acta Trop. 2020, 204, 105357. [Google Scholar] [CrossRef] [PubMed]
- Hebert, P.D.N.; Ratnasingham, S.; de Waard, J.R. Barcoding animal life: Cytochrome c oxidase subunit 1 divergences among closely related species. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270 (Suppl. 1), S96–S99. [Google Scholar] [CrossRef] [Green Version]
- Hebert, P.D.N.; Cywinska, A.; Ball, S.L.; deWaard, J.R. Biological identifications through DNA barcodes. Proc. R. Soc. London. Ser. B Biol. Sci. 2003, 270, 313–321. [Google Scholar] [CrossRef] [Green Version]
- Goldstein, P.Z.; DeSalle, R. Integrating DNA barcode data and taxonomic practice: Determination, discovery, and description. BioEssays News Rev. Mol. Cell. Dev. Biol. 2011, 33, 135–147. [Google Scholar] [CrossRef]
- Rozo-Lopez, P.; Mengual, X. Mosquito species (Diptera, Culicidae) in three ecosystems from the Colombian Andes: Identification through DNA barcoding and adult morphology. ZooKeys 2015, 513, 39–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tizard, J.; Patel, S.; Waugh, J.; Tavares, E.; Bergmann, T.; Gill, B.; Norman, J.; Christidis, L.; Scofield, P.; Haddrath, O.; et al. DNA Barcoding a unique avifauna: An important tool for evolution, systematics and conservation. BMC Evol. Biol. 2019, 19, 52. [Google Scholar] [CrossRef] [PubMed]
- Virgilio, M.; Backeljau, T.; Nevado, B.; De Meyer, M. Comparative performances of DNA barcoding across insect orders. BMC Bioinform. 2010, 11, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stallknecht, D.E.; Erickson, G.A. Antibodies to vesicular stomatitis New Jersey type virus in a population of white-tailed deer. J. Wildl. Dis. 1986, 22, 250–254. [Google Scholar] [CrossRef]
- Stallknecht, D.E.; Nettles, V.F.; Fletcher, W.O.; Erickson, G.A. Enzootic vesicular stomatitis New Jersey type in an insular feral swine population. Am. J. Epidemiol. 1985, 122, 876–883. [Google Scholar] [CrossRef]
- Stallknecht, D.E.; Fletcher, W.O.; Erickson, G.A.; Nettles, V.F. Antibodies to vesicular stomatitis New Jersey type virus in wild and domestic sentinel swine. Am. J. Epidemiol. 1987, 125, 1058–1065. [Google Scholar] [CrossRef]
- Mead, D.G.; Howerth, E.W.; Murphy, M.D.; Gray, E.W.; Noblet, R.; Stallknecht, D.E. Black fly involvement in the epidemic transmission of vesicular stomatitis New Jersey virus (Rhabdoviridae: Vesiculovirus). Vector-Borne Zoonotic Dis. 2004, 4, 351–359. [Google Scholar] [CrossRef]
- Tesh, R.B.; Peralta, P.H.; Johnson, K.M.; Tesh, R.B.; Peralta, P.H.; Johnson, K.M. Ecologic studies of vesicular stomatitis virus, I. prevalence of infection among animals and humans living in an area of endemic VSV activity. Am. J. Epidemiol. 1969, 90, 255–261. [Google Scholar] [CrossRef]
- Mead, D.G.; Ramberg, F.B.; Mare, C.J. Laboratory vector competence of black flies (Diptera: Simuliidae) for the Indiana serotype of vesicular stomatitis virus. Ann. N. Y. Acad. Sci. 2006, 916, 437–443. [Google Scholar] [CrossRef] [PubMed]
- Mead, D.G.; Gray, E.W.; Noblet, R.; Murphy, M.D.; Howerth, E.W.; Stallknecht, D.E. Biological transmission of vesicular stomatitis virus (New Jersey serotype) by Simulium vittatum (Diptera: Simuliidae) to domestic swine (Sus Scrofa). J. Med. Entomol. 2004, 41, 78–82. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mead, D.G.; Mare, C.J.; Cupp, E.W. Vector competence of select black fly species for vesicular stomatitis virus (New Jersey serotype). Am. J. Trop. Med. Hyg. 1997, 57, 42–48. [Google Scholar] [CrossRef] [PubMed]
- Rozo-Lopez, P.; Londono-Renteria, B.; Drolet, B.S. Venereal transmission of vesicular stomatitis virus by Culicoides sonorensis midges. Pathogens 2020, 9, 316. [Google Scholar] [CrossRef] [PubMed]
- Rozo-Lopez, P.; Londono-Renteria, B.; Drolet, B.S. Impacts of infectious dose, feeding behavior, and age of Culicoides sonorensis biting midges on infection dynamics of vesicular stomatitis virus. Pathogens 2021, 10, 816. [Google Scholar] [CrossRef]
- Letchworth, G.J.; Barrera, J.C.; Fishel, J.R.; Rodriguez, L. Vesicular stomatitis New Jersey virus RNA persists in cattle following convalescence. Virology 1996, 219, 480–484. [Google Scholar] [CrossRef]
- Taber, S.W. A new species of black sly (Diptera: Simuliidae) from Michigan. Southwest. Entomol. 2008, 33, 223–236. [Google Scholar] [CrossRef]
- Folmer, O.; Black, M.; Hoeh, W.; Lutz, R.; Vrijenhoek, R. DNA primers for amplification of mitochondrial cytochrome c oxidase subunit I from diverse metazoan invertebrates. Mol. Mar. Biol. Biotechnol. 1994, 3, 294–299. [Google Scholar] [CrossRef] [Green Version]
- Katoh, K.; Standley, D.M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 2013, 30, 772–780. [Google Scholar] [CrossRef] [Green Version]
- Darriba, D.; Posada, D.; Kozlov, A.M.; Stamatakis, A.; Morel, B.; Flouri, T. ModelTest-NG: A new and scalable tool for the selection of DNA and protein evolutionary models. Mol. Biol. Evol. 2020, 37, 291–294. [Google Scholar] [CrossRef] [Green Version]
- Flouri, T.; Izquierdo-Carrasco, F.; Darriba, D.; Aberer, A.J.; Nguyen, L.-T.; Minh, B.Q.; Von Haeseler, A.; Stamatakis, A. The phylogenetic likelihood library. Syst. Biol. 2015, 64, 356–362. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stamatakis, A. RAxML version 8: A tool for Phylogenetic analysis and post-analysis of large phylogenies. Bioinformatics 2014, 30, 1312–1313. [Google Scholar] [CrossRef] [PubMed]


| Species Name | State | County | Location |
|---|---|---|---|
| S. argus | AZ | Pima County | Arivaca Creek |
| S. bivittatum | AZ | Pima County | Cienaga Creek |
| S. encisoi | AZ | Pima County | Arivaca Creek |
| S. griseum | NM | Valencia County | Belen irrigation canal |
| S. mediovittatum | TX | Kinney County | Pinto Creek |
| S. meridionale | NM | Doña Ana County | Rio Grande |
| S. notatum | AZ | Yavapai County | Santa Maria River |
| S. paynei | AZ | Pima County | Arivaca Creek |
| S. robynae | TX | Brewster County | Rio Grande |
| S. trivittatum | TX | Kinney County | Pinto Creek |
| S. vittatum | AZ | Pima County | Cienaga Creek |
| S. argus | S. meridionale | S. mediovittatum | S. robynae | |||||
|---|---|---|---|---|---|---|---|---|
| To Voucher Sequence | Between Samples | To Voucher Sequence | Between Samples | To Voucher Sequence | Between Samples | To Voucher Sequence | Between Samples | |
| Max | 97.60% | 100% | 98.90% | 100% | 97.80% | 100% | 97.40% | 99.70% |
| Min | 95.90% | 96.50% | 96.30% | 95.20% | 94.30% | 98.10% | 95.60% | 95.20% |
| Avg | 96.70% | 97.80% | 97.90% | 98.50% | 95.40% | 99.40% | 96.90% | 98.10% |
| ±SD | 0.83% | 1.05% | 0.57% | 1.02% | 0.88% | 0.37% | 0.42% | 0.97% |
| March 2020 | April 2020 | May 2020 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Black Fly Species | Pools Screened 1 | rRT-PCR Pos. | Ct Range | Sequences Amplified | Pools Screened | rRT-PCR Pos. | Ct Range | Sequences Amplified | Pools Screened | rRT-PCR Pos. | Ct Range | Sequences Amplified |
| Simulium sp. | − | − | − | − | 8 | 0 | − | 0 | 13 | 2 (15.4%) | 30.1–32.5 | 0 |
| S. argus | − | − | − | − | 3 | 0 | − | 0 | − | − | − | − |
| S. mediovittatum | − | − | − | − | 5 | 3 (60%) | 30.1–30.4 | 2 (66.7%) | 8 | 3 (37.5%) | 30.1–31.6 | 2 (66.7%) |
| S. meridionale | − | − | − | − | 14 | 0 | − | 0 | 12 | 1 (8.3%) | 31.5 | 0 |
| S. notatum/ griseum | − | − | − | − | 1 | 1 (100%) | 29.82 | 1 (100%) | − | − | − | − |
| S. robynae | 1 | 0 | − | 0 | 3 | 0 | − | 0 | 9 | 1 (11.1%) | 27.0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Young, K.I.; Valdez, F.; Vaquera, C.; Campos, C.; Zhou, L.; Vessels, H.K.; Moulton, J.K.; Drolet, B.S.; Rozo-Lopez, P.; Pelzel-McCluskey, A.M.; et al. Surveillance along the Rio Grande during the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies. Pathogens 2021, 10, 1264. https://doi.org/10.3390/pathogens10101264
Young KI, Valdez F, Vaquera C, Campos C, Zhou L, Vessels HK, Moulton JK, Drolet BS, Rozo-Lopez P, Pelzel-McCluskey AM, et al. Surveillance along the Rio Grande during the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies. Pathogens. 2021; 10(10):1264. https://doi.org/10.3390/pathogens10101264
Chicago/Turabian StyleYoung, Katherine I., Federico Valdez, Christina Vaquera, Carlos Campos, Lawrence Zhou, Helen K. Vessels, J. Kevin Moulton, Barbara S. Drolet, Paula Rozo-Lopez, Angela M. Pelzel-McCluskey, and et al. 2021. "Surveillance along the Rio Grande during the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies" Pathogens 10, no. 10: 1264. https://doi.org/10.3390/pathogens10101264
APA StyleYoung, K. I., Valdez, F., Vaquera, C., Campos, C., Zhou, L., Vessels, H. K., Moulton, J. K., Drolet, B. S., Rozo-Lopez, P., Pelzel-McCluskey, A. M., Peters, D. C., Rodriguez, L. L., & Hanley, K. A. (2021). Surveillance along the Rio Grande during the 2020 Vesicular Stomatitis Outbreak Reveals Spatio-Temporal Dynamics of and Viral RNA Detection in Black Flies. Pathogens, 10(10), 1264. https://doi.org/10.3390/pathogens10101264






